Abstract
Purpose of review
During critical illness, alterations of intestinal blood supply and inflammatory activation can result in severe intestinal hypoxia (limited oxygen availability). Conditions of hypoxia lead to the activation of a transcriptional program that is under the control of the transcription factor hypoxia-inducible factor (HIF). In many instances, HIF-dependent alterations of gene expression represent endogenous adaptive responses that dampen pathologic inflammation and could be targeted to treat intestinal injury.
Recent findings
Post-translational stabilization of the HIF transcription factor and corresponding changes in gene expression are central to the resolution of intestinal injury. Examples for such responses that we discuss in this review include hypoxia-elicited increases in extracellular adenosine production and signaling, particularly through the A2B adenosine receptor, and intestinal protection provided by hypoxia-inducible netrin-1.
Summary
The present review focuses on HIF-elicited anti-inflammatory pathways that result in intestinal protection during critical illness. Many of these pathways represent novel therapeutic targets for attenuating multiorgan failure and critical illness. Whereas these therapeutic approaches are currently being investigated in cell culture models or in genetic mouse models, we are optimistic that at least some of these novel targets can be translated from bench to bedside in the near future.
Keywords: A2BAR, adenosine receptor, HIF, hypoxia-inducible factor, netrin-1, PHD, prolyl hydroxylase
INTRODUCTION
Intestinal injury significantly contributes to critical illness, sepsis and multiorgan failure [1▪,2–9]. For example, recent studies from the laboratory of Dr H. Thomas Lee, Columbia University, provide strong evidence for a functional role of intestinal activation in the pathogenesis of multiorgan failure. During ischemia and reperfusion injury of the liver or the kidneys, activation of intestinal inflammatory responses sets a spiral in motion that drives multi-organ failure. Indeed, ischemia and reperfusion to peripheral organs (such as the liver) results in activation of intestinal Paneth cells, and subsequent release of cytokines such as IL-17, causing multi-organ failure [10▪▪]. These exciting studies highlight the central role of intestinal injury in the pathogenesis of sepsis and multiorgan failure. As such, therapeutic strategies that would dampen intestinal inflammation and contribute to the maintenance of the gastrointestinal barrier function and its vascular support system would be central to the treatment of sepsis, and multiorgan failure such as acute kidney injury or acute lung injury [11–14].
During intestinal injury, several mechanisms result in hypoxia of the intestinal mucosa. This leads to the activation of a hypoxia-elicited transcriptional program that is critical for intestinal adaptation to conditions of limited oxygen availability by promoting anti-inflammatory responses and the resolution of mucosal injury. It is important to point out that hypoxia and inflammation share an interdependent relationship [15▪▪,16,17]. On one hand, conditions of hypoxia are typically associated with an inflammatory phenotype, characterized by inflammatory cell accumulation and leakage across epithelial and vascular barriers [8,18▪,19▪,20–28]. Simultaneously, inflammation itself frequently triggers profound tissue hypoxia [15▪▪]. This is due to an imbalance in oxygen demand and supply of inflammatory lesions. Inflamed tissues are characterized by significant increases in their oxygen consumption, both by inflamed resident cells such as epithelia or vascular endothelia, and also by recruited inflammatory cells, such as neutrophils or macrophages [1▪,29–33]. Simultaneously, supply with metabolites such as nutrients and oxygen is decreased due to vascular occlusion or thrombosis [5,34–38]. Together, these alterations in metabolic supply and demand result in inflammation-associated tissue hypoxia. Particularly mucosal organs such as the intestine are particularly prone to develop hypoxia-induced inflammation [39,40]. This is due to their large surface area in conjunction with their complex vascular supply system [3,41,42]. Indeed, experimental studies from the laboratory of Sean Colgan, University of Colorado, were among the first studies to demonstrate that intestinal inflammation is associated with profound tissue hypoxia. For the purpose of these studies, Dr Colgan’s research team utilized nitro-imidazole compounds that accumulate in hypoxic tissues and can be stained with antibodies [43]. Interestingly, these studies revealed that already at baseline conditions the intestinal mucosa stains with nitro-imidazole compounds, indicating some degree of hypoxia. Dr Colgan and his team refer to this observation as ‘physiologic hypoxia of the mucosal epithelium’ [15▪▪]. Indeed, this finding is not too surprising considering the fact that the intestinal lumen is anaerobic leading to an extremely steep oxygen gradient across the epithelial monolayer of the intestinal epithelium. However, nitro-imidazole staining increases profoundly in animals with experimentally induced intestinal inflammation, demonstrating that inflamed tissues become profoundly hypoxic [44].
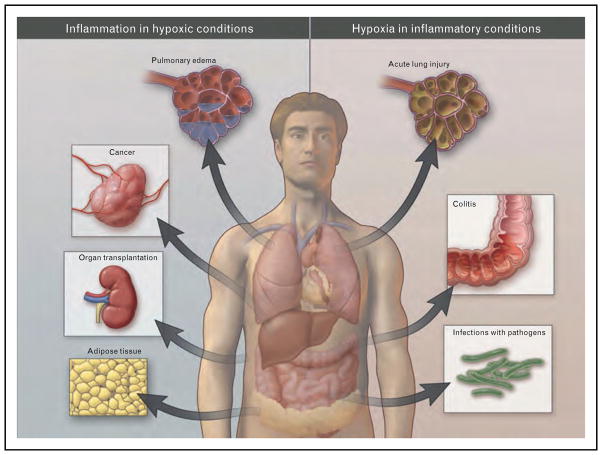
The interdependent relationship between hypoxia and inflammation plays an important role in many diseases. Examples for disease mechanisms characterized by hypoxia-induced inflammation or by inflammation causing tissue hypoxia are summarized in Fig. 1.
FIGURE 1.
Overview of clinical conditions characterized primarily by tissue hypoxia resulting in inflammatory changes (left), or inflammatory diseases that lead to tissue hypoxia (right; from the New England Journal of Medicine with permission [19▪]).
TRANSCRIPTIONAL RESPONSES TO HYPOXIA
Hypoxia as occurs during intestinal ischemia or inflammation results in the activation of a transcriptional program. This transcriptional pathway involves a key transcription factor – hypoxia-inducible factor (HIF) – which is degraded under nor-moxic conditions, and is stabilized when oxygen availability is limited [45,46]. Molecular studies demonstrated that HIF is a hetero-dimer consisting of two subunits, HIF-1α and HIF-1β. Whereas HIF-1β is stably expressed, HIF-1α is highly regulated on a post-translational level. During conditions of normal oxygen concentrations, this regulatory pathway results in immediate destruction of HIF-1α via the proteasomal pathway. In fact, hydroxylation of prolyl residues by prolyl hydroxylases (PHDs) results in degradation of HIF-1α by binding of the von Hippel Lindau gene product (pVHL) followed by ubiquitination and proteasomal destruction [47]. In addition, factor inhibiting HIF (FIH)-dependent hydroxylation of asparagyl residues prevents co-activator binding (e.g. p300) to HIF-1α, as an additional inhibitory pathway for HIF during nor-moxic conditions [48,49]. In contrast, PHDs and FIH are inactive during hypoxic conditions, allowing stabilization of the HIF hetero-dimer, and binding of HIF to the promoter region of hypoxia-regulated genes. In fact, HIF binds to a specific consensus sequence within the promoter region of hypoxia-driven genes [so called hypoxia-responsive element (HRE)], resulting in induction or repression of gene transcription [50]. Most frequently, HIF binding results in significant induction of gene transcription, for example in the case of erythropoietin, or vascular endothelial growth factor [51]. Whereas the molecular mechanism that determines if HIF functions as inducer or repressor of gene expression remains unclear, several examples have provided evidence that HIF can also mediate gene repression under hypoxia conditions, for example in the case of the peroxisome proliferator-activated receptor α [52], the adenosine kinase [53] or the equilibrative nucleoside transporter ENT1 [21] or ENT2 [54]. HIF-dependent gene regulation involves a large array of genes important in adaptation to hypoxia, including genes central for anaerobic metabolism (e.g. glycolytic enzymes) [51,55], mitochondrial functions [56], angiogenesis (e.g. vascular endothelial growth factor) [51,57], attenuation of inflammation (e.g. the anti-inflammatory guidance molecule netrin-1) [40] or erythropoiesis (e.g. erythropoietin) [58].
It is important to point out that oxygen sensing does not occur through HIFs, but through a group of oxygen-dependent enzymes that regulate the stability of the α subunit of HIF – the so called PHDs. Under well oxygenated conditions, HIFα becomes hydroxylated at one (or both) of two highly conserved prolyl residues by members of the PHD domain family (also called EgIN family) [46,59]. Hydroxylation of either of these prolyl residues generates a binding site for the pVHL tumor suppressor protein, which is a component of a ubiquitin ligase complex. As a result, HIFα is polyubiquitylated and subjected to proteasomal degradation when oxygen is available. The PHD proteins belong to the Fe(II) and 2-oxoglutarate-dependent oxygenase superfamily, whose activity is absolutely dependent on oxygen. Accordingly, the rate of HIF hydroxylation is suppressed by hypoxia. Under low oxygen conditions, or in cells lacking functional pVHL, HIFα accumulates, dimerizes with an HIFβ family member, translocates to the nucleus, and transcriptionally activates different genes, including genes involved in erythropoiesis, angiogenesis, autophagy, and energy metabolism [45–47,49,60,61]. It is important to point out that pharmacologic inhibitors of PHDs result in the nor-moxic stabilization of HIF. Indeed, such compounds have been examined in patients; for example, a recent study in patients with renal anemia demonstrated that PHD inhibitors that function to activate HIF can be used to reverse anemia. As such, the use of pharmacologic HIF activators for the treatment of critical illness is imminent.
HYPOXIA-INDUCIBLE FACTOR-DEPENDENT GENE PRODUCTS IN INTESTINAL ISCHEMIA AND INFLAMMATION
Hypoxia-inducible factor stabilization during gut ischemia or inflammation increases adenosine signaling pathways, thereby promoting the resolution of intestinal injury and inflammation.
Extracellular adenosine generation and signaling
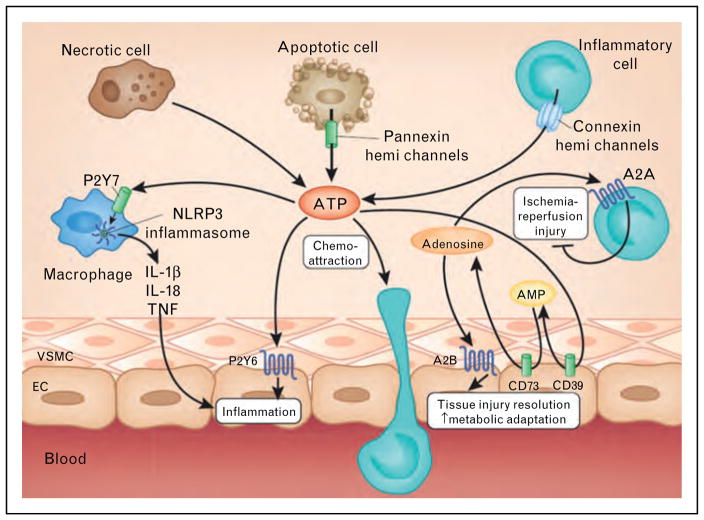
An important example for a hypoxia-elicited gut protection comes from the signaling molecule adenosine. In the extracellular compartment, adenosine mainly stems from precursor molecules such as ATP or ADP [2,22,26,31]. Whereas ATP can function as a signaling molecule itself and has been implicated as inflammatory mediator and danger signal [62,63], its enzymatic conversion to adenosine, and adenosine signaling events have been implicated in the resolution of intestinal injury and inflammation. For example, ATP conversion to adenosine occurs in a two-step process. The first step is the conversion of ATP or ADP to AMP. This process is under the enzymatic control of the ectoapyrase CD39, a hypoxia-induced enzyme that is expressed on the extracellular surface of multiple cells [64]. Mice with targeted gene deletion of cd39 experience a more severe phenotype during intestinal inflammation [8,65] or intestinal ischemia [66]. Similarly, cd39−/− mice experience more severe organ injury in other models of ischemia and inflammation, including the kidneys [67], the heart [32,68], the liver [64], the lungs [12,69], the vasculature [8,22,24,25] or the brain [70]. Together, these studies highlight that extracellular conversion ATP/ADP breakdown is hypoxia-stimulated and serves as a control mechanism to promote intestinal injury resolution (Fig. 2).
FIGURE 2.
Extracellular signaling of ATP and adenosine during ischemia or inflammation. Multiple cell types release ATP during ischemia and reperfusion (e.g. spillover from necrotic cells or controlled release through pannexin hemichannels from apoptotic cells or connexin hemichannels from activated inflammatory cells). Subsequent binding of ATP to P2 receptors enhances pathological inflammation and tissue injury, for example, through P2X7-dependent Nlrp3 inflammasome activation and P2Y6-dependent enhancement of vascular inflammation. ATP can be rapidly converted to adenosine through the ecto-apyrase CD39 (conversion of ATP to AMP) and subsequently by the ecto-5′ nucleotidase CD73 (conversion of AMP to adenosine). Adenosine signaling dampens sterile inflammation, enhances metabolic adaptation to limited oxygen availability and promotes the resolution of injury through activation of A2A adenosine receptors expressed on inflammatory cells and activation of A2B adenosine receptors expressed on tissue-resident cells (e.g. cardiac myocytes, vascular endothelia or intestinal epithelia). EC, endothelial cell; VSMC, vascular smooth muscle cell. (from Nature Medicine with permission [18▪]).
The second step of extracellular adenosine generation signaling involves the enzymatic conversion of AMP to adenosine which is catalyzed by the 5′-ectonucleodidase CD73 – a GPI-anchored enzyme that is expressed on most cell types on their extracellular surface [11,12,28,29,71–76]. Similar to CD39, previous studies had shown that CD73 is induced by hypoxia, and that hypoxia-dependent transcriptional increases of CD73 transcript, protein and function are under the control of HIF [8]. Recent studies also addressed the role of CD73 in intestinal ischemia and reperfusion injury [75]. Interestingly, pharmacological inhibition or targeted gene deletion of CD73 significantly enhanced not only local intestinal injury, but also secondary organ injury, following intestinal ischemia and reperfusion, as measured by intestinal and lung myeloperoxidase, aspartate and alanine aminotransferase, IL-1, IL-6, and histological injury. These studies also revealed that adenosine tissue levels were increased with intestinal ischemia and reperfusion injury. In contrast, cd73−/− mice had lower adenosine levels at baseline and no increase with ischemia–reperfusion injury. Again, other studies indicate that cd73−/− mice are prone to intestinal inflammation [77]. Together, these studies highlight that CD73-dependent adenosine production is under the control of hypoxia-signaling and serves as an endogenous anti-inflammatory pathway to dampen intestinal inflammation and injury.
Other studies implicate adenosine receptor signaling in gut protection from ischemia and inflammation. Extracellular adenosine can signal through four distinct adenosine receptors, the A1, A2A, A2B or the A3AR. Profiling studies of mucosal scrapings following murine ischemia and reperfusion demonstrated selective induction of the A2BAR transcript [78]. Moreover, gene-targeted mice for the A2BAR showed more profound intestinal ischemia–reperfusion injury compared with controls. In contrast, A2AAR−/− mice exhibited no differences in intestinal injury compared with littermate controls. In addition, selective inhibition of the A2BAR resulted in enhanced intestinal inflammation and injury during ischemia–reperfusion. Furthermore, A2BAR agonist treatment (BAY 60–6583) [13,20,27,78–82] protected from intestinal injury, inflammation, and permeability dysfunction in wild-type mice, whereas the therapeutic effects of BAY 60–6583 were abolished following targeted A2BAR gene deletion. Taken together, these studies demonstrate the A2BAR as a novel therapeutic target for protection during gastrointestinal ischemia and reperfusion. Other studies demonstrate that the A2BAR is induced by hypoxia, and this pathway is under the control of HIF [6,74,81]. Based on previous studies indicating an anti-inflammatory role for HIF-1-elicited enhancement of extracellular adenosine production via CD73 and signaling through the A2BAR, a recent study targeted HIF-1 during intestinal ischemia and reperfusion using pharmacological or genetic approaches [74]. Initial studies with pharmacological HIF activation indicated attenuation of intestinal injury with dimethyloxallyl glycine (DMOG – a well characterized HIF activator) treatment. Moreover, DMOG treatment was associated with induction of CD73 transcript and protein, DMOG protection was abolished in cd73−/− mice. Similarly, DMOG treatment enhanced A2BAR transcript and protein levels, whereas DMOG protection was abolished in A2BAR−/− mice. Finally, studies of mice with conditional HIF-1α deletion in intestinal epithelia or pharmacological inhibition of HIF-1 with 17-(dimethylaminoethylamino)-17-deme-thoxygeldanamycin revealed enhanced tissue injury during ischemia–reperfusion. These studies indicated a tissue-protective role of HIF-dependent enhancement of intestinal adenosine generation and signaling during intestinal ischemia–reperfusion. Moreover, these findings are also consistent with studies indicating a tissue-protective and anti-inflammatory role of HIF signaling in models of intestinal inflammation [17,44,83–85,86▪▪].
In addition to enhancing extracellular adenosine production and signaling, HIF has also been implicated in enhancing the extracellular signaling effects of adenosine by transcriptionally repressing its uptake mechanisms and its metabolism via repression of equilibrative nucleoside transporters [21,38,54,87,88] and the adenosine kinase – an intracellular enzyme responsible for adenosine conversion to AMP [53]. Taken together, these studies highlight that conditions of intestinal hypoxia –such as those which occur during gut ischemia or inflammation – are associated with the stabilization of the HIF transcription factor. In turn, HIF stabilization drives a pathway that increases the extra-cellular production and signaling effects of adenosine, thereby promoting the resolution of intestinal injury and inflammation.
Neuronal guidance molecule netrin-1 in intestinal injury
Netrin-1 is a neuronal guidance molecule that is known for its functions in brain development [89]. However, previous studies had linked netrin-1 to the coordination of inflammatory responses [39,90]. Given that mucosal surfaces are particularly prone to hypoxia-elicited inflammation, recent studies sought to determine the function of netrin-1 in hypoxia-induced inflammation of the intestine [40]. These studies revealed HIF-1-dependent induction of expression of the gene encoding netrin-1 (Ntn1) in hypoxic epithelia. Neutrophil transepithelial migration studies showed that by engaging A2B adenosine receptor A2BAR on neutrophils, netrin-1 attenuated neutrophil transmigration. Exogenous netrin-1 suppressed hypoxia-elicited inflammation of the intestine in wild-type but not in A2BAR-deficient mice, and inflammatory hypoxia was enhanced in Ntn1+/− mice relative to that in Ntn1+/+ mice. Together these studies demonstrate that HIF-1-dependent induction of netrin-1 attenuates hypoxia-elicited inflammation at mucosal surfaces [40]. Other studies also provide evidence for a role of netrin-1 signaling in attenuating intestinal inflammation [39]. Interestingly, both studies indicate that this protection involves signaling events through the A2BAR; at present it is not clear how netrin-1 can function through the A2BAR. Whereas direct binding of netrin-1 to the adenosine binding site of the A2BAR and concomitant signaling appears more unlikely, ongoing studies indicate that this could potentially involve an allosteric enhancement of A2BAR signaling effects.
CONCLUSION
Experimental studies utilizing murine models of intestinal ischemia and inflammation in combination with cell culture studies provide strong evidence that intestinal injury is associated with profound hypoxia of the apical aspects of the mucosa [1▪,3,5,15▪▪,17,18▪,19▪,31,35–37]. Intestinal hypoxia is associated with the post-translational stabilization of the transcription factor HIF. Indeed, multiple studies demonstrate a protective role of HIF stabilization in models of intestinal inflammation or injury [1▪,4–9,17,44,54,74,83–85,86▪▪]. The HIF-elicited protection involves the transcriptional induction of many gene products [10▪▪]. Recent studies have identified extracellular adenosine production and signaling as a pathway that is under the direct control of HIF, and provides potent protection from intestinal injury [8,11,12,22,24,25,28,29, 32,64,67–69,71–76,91–94]. Similarly, signaling events through the neuronal guidance molecule netrin-1 have been shown to be transcriptionally induced by HIF, and are associated with mucosal protection from hypoxia-induced inflammation and pathologic intestinal inflammation. These experimental studies highlight that therapeutic strategies including HIF activators, pharmacologic means of enhancing extracellular adenosine production or signaling (particularly through the A2BAR) could be considered for the treatment of patients suffering from critical illness in order to promote the resolution of gut injury and to dampen pathologic intestinal inflammation [3,6,13,20,24,25, 27,30,35,37,39,71,74,78–82,92,94–96]. Presently, these therapeutic inventions have been examined in cell culture models, or in murine models of intestinal injury. We are hopeful, that at least some of these therapeutic targets can be translated from bench to bedside, and will eventually be introduced into the treatment of patients suffering from critical illness.
KEY POINTS.
Intestinal injury plays a key role in the pathogenesis of sepsis and multiorgan failure. Recent studies indicate that this could involve activation of intestinal Paneth cells and subsequent cytokine release.
During intestinal injury (ischemia, inflammation), the intestinal mucosa becomes profoundly hypoxic resulting in the post-translational stabilization of the hypoxia-adaptive transcription factor HIF.
HIF stabilization is associated with a transcriptional program that enhances hypoxia tolerance, attenuates intestinal inflammation and promotes the resolution of injury.
Pharmacologic strategies to achieve HIF stabilization (e.g. PHD inhibitor) are protective during intestinal inflammation or ischemia and reperfusion.
HIF-target genes that have been implicated in intestinal protection include the extracellular production of adenosine and its signaling events through the A2BAR. These resemble novel pharmacologic targets for the prevention or treatment of intestinal injury during critical illness.
The evidence for these findings discussed in this review comes mostly from studies in tissue culture models, or murine models of intestinal injury. It will be critical to translate these experimental findings from bench to bedside for the treatment of critical illness.
Footnotes
Conflicts of interest
The present review is supported by U.S. National Institutes of Health grant R01-HL0921, R01-DK083385 and R01HL098294 to HKE and a grant by the Crohn’s and Colitis Foundation of America (CCFA) to H.K.E., and grants by the Juvenile Diabetes Foundation (JDF) and the American Heart Association (AHA) to Almut Grenz. None of the authors has a conflict of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 218).
- 1▪.Colgan SP, Eltzschig HK. Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol. 2012;74:7.1–7.23. doi: 10.1146/annurev-physiol-020911-153230. Comprehensive review that outlines the relationship between hypoxia signaling and extracellular adenosine generation and signaling during intestinal injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111:904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Rivera-Nieves J, Colgan SP. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin Ther Targets. 2009;13:1267–1277. doi: 10.1517/14728220903241666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann H, Eltzschig HK, Wurz H, et al. Hypoxia-independent activation of HIF-1 by enterobacteriaceae and their siderophores. Gastroenterology. 2008;134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr Opin Anaesthesiol. 2011;24:363–369. doi: 10.1097/ACO.0b013e32834873fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kong T, Westerman KA, Faigle M, et al. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006;20:2242–2250. doi: 10.1096/fj.06-6419com. [DOI] [PubMed] [Google Scholar]
- 7.Kuhlicke J, Frick JS, Morote-Garcia JC, et al. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE. 2007;2:e1364. doi: 10.1371/journal.pone.0001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng W, Kuhlicke J, Jackel K, et al. Hypoxia inducible factor-1 (HIF-1)-mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium. FASEB J. 2009;23:204–213. doi: 10.1096/fj.08-110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪▪.Park SW, Kim M, Brown KM, et al. Paneth cell-derived IL-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011;53:1662–1675. doi: 10.1002/hep.24253. This is the first study demonstrating that intestinal Paneth cells can be activated during ischemia and reperfusion of peripheral organs, and provides novel mechanistic insight on the central role of intestinal injury in multiorgan failure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckle T, Fullbier L, Grenz A, et al. Usefulness of pressure-controlled ventilation at high inspiratory pressures to induce acute lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2008;295:L718–L724. doi: 10.1152/ajplung.90298.2008. [DOI] [PubMed] [Google Scholar]
- 12.Eckle T, Fullbier L, Wehrmann M, et al. Identification of ectonucleotidases CD39 and CD73 in innate protection during acute lung injury. J Immunol. 2007;178:8127–8137. doi: 10.4049/jimmunol.178.12.8127. [DOI] [PubMed] [Google Scholar]
- 13.Eckle T, Grenz A, Laucher S, et al. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- 15▪▪.Colgan SP, Taylor CT. Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol. 2010;7:281–287. doi: 10.1038/nrgastro.2010.39. Very elegant review highlighting the functional role of HIF and pharmacologic HIF activators during intestinal inflammation as occurs during inflammatory bowel disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586:4055–4059. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor CT, Colgan SP. Hypoxia and gastrointestinal disease. J Mol Med. 2007;85:1295–1300. doi: 10.1007/s00109-007-0277-z. [DOI] [PubMed] [Google Scholar]
- 18▪.Eltzschig HK, Eckle T. Ischemia and reperfusion: from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. Current update on molecular mechanisms implicated in ischemia and reperfusion injury, and how these findings can be translated from bench to bedside. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. Current update on the relationship between hypoxia and inflammation, and several examples how this relationship plays out in a clinical setting, including ischemia and reperfusion, acute lung injury, infections with pathogens and cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckle T, Faigle M, Grenz A, et al. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eltzschig HK, Abdulla P, Hoffman E, et al. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99:1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Faigle M, Knapp S, et al. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108:1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eltzschig HK, Ibla JC, Furuta GT, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eltzschig HK, Thompson LF, Karhausen J, et al. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: coordination by extracellular nucleotide metabolism. Blood. 2004;104:3986–3992. doi: 10.1182/blood-2004-06-2066. [DOI] [PubMed] [Google Scholar]
- 26.Faigle M, Seessle J, Zug S, et al. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE. 2008;3:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schingnitz U, Hartmann K, Macmanus CF, et al. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184:5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thompson LF, Eltzschig HK, Ibla JC, et al. Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med. 2004;200:1395–1405. doi: 10.1084/jem.20040915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colgan SP, Eltzschig HK, Eckle T, et al. Physiological roles of 5′-ectonu-cleotidase (CD73) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frick JS, MacManus CF, Scully M, et al. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. J Immunol. 2009;182:4957–4964. doi: 10.4049/jimmunol.0801324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Macmanus CF, Colgan SP. Neutrophils as sources of extra-cellular nucleotides: functional consequences at the vascular interface. Trends Cardiovasc Med. 2008;18:103–107. doi: 10.1016/j.tcm.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eltzschig HK, Kohler D, Eckle T, et al. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113:224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 34.Hart ML, Kohler D, Eckle T, et al. Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 2008;28:1477–1483. doi: 10.1161/ATVBAHA.108.169219. [DOI] [PubMed] [Google Scholar]
- 35.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. 2011;1808:1329–1339. doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bauerle JD, Grenz A, Kim JH, et al. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol. 2011;22:14–20. doi: 10.1681/ASN.2009121217. [DOI] [PubMed] [Google Scholar]
- 37.Koeppen M, Eckle T, Eltzschig HK. Interplay of hypoxia and A2B adenosine receptors in tissue protection. Adv Pharmacol. 2011;61:145–186. doi: 10.1016/B978-0-12-385526-8.00006-0. [DOI] [PubMed] [Google Scholar]
- 38.Grenz A, Homann D, Eltzschig HK. Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal. 2011;15:2221–2234. doi: 10.1089/ars.2010.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aherne CM, Collins CB, Masterson JC, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2011 doi: 10.1136/gutjnl-2011-300012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberger P, Schwab JM, Mirakaj V, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 41.Collins CB, Aherne CM, Kominsky D, et al. Retinoic acid attenuates ileitis by restoring the balance between T-helper 17 and T regulatory cells. Gastro-enterology. 2011;141:1821–1831. doi: 10.1053/j.gastro.2011.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins CB, Aherne CM, McNamee EN, et al. Flt3 ligand expands CD103+ dendritic cells and FoxP3+ T regulatory cells, and attenuates Crohn’s-like murine ileitis. Gut. 2011 doi: 10.1136/gutjnl-2011-300820. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karhausen J, Haase VH, Colgan SP. Inflammatory hypoxia: role of hypoxia-inducible factor. Cell Cycle. 2005:4. [PubMed] [Google Scholar]
- 44.Karhausen J, Furuta GT, Tomaszewski JE, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 46.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 47.Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol. 2007;2:145–173. doi: 10.1146/annurev.pathol.2.010506.092049. [DOI] [PubMed] [Google Scholar]
- 48.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15:2675–2686. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraisl P, Aragones J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 50.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 51.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 52.Narravula S, Colgan SP. Hypoxia-inducible factor 1-mediated inhibition of peroxisome proliferator-activated receptor alpha expression during hypoxia. J Immunol. 2001;166:7543–7548. doi: 10.4049/jimmunol.166.12.7543. [DOI] [PubMed] [Google Scholar]
- 53.Morote-Garcia JC, Rosenberger P, Kuhlicke J, et al. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 54.Morote-Garcia JC, Rosenberger P, Nivillac NM, et al. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 55.Semenza GL, Roth PH, Fang HM, et al. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757–23763. [PubMed] [Google Scholar]
- 56.Fukuda R, Zhang H, Kim JW, et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 57.Carmeliet P, Baes M. Metabolism and therapeutic angiogenesis. N Engl J Med. 2008;358:2511–2512. doi: 10.1056/NEJMcibr0802500. [DOI] [PubMed] [Google Scholar]
- 58.Wang G, Jiang B, Rue E, et al. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. PNAS. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schofield CJ, Ratcliffe PJ. Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:343–354. doi: 10.1038/nrm1366. [DOI] [PubMed] [Google Scholar]
- 60.Aragones J, Fraisl P, Baes M, et al. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11–22. doi: 10.1016/j.cmet.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 62.Riegel AK, Faigle M, Zug S, et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood. 2011;117:2548–2555. doi: 10.1182/blood-2010-10-313957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 64.Hart ML, Gorzolla IC, Schittenhelm J, et al. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J Immunol. 2010;184:4017–4024. doi: 10.4049/jimmunol.0901851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Friedman DJ, Kunzli BM, ARYI, et al. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A. 2009;106:16788–16793. doi: 10.1073/pnas.0902869106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guckelberger O, Sun XF, Sevigny J, et al. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb Haemost. 2004;91:576–586. doi: 10.1160/TH03-06-0373. [DOI] [PubMed] [Google Scholar]
- 67.Grenz A, Zhang H, Hermes M, et al. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- 68.Kohler D, Eckle T, Faigle M, et al. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation. 2007;116:1784–1794. doi: 10.1161/CIRCULATIONAHA.107.690180. [DOI] [PubMed] [Google Scholar]
- 69.Reutershan J, Vollmer I, Stark S, et al. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB J. 2009;23:473–482. doi: 10.1096/fj.08-119701. [DOI] [PubMed] [Google Scholar]
- 70.Pinsky DJ, Broekman MJ, Peschon JJ, et al. Elucidation of the thromboregulatory role of CD39/ectoapyrase in the ischemic brain. J Clin Invest. 2002;109:1031–1040. doi: 10.1172/JCI10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckle T, Krahn T, Grenz A, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 72.Grenz A, Zhang H, Eckle T, et al. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- 73.Haeberle HA, Durrstein C, Rosenberger P, et al. Oxygen-independent stabilization of hypoxia inducible factor (HIF)-1 during RSV infection. PLoS ONE. 2008;3:e3352. doi: 10.1371/journal.pone.0003352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart ML, Grenz A, Gorzolla IC, et al. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5′-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 75.Hart ML, Henn M, Kohler D, et al. Role of extracellular nucleotide phosphohydrolysis in intestinal ischemia-reperfusion injury. FASEB J. 2008;22:2784–2797. doi: 10.1096/fj.07-103911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hart ML, Much C, Gorzolla IC, et al. Extracellular adenosine production by ecto-5′-nucleotidase protects during murine hepatic ischemic preconditioning. Gastroenterology. 2008;135:1739–1750. e3. doi: 10.1053/j.gastro.2008.07.064. [DOI] [PubMed] [Google Scholar]
- 77.Louis NA, Robinson AM, MacManus CF, et al. Control of IFN-alphaA by CD73: implications for mucosal inflammation. J Immunol. 2008;180:4246–4255. doi: 10.4049/jimmunol.180.6.4246. [DOI] [PubMed] [Google Scholar]
- 78.Hart ML, Jacobi B, Schittenhelm J, et al. Cutting Edge: A2B Adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182:3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 79.Chen H, Yang D, Carroll SH, et al. Activation of the macrophage A2b adenosine receptor regulates tumor necrosis factor-alpha levels following vascular injury. Exp Hematol. 2009;37:533–538. doi: 10.1016/j.exphem.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Grenz A, Osswald H, Eckle T, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eckle T, Kohler D, Lehmann R, et al. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 82.Zhang Y, Dai Y, Wen J, et al. Detrimental effects of adenosine signaling in sickle cell disease. Nat Med. 2011;17:79–86. doi: 10.1038/nm.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cummins EP, Seeballuck F, Keely SJ, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 84.Keely S, Glover LE, Macmanus CF, et al. Selective induction of integrin {beta}1 by hypoxia-inducible factor: implications for wound healing. FASEB J. 2009;23:1338–1346. doi: 10.1096/fj.08-125344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robinson A, Keely S, Karhausen J, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86▪▪.Tambuwala MM, Cummins EP, Lenihan CR, et al. Loss of prolyl hydroxylase-1 protects against colitis through reduced epithelial cell apoptosis and increased barrier function. Gastroenterology. 2010;139:2093–2101. doi: 10.1053/j.gastro.2010.06.068. Very important study showing for the first time a functional role of prolyl hydroxylase PHD1 in intestinal inflammation, thereby providing mechanistic insight into how HIF activating PHD inhibitors protect from intestinal inflammation. [DOI] [PubMed] [Google Scholar]
- 87.Loffler M, Morote-Garcia JC, Eltzschig SA, et al. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27:1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 88.Grenz A, Bauerle JD, Dalton JH, et al. Equilibrative nucleoside transporter ENT1 regulates postischemic blood-flow during acute kidney injury in mice. J Clin Invest. 2012 doi: 10.1172/JCI60214. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Serafini T, Colamarino SA, Leonardo ED, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 90.Grenz A, Dalton JH, Bauerle JD, et al. Partial netrin-1 deficiency aggravates acute kidney injury. PLoS One. 2011;6:e14812. doi: 10.1371/journal.pone.0014812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eltzschig HK, Weissmuller T, Mager A, et al. Nucleotide metabolism and cell-cell interactions. Methods Mol Biol. 2006;341:73–87. doi: 10.1385/1-59745-113-4:73. [DOI] [PubMed] [Google Scholar]
- 92.Grenz A, Zhang H, Weingart J, et al. Lack of effect of extracellular adenosine generation and signalling on renal erythropoietin secretion during hypoxia. Am J Physiol Renal Physiol. 2007:00243. doi: 10.1152/ajprenal.00243.2007. 2007. [DOI] [PubMed] [Google Scholar]
- 93.Tapper E, Schmelzle M, Eltzschig HK, et al. Erratum to: role of CD73 and extracellular adenosine in disease. Purinergic Signal. 2011 doi: 10.1007/s11302-011-9265-4. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wen J, Grenz A, Zhang Y, et al. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J. 2011;25:2823–2830. doi: 10.1096/fj.11-181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Csoka B, Nemeth ZH, Rosenberger P, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. 2010;185:542–550. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Koeppen M, Eckle T, Eltzschig HK. Selective deletion of the A1 adenosine receptor abolishes heart-rate slowing effects of intravascular adenosine in vivo. PLoS One. 2009;4:e6784. doi: 10.1371/journal.pone.0006784. [DOI] [PMC free article] [PubMed] [Google Scholar]