Abstract
The objective of this review is to point out some important facts that we don’t know about endogenous cannabinoids — lipid-derived signaling molecules that activate CB1 cannabinoid receptors and play key roles in motivation, emotion and energy balance. The first endocannabinoid substance to be discovered, anandamide, was isolated from brain tissue in 1992. Research has shown that this molecule is a bona fide brain neurotransmitter involved in the regulation of stress responses and pain, but the molecular mechanisms that govern its formation and the neural pathways in which it is employed are still unknown. There is a general consensus that enzyme-mediated cleavage, catalyzed by fatty acid amide hydrolase (FAAH), terminates the biological actions of anandamide, but there are many reasons to believe that other as-yet-unidentified proteins are also involved in this process. We have made significant headway in understanding the second arrived in the endocannabinoid family, 2-arachidonoyl-sn-glycerol (2-AG), which was discovered three years after anandamide. Researchers have established some of the key molecular players involved in 2-AG formation and deactivation, localized them to specific synaptic components, and showed that their assembly into a multi-molecular protein complex (termed the ‘2-AG signalosome’) allows 2-AG to act as a retrograde messenger at excitatory synapses of the brain. Basic questions that remain to be answered pertain to the exact molecular composition of the 2-AG signalosome, its regulation by neural activity and its potential role in the actions of drugs of abuse such as Δ9-THC and cocaine.
When anandamide was isolated from pig brain in 1992 and identified as the first endogenous marijuana-like substance (Devane et al., 1992), the neuroscience community was quick to realize the significance of this finding (Barinaga, 1992). Only two years earlier, the molecular characterization of a cell-surface receptor that recognizes marijuana’s main psychoactive constituent, Δ9-tetrahydrocannabinol (Δ9-THC)(Devane et al., 1988; Matsuda et al., 1990), had finally answered the longstanding question of how this compound exerts its unique brand of pharmacological effects – a combination of euphoria, calmness, appetite stimulation, sensory alterations and analgesia (Iversen, 2000). The isolation of anandamide promised to unlock the door to the brain neurotransmitter system hijacked by Δ9-THC. Past experience with peptide transmitters suggested that this expectation was not unrealistic: for example, the identification of the enkephalins (Hughes et al., 1975) and their receptors (Pert and Snyder, 1973; Terenius, 1973) had quickly led to the anatomical mapping of opioidergic pathways in the central nervous system (CNS) (Pert et al., 1976), a key step toward uncovering the roles of opioid peptides in the regulation of brain function and behavior. Therefore, optimism that similar advances would ensue from the discovery of anandamide was reasonably justified.
Indeed, a great deal has been learned in the years following that discovery: there is now convincing evidence that anandamide is a bona fide neurotransmitter (Di Marzo et al., 1994; Giuffrida et al., 1999) involved in the control of synaptic plasticity in the amygdala (Puente et al., 2011), the modulation of stress responses (Bortolato et al., 2007; Dlugos et al., 2012; Gobbi et al., 2005; Gunduz-Cinar et al., 2012; Hill et al., 2010; Khaturia et al., 2003), and the processing of central and peripheral pain signals (Hohmann et al., 2005; Clapper et al., 2010). Despite this progress, however, we still don’t fully understand how anandamide is produced in neurons, in which neural pathways it acts as a neurotransmitter, and what physiological stimuli trigger its release.
Why has anandamide proved to be such a hard nut to crack? How does this lipid-derived molecule differ from 2-arachidonoyl-sn-glycerol (2-AG), a more recent addition to the endocannabinoid lipid family (Mechoulam et al., 1995; Sugiura et al., 1995) for which we have obtained, within a few years of its discovery, a reasonably detailed knowledge of metabolism (Bisogno et al. 2003; Dinh et al., 2001; Stella et al., 1997) and synaptic localization (Katona et al., 2006; Nyilas et al., 2009)? Answering this question may provide important insights, not only on the biochemical properties and signaling functions of anandamide, but also on endocannabinoid transmission at large.
2-AG formation – a new trick for an old dog
Neurons produce 2-AG through a multifunctional lipid pathway that starts with the cleavage of phosphatidylinositol-4,5-bisphosphate (PIP2) and ends (at least temporarily) with the transient accumulation of non-esterified arachidonic acid in cell membranes (Figure 1). Individual checkpoints along this route can each generate separate sets of signaling molecules (for review, see Piomelli et al, 2007).
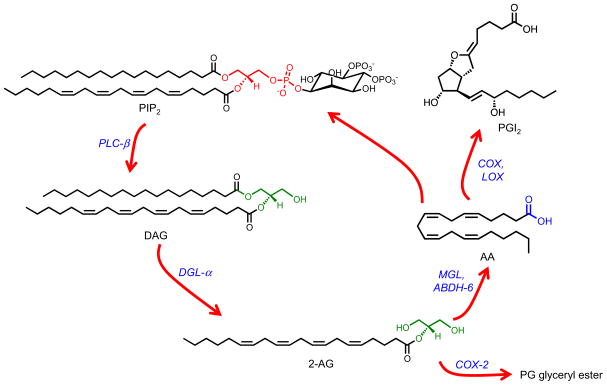
Figure 1. Formation and deactivation of 2-AG in brain neurons.
Receptor-operated phospholipase C-β (PLC-β) converts phosphatidylinositol-4,5-bisphosphate (PIP2) into 1,2-diacylglycerol (DAG). DAG is hydrolyzed by diacylglycerol lipase-α (DGL-α) forming 2-AG. 2-AG is subjected to hydrolytic cleavage catalyzed by either monoacylglycerol lipase (MGL) or α/β hydrolase domain-containing protein 6 (ABHD-6). Additionally, 2-AG can be dioxygenated by cyclooxygenase-2 (Cox-2) to yield a family of non-endocannabinoid prostaglandin (PG) glyceryl esters. Free arachidonic acid (AA) is converted into the eicosanoid family of compounds, for example prostacyclin (PGI2), by cyclooxygenase or lipoxygenase enzymes.
The first checkpoint is represented by the β–isoform of phospholipase C (PLC-β), which can be activated by various G protein-coupled receptors (e.g. the type-1 metabotropic glutamate receptor, mGluR5) and converts PIP2 into the second messenger 1,2-diacylglycerol (DAG)(Bennett et al., 1988). DAG regulates the activity of protein kinase C and other cellular effectors, but also serves as substrate for two functionally distinct enzymes: diacylglycerol kinase, which generates the intracellular signaling lipid (and metabolic intermediate), phosphatidic acid; and diacylglycerol lipase-α (DGL-α), which hydrolyses DAG forming 2-AG (Bisogno et al., 2003; Stella et al., 1997). In addition to serving as an endocannabinoid agonist, 2-AG can be also dioxygenated by cyclooxygenase-2 (Cox-2) to yield a family of prostaglandin (PG) glyceryl esters, which do not engage cannabinoid receptors yet display significant biological activities (Kozak et al., 2000). Moreover, 2-AG can be cleaved by hydrolases such as monoacylglycerol lipase (MGL) (Dinh et al., 2001) to produce non-esterified arachidonic acid (Figure 1). Like other polyunsaturated fatty acids, free arachidonate is either immediately reinserted into membrane phospholipids (part of a process known as ‘phospholipid remodeling’) or utilized for the production of eicosanoids (e.g., Cox-2-derived prostaglandins) (Piomelli et al, 2007).
The key reactions in the multifunctional pathway outlined above – and particularly the sequential contributions of PLC, DGL and MGL activities to the release of arachidonic acid from membrane phospholipids – have been known for decades (Allen et al., 1992; Bell et al., 1979). This prior knowledge greatly facilitated the molecular cloning of the main enzymes involved in 2-AG production (Bennett et al., 1988; Bisogno et al., 2003; Dinh et al., 2001), which in turn made possible the identification of the supra-molecular protein complex (‘signalosome’) that enables 2-AG signaling at excitatory synapses of the brain (Jung et al., 2012a). We will come back to the possible functions of the 2-AG signalosome later on in this article.
The trouble with anandamide
In contrast with 2-AG, the reactions leading to the production of anandamide are relatively unprecedented in lipid biochemistry. This state of affairs is not surprising, if we consider that anandamide and other members of its chemical family – the amides of ethanolamine with long-chain fatty acids (known as N-acylethanolamines or fatty acid ethanolamides [FAEs]) – were initially dismissed as being terminal products of post mortem tissue degradation rather than physiologically meaningful signaling molecules (Schmid et al., 1995; Kempe et al., 1996). Indeed, the functional significance of anandamide remained controversial until the mechanisms underlying the production and deactivation of this compound were outlined using primary cultures of rat brain neurons (Cadas et al., 1996; Cadas et al., 1997; Di Marzo et al., 1994) and its activity-dependent release in the CNS was demonstrated by microdialysis in freely moving rats (Giuffrida et al., 1999).
Figure 2 illustrates the three enzyme pathways that have been implicated so far in the mobilization of anandamide. The ‘canonical route’, shown in the center of the figure, was elaborated in 1994–1997 (Cadas et al., 1996; Cadas et al., 1997; Di Marzo et al., 1994) based on work conducted on non-endocannabinoid ethanolamides of saturated and monounsaturated fatty acids, such as oleoylethanolamide (OEA) and palmitoylethanolamide (PEA) (reviewed in Piomelli, 2013; Schmid et al., 1990). This model postulates that anandamide – which incorporates in its structure the polyunsaturated fatty acid, arachidonate – is released by hydrolysis of the phospholipid precursor, N-arachidonoyl-phosphatidylethanolamine (N-arachidonoyl-PE), catalyzed by a unique phospholipase D (PLD) that preferentially recognizes N-acyl-substituted PE species (collectively called NAPEs) over other more common phospholipids (reviewed in Ueda et al., 2013).
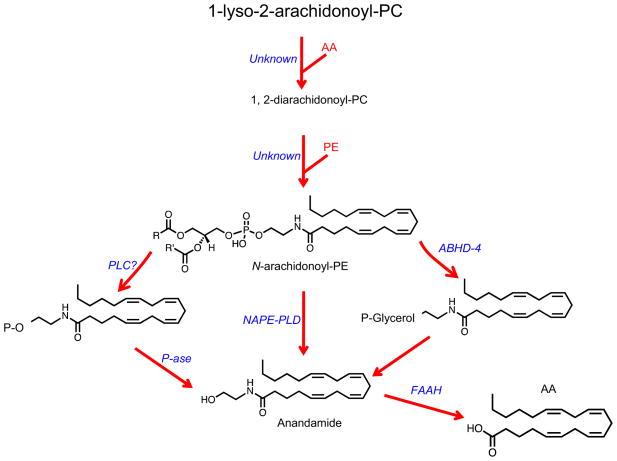
Figure 2. Formation and deactivation of anandamide in brain neurons.
The ‘canonical route’ of anandamide biosynthesis is shown in the center. According to this model, anandamide is released by hydrolysis of the phospholipid precursor, N-arachidonoyl-phosphatidylethanolamine (N-arachidonoyl-PE), catalyzed by a selective phospholipase D (PLD). N-arachidonoyl-PE is produced through a two-step reaction in which arachidonic acid (AA) is transferred from the sn-2 position of a phospholipid to the sn-1 position of lyso-phosphatidylcholine (PC), producing diarachidonoyl-PC. The sn-1 arachidonoyl chain of diarachidonoyl-PC is then transferred to the free amino group of PE, generating N-arachidonoyl-PE. Two additional routes of anandamide biosynthesis have been described. Left: an as-yet-uncharacterized phospholipase C (PLC) converts N-arachidonoyl-PE into phospho-anandamide, which is then dephosphorylated by a phosphatase (P-ase) forming anandamide. Right: N-arachidonoyl-PE is hydrolyzed by α/β hydrolase domain-containing protein 4 (ABHD-4), forming glycerophospho-anandamide, which generates anandamide after losing the glycerophosphate group. Anandamide is degraded intracellularly by the serine amidase, fatty acid amide hydrolase (FAAH).
The levels of NAPEs containing saturated and monounsaturated fatty acyl chains at their amino position, which do not generate anandamide, can be readily measured in non-stimulated neurons (Astarita and Piomelli, 2009; Cadas et al., 1997; Di Marzo et al., 1994). By contrast, concentrations of the anandamide precursor, N-arachidonoyl-PE, are vanishingly low under resting conditions, but rapidly and transiently increase when neurons are exposed to stimuli that elevate intracellular calcium levels (Cadas et al., 1996; Cadas et al., 1997; Di Marzo et al., 1994; Stella and Piomelli, 2001). For N-arachidonoyl-PE biosynthesis to occur, however, a sequence of two distinct enzyme-mediated reactions must take place. First, arachidonic acid must be transferred from the sn-2 position of various phospholipids, where it normally resides, to the sn-1 position of lyso-phosphatidylcholine (PC), producing a low-abundance PC species that incorporates arachidonic acid at both sn-1 and sn-2 positions. The newly formed diarachidonoyl-PC quickly gives away its sn-1 acyl chain to the free amino group of PE, generating N-arachidonoyl-PE (Figure 2). The sequential formation of diarachidonoyl-PC and N-arachidonoyl-PE is strictly calcium-dependent and represents the rate-limiting step in the production of anandamide by intact neurons (Cadas et al., 1997). This reaction also provides the only known selectivity filter in anandamide formation, because NAPE-PLD, which catalyzes the next and last step in this cascade, does not distinguish among NAPEs that contain different N-acyl substituents (Ueda et al., 2013). Despite their functional importance, the enzymes (or enzyme) responsible for the concerted production of diarachidonoyl-PC and N-arachidonoyl-PE have not been identified yet (Ueda et al., 2013). This knowledge gap thwarts our attempts to understand the physiological mechanisms governing anandamide release, and denies us the possibility to define neuronal populations in the CNS that utilize this lipid substance as a neurotransmitter.
Detours in the path to anandamide
It is quite common for lipid-derived messengers to be produced through multiple biogenetic pathways and anandamide does not make an exception to this rule (Piomelli et al., 2007). Two detours from its canonical biosynthesis have been proposed, both of which utilize N-arachidonoyl-PE as a starting point and substitute NAPE-PLD with different lipid hydrolases (Figure 2).
Macrophages exposed to the bacterial toxin, lipopolysaccharide (LPS), emit a burst of lipid mediators that include anandamide (Liu et al., 2006) along with other bioactive derivatives of arachidonic acid (Dennis et al., 2010). This response is unlikely to be mediated by NAPE-PLD – the expression of which is, in fact, strongly suppressed by LPS (Zhu et al., 2011) – but rather appears to require the consecutive action of two as-yet-uncharacterized enzymes: a PLC activity that converts N-arachidonoyl-PE into phospho-anandamide, and a phosphatase activity that cleaves the latter into anandamide and free phosphate (Liu et al., 2006) (Figure 2). In addition to this PLC-initiated mechanism, N-arachidonoyl-PE may be also subjected to a double deacylation at its sn-1 and sn-2 positions, catalyzed by the enzyme α/β hydrolase domain-containing protein 4 (ABHD-4), to produce glycerophospho-anandamide (Simon and Cravatt, 2006). This intermediate may be transformed into anandamide by cleavage of its phosphodiester bond, catalyzed by the phosphodiesterase GDE1, and release of glycerol phosphate (Simon and Cravatt, 2008).
The existence of multiple routes of anandamide production might reflect the diversity of physiological stimuli that are able to mobilize this endocannabinoid – which include, in neural cells, membrane depolarization, intracellular calcium transients, and dopamine D2 receptor activation (Di Marzo et al., 1994; Giuffrida et al., 1999; Liu et al., 2006; Lourenço et al., 2011; Steffens et al., 2003; Stella and Piomelli, 2001). However, it is important to point out that each of the three pathways described above attributes a central place to the enzyme system that catalyzes the formation of diarachidonoyl-PC and N-arachidonoyl-PE. Since this is the only system that directs FAE biosynthesis toward the selective production of anandamide, its molecular characterization will be as crucial to our understanding of endocannabinoid signaling as the identification of tyrosine and tryptophan hydroxylases was to the elucidation of aminergic transmission.
Anandamide degradation
The molecular mechanisms utilized by neural cells to degrade anandamide are reasonably well understood. The compound is a preferred endogenous substrate for the intracellular serine amidase, fatty acid amide hydrolase (FAAH), which catalyzes the cleavage of various long-chain fatty acid amides (Cravatt et al., 1996; Désarnaud et al., 1995; Hillard et al., 1995; Ueda et al., 1995a). Other lipid hydrolases, such as N-acylethanolamine acid amidase (NAAA) (Ueda et al., 2010) and acid ceramidase (AC) (Li et al., 1998; Park and Schuchman, 2006) are also able to hydrolyze lipid amide bonds, but show little or no affinity for anandamide and are unlikely to play an important role in its deactivation. Consistent with this view, genetic or pharmacological interventions that interrupt FAAH activity cause a profound enhancement in anandamide-mediated signaling at CB1 cannabinoid receptors (Cravatt et al, 2001; Kathuria et al., 2003), whereas blockade of NAAA or AC exerts no such effect (Realini et al., 2013; Sasso et al., 2012). Anandamide can be also metabolized by lipoxygenases and cyclooxygenases to oxygen-containing products that display significant biological activities at non-cannabinoid targets (Ueda et al., 1995b; Starovicz et al., 2013; Woodward et al., 2008). An important unanswered question is whether these reactions represent an alternative path for anandamide deactivation (Kim and Alger, 2004) or, rather, provide a mechanism for the generation of distinct classes of lipid mediators (Starovicz et al., 2013; Woodward et al., 2008).
2-AG degradation
We mentioned before that the serine lipase MGL catalyzes the cleavage of 2-AG into glycerol and arachidonic acid, which is then recycled into membrane phospholipids or transformed into the eicosanoid family of lipid mediators (Piomelli et al., 2007). There is a general consensus that MGL accounts for the majority of the 2-AG-hydrolyzing activity present in the rodent brain (Blankmann et al., 2007; Dinh et al., 2004). This assessment is supported by converging results obtained with MGL inhibitors such as URB602 (Hohmann et al., 2005) and JZL-184 (Long et al., 2009), MGL-deficient mice (Schlosburg et al., 2010) and genetically modified mice that over-express MGL (Jung et al., 2012b). Two additional serine hydrolases, ABDH-6 and ABDH-12 (Blankmann et al., 2007), are also able to catalyze the hydrolysis of 2-AG in broken cell preparations, but their roles in terminating the actions of 2-AG in live animals are uncertain. Evidence confirming such a role has been obtained for ABDH-6, the inhibition of which prolongs the effects of 2-AG at brain synapses (Jung et al., 2012a; Marrs et al., 2010). By contrast, recent data indicate that ABDH-12 is primarily involved in the degradation of lysophosphatidylserine, rather than 2-AG (Blankman et al., 2013). In addition to MGL-mediated hydrolysis, 2-AG is metabolized by Cox-2 to form bioactive PG glyceryl esters (Kozak et al., 2000). Thus, as in the case of anandamide, it appears that neural cells can steer 2-AG toward alternative fates of metabolic activation (e.g., formation of PG glyceryl esters) or deactivation (e.g., hydrolysis followed by arachidonic acid re-esterification into phospholipids). The selection between these two paths is likely to depend on the cells’ signaling needs, but how such selection is made and enforced is entirely unknown.
Accessing the degradation machinery
The endocannabinoids are released into the extracellular medium (Buczynski et al., 2013; Giuffrida et al., 1999) and exert the majority of their effects by binding to CB1 receptors present on the surface of presynaptic nerve terminals (Mackie, 2008). How do these lipid substances come in contact with the enzymes responsible for their degradation? It turns out that answering this question is, again, relatively straightforward for 2-AG, but much more complex (and controversial) for anandamide.
In the brain, MGL is primarily found in axon terminals and is almost equally distributed between the cytosol and the inner aspect of the presynaptic cell membrane (Dinh et al., 2001; Dinh et al., 2004; Gulyas et al., 2004). This localization indicates that MGL can readily access the pool of 2-AG that interacts with CB1 receptors and, therefore, can efficiently catalyze the hydrolysis of this compound at its main site of action. Another important enzyme may assist MGL in completing this task: pharmacological studies in a neural cell line suggest that the MGL-mediated degradation of 2-AG is driven by the subsequent condensation of arachidonic acid with coenzyme A (Beltramo et al. 2000), a pivotal step in phospholipid remodeling catalyzed by acyl-CoA synthetase (Farooqui et al., 2000).
Unlike MGL, FAAH is found in dendritic spines, where the enzyme resides in the membrane of mitochondria and other intracellular organelles (Gulyas et al., 2004). How does anandamide cover the distance that separates its site of action (presynaptic CB1 receptors) from its site of degradation (postsynaptic organelles)? Like other lipid molecules, anandamide can diffuse passively through the lipid bilayer, but there is substantial evidence that this process is accelerated by a rapid and selective carrier system present in both neurons and glial cells. This system exhibits three identifying features of a carrier-mediated transport. First, saturation kinetics: plots of the initial rate of [3H]anandamide accumulation against extracellular anandamide concentrations yield apparent Michaelis constants that are similar to those measured for other known neurotransmitter uptake systems (Beltramo et al., 1997; Piomelli et al., 1999). Second, substrate specificity: rat brain neurons and other cells in culture internalize [3H]anandamide, but not closely related analogs (Beltramo et al., 1997; Piomelli et al., 1999). Third, selective inhibition: a variety of natural and synthetic compounds, including chiral analogs of anandamide, block [3H]anandamide transport in a competitive and stereospecific manner (Di Marzo et al., 2004; Piomelli et al., 1999). Moreover, anandamide transport is clearly distinct from FAAH, since FAAH inhibitors do not interfere with its activity (Beltramo et al., 1997; Fegley et al., 2004). Even though this pharmacological evidence supports the existence of a selective anandamide transport system, the molecular identity of the transporter (or transporters) involved is still controversial. Several intracellular proteins that bind anandamide and might facilitate its movements across the cytosol, have been identified. However, the contribution of these proteins to the termination of anandamide signaling remains disputed (Fu et al., 2012; Berger et al., 2012)
A diffuse release mechanism?
The phospholipids that serve as precursors for anandamide and 2-AG can undergo rapid lateral movements in cell membranes. This high degree of mobility raises the question of whether endocannabinoid production might occur throughout the surface of neuronal cells. This possibility, though inconsistent with classical synaptic transmission, is not unprecedented in brain signaling. Modulatory substances such as adenosine and nitric oxide, which are not stored in synaptic vesicles, are thought to be released from non-synaptic sites and to act as diffusible autocrine or paracrine messengers. The slow time-course of anandamide production, which typically unfolds over a period of many minutes, suggests that this compound might operate through a similar mechanism (Giuffrida et al., 1999; Hohmann et al., 2005). The preferred localization of NAPE-PLD to presynaptic nerve endings (Egertova et al., 2008) does not negate this hypothesis because, as discussed above, it is still unclear whether NAPE-PLD is necessary for anandamide production.
Irrespective of whether it might be utilized by anandamide or not, the diffuse release mechanism outlined above is incompatible with endocannabinoid-mediated retrograde signaling at central synapses, which is known to be subjected to strict spatial and temporal constraints (Castillo, 2012; Katona and Freund, 2012). There is growing evidence, indeed, that this rapid, point-to-point signaling process is mediated by 2-AG, rather than anandamide, and is made possible by the existence of an anatomically defined structure, the ‘2-AG signalosome’, dedicated to the production and release of this endocannabinoid (Figure 3).
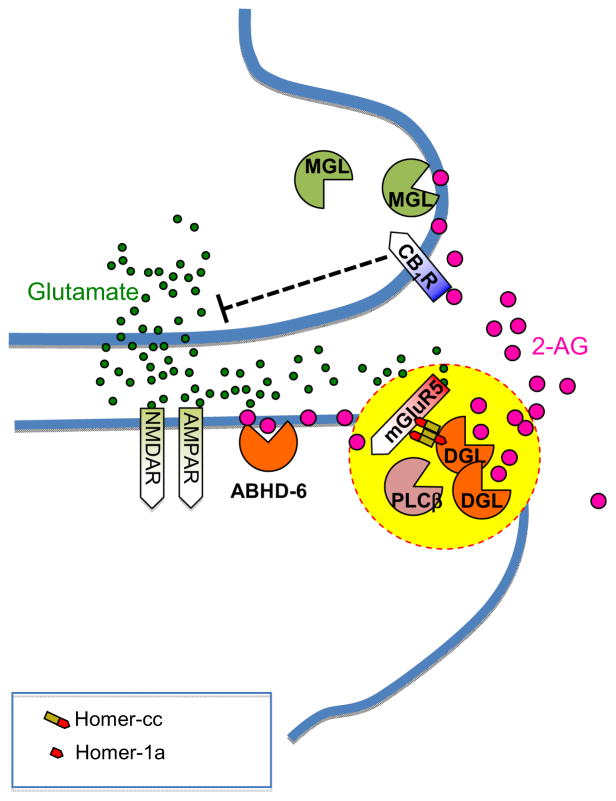
Figure 3. Schematic model of the 2-AG signalosome.
This supra-molecular complex, selectively localized to the perisynaptic zone of the dendritic spine, connects in a single functional unit three key proteins involved in 2-AG production at excitatory synapses of the brain – mGluR5 metabotropic glutamate receptors, phospholipase C-β (PLC-β) and diacylglycerol-α (DGL-α). Evidence indicates that these proteins may be held together by the scaffolding proteins Homer-cc and Homer 1a. The proximity of mGluR5 to PLC-β and DGL-α allows for the rapid accumulation of 2-AG, which travels across the synaptic cleft to activate CB1 receptors on axon terminals. The 2-AG that reaches presynaptic terminals may be quickly hydrolyzed by monoacylglycerol lipase (MGL), while the 2-AG that fails to reach the terminals may be degraded by α/β hydrolase domain-containing protein 6 (ABHD-6). Other abbreviations: AMPAR, AMPA receptors; NMDAR, NMDA receptors.
The 2-AG signalosome was identified at excitatory synapses of the ventral striatum and prefrontal cortex (Jung et al., 2012a), but is likely to be present in other regions of the mammalian CNS (Katona et al., 2006; Matyas et al., 2008; Nyilas et al., 2009). Selectively localized to a portion of the dendritic spine that borders the postsynaptic density, this supra-molecular complex connects in a single functional unit, held together by Homer proteins, three key players in 2-AG production: mGluR5, PLC-β and DGL-α (Jung et al., 2007; Jung et al., 2012a). When glutamate secreted by excitatory axon terminals binds to mGluR5, the physical proximity of this receptor to PLC-β and DGL-α enables the rapid hydrolysis of preformed PIP2 and the local accumulation of 2-AG. Driven by this concentration spike, 2-AG escapes the postsynaptic membrane, crosses the synaptic cleft and engages CB1 receptors on axon terminals, reducing calcium channel activity and suppressing glutamate release (Mackie, 2008). The pool of 2-AG that reaches presynaptic terminals can be quickly degraded by MGL (Dinh et al., 2001; Dinh et al., 2004; Gulyas et al., 2004), while the portion that remains associated with the spine is probably cleared by ABHD-6 (Marrs et al., 2010) (Figure 3). It is important to point out that this structural arrangement is restricted to excitatory synapses, which contain relatively low levels of CB1 receptors (Marsicano and Lutz, 1999). Inhibitory synapses formed by cholecystokinin-containing GABAergic interneurons – where retrograde signaling has been also demonstrated (Castillo et al., 2012; Katona and Freund, 2012) and CB1 is extremely abundant (Katona et al., 1999) – must regulate endocannabinoid signaling through a distinct mechanism, which remains unknown.
Conclusions
Twenty years after the discovery of anandamide, many aspects of the natural history of this substance remain mysterious. The intracellular enzyme responsible for its deactivation, FAAH, has been identified (Cravatt et al., 1996; Désarnaud et al., 1995; Hillard et al., 1995; Ueda et al., 1995) and potent and selective inhibitors of its activity have been disclosed (for review, see Piomelli, 2005). This has made possible, in turn, to unmask important functions served by anandamide in the control of stress-coping responses (Bortolato et al., 2007; Dlugos et al., 2012; Gobbi et al., 2005; Gunduz-Cinar et al., 2012; Hill et al., 2010; Khaturia et al., 2003) and pain initiation (Clapper et al., 2010; Hohmann et al., 2005). Despite these advances, we still need to define the enzyme system through which anandamide is produced in neurons, the physiological stimuli that trigger its formation, and the neural pathways that utilize it as a modulatory transmitter. Without this information, our understanding of anandamide-mediated endocannabinoid signaling will remain tentative.
Thanks to decades of research on the biochemistry of arachidonic acid, we know a lot more about 2-AG than we do about anandamide. The enzymes involved in the production and deactivation of this glycerol ester have been molecularly cloned (Bisogno et al., 2003; Dinh et al., 2004) and substantial progress has been made on the localization of these proteins in the CNS (Castillo et al., 2012; Katona and Freund, 2012). Selective chemical probes targeting 2-AG elimination have also been disclosed (Hohmann et al., 2005; Long et al., 2009). Nevertheless, several fundamental questions remain unanswered. Most urgent among them, possibly, are those pertaining to the organization of the 2-AG signalosome. What is the exact molecular composition of this signaling complex, and how is it assembled? Can neural activity alter its organization? In addition to mGluR5, do other neurotransmitter receptors coupled to PLC-β activation (e.g., certain muscarinic receptor subtypes) form signalosome-like structures at CNS synapses? And, last but not least, are the effects of drugs of abuse such as Δ9-THC and cocaine linked to short-term or long-term changes in the structure and function of this complex? As we start addressing these questions, we can be sure of one thing: the endocannabinoids are still not done surprising us.
Highlights.
This article outlines several unanswered questions about the endocannabinoid system
The enzymes involved in anandamide biosynthesis are unknown
This knowledge gap makes it difficult to localize neurons that use anandamide as a transmitter
The enzymes involved in 2-AG biosynthesis and degradation have been identified
2-AG production is localized to a signaling complex, whose exact composition remain unknown
Acknowledgments
Work in the Piomelli lab is funded by NIDA grants R01 DA012413 and DP1 DA031387.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen AC, Gammon CM, Ousley AH, McCarthy KD, Morell P. Bradykinin stimulates arachidonic acid release through the sequential actions of an sn-1 diacylglycerol lipase and a monoacylglycerol lipase. 1992;58:1130–1139. doi: 10.1111/j.1471-4159.1992.tb09372.x. [DOI] [PubMed] [Google Scholar]
- Astarita G, Piomelli D. Lipidomic analysis of endocannabinoid metabolism in biological samples. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2755–2767. doi: 10.1016/j.jchromb.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barinaga M. Pot, heroin unlock new areas for neuroscience. Science. 1992;258:1882–1884. doi: 10.1126/science.1335165. [DOI] [PubMed] [Google Scholar]
- Bell RL, Kennerly DA, Stanford N, Majerus PW. Diglyceride lipase: a pathway for arachidonate release for human platelets. Proc Natl Acad Sci USA. 1979;76:3238–3241. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- Beltramo M, Piomelli D. Carrier-mediated transport and enzymatic hydrolysis of the endogenous cannabinoid 2-arachidonylglycerol. Neuroreport. 2000;11:1231–1235. doi: 10.1097/00001756-200004270-00018. [DOI] [PubMed] [Google Scholar]
- Bennet CF, Balcarek JM, Varricchio A, Crooke ST. Molecular cloning and complete amino-acid sequence of for a phosphoinositide-specific phospholipase C. 1988;334:268–270. doi: 10.1038/334268a0. [DOI] [PubMed] [Google Scholar]
- Berger WT, Ralph BP, Kaczocha M, Sun J, Balius TE, Rizzo RC, Haj-Dahmane S, Ojima I, Deutsch DG. Targeting fatty acid binding protein (FABP) anandamide transporters - a novel strategy for development of anti-inflammatory and anti-nociceptive drugs. PLoS One. 2012;7:e50968. doi: 10.1371/journal.pone.0050968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Long JZ, Trauger SA, Siuzdak G, Cravatt BF. ABHD12 controls brain lysophosphatidylserine pathways that are deregulated in a murine model of the neurodegenerative disease PHARC. Proc Natl Acad Sci USA. 2013;110:1500–1505. doi: 10.1073/pnas.1217121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Buczynski MV, Polis IY, Parsons LH. The volitional nature of nicotine exposure alters anandamide and oleoylethanolamide levels in the ventral tegmental area. Neuropsychopharmacology. 2013;38:574–584. doi: 10.1038/npp.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, Gaillet S, Beltramo M, Venance L, Piomelli D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J Neurosci. 1996;16:3934–3942. doi: 10.1523/JNEUROSCI.16-12-03934.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadas H, di Tomaso E, Piomelli D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J Neurosci. 1997;17:1226–1242. doi: 10.1523/JNEUROSCI.17-04-01226.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chávez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, Duranti A, Tontini A, Sanchini S, Sciolino NR, Spradley JM, Hohmann AG, Calignano A, Mor M, Tarzia G, Piomelli D. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–1270. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis EA, Deems RA, Harkewicz R, Quehenberger O, Brown HA, Milne SB, Myers DS, Glass CK, Hardiman G, Reichart D, Merril AH, Jr, Sullards MC, Wang E, Murphy RC, Raetz CRH, Garret TA, Guan Z, Ryan AC, Russel DW, McDonald JG, Thompson BM, Shaw WA, Sud M, Zhao Y, Gupta S, Maurya MR, Fahy E, Subramaniam S. A mouse macrophage lipidome. J Biol Chem. 2010;285:39976–39985. doi: 10.1074/jbc.M110.182915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désarnaud F, Cadas H, Piomelli D. Anandamide amidohydrolase activity in rat brain microsomes. Identification and partial characterization. J Biol Chem. 1995;270:6030–6035. doi: 10.1074/jbc.270.11.6030. [DOI] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. 1988;34:605–613. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DiMarzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, Sensi SL, Kathuria S, Piomelli D. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci USA. 2002;99:10819–10824. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh TP, Kathuria S, Piomelli D. RNA interference suggests a primary role for monoacylglycerol lipase in the degradation of the endocannabinoid 2-arachidonoylglycerol. Mol Pharmacol. 2004;66:1260–1264. doi: 10.1124/mol.104.002071. [DOI] [PubMed] [Google Scholar]
- Dlugos A, Childs E, Stuhr KL, Hillard CJ, de Wit H. Acute stress increases circulating anandamide and other N-acylethanolamines in healthy humans. Neuropsychopharmacology. 2012;37:2416–2427. doi: 10.1038/npp.2012.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertová M, Simon GM, Cravatt BF, Elphick MR. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 2008;506:604–615. doi: 10.1002/cne.21568. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA, Farooqui T. Deacylation and reacylation of neural membrane glycerophospholipids. J Mol Neurosci. 2000;14:123–135. doi: 10.1385/jmn:14:3:123. [DOI] [PubMed] [Google Scholar]
- Fegley D, Kathuria S, Mercier R, Li C, Goutopoulos A, Makriyannis A, Piomelli D. Anandamide transport is independent of fatty-acid amide hydrolase activity and is blocked by the hydrolysis-resistant inhibitor AM1172. Proc Natl Acad Sci U S A. 2004;101:8756–8761. doi: 10.1073/pnas.0400997101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Bottegoni G, Sasso O, Bertorelli R, Rocchia W, Masetti M, Guijarro A, Lodola A, Armirotti A, Garau G, Bandiera T, Reggiani A, Mor M, Cavalli A, Piomelli D. A catalytically silent FAAH-1 variant drives anandamide transport in neurons. Nat Neurosci. 2012;15:64–69. doi: 10.1038/nn.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodríguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas Al, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, Boscia F, Freund TF. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–458. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Gunduz-Cinar O, MacPherson KP, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie TS, Gorka AX, Alapaguja SO, Nikas SP, Makriyannis A, Poulton R, Patel S, Hariri AR, Caspi A, Moffit TE, Kunos G, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psych. 2012 Jun 12; doi: 10.1038/mp.2012.72. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McLaughlin RJ, Bingham B, Shrestha L, Lee T, TY, Gray JM, Hillard CJ, Gorzalka BB, Viau V. Endogenous cannabinoid signaling is essential for stress adaption. Proc Natl Acad Sci USA. 2010;107:9406–9411. doi: 10.1073/pnas.0914661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Wilkison DM, Edgemond WS, Campbell WB. Characterization of the kinetics and distribution of N-arachidonylethanolamine (anandamide) hydrolysis by rat brain. Biochim Biophys Acta. 1995;1257:249–256. doi: 10.1016/0005-2760(95)00087-s. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, Mangieri R, Krey JF, Walker JM, Holmes PV, Crystal JD, Duranti A, Tontini A, Mor M, Tarzia G, Piomelli D. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hugues J, Smith TW, Kosterlitz HW, Fothergill LA, Morgan BA, Morris HR. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–580. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- Iversen LL. The science of marijuana. Oxford University Press; 2000. [Google Scholar]
- Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–621. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- Jung KM, Sepers M, Henstridge CM, Lassalle O, Neuhofer D, Martin H, Ginger M, Frick A, DiPatrizio NV, Mackie K, Katona I, Piomelli D, Manzoni OJ. Uncoupling of the endocannabinoid signaling complex in a mouse model of fragile X syndrome. Nat Commun. 2012a;3:1080. doi: 10.1038/ncomms2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Clapper JR, Fu J, D’Agostino G, Guijarro A, Thongkham D, Avanesian A, Astarita G, DiPatrizio NV, Frontini A, Cinti S, Diano S, Piomelli D. 2-arachidonoylglycerol signaling in forebrain regulates systemic energy metabolism. Cell Metab. 2012b;15:299–310. doi: 10.1016/j.cmet.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlágh B, Sík A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Freund TF. Multiple functions of endocannabinoid signaling in the brain. Annu Rev Neurosci. 2012;35:529–558. doi: 10.1146/annurev-neuro-062111-150420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe K, Hsu FF, Bohrer A, Turk J. Isotope dilution mass spectrometric measurements indicate that arachidonylethanolamide, the proposed endogenous ligand of the cannabinoid receptor, accumulates in rat brain tissue post mortem but is contained at low levels in or is absent from fresh tissue. J Biol Chem. 1996;271:17287–17295. doi: 10.1074/jbc.271.29.17287. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–698. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–33749. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Li CM, Hong SB, Kopal G, He X, Linke T, Hou WS, Koch J, Gatt S, Sandhoff K, Schuchman EH. Cloning and characterization of the full-length cDNA and genomic sequences encoding murine acid ceramidase. Genomics. 1998;50:267–274. doi: 10.1006/geno.1998.5334. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci USA. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenço J, Matias I, Marsicano G, Mulle C. Pharmacological activation of kainate receptors drives endocannabinoid mobilization. J Neurosci. 2011;31:3243–3248. doi: 10.1523/JNEUROSCI.3512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–4. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, Coy J, Bodor AL, Muccioli GG, Hu SS, Woodruff G, Fung S, Lafourcade M, Alexander JP, Long JZ, Li W, Xu C, Möller T, Mackie K, Manzoni OJ, Cravatt BF, Stella N. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–957. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Lutz B. Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur J Neurosci. 1999;11:4213–4225. doi: 10.1046/j.1460-9568.1999.00847.x. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Mátyás F, Urbán GM, Watanabe M, Mackie K, Zimmer A, Freund TF, Katona I. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, Hohmann AG, Katona I. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29:1964–1978. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Schuchman EH. Acid ceramidase and human disease. Biochim Biophys Acta. 2006;1758:2133–2138. doi: 10.1016/j.bbamem.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Pert CB, Snyder SH. Opiate receptor: demonstration in nervous tissue. Science. 1973;179:1011–1014. doi: 10.1126/science.179.4077.1011. [DOI] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc Natl Acad Sci U S A. 73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D, Beltramo M, Glasnapp S, Lin SY, Goutopoulos A, Xie XQ, Makriyannis A. Structural determinants for recognition and translocation by the anandamide transporter. Proc Natl Acad Sci U S A. 1999;96:5802–5807. doi: 10.1073/pnas.96.10.5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli D. The endocannabinoid system: a drug discovery perspective. Curr Opin Investig Drugs. 2005;6:672–679. [PubMed] [Google Scholar]
- Piomelli D, Astarita G, Rapaka R. A neuroscientist’s guide to lipidomics. Nat Rev Neurosci. 2007;8:743–754. doi: 10.1038/nrn2233. [DOI] [PubMed] [Google Scholar]
- Piomelli D. A fatty gut feeling. Trends Endocrinol Metab. 2013 Apr 5; doi: 10.1016/j.tem.2013.03.001. pii: S1043–2760(13)00047–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puente N, Cui Y, Lassalle O, Lafourcade M, Georges F, Venance L, Grandes P, Manzoni OJ. Polymodal activation of the endocannabinoid system in the extended amygdala. Nat Neurosci. 2011;14:1542–1549. doi: 10.1038/nn.2974. [DOI] [PubMed] [Google Scholar]
- Realini N, Solorzano C, Pagliuca C, Pizzirani D, Armirotti A, Luciani R, Costi MP, Bandiera T, Piomelli D. Discovery of highly potent acid ceramidase inhibitors with in vitro tumor chemosensitizing activity. Sci Rep. 2013;3:1035. doi: 10.1038/srep01035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasso O, Moreno-Sanz G, Martucci C, Realini N, Dionisi M, Mengatto L, Duranti A, Tarozzo G, Tarzia G, Mor M, Bertorelli R, Reggiani A, Piomelli D. Antinociceptive effects of the N-acylethanolamine acid amidase inhibitor ARN077 in rodent pain models. Pain. 2013;154:350–360. doi: 10.1016/j.pain.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, Kinsey SG, Nguyen PT, Ramesh D, Booker L, Burston JJ, Thomas EA, Selley DE, Sim-Selley LJ, Liu QS, Lichtman AH, Cravatt BF. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid HH, Schmid PC, Natarajan V. N-acylated glycerophospholipids and their derivatives. Prog Lipid Res. 1990;29:1–43. doi: 10.1016/0163-7827(90)90004-5. [DOI] [PubMed] [Google Scholar]
- Schmid PC, Krebsbach RJ, Perry SR, Dettmer TM, Maasson JL, Schmidt HH. Occurrence and post mortem generation of anandamide and other long-chain N-acylethanolamines in mammalian brain. FEBS Lett. 1995;375:117–120. doi: 10.1016/0014-5793(95)01194-j. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acylethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9349. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Makuch W, Korostynski M, Malek N, Slezak M, Zychowska M, Petrosino S, De Petrocellis L, Cristino L, Przewlocka B, Di Marzo V. Full inhibition of spinal FAAH leads to TRPV1-mediated analgesic effects in neuropathic rats and possible lipoxygenase-mediated remodeling of anandamide metabolism. PLoS One. 2013;8:e60040. doi: 10.1371/journal.pone.0060040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens M, Feuerstein TJ, van Velthoven V, Schnierle P, Knörle R. Quantitative measurement of depolarization-induced anandamide release in human and rat neocortex. Naunyn Schmiedebergs Arch Pharmacol. 2003;368:432–436. doi: 10.1007/s00210-003-0817-1. [DOI] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Stella N, Piomelli D. Receptor-dependent formation of endogenous cannabinoids in cortical neurons. Eur J Pharmacol. 2001;425:189–196. doi: 10.1016/s0014-2999(01)01182-7. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, Iton K, Yamashita A, Waku K. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Terenius L. Characteristics of the “receptor” for narcotic analgesics in synaptic plasma membrane fraction from rat brain. Acta Pharmacol Toxicol (Copenh) 1973;33:377–384. doi: 10.1111/j.1600-0773.1973.tb01539.x. [DOI] [PubMed] [Google Scholar]
- Ueda N, Kurahashi Y, Yamamoto S, Tokunaga T. Partial purification and characterization of the porcine brain enzyme hydrolyzing and synthesizing anandamide. J Biol Chem. 1995a;270:23823–23827. doi: 10.1074/jbc.270.40.23823. [DOI] [PubMed] [Google Scholar]
- Ueda N, Yamamoto K, Kurahashi Y, Yamamoto S, Ogawa M, Matsuki N, Kudo I, Shinkai H, Shirakawa E, Tokunaga T. Oxygenation of arachidonylethanolamide (anandamide) by lipoxygenases. Adv Prostaglandin Thromboxane Leukot Res. 1995b;23:163–165. [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolyzing acid amidase (NAAA) 2010;49:299–315. doi: 10.1016/j.plipres.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Ueda N, Tsuboi K, Uyama T. Metabolism of endocannabinoids and related N-acylethanolamines: Canonical and alternative pathways. FEBS J. 2013;280:1874–1894. doi: 10.1111/febs.12152. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Woodward DF, Carling RW, Cornell CL, Fliri HG, Martos JL, Pettit SN, Liang Y, Wang JW. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol Ther. 2008;120:71–80. doi: 10.1016/j.pharmthera.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Zhu C, Solorzano C, Sahar S, Realini N, Fung E, Sassone-Corsi P, Piomelli D. Proinflammatory stimuli control N-acylphosphatidylethanolamine-specific phospholipase D expression in macrophages. Mol Pharmacol. 2011;79:786–792. doi: 10.1124/mol.110.070201. [DOI] [PMC free article] [PubMed] [Google Scholar]