Abstract
Objective
Our previous findings support an additive effect of cocaine to HIV-infection in the development of pulmonary arteriopathy through enhanced proliferation of human pulmonary smooth muscle cells. We now examined the role of anti-proliferative bone morphogenetic protein receptor (BMPR) axis in HIV-protein and cocaine mediated pulmonary smooth muscle hyperplasia.
Approach and Results
Stimulation of BMPR axis resulted in attenuation of synergistic increase in the proliferation of human pulmonary arterial smooth muscle cells in response to cocaine and HIV protein, Trans-Activator of Transcription (Tat). Interestingly, an increase in mRNA but decrease in protein levels of BMPR with correlated decrease in the activation of SMAD1/5/8 and Id1 gene expression was observed on combined treatment with cocaine and Tat compared to untreated cells at all-time points tested. Although longer exposure to either cocaine or Tat alone also resulted in a significant decrease in the BMPR protein expression, the abrogation on combined treatment was still significantly more compared to mono-treatments. Significant increase in mRNA but down-modulation of BMPR protein expression was also observed in the lung extracts from HIV-infected intravenous drug users (HIV+IVDU) when compared with HIV-infected non-IVDUs (HIV) or un-infected IVDUs (IVDU). Furthermore, significant decrease in BMPR protein expression was also observed in HIV or IVDUs compared to normal controls that correlated with in-vitro findings on chronic exposure to cocaine or HIV protein alone.
Conclusion
Simultaneous exposure of pulmonary smooth muscle cells to viral protein(s) and cocaine exacerbates down-regulation of BMPR axis that may results in enhanced pulmonary vasculature aberrations in HIV+IVDUs.
Keywords: Tat, SMAD, BMP, IVDU, Nef, gp-120
Pulmonary arterial hypertension is one of the most common non-infectious complications of HIV-infection1 with approximately 1000 times higher incidence in HIV-infected patients compared with the general population2. The probability of survival reduces to one half in the individuals who develop HIV-related PAH (HRPAH) compared with HIV-infected individuals without PAH3. Despite major clinical advances in therapy over the past few years, the prognosis of HRPAH remains poor and is similar to that of some advanced cancers. Furthermore, while it is evident from other case reports that the abuse of cocaine and other stimulants is a possible risk factor in the development of PAH4-6, intravenous drug use (IVDU) was found to be one of the major risk factors for HIV-infection in the HRPAH patients7. Our recent study showing enhanced pulmonary vascular remodeling in HIV-infected lung tissues from IV heroin and/or cocaine abusers indicates that IVDU and HIV-1 potentially act in concert to cause pulmonary arteriopathy8. However, it is still not clear how illicit drugs and HIV-infection either alone or in combination can cause the vascular dys-regulation associated with increased pulmonary vascular resistance and cardiac dysfunction.
The possibility of direct HIV-infection of pulmonary vasculature cells leading to HRPAH development is unlikely since HIV-1 RNA or DNA is not found in the pulmonary vessels of human lung tissues9. Studies demonstrate that the direct action of HIV-proteins released by the infected lymphocytes and macrophages play a major role in the development of HRPAH10. Recently, we11 showed that pulmonary vascular remodeling develops in the presence of HIV-1 proteins without an active infection, leading to pulmonary hypertension1 in a non-infectious HIV-transgenic rat model. The pulmonary arterial smooth muscle cells (PASMCs) are key players in the pathogenesis of all forms of PAH vascular remodeling. The exposure of PASMCs to viral proteins and growth factors after damage to the endothelial monolayer leads to smooth muscle hypertrophy and proliferation. However, the cellular and molecular mechanisms underlying the thickening of blood vessels are poorly defined.
While bone morphogenetic protein receptor (BMPR)-2 mutations have been associated with familial PAH; many studies suggest that a critical reduction in the expression of BMPRs may be important in the pathogenesis of PAH12. Bone morphogenetic protein-2 or -4 on binding to BMPR negatively regulates smooth muscle cell growth and proliferation13. The BMP ligands bind to heteromeric complexes of BMPR-1A or BMPR-1B with BMPR-2 resulting in the phosphorylation of Regulatory-Sma and MAD Related Family proteins (R-SMAD). Activated RSMADs: SMAD-1/5/8 then form complex with SMAD4 that translocate to the nucleus and regulate the transcription of BMP/SMAD-responsive anti-proliferative genes14.
HIV-protein Tat, the transactivating factor of HIV-1 is actively secreted by infected cells15 and acts as an angiogenic and oncogenic factor by promoting growth, migration and production of growth factors in various cell-types12, 15. In our previous findings, we have shown that cocaine synergizes with HIV-Tat to promote proliferation of PASMCs8. In this study we partially defined the mechanism(s) mediating this increased proliferation and enhanced pulmonary vascular remodeling in HIV-infected IVDUs by examining the alterations in the anti-proliferative BMP/BMPR axis. We here report significantly more attenuation in the BMPR protein expression in PASMCs on combined treatment with HIV-Tat and cocaine compared to either treatment alone, concomitant with abrogation of BMPR downstream signaling and anti- proliferative Id1 gene expression. Furthermore, to the best of our knowledge this is the first report demonstrating significant down-modulation of BMPR expression in the lungs from HIV infected IVDUs compared to HIV-infected non-drug users or un-infected IVDUs. Some of the results of these studies have been previously reported in the form of abstract.
MATERIAL AND METHODS
Materials and Methods are available in the online-only Supplement.
RESULTS
BMP-2 stimulation and BMPR-2 over expression results in attenuation of Tat and cocaine mediated synergistic increase in proliferation of PASMCs
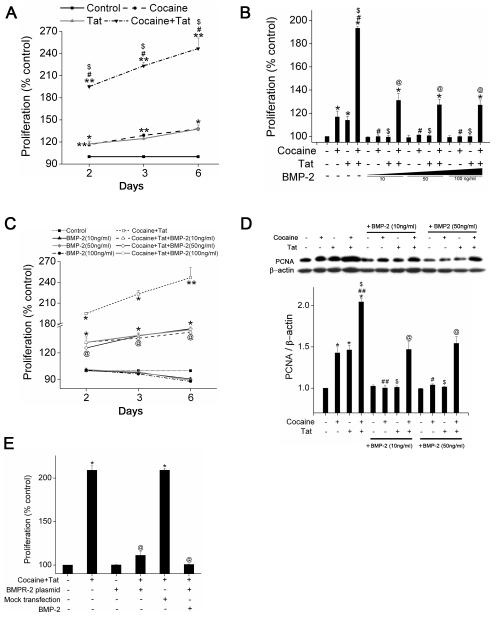
We earlier8 reported that cocaine or Tat treatment alone significantly increases PASMC proliferation and this effect is further enhanced on simultaneous cocaine and Tat exposure. Since bone morphogenetic proteins on binding to BMPR negatively regulate PASMC growth and proliferation13, we next wanted to elucidate if cocaine and Tat mediated enhanced proliferation of PASMCs involves alterations in this anti-proliferative BMPR axis. To begin with, we exposed the cells to cocaine and/or Tat for 2, 3 and 6 days and saw a gradual increase in the PASMC proliferation on cocaine or Tat treatment alone compared to untreated cells. As expected combined treatment with cocaine and Tat resulted in synergistic increase in cell proliferation, with maximum fold increase observed overtime when compared with either of the mono-treatments (Figure 1A). We then tested if stimulation with BMP-2 could prevent the cocaine and/or Tat mediated enhanced SMC proliferation. As represented in Figure 1B, BMP-2 could completely prevent an increase in cell proliferation induced by either cocaine or Tat treatment alone as early as 48h post-treatment at all concentrations tested. However, pre-treatment with BMP-2 could significantly reduce but was not able to completely abrogate the synergistic increase in cell proliferation after combined treatment with cocaine and Tat, even at higher concentrations and longer exposure (Figure 1B, C).Furthermore, western blot analysis of proliferation marker, PCNA ( Figure 1D) also confirmed the inability of BMP-2 to completely prevent the cocaine-Tat triggered proliferation. This could be due to the alterations in the BMPR expression on combined treatment with Tat and cocaine, resulting in lower availability of receptors for ligand binding. Therefore, to next explore the importance of BMPR in cocaine-Tat mediated SMC proliferation, we over-expressed BMPR-2 by transiently transfecting HPASMCs with BMPR-2 expression vector. BMPR-2 over-expression was first confirmed by western blot analysis of transfected cells (Supplementary Figure I). Cell proliferation analysis showed significant reduction in proliferation of cells transfected with BMPR-2 expression vector on treatment with Tat and cocaine compared to the treatment of mock transfected cells (Figure 1E). The fold increase in the cell proliferation of mock transfected cells in response to cocaine and Tat treatment was comparable to that obtained on treatment of un-transfected control cells with cocaine and Tat. Importantly, BMP-2 treatment of BMPR-2 over-expressing cells could completely abrogate the cocaine-Tat mediated enhanced proliferation. These findings clearly confirm the involvement of BMPR axis dysfunction in Tat and cocaine mediated augmentation of PASMC proliferation.
Figure 1. Attenuation of Tat and/or cocaine mediated increased proliferation of HPASMCs on activation of BMP/BMPR axis.
A. HPASMCs (3×103/well) were seeded in 96 well plate. After 48h, the medium was replaced with 0.1% serum containing medium followed by cocaine (1μM) and/or Tat (25ng/ml) treatment for indicated time periods followed by MTS cell proliferation assay. All values are mean ±SD of three independent experiments. *p< 0.01, **p<0.001 vs. control, #p<0.001 vs. cocaine, $p<0.001 vs. Tat. Cell proliferation analyses of cocaine and/or Tat treated HPASMCs in the presence or absence of pre-treatment with BMP-2 (10, 50 or 100ng/ml) at 48h (B) and at 2-6 days (C) post-treatment. All values are mean ±SD of three independent experiments. *p< 0.001 vs. control, #p<0.001 vs. cocaine, $p<0.001 vs. Tat, @p<0.001 vs. combined cocaine and Tat. D. Quiescent HPASMCs were treated with cocaine and/or Tat in the presence or absence of 10 or 50ng/ml BMP-2 for 48h followed by protein extraction. Western blot was used to determine the cell proliferation using PCNA as the marker. Lower panel is the densitometric analysis of blots from three independent experiments (mean+SEM). *p< 0.001 vs. control, #p<0.01, ##p<0.001 vs. cocaine, $p<0.001 vs. Tat, @p<0.001 vs. cocaine and Tat. E. BMPR-2 over expression significantly reduced the cocaine and Tat-mediated smooth muscle proliferation. The HPASMCs plated in 96 wells were serum-starved for 24h before transfection with BMPR-2 expression vector. At 24h post-transfection cells were treated with cocaine, Tat and/or BMP-2 (10ng/ml) for 48h followed by MTS assay. All values are mean ±SD of three independent experiments. *p<0.001 vs. control, @p<0.001 vs. cocaine and Tat.
Combined treatment of PASMCs with Tat and cocaine results in increased BMPR mRNA expression
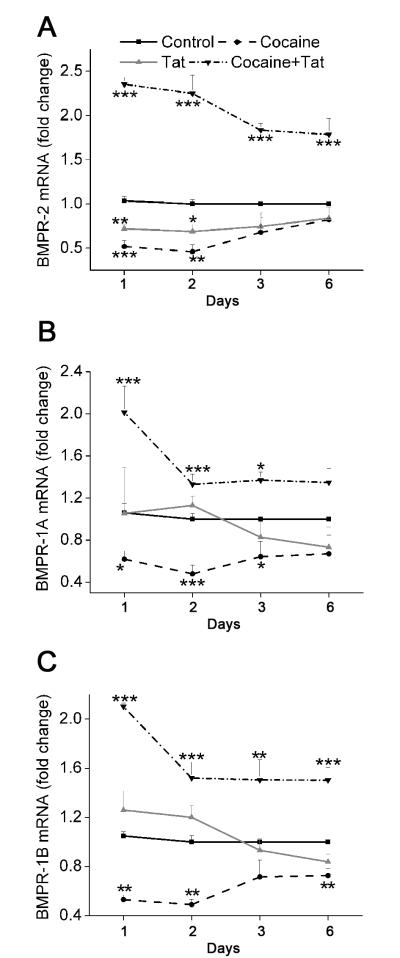
The above mentioned findings led us to investigate the expression of heteromeric BMP receptor complex in relation to Tat and/or cocaine mediated increase in proliferation. The HIV-Tat protein has been found to repress the BMPR-2 gene expression in monocytic cell line, resulting in inhibition of BMP responsive downstream signaling16. Similar to these findings we also observed a significant decrease in the BMPR-2 mRNA expression on analysis of total RNA isolated from HPASMCs treated with Tat (Figure 2A). In addition, we also found a decrease of BMPR-2 mRNA in cells exposed to only cocaine for 1, 2, 3 or 6 days. However cells treated with both Tat and cocaine, showed a significant increase in BMPR-2 mRNA compared to untreated cells at all time intervals tested. In addition, expression of both BMPR-1A and -1B also decreased after treatment with cocaine alone whereas Tat treatment did not result in significant changes at any of the time points tested (Figure 2B, C). Interestingly, similar to the effect on BMPR-2 expression, combined treatment with both Tat and cocaine resulted in significant increase in BMPR-1A and -1B expression (Figure 2B, C) compared to untreated control with higher difference in the expression observed at 24h post-treatment compared to later time points.
Figure 2. Increased BMPR-2, -1A and -1B mRNA expression in HAPSMCs on combined treatment with Tat and cocaine.
Quiescent HPASMCs were treated with 1μM cocaine and/or 25ng/ml Tat for 2, 3 and 6 days. Quantitative mRNA analysis of BMP receptor-2 (A), -1A (B) and -1B (C) was carried out by Real-Time RT-PCR using the SYBR Green detection method. All values are mean ± SD of at least three independent experiments, *p≤0.05, **p≤0.01, ***p<0.001 compared to untreated control.
Combined treatment with Tat and cocaine results in greater attenuation of BMPR protein expression compared to mono-treatments
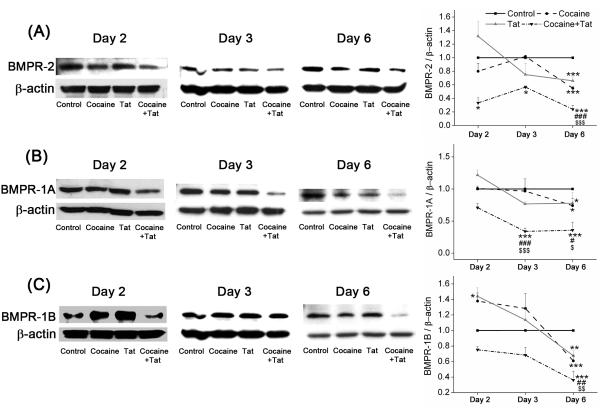
After observing an increase in the mRNA levels of BMPRs in HPASMC following treatment with cocaine and Tat, the protein expression was determined by western blot analysis at 2, 3 or 6 days post-treatment. We observed a significant decrease in the BMPR-2 protein levels following simultaneous exposure to both Tat and cocaine compared to untreated cells at all time-points tested (Figure 3). On the contrary, although not significant, but trend towards increase in the BMPR-2 (Figure 3A) expression was observed following 48h treatment with Tat alone. However, a downward trend in BMPR-2 expression was seen on treatment with cocaine alone when compared to untreated control. Nevertheless, a significant reduction in BMPR-2 expression was observed as the cocaine or Tat exposure time increased from 2 to 6 days. However, this cocaine or Tat mono-treatment mediated attenuation of BMPR protein levels on longer exposure was still significantly less compared to that observed on the combined treatment.
Figure 3. Decreased BMPR-2, -1A and -1B protein levels on combined treatment with Tat and cocaine.
Quiescent HPASMCs treated with cocaine (1μM) and/or Tat (25ng/ml) for 2, 3 and 6 days were lysed using RIPA lysis buffer and then used for Western blot analysis. Representative images of Western blots and densitometry analysis using NIH Image J software of BMPR-2 (A), BMPR-1A (B) and BMPR-1B (C) are shown. All values are mean ±SEM of at least three independent experiments, *p<0.05 **p<0.01, ***p<0.001 compared to untreated control, #p<0.05, ##p<0.01, ###p<0.001 compared to cocaine, $p<0.05, $$p<0.01, $$$p<0.001 compared to Tat.
Furthermore, decrease in BMPR-1A and -1B was also observed on combined treatment with Tat and cocaine at all time-points tested (Figure 3B and C). Similar to BMPR-2 findings, significant increase in BMPR-1B (Figure 3C) expression was observed following 48h treatment with Tat alone. Interestingly, an increase in BMPR-1B protein expression was also found on treatment of cells with cocaine alone compared to untreated control (Figure 3C). BMPR-1B has earlier been reported to be involved in the enhanced proliferation of PASMC isolated from primary pulmonary hypertension patients in response to BMP-2/-7 ligands17 and we also observed significantly higher secretion of BMP-2, -7 ligands in response to cocaine or Tat mono-treatments (Supplementary Figure II). However, just like BMPR-2 expression, cocaine or Tat mono-treatment for 6 days also resulted in significant reduction of BMPR-1A and -1B protein expression compared with untreated controls with the protein levels still significantly higher when compared with the combined treatment.
Cocaine exposure results in the repression of BMPR mediated downstream signaling in HIV-Tat treated PASMCs
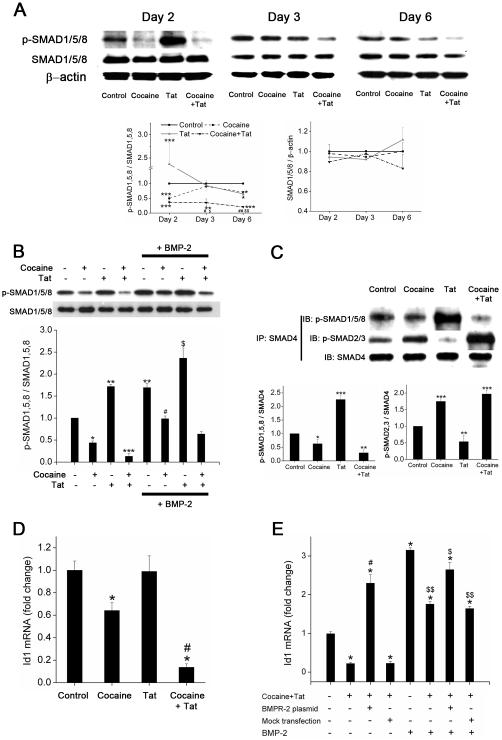
Binding of ligands to BMPR results in phosphorylation of R-SMADs (SMAD1/5/8) followed by their complex formation with co-SMAD4. The nuclear translocation of this complex then leads to the regulation of BMP/SMAD-responsive anti-proliferative genes. As illustrated in Figure 4A, combined treatment of cells with cocaine and Tat for 2, 3 or 6 days resulted in significant repression in the levels of p-SMAD1/5/8 when compared with untreated cells. Initially at 48h post-treatment, HIV-Tat exposure resulted in significant increase of p-SMAD1/5/8 expression. However, this increase in activation of SMAD1/5/8 came back to control levels at 3 days post-treatment followed by significant decrease on longer exposure of 6 days. Interestingly, cocaine alone exhibited a significant reduction in the phosphorylation of SMAD1/5/8 compared to untreated control at 2 and 6 days of post-treatment. However, similar to BMPR protein expression, levels of p-SMAD1/5/8 on combined treatments were still significantly less compared to mono-treatments at 3 and 6 days post-treatment.
Figure 4. Abrogation of BMP receptor-mediated downstream signaling on exposure of Tat treated HPASMCs to cocaine.
A. Cellular extracts of HPASMCs treated with cocaine (1μM) and/or Tat (25ng/ml) for 2, 3 and 6 days were analyzed for phosphorylated (p)-SMAD1/5/8 and total SMAD1/5/8 protein expression by Western blot. Histogram represents densitometric analysis of three independent experiments. Values are mean ±SEM. *p<0.05, **p<0.01, ***p<0.001 compared to untreated control, #p<0.05, ##p<0.01 compared to cocaine treatment alone, $p<0.05, $$p<0.01 compared to Tat treatment alone. B. Cellular extracts of HPASMCs treated with cocaine (1μM) and/or Tat (25ng/ml) for 48h stimulated with or without BMP-2 (10ng/ml) 30min prior to cell lysis were analyzed for phosphorylated (p)-SMAD1/5/8 and total SMAD1/5/8 protein expression by Western blot. Histogram represents densitometric analysis of three independent experiments. Values are mean ±SEM. *p<0.05, **p<0.01, ***p<0.001 compared to untreated control, #p<0.05 compared to cocaine treatment alone, $p<0.01 compared to Tat treatment alone. C. Immunoprecipitation (IP) of protein extract from cells treated with cocaine and/or Tat for 48h using total SMAD4 antibody followed by immunoblotting (IB) with antibodies against p-SMAD1/5/8 or p-SMAD2/3. Graphs (lower panel) represent densitometric analysis of complex formation (mean ±SEM) from three independent experiments. *p<0.05, **p<0.01, ***p<0.001 compared to untreated control. D. Decreased anti-proliferative Id1 target gene expression in HPASMCs on combined treatment with Tat and cocaine for 48 hours. Quantitative analysis of BMP target gene Id1 was carried out by Real-Time RT-PCR using the SYBR Green detection method. All values are mean ±SD of at least three independent experiments. *p≤0.001 compared to untreated control, #p<0.001 compared to cocaine alone. E. Up-regulation of anti-proliferative-Id1 gene in cocaine and Tat treated HPASMCs on BMPR-2 over expression. Serum starved, BMPR-2 transfected HPASMCs were treated with cocaine and Tat for 48h followed by stimulation with BMP-2 (10ng/ml). Total RNA was then isolated after 30min for RT-PCR analysis of Id1. All values are mean ±SD of three independent experiments. *p≤ 0.001 compared to treated versus untreated, #p≤0.001 compared to combined cocaine-Tat treatment, $p≤0.05, $$p≤0.001 compared with BMP-2 treatment.
It has been reported earlier that addition of BMP-2 activates SMAD1/5/8 and inhibits growth factor stimulated proliferation of human pulmonary smooth muscle cells18. Likewise, pre-stimulation with BMP-2 caused significant increase in p-SMAD1/5/8 level in cells exposed or unexposed to cocaine or Tat compared to non-BMP-2 stimulated cells. Also, presence of BMP-2 could rescue the reduction in the levels of p-SMAD1/5/8 on combined treatment with cocaine and Tat (Figure 4B). Further, the anti-proliferative effect of BMP-2 seen in Figure 1 could be reversed in cells lacking SMAD1/5/8 in case of mono- as well as combined-cocaine/Tat treatments, as observed in HPASMCs transiently transfected with siRNA against SMAD1/5/8 (Supplementary Figure III).
Alterations in the activation of SMAD1/5/8 were further confirmed by analysis of its co-immunoprecipitation with SMAD-4. As illustrated in Figure 4C, combined treatment of cells with cocaine and Tat for 48h resulted in significant repression in the levels of p-SMAD1/5/8-SMAD4 complex when compared with untreated cells. Treatment with Tat alone caused a significant increase whereas cocaine alone exhibited a significant reduction in the levels of SMAD1/5/8-SMAD4 complex compared to untreated control. Interestingly, contrasting effect was seen on the transforming growth factor (TGF)-β mediated R-SMAD-co-SMAD (p-SMAD2/3-SMAD4) complex formation when SMAD4 immuno-precipitated cell lysate was independently probed with p-SMAD2/3. Both, combined treatment of cocaine and Tat or treatment with cocaine alone resulted in significant increase in p-SMAD2/3-SMAD4 complex formation compared with untreated cells, whereas Tat alone caused significant reduction in the p-SMAD2/3-SMAD4 complex levels (Figure 4C).
Next to assess the effect of cocaine and Tat on the downstream BMPR target gene, total RNA from 48h treated HPASMCs was evaluated for the levels of anti-proliferative Id1 mRNA by real time RT-PCR. Concomitant with the levels of p-SMAD1/5/8, both combined treatment of cocaine and Tat and cocaine treatment alone expressed lower Id1 mRNA levels compared to untreated cells. Furthermore, the decrease in the Id1 mRNA expression on treatment with both cocaine and Tat (Figure 4D) was significantly more than cocaine treatment alone. Over-expression of BMPR-2 in HPASMCs prevented the cocaine and Tat mediated decrease in Id1 mRNA expression (Figure 4E) both in the absence and presence of BMP-2 stimulation. Overall, these results clearly demonstrate that cocaine and Tat together, negatively affect BMPR downstream signaling in HPASMCs that could lead to increased proliferation.
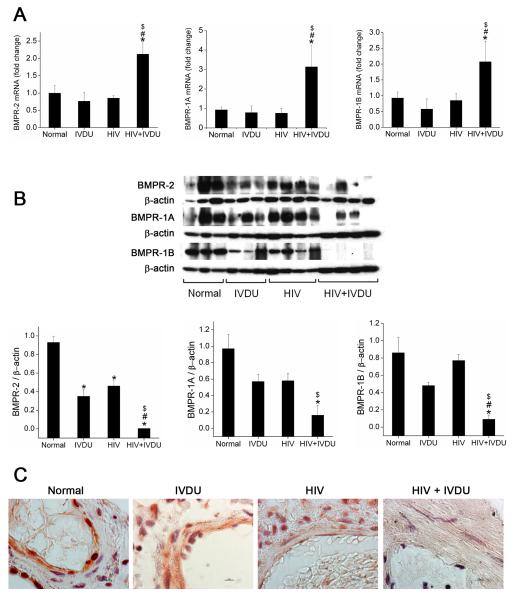
IVDU is associated with increase in BMPR mRNA expression in HIV-infected lungs compared to lung tissues from HIV-infected non drug users or un-infected IVDUs
Down-modulation of BMPR expression and downstream signaling pathways in response to HIV-Tat and cocaine treatment led us to explore the possible implication of this phenomenon in vivo. We evaluated BMPR expression in human lung tissues from HIV-infected IVDUs (HIV+IVDUs) that demonstrated enhanced pulmonary vascular remodeling compared to HIV-infected non-drug users (HIV) or un-infected IVDUs (IVDU) as reported in our previous study8. Similar to our in-vitro findings on BMPR mRNA expression in Tat and cocaine treated HPASMCs, human lungs from HIV+IVDU group demonstrated significant increase in BMPR-2; -1A and -1B mRNA expression (Figure 5A) when compared with lungs from HIV group, IVDU group or normal controls as seen by real time RT-PCR analysis. On the other hand, lungs from HIV or IVDU group did not show any changes in BMPR-2; -1A and -1B mRNA expression compared to normal lungs.
Figure 5. Increase in mRNA and decrease in protein expression of BMPRs in human lung tissues from HIV infected IVDUs.
A. Total RNA was extracted from frozen human lung tissues from HIV-infected individuals with (HIV+IVDU group, n=4) or without IVDU (HIV group, n=4) and uninfected individuals with (IVDU group, n=3) or without IVDU (normal group, n=3). Quantitative analysis of BMPR-2,-1A and -1B was carried out by Real-Time RT-PCR using the SYBR Green detection method. Mean+ SD, *p<0.05 compared to normal, #p<0.05 compared to IVDU, $p<0.05 compared to HIV group. B. Expression of BMPR-2, -1A and -1B was analyzed by Western blot of total protein extract obtained from frozen lung tissues. Upper panel shows the western blot image and lower panel represents the densitometric analysis of the immunoblots. Mean+ SEM, *p<0.05 compared with normal, #p<0.05 compared with IVDU, $p<0.05 compared with HIV. C. Representative photomicrographs of BMPR-2 immuno-histochemistry on paraffin-embedded lung sections from Normal, HIV+/-IVDUs are shown. Original magnification 100X, Scale bar: 100μm.
IVDU is associated with greater down-modulation of BMPR protein expression in HIV-infected lungs compared to lung tissues from HIV-infected non drug users or un-infected IVDUs
Western blot analysis of total lung extract revealed significant loss of BMPR-2 protein expression in HIV+ IVDU group compared to HIV or IVDU groups (Figure 5B). However, compared to normal group, a significant reduction in BMPR-2 protein expression was also observed in the protein extracts from IVDU or HIV group. Similarly, HIV+IVDU group had lower BMPR-1A and -1B protein expression compared to either HIV or IVDU groups. Maximum expression of BMPR-1A and -1B receptors was observed in the protein extract from normal lung tissues.
The loss of BMPR-2 expression in HIV+IVDU group was further confirmed by immunohistochemical analysis on paraffin embedded lung- sections from selective individuals. As shown in the representative images in Figure 5C and Supplementary Figure IV A, HIV+IVDU group had remarkably reduced BMPR-2 expression in the smooth muscle lining of the thickened arterial wall whereas BMPR-2 staining was distinctly seen in the arterial smooth muscle lining of lung sections from HIV or IVDU groups. However BMPR-2 staining in the vascular wall of HIV or IVDU group lung sections was notably less compared to the un-infected non-IVDU normal lung sections with maximum expression observed in case of normal lungs. Absence of staining in the negative control (Supplementary Figure IV B) validates the positive BMPR-2 expression in the representative sections
Chronic exposure of PASMCs to HIV-Tat in presence of cocaine results in greater reduction in BMPR protein expression compared to other HIV proteins
Since we observed a decrease in the BMPR protein expression in human lungs of HIV group, we speculated that there may be an involvement of other HIV-proteins in addition to Tat in down-modulating BMPR in HIV infected individuals. Hence we chronically exposed HPASMCs to either HIV protein: Nef or gp120 in the presence or absence of cocaine. As illustrated in Figure 6, chronic exposure to either Nef or gp-120 demonstrated significant reduction in BMPR-2, -1A and -1B protein expression compared to untreated cells. In case of BMPR-2 and -1A, a trend towards decrease in expression was observed in Nef or gp120 treated cells when compared to Tat treated cells (Figure 6A). However, maximum loss of BMPR expression was observed only on combined treatment with cocaine and Tat compared to all other treatments. These results correlate with our in-vivo findings of reduced protein expression of BMP receptors in lungs from HIV or IVDU groups. Overall, our in-vitro findings showing enhanced impairment of BMP signaling in cocaine and HIV-Tat treated HPASMCs correlate with increased loss of BMPR expression in the lungs from HIV-infected IVDUs compared to lungs exposed to either HIV or IVDU alone
Figure 6. Treatment of HPASMCs with Tat in presence of cocaine results in greater reduction in BMPR protein expression when compared with other HIV proteins.
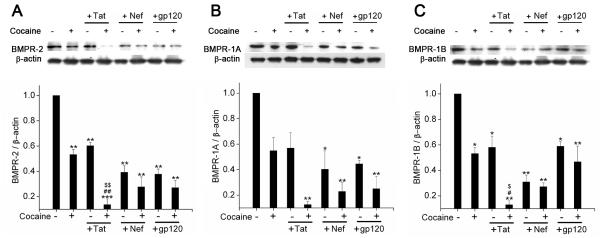
Quiescent HPASMCs treated with cocaine (1μM) and/or Tat (25ng/ml) / Nef (10ng/ml) / gp-120CM (100ng/ml) for 6 days were lysed using RIPA lysis buffer for Western blot analysis. Representative images of BMPR-2 (A), BMPR-1A (B) and BMPR-1B (C) with densitometry analysis of western blots are shown. All values are mean ±SEM of at least three independent experiments, *p<0.01, **p<0.001 compared to untreated control, #p<0.01, ##p<0.001 compared to cocaine alone, $p<0.01, $$p<0.001 compared to Tat treatment alone.
DISCUSSION
In this study we demonstrate that the synergistic increase in the proliferation of pulmonary smooth muscle cells in response to combined treatment with HIV-Tat and cocaine involves attenuation in the protein levels of BMPR-2, -1A and -1B. Consistent with the down-regulation of BMPR expression we observed significant decrease in the phosphorylation of Smad1/5/8 and expression of anti-proliferative Id1 gene. Furthermore, increase in the proliferation of HPASMCs in response to cocaine and Tat treatment was found to be diminished in cells over-expressing BMPR-2. In addition, we offer for the first time an in-vivo evidence of significantly more decrease in the expression of both type II and type I BMPRs in the lung tissues from HIV+IVDUs with enhanced pulmonary arteriopathy compared to HIV or IVDU groups as shown in our previous findings8.
Various studies on human lung tissues from idiopathic or heritable PAH have shown that impairment in BMPR-2 expression may critically contribute to the pathogenesis of PAH, irrespective of mutations in the gene13, 19. This indicates that other environmental factors could negatively affect BMP signaling predisposing smooth muscle cells to enhanced proliferation associated with PAH. Furthermore, reduction in BMPR expression and down-regulation of SMAD signaling has also been reported in the monocrotaline and chronic hypoxia-induced animal models of PAH20, 21. Similar to these findings, we in this study observed a significant decrease in the BMPR-2 and downward trend in BMPR-1A and -1B protein levels following simultaneous exposure of HPASMCs to both Tat and cocaine compared to untreated cells at early time points. However, chronic treatment with both Tat and cocaine later resulted in significant decrease in the protein expression of all BMPRs. Nevertheless, chronic 6 days treatment with either Tat or cocaine alone also resulted in significant decrease in BMPR protein levels compared to untreated controls. Chronic treatment of HPASMCs with other viral proteins such as Nef or gp-120 in presence or absence of cocaine also led to significant decrease in the BMPR expression. However, maximum decrease in BMPR protein levels at this time point was observed on simultaneous treatment with both HIV-Tat and cocaine.
On the contrary to the protein expression, combined treatment with cocaine and Tat resulted in significant increase in the mRNA expression of BMPR-2,-1A and -1B. Nevertheless, cocaine mono-treatment also led to a decrease in BMPR-2,-1A and -1B mRNA expression and this correlated with the decrease in protein expression observed after longer exposure. HIV-Tat mono-treatment showed decrease in the BMPR-2 mRNA expression with no significant alterations in the BMPR-1A, -1B mRNA expression at all time intervals. Our ex-vivo findings on human lungs from HIV+IVDUs also demonstrated decrease in BMPR protein expression with increase in mRNA expression. A number of proteins have previously been shown to have decreased expression in presence of elevated corresponding mRNA22, 23. This may be due to involvement of feedback regulation of BMPR mRNA expression in response to significant increase in the secretion of BMP ligands by PASMCs on treatment with Tat or cocaine alone and in response to no change in ligands on combined treatment (see supplementary Figure II). However the changes in BMPR mRNA expression in response to the BMP ligands did not correspond to the changes at the protein level. It could be that enhanced mRNA expression reflect a negative feedback loop coupled with post-transcriptional targeting of mRNA and regulation of protein expression by microRNAs24 or by ubiquitinated degradation of receptor proteins 25, which is a focus of our ongoing studies.
BMP ligands are known to have a higher affinity for BMPR-1A and -1B than BMPR-219. Distinct downstream signaling cascades are known to be activated depending on the expression level of ligands and receptors, ligand-receptor affinity or the presence of preformed type I/type II receptor complexes. Binding of ligands to preformed receptor complexes leads to activation of canonical SMAD-dependent signaling whereas ligand induced receptor complex formation results in activation of non-SMAD dependent mitogen activated protein kinase (MAPK) signaling26. The increased expression of BMP ligands on treatment with Tat or cocaine (see supplementary Figure II) may have led to first binding of ligands to type I receptors and then recruitment of type II receptor BMPR-2, resulting in activation of MAPK dependent pro-proliferative signaling26. Given that we did not observe significant reduction in expression of BMPRs on exposure to only Tat or cocaine at 2 days post-treatment, the above explanation may fit our findings of enhanced proliferation of PASMCs on treatment with either Tat or cocaine alone8. Interestingly, we also observed an increase in the protein levels of BMPR-1B on exposure to either Tat or cocaine alone at 2 days post-treatment. Takeda et al previously reported enhanced expression of BMPR-1B in PASMCs isolated from primary pulmonary hypertension (pph) patients compared to control cells17. They also demonstrated involvement of BMPR-1B in the stimulation of mitosis of pphPASMC through MAPK pathway in response to BMP-2 and -7. In addition, we are currently investigating the role of other inhibitory factors such as Smad-6, -7 or BAMBI that may also alter the downstream signaling cascade and change the cell fate in response to cocaine and/or Tat treatment.
Yang et al earlier found reduction in phosphorylation of Smad1/5 in the pulmonary arterial walls of familial and idiopathic PAH patients with and without underlying BMPR-2 mutations13. Likewise in this study, reduction in the expression of BMPRs was accompanied with the inhibition of SMAD1/5/8 phosphorylation and BMP/SMAD responsive Id1 gene expression on combined treatment with Tat and cocaine. Interestingly, treatment with cocaine alone also resulted in the reduction of SMAD1/5/8 activation and Id1 gene expression at early time point but without any changes in the BMPR-2 and -1A expression and in the presence of significant increase in the BMPR-1B expression at 2 days post-treatment. Cocaine has high affinity for sigma-1 receptors and binding results in translocation of these receptors to plasma membrane where they are known to activate other receptors or kinase(s) including Src family kinase (SFK)27. On the other hand, Src tyrosine kinase is known to interact with cytosolic terminal of BMPR-2 and is negatively regulated by BMP signaling28. However, it is not known if activation of SFK can conversely inhibit BMP signaling through interaction with BMPR-2 or by crosstalk with SMAD1/5/8. Furthermore, the enhanced levels of phosphorylated SMAD2/3 complexed with co-SMAD4 compared to phosphorylated SMAD1/5/8 in the presence of cocaine, suggest activation of an alternative TGF-β receptor-SMAD2/3 dependent pathway on cocaine treatment that may have competitively inhibited the binding of co-SMAD4 with phosphorylated SMAD1/5/8. Possibility of activation of this alternative pathway in presence of cocaine is further supported by earlier evidence of phosphorylation of TGF-β type II receptor by Src during oncogenic signaling in mammary epithelial cells29. Moreover, increased expression of phosphorylated Smad2 in small pulmonary arteries of patients with idiopathic PAH has been shown earlier30, indicating that a failure of BMPR-2/Smad1, 5 signaling leads to increased signaling via TGF-β/ALK-5/Smad2, 3 axis. In addition, Smad1 is known to physically interact with Smad3 and this may prevent the phosphorylation of SMAD319
We earlier demonstrated that Tat and cocaine mediated proliferation of PASMCs is associated with activation of platelet-derived growth factor-β receptor and could be blocked by a PDGF tyrosine kinase inhibitor, imatinib8. Interestingly, PDGF is able to antagonize the BMP and TGF signaling pathways in vascular smooth muscle cells by repressing the SMAD protein expression31 and deficiency in BMPR activity is associated with enhanced activity of PDGF signaling pathway32. In our previous findings, we reported enhanced pulmonary vascular remodeling and PDGF expression in HIV+IVDUs compared with lungs from HIV-infected non-drug users or un-infected IVDUs. Now in our current findings we demonstrate significant greater loss of BMPR-1A, -1B and -2 expressions in these human lung tissues from HIV-infected IVDUs compared to HIV or IVDU group. Therefore, it may be that interplay between PDGF and BMP signaling pathways in response to illicit drugs and HIV-proteins results in exacerbated vascular remodeling in the lungs from HIV infected IVDUs.
Supplementary Material
SIGNIFICANCE.
This article focuses on the alterations in anti-proliferative BMPR axis during HIV-protein(s) and cocaine mediated pulmonary smooth muscle hyperplasia. Our study indicates significantly more attenuation in the BMPR expression and downstream signaling pathway on combined exposure to HIV-protein(s) and cocaine compared to any one exposure. Understanding the effect of viral-cocaine interactions on this anti-proliferative pathway will subsequently be critical for the development of novel therapeutic strategies aimed at abrogating PAH associated with HIV and/or drug abuse, in particular, and all types of arteriopathy in general.
ACKNOWLEDGEMENTS
We are grateful to Haihua Gu for his help in the analysis of BMP ligands using ELISA.
SOURCES OF FUNDING This work was supported by NIH grants: 1R01DA034542 and 1R03DA031589 and American Heart Association’s Scientist Development grant: 11SDG7500016 awarded to N.K.D.
Footnotes
DISCLOSURES: None
REFERENCES
- 1.Lund AK, Lucero J, Herbert L, Liu Y, Naik JS. Human immunodeficiency virus transgenic rats exhibit pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2011;301:L315–326. doi: 10.1152/ajplung.00045.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaro G, Lipshultz SE. Pathogenesis of hiv-associated cardiomyopathy. Ann N Y Acad Sci. 2001;946:57–81. doi: 10.1111/j.1749-6632.2001.tb03903.x. [DOI] [PubMed] [Google Scholar]
- 3.Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, Hirschel B, Luthy R. Hiv-associated primary pulmonary hypertension. A case control study. Swiss hiv cohort study. Am J Respir Crit Care Med. 1997;155:990–995. doi: 10.1164/ajrccm.155.3.9117037. [DOI] [PubMed] [Google Scholar]
- 4.Yakel DL, Jr., Eisenberg MJ. Pulmonary artery hypertension in chronic intravenous cocaine users. Am Heart J. 1995;130:398–399. doi: 10.1016/0002-8703(95)90459-x. [DOI] [PubMed] [Google Scholar]
- 5.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. Hiv-related pulmonary hypertension: Analytic review of 131 cases. Chest. 2000;118:1133–1141. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 6.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest. 2006;130:1657–1663. doi: 10.1378/chest.130.6.1657. [DOI] [PubMed] [Google Scholar]
- 7.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, Le Gall C, Parent F, Garcia G, Herve P, Barst RJ, Simonneau G. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med. 2003;167:1433–1439. doi: 10.1164/rccm.200204-330OC. [DOI] [PubMed] [Google Scholar]
- 8.Dhillon NK, Li F, Xue B, Tawfik O, Morgello S, Buch S, Ladner AO. Effect of cocaine on human immunodeficiency virus-mediated pulmonary endothelial and smooth muscle dysfunction. Am J Respir Cell Mol Biol. 2011;45:40–52. doi: 10.1165/rcmb.2010-0097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanmogne GD, Kennedy RC, Grammas P. Analysis of human lung endothelial cells for susceptibility to hiv type 1 infection, coreceptor expression, and cytotoxicity of gp120 protein. AIDS Res Hum Retroviruses. 2001;17:45–53. doi: 10.1089/088922201750056771. [DOI] [PubMed] [Google Scholar]
- 10.Humbert M, Montani D, Perros F, Dorfmuller P, Adnot S, Eddahibi S. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul Pharmacol. 2008;49:113–118. doi: 10.1016/j.vph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Mermis J, Gu H, Xue B, Li F, Tawfik O, Buch S, Bartolome S, O’Brien-Ladner A, Dhillon NK. Hypoxia-inducible factor-1 alpha/platelet derived growth factor axis in hiv-associated pulmonary vascular remodeling. Respir Res. 2011;12:103. doi: 10.1186/1465-9921-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barillari G, Ensoli B. Angiogenic effects of extracellular human immunodeficiency virus type 1 tat protein and its role in the pathogenesis of aids-associated kaposi’s sarcoma. Clin Microbiol Rev. 2002;15:310–326. doi: 10.1128/CMR.15.2.310-326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 14.Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- 15.Ensoli B, Buonaguro L, Barillari G, Fiorelli V, Gendelman R, Morgan RA, Wingfield P, Gallo RC. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 tat protein on cell growth and viral transactivation. J Virol. 1993;67:277–287. doi: 10.1128/jvi.67.1.277-287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldwell RL, Gadipatti R, Lane KB, Shepherd VL. Hiv-1 tat represses transcription of the bone morphogenic protein receptor-2 in u937 monocytic cells. J Leukoc Biol. 2006;79:192–201. doi: 10.1189/jlb.0405194. [DOI] [PubMed] [Google Scholar]
- 17.Takeda M, Otsuka F, Nakamura K, Inagaki K, Suzuki J, Miura D, Fujio H, Matsubara H, Date H, Ohe T, Makino H. Characterization of the bone morphogenetic protein (bmp) system in human pulmonary arterial smooth muscle cells isolated from a sporadic case of primary pulmonary hypertension: Roles of bmp type ib receptor (activin receptor-like kinase-6) in the mitotic action. Endocrinology. 2004;145:4344–4354. doi: 10.1210/en.2004-0234. [DOI] [PubMed] [Google Scholar]
- 18.Wong GA, Tang V, El-Sabeawy F, Weiss RH. Bmp-2 inhibits proliferation of human aortic smooth muscle cells via p21cip1/waf1. American journal of physiology. Endocrinology and metabolism. 2003;284:E972–979. doi: 10.1152/ajpendo.00385.2002. [DOI] [PubMed] [Google Scholar]
- 19.Morrell NW. Pulmonary hypertension due to bmpr2 mutation: A new paradigm for tissue remodeling? Proceedings of the American Thoracic Society. 2006;3:680–686. doi: 10.1513/pats.200605-118SF. [DOI] [PubMed] [Google Scholar]
- 20.McMurtry MS, Bonnet S, Michelakis ED, Bonnet S, Haromy A, Archer SL. Statin therapy, alone or with rapamycin, does not reverse monocrotaline pulmonary arterial hypertension: The rapamcyin-atorvastatin-simvastatin study. American journal of physiology. Lung cellular and molecular physiology. 2007;293:L933–940. doi: 10.1152/ajplung.00310.2006. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H, Goto N, Kojima Y, Tsuda Y, Morio Y, Muramatsu M, Fukuchi Y. Downregulation of type ii bone morphogenetic protein receptor in hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2006;290:L450–458. doi: 10.1152/ajplung.00206.2005. [DOI] [PubMed] [Google Scholar]
- 22.Reichrath J, Welter C, Mitschele T, Classen U, Meineke V, Tilgen W, Seifert M. Different expression patterns of calpain isozymes 1 and 2 (capn1 and 2) in squamous cell carcinomas (scc) and basal cell carcinomas (bcc) of human skin. The Journal of pathology. 2003;199:509–516. doi: 10.1002/path.1308. [DOI] [PubMed] [Google Scholar]
- 23.Wei X, Guo W, Wu S, Wang L, Huang P, Liu J, Fang B. Oxidative stress in nsc-741909-induced apoptosis of cancer cells. Journal of translational medicine. 2010;8:37. doi: 10.1186/1479-5876-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: New concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J, Crilley TK, Butler LM, Blackbourn DJ, Nash GB, Lehner PJ, Morrell NW. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type ii. The Journal of biological chemistry. 2010;285:37641–37649. doi: 10.1074/jbc.M110.132415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (bmp) receptor oligomerization determines different bmp-2 signaling pathways. J Biol Chem. 2002;277:5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- 27.Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends Pharmacol Sci. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong WK, Knowles JA, Morse JH. Bone morphogenetic protein receptor type ii c-terminus interacts with c-src: Implication for a role in pulmonary arterial hypertension. American journal of respiratory cell and molecular biology. 2005;33:438–446. doi: 10.1165/rcmb.2005-0103OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galliher AJ, Schiemann WP. Src phosphorylates tyr284 in tgf-beta type ii receptor and regulates tgf-beta stimulation of p38 mapk during breast cancer cell proliferation and invasion. Cancer research. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 30.Richter A, Yeager ME, Zaiman A, Cool CD, Voelkel NF, Tuder RM. Impaired transforming growth factor-beta signaling in idiopathic pulmonary arterial hypertension. American journal of respiratory and critical care medicine. 2004;170:1340–1348. doi: 10.1164/rccm.200311-1602OC. [DOI] [PubMed] [Google Scholar]
- 31.Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A. Molecular basis for antagonism between pdgf and the tgfbeta family of signalling pathways by control of mir-24 expression. The EMBO journal. 2010;29:559–573. doi: 10.1038/emboj.2009.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, Schellong S, Urashima T, Wang L, Morrell NW, Rabinovitch M. An antiproliferative bmp-2/ppargamma/apoe axis in human and murine smcs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–1857. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.