Abstract
In women, the corpus luteum is the source of circulating relaxin. No previous studies have addressed whether the corpus luteum is also a relaxin target organ. We determined relaxin receptor LGR7 mRNA expression in human term pregnancy corpora lutea and non-human primate corpora lutea obtained during the menstrual cycle. Real-time RT-PCR demonstrated the expression of LGR 7 mRNA in both human and rhesus monkey corpora lutea. Rhesus monkey corpora lutea were obtained from naturally cycling animals following documented LH surges at early, mid, mid-late and late luteal phases. Luteal expression of LGR7 mRNA did not show temporal variation. Since the primate corpus luteum is LH dependent, we assessed LGR7 mRNA expression in corpora lutea from rhesus monkeys treated with a GnRH antagonist which significantly suppressed pituitary LH levels. GnRH antagonist treatment, which also inhibits both progesterone and relaxin production, resulted in a five fold increase in luteal LGR7 mRNA expression. These data suggest that luteal LGR7 mRNA expression may be regulated by relaxin and/or LH, and that the primate corpus luteum is a target organ for relaxin.
Keywords: RXFP1, LGR7, rhesus monkey, relaxin, corpus luteum, relaxin receptor
Introduction
In women and non-human primates, normal function of the ovarian corpus luteum (CL) is required for establishment and maintenance of early pregnancy. Various structural and endocrinological changes occur in the ovary to allow for proper luteal formation, maintenance, and regression. The precise mechanisms involved in the regulation of the primate CL are not precisely established. Luteinizing hormone (LH) is required for luteal formation and maintenance of luteal steroidogenesis.
Relaxin is an important agent involved in remodeling of connective tissue in mammalian reproductive tract organs. We have previously demonstrated that relaxin is a product of the human CL and that the human CL is the major source of circulating relaxin during pregnancy.1 Although previous investigators have reported that LGR7 (RXFP1) mRNA is present in the human ovary, it has not been established whether the CL expresses a relaxin receptor.2,3 The presence of a relaxin receptor in luteal cells would suggest that it is a target organ for relaxin which may have an auto-regulatory role. Our hypotheses were that the relaxin receptor, LGR7 mRNA, is expressed in human and non-human primate corpora lutea, and that LGR7 mRNA expression is hormonally regulated, specifically by LH. We opted to address our questions using a rhesus monkey model, since the endocrinology of the menstrual cycle in the rhesus monkey is identical to that of the human.
Materials and Methods
Animal protocols were carried out at the Oregon National Primate Research Center in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals. To determine whether the CL expresses LGR7, and to determine if LGR7 expression is regulated during luteal development, rhesus monkey (Macaca mulatta) corpora lutea were obtained from naturally cycling animals following documented LH surges at four time points during the luteal phase of the menstrual cycle. These time points represent different stages of luteal function: days 3-5, early stage; days 7-8, mid-luteal stage; days 10-12, mid-late stage; and days 14-16, late stage; as described previously.4 To study the role of LH, rhesus monkeys were untreated (controls, n=3) or received the GnRH antagonist, Antide, (3mg/kg body weight, Salk Institute for Biological Studies, San Diego, CA) (n=3) on days 6, 7, and 8 of the luteal phase of the menstrual cycle to suppress pituitary LH, and corpora lutea were removed 24 hours later as described previously.5 This dose and duration of the GnRH antagonist (Antide) has been previously shown to reduce progesterone levels to below 1 ng/mL. Total cellular RNAs from all tissues were isolated using the guanidinium-thiocyanate procedure and were reverse transcribed into cDNAs. Identical reactions were also performed omitting reverse transcriptase, as controls for false-positive results due to contamination with genomic DNA. Real-time PCR reactions were performed using SYBR Green qPCR Master Mix using the Rotor-Gene 3000 real-time PCR system (Corbett Robotics Inc, San Francisco, USA). Rhesus monkey skeletal muscle and human endometrium were used as the negative and positive tissue controls respectively. Primers previously verified to amplify a portion of the N- terminus region of the human LGR7 cDNA, and human beta-actin cDNA were used.6 The Beta-actin forward primer sequence was: 5′-3′: ACTCTTCCAGCCTTCCTTC spanning nucleotides 851-871 and the beta-actin reverse primer sequence was 5′-3′: ATCTCCTTCTGCATCCTGTC spanning nucleotides 1123-1004. The LGR7 forward primer sequence was: 5′GTGGAGACAACAATGGATGG 3′, (nucleotides 267-286). The LGR7 reverse primer sequence was 5′ AAGAAACCGATGGAACAGC 3′, (nucleotides 458-440). The 192 base pair LGR7 amplicon spans exons 2, 3, and 4. PCR amplification conditions were: 50 °C for two minutes, 95 °C for two minutes, followed by 40 cycles of 95 °C for 20 seconds, 55 °C for 20 seconds, and 72 °C for 20 seconds. The LGR7 standard, generated from RNA isolated from human endometrial glandular epithelial cells, reverse transcribed into cDNA and amplified by PCR, had a defined concentration of 2.65 fmoles/μl (330 pg/μl), as previously described.6 Standard curves were generated using serial dilutions of the LGR7 standard. Cycle thresholds (Ct) were generated for each reaction.
To assess the purity of the products of each PCR reaction, melt curve analyses were performed. To verify amplicon size, PCR products were assessed by electrophoretic analysis using 2% agarose gels. To compare the nucleotide sequences of the rhesus monkey amplicons produced in our reactions to that of the reported human sequence, PCR products were sequenced using the dye termination method with the ABI 3130 XL nucleotide sequencer in conjunction with ABI prism 3100 data collection software (Applied Biosystems, Foster City, CA). Nucleotide sequences were subjected to BLAST analysis (www.ncbi.nih.gov) to confirm identity. The amplicon sequence identified from the rhesus monkey CL was identical to the nucleotide sequence of ampilicons produced in RT-PCR reactions using these same primers in reactions programmed using RNAs from other rhesus monkey tissues. The nucleotide sequence was submitted to GENBANK (ACCESSION # DQ996538).
All comparisons were performed using JMP statistical software (SAS institute, Inc., Cary, NC) written for the Macintosh computer (Apple Computer Systems, Cupertino, Ca). Amounts of LGR7 mRNA were not normally distributed; therefore, analyses of these data were performed non-parametrically using the Kruskal-Wallis rank-sum testing.
Discussion
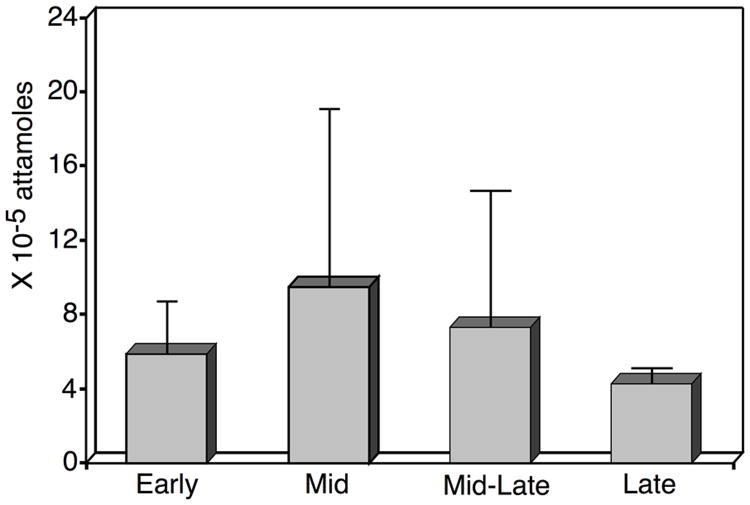
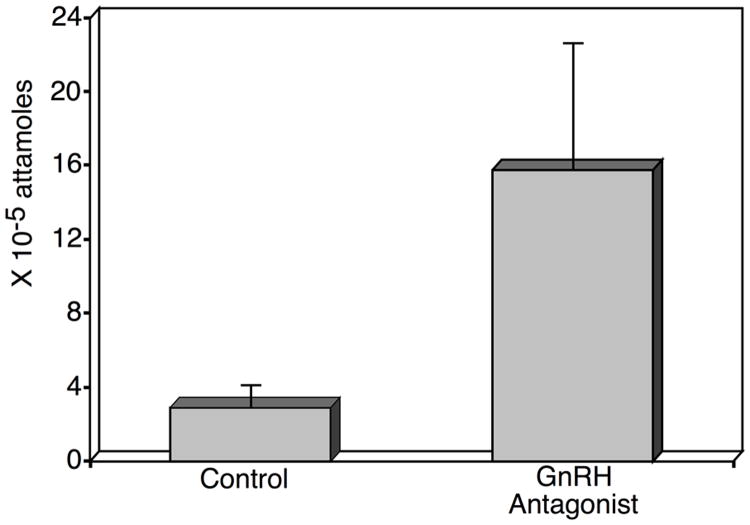
Our results demonstrated that LGR7 mRNA is expressed in human and rhesus monkey corpora lutea. Levels of LGR7 mRNA in rhesus monkey corpora lutea, depicted in figure 1, do not show temporal regulation with luteal development. Although it appears there may be a pattern of expression, there are no significant differences between LGR7 mRNA levels at any time points. The apparent pattern is likely due to larger variation between individual animals in the mid and mid-late stages. As shown in figure 2, GnRH antagonist treatment resulted in a significant increase (p = 0.01) in luteal LGR7 mRNA expression in the rhesus monkey. Therefore, we conclude that luteal expression of LGR7 mRNA at day 9 after 3 days of GnRH antagonist treatment, appears to be regulated by LH and likely by ovarian factors. LH inhibition is associated with increased luteal LGR7 mRNA expression, and presumably increased relaxin action in the rhesus monkey CL.
Figure 1.
LGR7 mRNA levels in corpora lutea obtained at various times during the luteal phase of the menstrual cycle. Corpora lutea are designated as early stage, (days 3-5), mid stage, (days 7-8), mid-late stage (days 10-11), and late stage (days 14-16) following a documented LH surge. Each bar represents the mean amount of LGR7 amplicon, (× 10-5 attamoles ± standard error), resulting from total RNAs, obtained from 3 animals at each time point, that were reverse transcribed into cDNAs, each of which were subjected to 5 PCR reactions.
Figure 2.
LGR7 mRNA levels (× 10-5 attamoles ± standard error) in corpora lutea obtained from rhesus monkeys that were either untreated (controls n=3 animals) or received a GnRH antagonist, (3mg/kg) (n=3 animals) on days 6, 7, and 8 of the luteal phase of the menstrual cycle, to suppress pituitary LH. Corpora lutea were removed 24 hours later, on day 9. Data are expressed as described for Figure 1.
Sequencing of rhesus monkey corpora lutea PCR products verified our previous findings regarding the nucleotide sequence of PCR products programmed with rhesus monkey endometrium RNA, using these same primers. The rhesus monkey amplicon sequence indicated 97% (187/192 bases) nucleotide sequence homology with the corresponding sequence of human LGR7 mRNA, which corresponds to a 95% (60/63) homology in amino acid sequence.
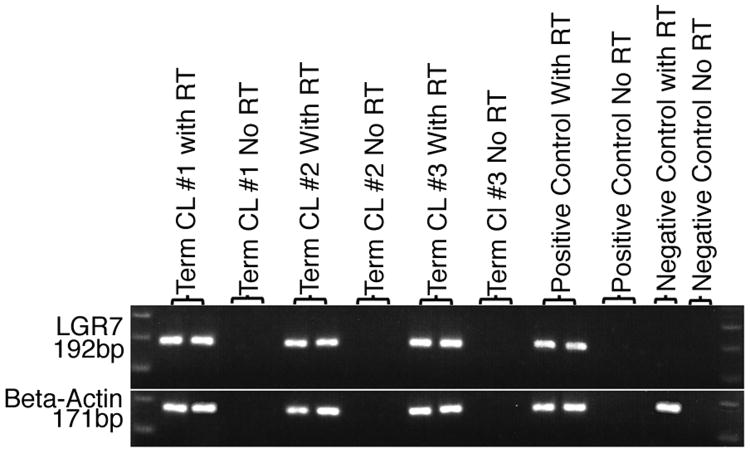
To verify that expression of LGR7 mRNA in rhesus monkey corpora lutea was not unique to this species, LGR7 mRNA expression in human corpora lutea was determined. As shown in figure 3, the expected 192 base pair LGR7 amplicon is clearly present in lanes with PCR products from reactions programmed with total RNA from human corpora lutea. These human corpora lutea were obtained from normal pregnancies at term during elective repeat cesarean section. No products are detectable from reactions programmed without the addition of reverse transcriptase. The 192 base pair LGR7 amplicon is detectable from reactions using RNA from the positive tissue control, human endometrium, and is undetectable from reactions using RNA from the negative control, rhesus monkey skeletal muscle.
Figure 3.
Expression of LGR7 mRNA in Human Term Pregnancy Corpora Lutea. Electrophoretic profiles of PCR products from reactions programmed by RNA isolated from 3 individual specimens (#1, #2, and #3) are shown. Lanes 1 and 20: 100 base pair DNA ladder. Lanes 2 and 3: Human CL #1, reactions with reverse transcriptase (RT). Lanes 4 and 5: Human CL #1, reactions without RT. Lanes 6 and 7: Human CL #2, reactions with RT. Lanes 8 and 9: Human CL #2, reactions without RT. Lanes 10 and 11: Human CL #3, reactions with RT. Lanes 12 and 13: Human CL #3, reactions without RT. Lanes 15, 16: Positive control with RT. Lane 17: Positive control without RT. Lane 18: Negative control with RT. Lane 19: Negative control without RT.
In summary, these data demonstrate that LGR7 mRNA is expressed in rhesus monkey and human corpora lutea. In concert with the fact that the CL produces relaxin, these data support the hypothesis that the CL is a relaxin target organ. Unvaried expression of LGR7 mRNA throughout the luteal phase makes elucidating relaxin's role in corpus luteum function difficult. To date, the mechanisms responsible for the demise of the human CL, luteal regression at the end of the menstrual cycle, have yet to be elucidated. Matrix metalloproteinases (MMPs) and relaxin (RLX) are reported to play an important role in tissue remodeling. Relaxin is a known regulator of MMPs in various reproductive tissues.7,8 In the rhesus monkey CL, MMP-2 and MMP-9 mRNA and protein expression are highest in the late luteal stage, compared to levels at earlier stages of luteal development.9
Bogan et al., who have recently identified differentially expressed genes in the rhesus monkey CL using tissue obtained from the identical model used in our studies, have shown that luteal relaxin mRNA levels increase thirty fold from the early to the late luteal phase.10 Expression of only one other gene, prostaglandin F2α receptor, had a more pronounced temporal difference. Increased luteal relaxin levels may be important during the late luteal phase, possibly involved in the connective tissue remodeling which occurs during regression of the corpus luteum at this developmental stage. Relaxin stimulation of MMP-2 and/or MMP-9 production may mediate relaxin's connective tissue actions. Enhanced understanding of the changes in gene expression which occur during the luteal phase may allow us to decipher the mechanisms involved in regulation of the structure and function of the primate corpus luteum.
Acknowledgments
Supported by NIH Grants HD22338, HD18185 and RR00163
References
- 1.Weiss G, O'Byrne EM, Steinetz BG. Relaxin: a product of the human corpus luteum of pregnancy. Science. 1976;194:948–9. doi: 10.1126/science.982052. [DOI] [PubMed] [Google Scholar]
- 2.Hsu SY, Nakabayashi K, Nishi S, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–4. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 3.Shirota K, Tateishi K, Koji T, et al. Early human preantral follicles have relaxin and relaxin receptor (LGR7), and relaxin promotes their development. J Clin Endocrinol Metab. 2005;90:516–21. doi: 10.1210/jc.2004-0130. [DOI] [PubMed] [Google Scholar]
- 4.Duffy DM, Wells TR, Haluska GJ, et al. The ratio of progesterone receptor isoforms changes in the monkey corpus luteum during the luteal phase of the menstrual cycle. Biol Reprod. 1997;57:693–9. doi: 10.1095/biolreprod57.4.693. [DOI] [PubMed] [Google Scholar]
- 5.Duffy DM, Stewart DR, Stouffer RL. Titrating luteinizing hormone replacement to sustain the structure and function of the corpus luteum after gonadotropin-releasing hormone antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab. 1999;84:342–9. doi: 10.1210/jcem.84.1.5362. [DOI] [PubMed] [Google Scholar]
- 6.Mazella J, Tang M, Tseng L. Disparate effects of relaxin and TGFbeta1: relaxin increases, but TGFbeta1 inhibits, the relaxin receptor and the production of IGFBP-1 in human endometrial stromal/decidual cells. Hum Reprod. 2004;19:1513–8. doi: 10.1093/humrep/deh274. [DOI] [PubMed] [Google Scholar]
- 7.Maruo N, Nakabayashi K, Wakahashi S, et al. Effects of recombinant H2 relaxin on the expression of matrix metalloproteinases and tissue inhibitor metalloproteinase in cultured early placental extravillous trophoblasts. Endocrine. 2007;32:303–10. doi: 10.1007/s12020-008-9034-5. [DOI] [PubMed] [Google Scholar]
- 8.Jeyabalan A, Kerchner LJ, Fisher MC, et al. Matrix metalloproteinase-2 activity, protein, mRNA, and tissue inhibitors in small arteries from pregnant and relaxin-treated nonpregnant rats. J Appl Physiol. 2006;100:1955–63. doi: 10.1152/japplphysiol.01330.2005. [DOI] [PubMed] [Google Scholar]
- 9.Young KA, Hennebold JD, Stouffer RL. Dynamic expression of mRNAs and proteins for matrix metalloproteinases and their tissue inhibitors in the primate corpus luteum during the menstrual cycle. Mol Hum Reprod. 2002;8:833–40. doi: 10.1093/molehr/8.9.833. [DOI] [PubMed] [Google Scholar]
- 10.Bogan RL, Murphy MJ, Stouffer RL, et al. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol. 2008;22:1260–73. doi: 10.1210/me.2007-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]