Abstract
A significant inter‐arm difference in systolic blood pressure (IADSBP) has recently been associated with worse cardiovascular outcomes. The authors hypothesized that part of this association is mediated by arterial stiffness, and examined the relationship between significant IADSBP and carotid‐femoral pulse wave velocity (CF‐PWV) in a sample from the Baltimore Longitudinal Study of Aging. Of 1045 participants, 50 (4.8%) had an IADSBP ≥10 mm Hg at baseline, and 629 had completed data from ≥2 visits (for a total of 1704 visits during 8 years). CF‐PWV was significantly higher in patients with an IADSBP ≥10 mm Hg (7.3±1.9 vs 8.2±2, P=.002). Compared with others, patients with IADSBP ≥10 mm Hg also had higher body mass index, waist circumference, and triglycerides; higher prevalence of diabetes; and lower high‐density lipoprotein (HDL) cholesterol (P<.001 for all). A significant association with IADSBP ≥10 mm Hg was observed for CF‐PWV in both cross‐sectional (odds ratio [OR], 1.19; 95% confidence interval [CI], 1.06–1.87; P=.01) and longitudinal (OR, 1.15; 95% CI, 1.03–1.29; P=.01) multivariate analyses. Female sex, Caucasian race, high body mass index (plus diabetes and low HDL cholesterol only cross‐sectionally) were other independent correlates of IADSBP ≥10 mm Hg. Significant IADSBP is associated with increased arterial stiffness in community‐dwelling older adults.
Hypertension guidelines have recommended routine bilateral blood pressure (BP) measurement for many years,1 but the justification has been poor and adoption in primary care practice negligible to date.2 Yet, an inter‐arm difference in systolic BP (IADSBP) is frequently encountered in clinical practice and may suggest the presence of a hidden congenital heart disease, aortic dissection, or atherosclerotic disease involving the supra‐aortic arteries.3 A stenosis of the supra‐aortic arteries has been associated with significant IADSBP in patients undergoing invasive angiography for cardiovascular procedures.4, 5, 6 Thus, an IADSBP ≥10 mm Hg has been recently proposed as a sign of potential supra‐aortic vascular disease, although a cutoff of ≥15 mm Hg is more specific for significant arterial stenosis with slightly lower sensitivity.4 Furthermore, several studies and meta‐analysis mostly involving individuals with high cardiovascular risk profile recently reported significant independent associations between an IADSBP ≥10 mm Hg and the presence of peripheral vascular disease, pre‐existing coronary artery disease7, 8 and increased cardiovascular morbidity and mortality.9, 10, 11 Reasons for these associations are mainly unknown, and may lie beyond the ability of the IADSBP to detect a stenotic artery.12, 13 Indeed, no study has ever confirmed the presence of a stenosis of supra‐aortic arteries among unselected patients with significant IADSBP and without overt atherosclerotic disease.
Carotid‐femoral pulse wave velocity (CF‐PWV) is considered the gold‐standard measurement of arterial stiffness,14 and several studies have demonstrated its strong ability to predict cardiovascular morbidity and mortality in the general population.15, 16 To gain insights into the association between IADSBP and adverse cardiovascular outcomes, we explored the hypothesis that IADSBP is cross‐sectionally and longitudinally associated with CF‐PWV. If this hypothesis is correct, we may hypothesize that part of the association between IADSBP and cardiovascular risk is mediated by arterial stiffness.
Methods
Selection of Study Population
The study sample for the present analysis was drawn from the Baltimore Longitudinal Study of Aging, an ongoing prospective study of normative aging in community‐dwelling volunteers who undergo approximately 3 days of medical, physiological, and psychological examinations at regular intervals.17 Participants are enrolled if they are healthy at baseline (eg, no evidence of diabetes, stroke, or heart disease), but remain in the study if disease develops. For the present analysis, we included 1045 participants with both sequential and simultaneous BP measurements and CF‐PWV available at the same study visit. Of these 1045 participants, only those with at least two prospective evaluations of simultaneous BP measurements were retained for longitudinal analysis. Participants lost at follow‐up (n=416), compared with those who were available, were younger, less likely to be African American and to have hypertension, but more likely to be obese. The remaining 629 participants had completed data from up to 8 visits (mean visits per participant=2.7, standard deviation [SD]=1, range 2–8), for a total of 1704 visits during 8 years. The mean interval between visits was 4.1 years (SD=1.6, range 1–8 years). All clinical variables used in the present analysis were routinely collected at each visit as per study protocol. The study protocol was approved by the Intramural Research Program of the National Institute on Aging and the institutional review board of the MedStar Health Research Institute (Baltimore, MD). All participants provided informed participation consent at each visit.
BP Measurements
Sequential Measurements
Brachial BP was measured by trained nurse practitioners according to a highly standardized protocol using two standard aneroid sphygmomanometers with appropriately sized cuffs. One sphygmomanometer was placed at each arm and, after at least 5 minutes of rest in the supine position, 3 BP measurements from each arm were taken, alternating right then left, and allowing 1 minute between each measurement on the same arm.
Simultaneous Measurements
Simultaneous bilateral brachial BP measurements were performed as part of the routine diagnostic procedure to assess bilateral ankle‐brachial index (ABI). After 10 minutes of rest in the supine position, 3 BP measurements were taken simultaneously and automatically using a validated automatic oscillometric device (Colin VP‐2000; Colin Medical Technology, Komaki, Japan). The ABI was calculated as the average of the 3 resting ankle systolic pressure readings divided by the average of the 3 resting brachial systolic pressure readings.
For both sequential and simultaneous measures of BP, values of systolic BP differing >25 mm Hg from the lowest measure at the same arm were removed from the analysis.18 Thirty‐one patients with 38 sequential recordings and 7 patients with 10 simultaneous recordings meeting these prespecified criteria were excluded from the original population of 1083 participants. For the purpose of this analysis, the IADSBP was calculated as the difference in systolic BP between the right and the left arm at each reading (first, second, and third) for both sequential and simultaneous BP measurements.
Definition and Prevalence of Significant IADSBP
In preliminary exploratory analysis, we confirmed that the prevalence of an IADSBP ≥10 mm Hg decreased, moving from the first to the third reading (Figure1). The decrease in prevalence was much more significant for sequential (from 18.2% to 11.4%; 6.8% decrease) compared with simultaneous measurements (from 8% to 6.1%; 1.9% decrease), and the prevalence of significant IADSBP was generally lower with simultaneous than sequential measurements (Figure), as previously reported.19 Bland‐Altman plots did not show any tendencies for the IADSBP at each reading to vary as a function of the mean systolic BP, although a wider spread of IADSBP values was generally observed for sequential as compared with simultaneous measurements and at the first as compared with the third set of readings (Figure S1). The first set of readings was then discarded, and for both the sequential and the simultaneous method, IADSBP was considered ≥10 mm Hg only if found by both the second and the third reading, to prevent overestimation and improve consistency. This resulted in a final prevalence of IADSBP ≥10 mm Hg of 3.2% with the sequential method and of 4.8% with the simultaneous method (50 of 1045 participants, 24 with right>left, 26 with left>right). For an IADSBP ≥15 mm Hg, the final prevalence rates were 0.5% and 1.1%, respectively. Given the results of our exploratory analysis and because it has been shown that simultaneous BP measurements are more reliable and reproducible than sequential ones,19 subsequent analyses were performed by considering as “cases” those participants with an IADSBP ≥10 mm Hg at both the second and the third simultaneous readings. The distribution of the mean IADSBP in the whole cross‐sectional sample is presented in Figure S2.
Figure 1.
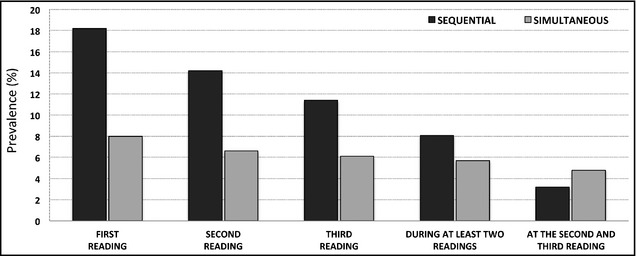
Prevalence of inter‐arm difference in systolic blood pressure (IADSBP) ≥10 mm Hg in the cross‐sectional study sample. The chart shows the prevalence of IADSBP ≥10 mm Hg at the first, second, and third blood pressure reading, separately for the sequential (black columns) and simultaneous (grey columns) method. The prevalence of IADSBP ≥10 mm Hg decreased moving from the first to the third sets of readings for both methods and when using combinations of these readings.
Between‐visit variations of IADSBP in the same patient were previously shown to be large,18, 20 and this finding was confirmed in our preliminary analysis. Of 1704 total longitudinal observations in 629 participants (Figure S3A), only 56 participants had an IADSBP ≥10 mm Hg. Of these 56 participants over 200 longitudinal observations (Figure S3B), 51 had an IADSBP ≥10 mm Hg at a single visit sometimes during the course of the study. Only 5 participants had an IADSBP ≥10 mm Hg at more than one visit (3 participants with right>left at 2 consecutive visits, 1 participant with left>right at 2 consecutive visits, and 1 participant with left>right at 2 separate visits [Figure S3B]).
Carotid‐Femoral Pulse Wave Velocity
Carotid‐femoral pulse wave velocity was measured after the patient had rested in the supine position in a quiet room for at least 10 minutes. Patients abstained from food and drinking coffee or other caffeine‐containing beverages for at least 45 minutes before the test. CF‐PWV was calculated as the distance traveled by the pulse wave divided by the time difference between the feet of carotid and femoral arterial waveforms gated to electrocardiography.14 Over the long follow‐up period, two devices were used to record CF‐PWV (2003–2010: Complior SP device; Artech Medical, Paris, France; starting 2011: SphygmoCor system, AtCor Medical, Sydney, New South Wales, Australia); therefore, CF‐PWV measures were standardized as previously described.21
Clinical Variables
To calculate clinical systolic and diastolic BP, the first set of readings was discarded and the second and third readings at both arms were averaged. This mean BP values were used to calculate the steady and pulsatile components of BP (expressed as mean BP=2/3 diastolic BP+1/3 systolic BP, and pulse pressure=systolic BP−diastolic BP, respectively) and to estimate the prevalence of hypertension. The latter was defined as the presence of a BP ≥140/90 mm Hg and/or the use of antihypertensive medications. Heart rate was recorded at the same time of BP measurement by the nurse practitioner. Body mass index (BMI) was calculated as weight divided by squared height (kg/m2) and a BMI ≥30 was used as an index of general obesity. Waist circumference, defined as the minimal abdominal circumference between the lower edge of the rib cage and the iliac crests, was measured according to a highly standardized procedure, and Adult Treatment Panel III (ATP III) criteria were used to estimate the prevalence of central obesity (waist circumference >102 cm in men and >88 cm in women).22 Fasting serum samples were drawn to assay plasma glucose, triglycerides, total cholesterol, and high‐density lipoprotein (HDL) cholesterol, while low‐density lipoprotein cholesterol concentrations were estimated using the Friedewald formula. Diabetes mellitus was defined by the American Diabetes Association criteria and/or the use of diabetes medications.23 Dyslipidemia was defined as total cholesterol ≥200 mg/dL and/or by use of lipid‐lowering treatment. Metabolic syndrome was diagnosed according to ATP III criteria.22 Estimated glomerular filtration rate, calculated by the simplified modification of diet in renal disease formula, was used to determine renal function, and renal failure was defined as glomerular filtration rate <60 mL/min/1.73 m2. Smoking status was ascertained by a questionnaire and participants were classified as ever smoker vs never smoker (<100 cigarettes in lifetime). Cardiovascular diseases were ascertained at each visit using a standardized questionnaire. Coronary artery disease was defined as a history of myocardial infarction, heart failure, current angina associated with a positive stress test, or previous cardiac procedures (including surgical or percutaneous cardiac revascularization). Peripheral arterial disease was determined by the presence of intermittent claudication and/or an ABI ≤0.9 in either leg.
Statistical Analysis
Continuous data are presented as mean±standard deviation and categorical data as percentages. Comparisons between participants with and without an IADSBP ≥10 mm Hg were performed using Student t test or the chi‐square test as appropriate. For cross‐sectional analyses, multivariate logistic regression models were used to investigate the relationship between CF‐PWV and an IADSBP ≥10 mm Hg after adjusting for variables that in univariate analysis were associated with an IADSBP ≥10 mm Hg (heart rate, BMI, diabetes, triglycerides, HDL) or known to potentially affect both IADSBP and CF‐PWV (age, sex, race, smoking, antihypertensive drugs, systolic BP). Collinearity was assessed calculating the variance inflation factor, which was considered acceptable when ≤2. Backward selection was used to select variables significantly associated with the outcome. The longitudinal relationship between PWV and an IADSBP ≥10 mm Hg was examined using generalized estimating equations with a standard (marginal) model,24 which consider the within‐person correlation when examining multiple observations per patient, do not require a balanced design and can be used for dichotomous outcomes such as the presence or absence of an IADSBP ≥10 mm Hg. A binary distribution, logit link, and exchangeable correlation matrix was used and odds ratios were calculated. Analyses were adjusted for the time‐varying values of age and other covariates included in previous cross‐sectional analysis. As the interaction between CF‐PWV and age was found to be not significant at either the P<.05 or P<.10 level, this coefficient was not included in the final model. A reduced model was obtained after removing variables that did not retain statistical significance. All analyses were performed using the SAS package (version 9.3; SAS Institute Inc, Cary, NC). Statistical significance was set at P<.05.
Results
Study Population
Descriptive characteristics of the cross‐sectional and longitudinal samples are shown in Table 1. Compared with the cross‐sectional sample, individuals retained for longitudinal analysis did not differ significantly at baseline, except for the slightly older age and higher prevalence of hypertension and renal failure. In the cross‐sectional sample, participants' mean age was 66 years, 51% were men, and 24% were African Americans. Mean BMI was 27±5 kg/m2, and the prevalence of obesity was 23%. About 20% of participants were diagnosed with diabetes, 47% with treated hypertension, and 74% with dyslipidemia, although mean systolic (118±15 mm Hg) and diastolic (66±9 mm Hg) BP values were within normal ranges and only 8% of the participants had BP values ≥140/90 mm Hg and 38% total cholesterol values ≥200mg/dL (Table 1).
Table 1.
Characteristics of Study Population for Cross‐Sectional and Longitudinal Analyses
| Cross‐Sectional Sample (n=1045) | IADSBP <10 mm Hg (n=995) | IADSBP ≥10 mm Hg (n=50) | P Value | Longitudinal Sample (n=629) | |
|---|---|---|---|---|---|
| Age, y | 66±13 | 66±13 | 64±14 | .27 | 68±12 |
| Follow‐up, y | – | – | – | – | 4.1±1.6 (1–8) |
| Number of visits | – | – | – | – | 2.7±1 (2–8) |
| Men, % | 51 | 51 | 42 | .21 | 50 |
| African American, % | 24 | 25 | 16 | .16 | 28 |
| Body mass index, kg/m2 | 27±5 | 27±4 | 31±6 | <.0001 | 27±4 |
| Waist circumference, cm | 91±12 | 91±12 | 100±14 | <.0001 | 92±11 |
| Total cholesterol, mg/dL | 191±37 | 191±37 | 193±43 | .76 | 192±38 |
| HDL cholesterol, mg/dL | 59±17 | 59±17 | 50±15 | <.001 | 59±17 |
| LDL cholesterol, mg/dL | 112±33 | 111±33 | 116±39 | .41 | 112±33 |
| Triglycerides, mg/dL | 104±59 | 102±59 | 134±51 | <.001 | 103±56 |
| Fasting plasma glucose, mg/dL | 92±17 | 92±17 | 95±14 | .25 | 95±18 |
| Glomerular filtration rate, mL/min/1.73 m2 | 77±17 | 76±18 | 78±20 | .53 | 74±17 |
| Mean systolic BP, mm Hg | 118±15 | 118±15 | 120±13 | .30 | 120±15 |
| Mean diastolic BP, mm Hg | 66±9 | 66±9 | 66±7 | .57 | 67±9 |
| Mean BP, mm Hg | 83±10 | 83±10 | 84±7 | .82 | 84±9 |
| Pulse pressure, mm Hg | 51±13 | 51±13 | 54±14 | .11 | 53±13 |
| Heart rate, beats per min | 66±10 | 65±9 | 69±12 | .004 | 65±10 |
| Carotid‐femoral pulse wave velocity, m/s | 7.4±1.9 | 7.3±1.9 | 8.2±2 | .002 | 7.2±1.8 |
| Ankle‐brachial index | 1.18±0.1 | 1.18±0.1 | 1.19±0.1 | .38 | 1.18±0.1 |
| Ankle‐brachial index ≤0.9, % | 2 | 2 | 0 | .34 | 2 |
| Hypertension, % | 47 | 47 | 46 | .93 | 53 |
| BP ≥140/90 mm Hg, % | 8 | 8 | 8 | .91 | 10 |
| Antihypertensive drugs, % | 44 | 44 | 44 | .99 | 50 |
| Diabetes, % | 19 | 18 | 42 | <.0001 | 22 |
| Smoking, ever, % | 45 | 45 | 34 | .12 | 47 |
| Dyslipidemia, % | 74 | 74 | 84 | .11 | 76 |
| Total cholesterol ≥200 mg/dL, % | 38 | 38 | 38 | .98 | 39 |
| Lipid‐lowering drugs, % | 42 | 42 | 42 | .98 | 44 |
| Renal failure, % | 17 | 17 | 18 | .81 | 21 |
| Obesity, % | 23 | 21 | 56 | <.0001 | 22 |
| Central obesity, % | 33 | 31 | 71 | <.0001 | 34 |
| Metabolic syndrome, % | 10 | 9 | 24 | <.001 | 10 |
| Coronary artery disease, % | 11 | 11 | 12 | .74 | 9 |
| Cardiac procedures, % | 6 | 5 | 8 | .38 | 7 |
| Peripheral arterial disease, % | 2 | 2 | 0 | .27 | 2 |
Abbreviations: BP, blood pressure; HDL, high‐density lipoprotein; IADSBP, inter‐arm difference in systolic blood pressure; LDL, low‐density lipoprotein.
Clinical Correlates of Significant IADSBP
Compared with participants with an IADSBP <10 mm Hg, those with an IADSBP ≥10 mm Hg had higher BMI, waist circumference and triglycerides, and lower HDL (Table 1). Accordingly, prevalence rates of diabetes, obesity, central obesity, and metabolic syndrome were also significantly higher in this group. No significant differences were found for age or systolic and diastolic BP and prevalence of hypertension and cardiovascular diseases. Heart rate and CF‐PWV were also significantly higher in patients with an IADSBP ≥10 mm Hg (Table 1).
Cross‐Sectional Relationship Between CF‐PWV and Significant IADSBP
Adjusting for multiple confounders, CF‐PWV was significantly associated with IADSBP ≥10 mm Hg (OR, 1.24; 95% CI, 1.05–1.48; P=.01 [Table 2). After backward selection of variables that did not reach a P value level ≤.05, CF‐PWV, together with female sex, Caucasian race, high BMI, diabetes, and low HDL persisted as independent correlates of an IADSBP ≥10 mm Hg (Table 2). No significant interaction was found between sex or race and other variables, and results remained substantially unchanged when mean BP or pulse pressure was substituted for systolic BP or obesity for BMI (data not shown).
Table 2.
Cross‐Sectional Association Between Predictors and an IADSBP ≥10 mm Hg
| Full Model | Reduced Model | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |||
| Pulse wave velocity, m/s | 1.24 | 1.05 | 1.48 | .01 | 1.19 | 1.01 | 1.38 | .03 |
| Age, y | 0.99 | 0.96 | 1.01 | .31 | ||||
| Male sex | 0.40 | 0.19 | 0.83 | .01 | 0.34 | 0.17 | 0.68 | .003 |
| African American race | 0.31 | 0.13 | 0.76 | .01 | 0.32 | 0.14 | 0.76 | .01 |
| Heart rate, beats per min | 1.01 | 0.98 | 1.04 | .59 | ||||
| Smoking, ever | 0.56 | 0.29 | 1.11 | .10 | ||||
| Body mass index, kg/m2 | 1.16 | 1.08 | 1.23 | <.0001 | 1.16 | 1.09 | 1.23 | <.0001 |
| Antihypertensive drugs | 0.69 | 0.35 | 1.38 | .30 | ||||
| Systolic BP | 1.01 | 0.98 | 1.03 | .63 | ||||
| Diabetes | 2.68 | 1.34 | 5.36 | .005 | 2.42 | 1.26 | 4.66 | .01 |
| Triglyceride, mg/dL | 1.00 | 0.99 | 1.01 | .78 | ||||
| HDL cholesterol, mg/dL | 0.98 | 0.95 | 1.00 | .09 | 0.97 | 0.95 | 0.99 | .03 |
Abbreviations: BP, blood pressure; CI, confidence interval; HDL, high‐density lipoprotein; IADSBP, inter‐arm difference in systolic blood pressure; OR, odds ratio.
Longitudinal Relationship Between CF‐PWV and Significant IADSBP
Longitudinal data in standard (marginal) format were analyzed using generalized estimating equation models24 adjusting for sex, race, and time‐varying age, heart rate, smoking, BMI, antihypertensive drugs, systolic BP, diabetes, triglycerides, and HDL cholesterol. Results of the reduced model showed that each unit increase of CF‐PWV, in terms of both difference between patients or within the same patients across subsequent assessments, was associated with IADSBP ≥10 mm Hg (OR, 1.15; 95% CI, 1.03–1.29; P=.01 [Table 3). Female sex, Caucasian race, and high BMI were also independently associated with an IADSBP ≥10 mm Hg, while the ORs for diabetes and HDL were in the expected direction but did not reach statistical significance (Table 3).
Table 3.
Longitudinal Association Between Predictors and an IADSBP ≥10 mm Hg
| Full Model | Reduced Model | |||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |||
| Pulse wave velocity, m/s | 1.17 | 1.04 | 1.31 | .007 | 1.15 | 1.03 | 1.29 | .01 |
| Age, y | 0.98 | 0.95 | 1.02 | .31 | ||||
| Male sex | 0.34 | 0.16 | 0.73 | .006 | 0.43 | 0.22 | 0.83 | .01 |
| African American race | 0.34 | 0.15 | 0.75 | .008 | 0.40 | 0.19 | 0.84 | .02 |
| Heart rate, beats per min | 1.01 | 0.98 | 1.04 | .61 | ||||
| Smoking, ever | 0.71 | 0.38 | 1.32 | .27 | ||||
| Body mass index, kg/m2 | 1.13 | 1.06 | 1.20 | <.001 | 1.16 | 1.09 | 1.23 | <.001 |
| Antihypertensive drugs | 0.97 | 0.51 | 1.88 | .94 | ||||
| Systolic BP | 1.01 | 0.99 | 1.03 | .20 | ||||
| Diabetes | 1.72 | 0.87 | 3.42 | .12 | ||||
| Triglycerides, mg/dL | 1.00 | 0.99 | 1.01 | .80 | ||||
| HDL cholesterol, mg/dL | 0.99 | 0.96 | 1.01 | .29 | ||||
Abbreviations: BP, blood pressure; CI, confidence interval; HDL, high‐density lipoprotein; IADSBP, inter‐arm difference in systolic blood pressure; OR, odds ratio.
Discussion
This is the first large‐scale study that explores the prevalence of significant IADSBP in community‐dwelling individuals with low cardiovascular risk and analyzes its cross‐sectional and longitudinal relationship with CF‐PWV.
Relationship Between IADSBP and CF‐PWV
Studies of patients undergoing angiography for other clinical reasons were able to confirm the presence of significant atherosclerosis involving the supra‐aortic arteries as the reason for the IADSBP.4, 5, 25 However, this kind of evidence has never been produced either by angiography or by Doppler ultrasonography in unselected individuals from the general population, for whom the pathological basis of a significant IADSBP remains unknown. For this reason, experts recommend that the definition of “arterial stenosis” should not be used in studies according to measurement of the inter‐arm difference alone.7 In searching for an explanation of the increasingly reported association between a significant IADSBP and poor cardiovascular outcomes,7 we tested and confirmed in multivariate cross‐sectional and longitudinal analyses an independent relationship between an IADSBP ≥10 mm Hg and CF‐PWV, a parameter considered the gold standard for noninvasive assessment of arterial stiffness.14 Our primary hypothesis, although not verified, is that ongoing endothelial dysfunction and arterial stiffening underlie the association between increased CF‐PWV and significant IADSBP. Previous studies have shown independent associations of significant IADSBP with other markers of atherosclerosis, namely carotid artery intima‐media thickness,12 coronary artery calcium score,12 brachial‐ankle pulse wave velocity,13 and an ABI ≤0.9.12, 13, 26 Compared with our population, despite their almost 10‐year younger age, these studies included individuals with worse cardiovascular profile, particularly because of the higher prevalence of hypertension and coronary artery disease (69% and 19%, respectively, in the study by Su and colleagues13), uncontrolled systolic BP (>140 mm Hg in up to 40% in the total cohort by Shadman and colleagues26), and lower HDL and higher triglycerides mean values.12, 13, 26 The results of our study are new because they were obtained in a healthier aging population and using an earlier marker of subclinical vascular damage such as CF‐PWV. Increased CF‐PWV has been demonstrated to be a strong predictor of cardiovascular morbility and mortality in the general population.15, 16 Our current findings raise the possibility that part of the association between an IADSBP ≥10 mm Hg and cardiovascular risk reported in the literature may be mediated by arterial stiffness.
We speculate that similar systolic BP in both arms may reflect a fine‐tuning process to maintain a state of homeostasis, and a significant IADSBP may be the result of a disturbance to this homeostasis, either by mechanical obstruction (as assumed so far) or dysfunction of fine‐tuning, of which endothelial function, arterial stiffness, and wave reflection may be a part. If there is any association between CF‐PWV and significant IADSBP beyond atherosclerosis, one may speculate that since the left and right subclavian arteries originate from different points of the aortic arch, by physics this should result in slightly different pressures, but this would be masked by proper endothelial function that will act on the different blood flow in each arm to bring the BP to target (more or less like an electronic stability control on a car, where brakes act differently on each wheel to keep the car balanced when braking). Endothelial dysfunction and consequent arterial stiffness would unmask these differences, which may also be accentuated by wave reflection that might present in one more than the other arm due to hemodynamic differences.
Other Clinical Correlates of IADSBP
In our multivariate analysis, an IADSBP ≥10 mm Hg was associated with increasing BMI, decreasing HDL, and presence of diabetes. Waist circumference, central adiposity, and metabolic syndrome were also significantly higher in participants with an IADSBP ≥10 mm Hg, but BMI (or obesity) was preferred for multivariate analyses because of the higher ORs and the lower correlation with other variables included in the models (especially sex and CF‐PWV). The associations with BMI,12, 13 diabetes,12 and HDL,26 all previously reported in other studies, suggest a potential causal relationship between unfavorable changes in body composition and lipid metabolism and significant IADSBP. In contrast with some previous reports,12, 13, 26 but in agreement with others,18, 27 hypertension (as well as continuous BP‐related parameters or antihypertensive medications) did not appear to influence IADSBP. The lower mean systolic and diastolic BP values (with only 8% of the participants having BP values ≥140/90 mm Hg) and the better cardiovascular risk profile of our population, as well as rigorous BP measurement protocol (requiring supine position, adequate resting time, and multiple readings) may partially explain these contrasting findings. Finally, the higher probability of significant IADSBP in women is consistent with what has already been reported in several other studies.12, 13, 26 The reason is unclear, and we do not have an explanation for the lower tendency of African American participants in our cohort to have an IADSBP ≥10 mm Hg. African Americans in our population were more likely to be hypertensive (56% vs 44%, P=.001) and receive antihypertensive drugs (52% vs 42%, P=.006) but also had higher HDL values (61±18 vs 58±17, P=.01) and lower triglycerides (90±51 vs 108±61, P<.001). The absence of a relationship between hypertension and the IADSBP in our analysis made us hypothesize that, among all factors, the effect of a favorable lipid profile in this race group may have overcome that of hypertension and in part explain our findings. However, further studies are needed to address the complex relationship between sex, race, and significant IADSBP.
Ascertainment of IADSBP
Despite the major emphasis placed on the prognostic value of a significant IADSBP,2 considerable debate exists regarding the reproducibility and generalizability of these findings to the general community.28 It has been shown that the more BP measurements are taken, the lower the prevalence of significant IADSBP becomes, and multiple readings appear necessary to overcome the initial so‐called white‐coat effect.29 In addition, published data clearly suggest that sequential measurement of BP overestimates the prevalence of IADSBP, and simultaneous measurement of both arms seems preferable.19 Our preliminary exploratory analysis confirmed these previous findings, plus the low consistency and reproducibility of IADSBP over time when looking at longitudinal trajectories of 629 participants in our study with more than two simultaneous BP evaluations.18, 20
In our analysis, we used multiple BP measurements taken simultaneously on both arms with an automated device to define participants with an IADSBP ≥10 mm Hg. Previous population studies used either sequential BP measurements to calculate the IADSBP,12 combined different sequential BP measurement protocols,26 or did not have multiple simultaneous BP readings to estimate the IADSBP,13 therefore, generally reporting higher prevalence than ours. Nevertheless, significant associations with subclinical markers of atherosclerosis were consistently reported.12, 13, 26 Reasons for this may presumably reside on the ability of the IADSBP estimated by sequential BP readings to capture some of the short‐term variability in BP, a measure that is per se independently associated with increased arterial stiffness30 and cardiovascular disease.31
Study Limitations
There are several limitations in this study. The majority of participants included in the current analysis were treated chronically with antihypertensive and lipid‐lowering medications. Although adjustments for these medications did not have any significant effect in our multivariate models, we cannot completely exclude the influence of these agents on the estimation of both the IADSBP and the CF‐PWV. In addition, central pulse pressure and wave reflection data were not available for most of the patients included in this analysis, and we could not explore the association between IADSBP and wave reflection parameters at the time of the study.
Although appropriate cuff size was chosen based on the arm circumference of each participant, larger arms of individuals with higher BMI increase the likelihood of inaccurate BP measurements. The same applies to larger bellies and/or breasts for the estimation of CF‐PWV.14
The low number of participants with IADSBP ≥10 mm Hg detected at baseline and in subsequent visits prevented us from performing further subanalysis stratified by either sex, race, or obesity, and different longitudinal models testing the association between CF‐PWV and the development of IADSBP ≥10 mm Hg among those with IADSBP <10 mm Hg at baseline. However, such a low prevalence and incidence were expected in a cohort of patients with a low cardiovascular risk profile. Moreover, we preferred to eliminate most potential sources of bias in the estimation of the IADSBP by considering only the second and third simultaneous and automated BP readings, thus determining a significant reduction in the prevalence of participants with an IADSBP ≥10 mm Hg. Despite this careful approach, the consistency and reproducibility of the simultaneous IADSBP in our longitudinal sample remained low. Nevertheless, the association between CF‐PWV and an IADSBP ≥10 mm Hg was consistent both in cross‐sectional and longitudinal analyses, although the sensitivity and specificity of this parameter for the detection of abnormal arterial compliance are probably limited.
Conclusions
We herein demonstrate a positive relationship between CF‐PWV and significant IADSBP in community‐dwelling healthy aging individuals. This finding may help explaining at least part of the association between IADSBP and poor cardiovascular outcomes reported in the literature. Indeed, besides its potential ability to detect obstructive disease of the supra‐aortic arteries, our results suggest that an IADSBP ≥10 mm Hg could potentially be considered as a marker of increased arterial stiffness and support the opportunity to assign individuals with a significant IADSBP to further cardiovascular assessment. For this purpose, IADSBP should be measured simultaneously and multiple times, and its low consistency and reproducibility over time should be taken into account.
Disclosures
None.
Supporting information
Figure S1. Relationship between inter‐arm difference in systolic blood pressure and mean systolic blood pressure.
Figure S2. Distribution of inter‐arm difference in systolic blood pressure in the cross‐sectional sample.
Figure S3. Longitudinal trajectories of inter‐arm difference in systolic blood pressure.
Source of Funding
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
J Clin Hypertens (Greenwich). 2013;15:880–887. DOI: 10.1111/jch.12178. ©2013 Wiley Periodicals, Inc.
References
- 1. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28:1462–1536. [DOI] [PubMed] [Google Scholar]
- 2. McManus RJ, Mant J. Do differences in blood pressure between arms matter? Lancet. 2012;379:872–873. [DOI] [PubMed] [Google Scholar]
- 3. Perloff D, Grim C, Flack J, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. [DOI] [PubMed] [Google Scholar]
- 4. English JA, Carell ES, Guidera SA, Tripp HF. Angiographic prevalence and clinical predictors of left subclavian stenosis in patients undergoing diagnostic cardiac catheterization. Catheter Cardiovasc Interv. 2001;54:8–11. [DOI] [PubMed] [Google Scholar]
- 5. Baribeau Y, Westbrook BM, Charlesworth DC, et al. Brachial gradient in cardiac surgical patients. Circulation. 2002;106:I11–I13. [PubMed] [Google Scholar]
- 6. Calligaro KD, Ascer E, Veith FJ, et al. Unsuspected inflow disease in candidates for axillofemoral bypass operations: a prospective study. J Vasc Surg. 1990;11:832–837. [PubMed] [Google Scholar]
- 7. Clark CE, Taylor RS, Shore AC, et al. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta‐analysis. Lancet. 2012;379:905–914. [DOI] [PubMed] [Google Scholar]
- 8. Orme S, Ralph SG, Birchall A, et al. The normal range for inter‐arm differences in blood pressure. Age Ageing. 1999;28:537–542. [DOI] [PubMed] [Google Scholar]
- 9. Clark CE, Taylor RS, Shore AC, Campbell JL. The difference in blood pressure readings between arms and survival: primary care cohort study. BMJ. 2012;344:e1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark CE, Powell RJ. The differential blood pressure sign in general practice: prevalence and prognostic value. Fam Pract. 2002;19:439–441. [DOI] [PubMed] [Google Scholar]
- 11. Clark CE, Campbell JL, Powell RJ. The interarm blood pressure difference as predictor of cardiovascular events in patients with hypertension in primary care: cohort study. J Hum Hypertens. 2007;21:633–638. [DOI] [PubMed] [Google Scholar]
- 12. Aboyans V, Kamineni A, Allison MA, et al. The epidemiology of subclavian stenosis and its association with markers of subclinical atherosclerosis: the multi‐ethnic study of atherosclerosis (MESA). Atherosclerosis. 2010;211:266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su HM, Lin TH, Hsu PC, et al. Association of interarm systolic blood pressure difference with atherosclerosis and left ventricular hypertrophy. PLoS ONE. 2012;7:e41173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laurent S, Cockcroft J, Van Bortel L, et al. European Network N‐i . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 15. Sutton‐Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well‐functioning older adults. Circulation. 2005;111:3384–3390. [DOI] [PubMed] [Google Scholar]
- 16. Mitchell GF, Hwang SJ, Vasan RS, et al. Arterial stiffness and cardiovascular events: the Framingham heart study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shock NW, Greulich RC, Andres RA, et al. Normal Human Aging: The Baltimore Longitudinal Study of Aging. NIH publication no. 84‐2450. Washington, DC: U.S. Government Printing Office; 1984:45. [Google Scholar]
- 18. Eguchi K, Yacoub M, Jhalani J, et al. Consistency of blood pressure differences between the left and right arms. Arch Intern Med. 2007;167:388–393. [DOI] [PubMed] [Google Scholar]
- 19. Verberk WJ, Kessels AG, Thien T. Blood pressure measurement method and inter‐arm differences: a meta‐analysis. Am J Hypertens. 2011;24:1201–1208. [DOI] [PubMed] [Google Scholar]
- 20. Kleefstra N, Houweling ST, Meyboom‐de Jong B, Bilo HJ. [Measuring the blood pressure in both arms is of little use; longitudinal study into blood pressure differences between both arms and its reproducibility in patients with diabetes mellitus type 2]. Ned Tijdschr Geneeskd. 2007;151:1509–1514. [PubMed] [Google Scholar]
- 21. AlGhatrif M, Strait J, Morrell C, et al. Age‐associated longitudinal changes in arterial stiffness trajectories and blood pressure within individuals of community dwelling population free (Abstract). J Am Coll Cardiol. 2013;61:E1381. [Google Scholar]
- 22. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 23. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Twisk JW. Longitudinal data analysis. A comparison between generalized estimating equations and random coefficient analysis. Eur J Epidemiol. 2004;19:769–776. [DOI] [PubMed] [Google Scholar]
- 25. Osborn LA, Vernon SM, Reynolds B, et al. Screening for subclavian artery stenosis in patients who are candidates for coronary bypass surgery. Catheter Cardiovasc Interv. 2002;56:162–165. [DOI] [PubMed] [Google Scholar]
- 26. Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. [DOI] [PubMed] [Google Scholar]
- 27. Lane D, Beevers M, Barnes N, et al. Inter‐arm differences in blood pressure: when are they clinically significant? J Hypertens. 2002;20:1089–1095. [DOI] [PubMed] [Google Scholar]
- 28. Kleefstra N, Houweling ST, Bilo HJ. Interarm blood pressure difference and vascular disease. Lancet. 2012;380:23; author reply 24–25. [DOI] [PubMed] [Google Scholar]
- 29. Verberk WJ, Kroon AA, Thien T, et al. Prevalence of the white‐coat effect at multiple visits before and during treatment. J Hypertens. 2006;24:2357–2363. [DOI] [PubMed] [Google Scholar]
- 30. Schillaci G, Bilo G, Pucci G, et al. Relationship between short‐term blood pressure variability and large‐artery stiffness in human hypertension: findings from 2 large databases. Hypertension. 2012;60:369–377. [DOI] [PubMed] [Google Scholar]
- 31. Rothwell PM, Howard SC, Dolan E, et al. Prognostic significance of visit‐to‐visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Relationship between inter‐arm difference in systolic blood pressure and mean systolic blood pressure.
Figure S2. Distribution of inter‐arm difference in systolic blood pressure in the cross‐sectional sample.
Figure S3. Longitudinal trajectories of inter‐arm difference in systolic blood pressure.


