Abstract
Purpose
Recent studies suggest a link between brown adipose tissue (BAT) and bone. The purpose of our study was to investigate the effects of BAT on femoral bone structure.
Materials and Methods
We studied 105 patients (19 m, 86 f, mean age 45.5±16.1 y) who underwent F18-FDG positron emission tomography/computed tomography (PET/CT) for benign etiologies (n=20) or follow-up of successfully treated malignancies (n=85); mean time between PET/CT and last form of treatment was 14.8±18.0 months. BAT volume by PET/CT; femoral bone structure by CT (total femoral cross-sectional area (CSA), cortical CSA); thigh muscle CSA and thigh subcutaneous fat CSA by CT were assessed.
Results
There were positive correlations between BAT volume and total femoral CSA and cortical CSA, independent of age, BMI and history of malignancy (P<0.05). BAT volume correlated positively with thigh muscle CSA and thigh fat CSA (p<0.05). When total femoral CSA was entered as a dependent variable and BAT volume, age and BMI as independent variables in a forward stepwise regression model, BAT volume was the only predictor of total femoral CSA. When femoral cortical CSA was entered as a dependent variable and BAT volume, age and BMI as independent variables, BAT volume was the only predictor of femoral cortical CSA.
Conclusion
BAT volume is a positive predictor of femoral bone structure and correlates positively with thigh muscle and subcutaneous fat, possibly mediated by muscle. These results provide further evidence of a positive effect of BAT on bone.
Keywords: brown adipose tissue (BAT), bone, structure, muscle, fat
Introduction
Recent studies have suggested a positive link between brown adipose tissue (BAT) and bone (1-4). We have previously shown a positive correlation of cold-stimulated BAT and BMD in young normal-weight women and women with anorexia nervosa (AN) and identified preadipocyte factor 1 (Pref-1) and insulin-like growth factor-binding protein 2 (IGFBP-2) as possible negative predictors of BAT in this population (1, 2). In a study in children who were treated successfully for malignancies, BAT volume correlated positively with femoral cross sectional area, cortical area and thigh muscle area (4). The contribution of muscle as a determinant of bone structure decreased the contribution of BAT, suggesting that the BAT-bone connection could be in part mediated by muscle (4). BAT and muscle cells arise from a common precursor cell and several regulators of cell fate switch between myocytes and brown adipocytes have been recently identified (5-8). No association between BAT and muscle mass was found in our previous study in young non-obese women, which may have been secondary to the small number of subjects and the low range of muscle areas (1). Furthermore, children have larger areas of BAT than adults and muscle mass increases during puberty (9), therefore, data in children cannot be extrapolated to adults. The purpose of this study was to investigate the effect of BAT on femoral bone structure and muscle mass in adult men and women. We hypothesized that BAT would be a positive predictor of bone structure and muscle mass in adults.
1. Materials and Methods
The study was approved by Partners Healthcare Institutional Review Board and complied with Health Insurance Portability and Accountability Act guidelines, with exemption status for individual informed consent. A retrospective search was performed of 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) examinations obtained at our institution from January 2005 to June 2013. Inclusion criteria comprised subjects older than 18 years who were successfully treated for malignancies or had no history of malignancy and who were BAT positive on PET/CT. We excluded subjects with diabetes mellitus, chronic renal disease, or other chronic disease that could influence bone metabolism. In addition, we excluded patients who were on glucocorticoids and osteoporosis medication at the time of PET/CT. None of the patients had radiation therapy to the lower extremities.
2.1. 18F-FDG-PET/CT
The PET/CT studies were performed on an integrated PET/CT scanner (Siemens Biograph 16 or 64, Siemens, Erlangen, Germany or GE Healthcare discovery, Milwaukee, Wisconsin, USA), with a 16 or 64-slice CT and a full-ring HI-REZ LSO PET. Patients fasted 6 hours before the exam. Blood glucose levels were measured upon arrival, and patients were only injected with 18F-FDG if the blood glucose was less than or equal to 250 mg/dl.
18F-FDG was produced using an on-site 230 MeV isochronous cyclotron. The dose injected was based on patient's body mass index (BMI). Patients with BMI less than 30 were given 15 mCi, patients with BMI between 30.1 and 44 were given 20 mCi, and patients with BMI greater than 44.1 were given 25 mCi. After injection, the patient relaxed in a semi-reclined chair for at least 45 minutes. Attenuation correction CT obtained in mid-expiration phase without intravenous contrast (slice thickness 5 mm; table feed per rotation, 18 mm; time per table rotation, 0.5 s; tube voltage, 120 kVp; tube current, 11 mAs; field of view, 20 cm) and PET images were acquired with the patient's arms over the head. 3D mode PET images were obtained from the skull base to the mid-thigh, with 6-8 bed positions lasting 3-7 minutes each. Images were reconstructed to a slice thickness of 2.4 mm.
2.2 Image analysis
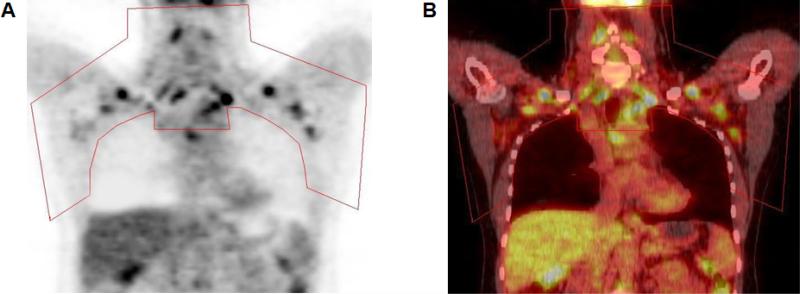
Semiquantitative and qualitative evaluation of PET images was performed as previously described (1) and the volume of BAT (ml) was quantified using PET-CT Viewer shareware (10, 11) (Figure 1).
Figure 1.
Quantification of brown adipose tissue in regions of interest (red outline) in images obtained with positron-emission tomography (PET) (A) and fused PET–CT (B).
Femoral bone structure was determined in the right proximal thigh by CT, which was obtained as part of the PET/CT. Measurements were obtained 10 cm below the femoral head, which corresponded to the proximal femoral metadiaphysis. The following parameters were obtained: total femoral cross-sectional area (CSA) (mm2) and femoral cortical CSA (mm2). Thigh muscle CSA and thigh subcutaneous adipose tissue (SAT) CSA (mm2) were also determined. Images were analyzed using Alice software (version 4.3.9 Parexel, Waltham, MA).
2.3. Statistical Analysis
Statistical analysis was performed using JMP (SAS Institute, Carry, NC) software. Variables were tested for normality of distribution using the Shapiro-Wilk test. Variables that were not normally distributed were log transformed. Linear regression analysis between BAT volume and measures of femoral bone structure and thigh muscle and SAT was performed. Standard least squares modeling was used to control for age, BMI, history of malignancy, and gender. Separate analysis for male and female subjects was also performed. Forward stepwise regression modeling was also performed to determine the strongest predictor of femoral CSA and femoral cortical femoral CSA. P ≤ 0.05 was used to denote significance and p ≤ 0.1 was used to denote a trend.
2. Results
The study group comprised 105 patients (19 men, 86 women) with a mean age of 45.5±16.1 years (range 19 to 77 years) and a mean BMI of 25.3±4.8 kg/m2 (range 15.7 to 48.9 kg/m2) who underwent PET/CT for benign etiologies (n=20; lung nodules, n=17; unexplained adenopathy n=1; neurofibromatosis type 1, n=1; Ollier's disease, n=1) or follow-up of successfully treated malignancies (n=85; lymphoma, n= 36; gastrointestinal cancers, n=13; genitourinary cancers, n=5; melanoma, n=10; breast cancer, n=7; head and neck cancer, n=10; and lung cancer, n=4) and had no evidence of active disease at time of PET/CT. The mean time between PET/CT and last form of treatment was 14.8±18.0 months (range 1 to 72 months).
All subjects were BAT positive on PET/CT (mean volume 16.8±15.0 ml, range: 2.4 to 68.6 ml) as per inclusion criteria. Forty-one subjects were scanned in the spring and summer and 64 subjects in the fall and winter. There was no association between the seasons in which the scan was performed and the amount of BAT (p=0.8). Subjects without a history of malignancy had a trend toward higher BAT volume compared to the group with a history of malignancy (21.0±16.2 vs 15.9±14.7 ml, p=0.1).
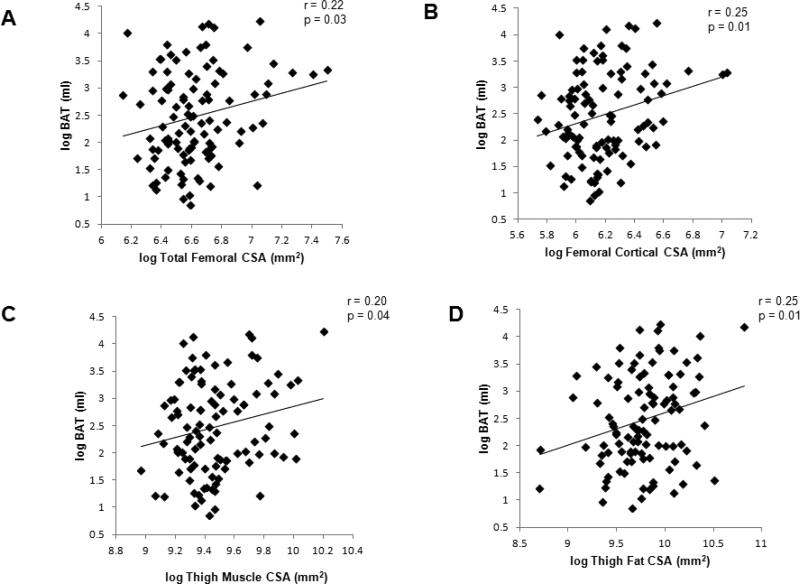
There were positive correlations between BAT volume and femoral structure (Figure 2). BAT volume correlated positively with total femoral CSA (r=0.22, p=0.03) and femoral cortical CSA (r=0.25, p=0.01), which remained significant after controlling for age, BMI and history of malignancy (p=0.05 and p=0.03, respectively), but lost significance after controlling for gender (p=0.2 and p=0.1, respectively). BAT volume correlated positively with thigh muscle CSA (r=0.20, p=0.04), independent of age and history of malignancy (p=0.06). However, the association lost significance after controlling for BMI and gender (p=0.5). BAT volume correlated positively with thigh SAT CSA (r=0.25, p=0.01), independent of age, gender, BMI and history of malignancy (p=0.0009).
Figure 2.
Regression analyses of brown adipose tissue (BAT) volume on total femoral cross sectional area (CSA) (A), femoral cortical CSA (B), thigh muscle CSA (C), and thigh subcutaneous adipose tissue (SAT) CSA (D). There were positive correlations with BAT and femoral bone structure, thigh muscle and thigh SAT.
Men had significantly more BAT than women (23.3±19.2 vs 15.4±13.7 ml, p=0.04). Mean age in the male group was lower compared to the female group (40.1±15.9 vs 46.7±16.0 kg/m2, p=0.1) (trend) and BMI was similar between the groups (p=0.4). Within the male group alone, there was a trend toward a positive correlation between BAT and total femoral CSA (r=0.33, p=0.1), independent of age, history of malignancy, and BMI (p=0.08). There was no significant correlation between BAT and femoral cortical CSA (r=0.31, p=0.2), however, the association became a trend after controlling for age, history of malignancy, and BMI (p=0.1). There was a positive correlation between BAT and thigh SAT CSA (r=0.62, p=0.005), independent of age, history of malignancy, and BMI (p=0.004). There was no association between BAT and thigh muscle CSA (p=0.4).
Within the female group alone, there was a trend toward a positive correlation between BAT and femoral cortical CSA (r=0.2, p=0.1), which lost significance after controlling for age, history of malignancy, and BMI (p=0.2). There was no association between BAT and total femoral CSA (p=0.4). There was a positive correlation between BAT and thigh SAT CSA (r=0.28, p=0.009), independent of age, history of malignancy, and BMI (p=0.03). There was no association between BAT and thigh muscle CSA (p=0.5).There was no association between BAT volume and age in the group as a whole (p=0.7). However, in the group without history of malignancy, there was a trend toward an inverse association between BAT and age (r= −0.40, p=0.08) while no such association was found in the subjects with history of malignancy (p=0.7). There was a trend of a positive correlation between BAT volume and BMI (r= 0.18, p= 0.07) in the group as a whole; however, within the groups with and without history of malignancy, the association was no longer significant (p=0.2 for both associations). Muscle area correlated positively with total and cortical femoral CSA (r= 0.68 and 0.67, p≤0.0001).
When total femoral CSA was entered as a dependent variable and BAT volume, age and BMI as independent variables in a forward stepwise regression model, BAT volume was the only predictor of femoral density (p=0.03).
When femoral cortical CSA was entered as a dependent variable and BAT volume, age and BMI as independent variables, BAT volume was the only predictor of femoral CSA (p=0.01).
The addition of muscle CSA as an independent variable in both models significantly decreased the contribution of BAT and muscle CSA became the only predictor of femoral bone density and femoral CSA.
3. Discussion
Our study is the first to show a positive association between BAT and femoral bone structure and muscle area in adult patients. This association was both age and BMI independent. BAT volume was a positive predictor of both total femoral CSA and femoral cortical CSA. In addition we demonstrate a positive effect of BAT on thigh muscle and thigh SAT.
Recent studies have shown a possible link between BAT and bone formation (1-4, 12-14). Using FoxC2AD+/Tg mice, a well-established model for induction of BAT, Rahman et al demonstrated that BAT produces factors that induce osteoblast differentiation and osteocyte support for bone formation and bone turnover (13). In addition, bone morphogenetic protein (BMP) 7, a member of the BMP family, which are stimulators of osteoblastogenesis, has been identified as a promoter of BAT differentiation through suppression of early adipogenic inhibitors, such as Pref-1 (14, 15). We have previously shown higher BMD and lower Pref-1 levels in young non-obese BAT positive women compared to BAT negative women (1) suggesting that BAT may be involved in the regulation of stem cell differentiation into the bone lineage at the expense of adipogenesis.
In an animal model of heterotopic ossification, injection of BMP2 into mouse muscle triggered a BAT induced hypoxic gradient leading to bone formation (12), implying a link between BAT, muscle and bone. A common origin of BAT and skeletal muscle precursors has been identified, which can give rise to both skeletal muscle and BAT (5-8). Concordant with this, Gilsanz et al found increased muscle volume in children with positive BAT compared to children without BAT, independent of age, sex, and BMI (16). We did not find an association between muscle and BAT in our prior study in young, non-obese women, which may have been due to the low range of muscle areas as all of our women were thin (1). In the current study, we therefore studied patients with a wide range of BMI and muscle areas and show a positive correlation between BAT and muscle in a large cohort of female and male patients. Importantly, there were positive correlations between BAT and femoral bone structure, independent of age, BMI, and history of malignancy. However, despite the significant correlations the variances contributed by BAT suggest a small effect size.
Interestingly, we also observed a positive correlation between BAT and thigh SAT. Although abdominal fat accumulation is associated with increased cardiometabolic risk, extremity SAT appears to be relatively protective (17, 18). The increased cardiometabolic risk of abdominal vs. extremity SAT has been attributed in part to higher expression of inflammatory cytokines within abdominal fat and elevated portal vein free fatty acid levels (19, 20), while extremity SAT acts as a buffer for dietary lipid influx, protecting other tissues from lipid overflow (18).
Separate analyses in men and women showed more BAT in men and stronger correlations between femoral structure and BAT in men compared to women, despite smaller number of male subjects in our study. This suggests that the BAT-bone connection might be stronger in men. However, larger studies will be necessary to confirm these findings.
We did not find an inverse association between BAT and age in the group as a whole as has been previously reported (11); however, there was an inverse association between BAT and age in the group without history of malignancy. This suggests that the history of malignancy might influence such an association. We found a trend towards a positive association between BAT and BMI in the group as a whole; however, within the groups with and without history of malignancy, the association was no longer significant, which also suggests that the history of malignancy might influence such an association.
Our study had several limitations. First is the retrospective nature of our study and second the heterogeneous subject population. However, we only included patients who had no history of malignancy or were successfully treated for malignancy and had no evidence of active disease at the time of PET/CT, but we acknowledge that a history of malignancy likely plays a role in the relationship between BAT and bone We also excluded patients with chronic disease or medication use that could influence bone metabolism. In addition, we controlled for disease status (history of malignancy) in our regression analyses. Strengths of our study include the large number of subjects and detailed quantification of BAT, femoral structure and thigh body composition.
In conclusion, our study shows positive correlations between BAT and femoral bone structure as well as muscle and thigh fat in adults with and without a history of malignancy, providing further evidence that BAT is involved in the regulation of bone formation, possibly mediated by muscle.
Highlights.
Brown adipose tissue (BAT) is a positive predictor of femoral bone structure.
BAT correlates positively with thigh muscle and thigh subcutaneous fat. Thigh fat is thought to be protective of cardiovascular risk
Acknowledgments
Funding: This work was supported in part by National Institutes of Health Grant R24 DK084970
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflict of interest to declare.
References
- 1.Bredella MA, Fazeli PK, Freedman LM, et al. Young women with cold-activated brown adipose tissue have higher bone mineral density and lower Pref-1 than women without brown adipose tissue: a study in women with anorexia nervosa, women recovered from anorexia nervosa, and normal-weight women. J Clin Endocrinol Metab. 2012;97:E584–590. doi: 10.1210/jc.2011-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredella MA, Fazeli PK, Lecka-Czernik B, Rosen CJ, Klibanski A. IGFBP-2 is a negative predictor of cold-induced brown fat and bone mineral density in young non-obese women. Bone. 2013;53:336–339. doi: 10.1016/j.bone.2012.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee P, Brychta RJ, Collins MT, et al. Cold-activated brown adipose tissue is an independent predictor of higher bone mineral density in women. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ponrartana S, Aggabao PC, Hu HH, Aldrovandi GM, Wren TA, Gilsanz V. Brown adipose tissue and its relationship to bone structure in pediatric patients. J Clin Endocrinol Metab. 2012;97:2693–2698. doi: 10.1210/jc.2012-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elabd C, Chiellini C, Carmona M, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009;27:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 6.Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell Metab. 2010;11:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilsanz V, Smith ML, Goodarzian F, Kim M, Wren TA, Hu HH. Changes in brown adipose tissue in boys and girls during childhood and puberty. J Pediatr. 2012;160:604–609. e601. doi: 10.1016/j.jpeds.2011.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaras L, Tal I, Palmer MR, Parker JA, Kolodny GM. Shareware program for nuclear medicine and PET/CT PACS display and processing. AJR Am J Roentgenol. 2007;188:W565–568. doi: 10.2214/AJR.06.1058. [DOI] [PubMed] [Google Scholar]
- 11.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olmsted-Davis E, Gannon FH, Ozen M, et al. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–632. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahman S, Lu Y, Czernik PJ, Rosen CJ, Enerback S, Lecka-Czernik B. Inducible Brown Adipose Tissue, or Beige Fat, Is Anabolic for the Skeleton. Endocrinology. 2013 doi: 10.1210/en.2012-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng YH, Kokkotou E, Schulz TJ, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Schulz TJ, Espinoza DO, et al. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilsanz V, Chung SA, Jackson H, Dorey FJ, Hu HH. Functional brown adipose tissue is related to muscle volume in children and adolescents. J Pediatr. 158:722–726. doi: 10.1016/j.jpeds.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snijder MB, van Dam RM, Visser M, Seidell JC. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol. 2006;35:83–92. doi: 10.1093/ije/dyi253. [DOI] [PubMed] [Google Scholar]
- 18.Snijder MB, Visser M, Dekker JM, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–308. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 19.Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. [PubMed] [Google Scholar]
- 20.Poulain-Godefroy O, Lecoeur C, Pattou F, Fruhbeck G, Froguel P. Inflammation is associated with a decrease of lipogenic factors in omental fat in women. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1–7. doi: 10.1152/ajpregu.00926.2007. [DOI] [PubMed] [Google Scholar]