Abstract
Three adhesion complexes span the sarcolemma and facilitate critical connections between the extracellular matrix and the actin cytoskeleton: the dystrophin- and utrophin-glycoprotein complexes and α7β1 integrin. Loss of individual protein components results in a loss of the entire protein complex and muscular dystrophy. Muscular dystrophy is a progressive, lethal wasting disease characterized by repetitive cycles of myofiber degeneration and regeneration. Protein replacement therapy offers a promising approach for the treatment of muscular dystrophy. Recently, we demonstrated that sarcospan facilitates protein-protein interactions amongst the adhesion complexes and is an important therapeutic target. Here, we review current protein replacement strategies, discuss the potential benefits of sarcospan expression, and identify important experiments that must be addressed for sarcospan to move to the clinic.
Keywords: Duchenne, dystrophin, muscle, integrin, laminin-binding, mdx, muscular dystrophy, sarcolemma, sarcospan, utrophin
Introduction
Duchenne muscular dystrophy (DMD) is a progressive muscle wasting disease caused by mutations in the dystrophin gene leading to a loss of the dystrophin-glycoprotein complex (DGC) at the sarcolemma [1]. DMD occurs in approximately 1 in 3,500 male children, leading to respiratory or cardiac failure in the second decade of life. In 1986, the gene encoding dystrophin was identified, and mutations in the dystrophin gene were determined to be responsible for DMD [2, 3]. While dystrophin was identified over 25 years ago, there is still no cure for the disease [2]. The N-terminus of dystrophin binds directly to filamentous actin (F-actin) in the cytoplasm of the myofiber and the C-terminus of dystrophin is associated with a group of proteins including neuronal nitric oxide synthase (nNOS), α-syntrophin, α-dystrobrevin, and β-dystroglycan (DG) [4, 5]. Dystrobrevin binds to the intermediate filament protein syncoilin, which provides a connection between desmin and the DGC that is thought to be important for maintaining mechanical strength and structural organization necessary for muscle contractions [6]. nNOS requires binding to both dystrophin and syntrophin for sarcolemmal localization and produces nitric oxide, which stimulates blood flow necessary to meet the metabolic demands of healthy muscle [7-11]. The DGC functions to stabilize the sarcolemma during muscle contractions by providing a critical connection between the extracellular matrix (ECM) and the intracellular actin cytoskeleton [12]. α-DG, a peripheral membrane protein, provides the connection between the transmembrane β-DG and laminin-211 in the ECM [13, 14]. The central mucin domain of α-DG is heavily glycosylated and the extent of glycosylation determines the affinity of DGC attachment to the ECM [5, 13-18]. The sarcoglycan-sarcospan (SG-SSPN) subcomplex stabilizes α-DG's association with β-DG at the cell surface [19-21]. Loss of the DGC in DMD renders the sarcolemma susceptible to membrane ruptures, which initiates the cycles of myofiber degeneration and regeneration characteristic of DMD [1, 12, 15, 22].
Many forms of muscular dystrophy result from a loss of muscle cell attachment to its surrounding ECM. In addition to the DGC, two adhesion complexes span the sarcolemma and facilitate this connection: the utrophin-glycoprotein complex (UGC) and α7β1 integrin. The UGC is homologous to the DGC, where utrophin replaces dystrophin [23, 24]. α7β1 integrin is the main heterodimeric integrin expressed in adult skeletal muscle [25-27]. Mutations in genes encoding the protein components of the DGC/UGC, α7β1 integrin, and ECM cause various forms of muscular dystrophy, which are classified based on the severity of disease and the muscle groups predominantly affected. Autosomal recessive limb-girdle muscular dystrophies (AR-LGMDs) encompass a wide array of genetic disorders and disease onset typically occurs between 10-30 years of age. Mutations in α-, β-, γ-, and δ-SG and mutations in glycosyltransferases resulting in the hypoglycosylation of α-DG cause AR-LGMD subtypes 2C-2F, 2K, and 2N-2O respectively [28-37]. Congenital muscular dystrophies (CMDs) are an autosomal recessive disease characterized by severe muscular dystrophy with onset occurring within the first year of life. Various forms of CMD are also caused by hypoglycosylation of α-DG due to mutations in glycosyltransferases as well as mutations in laminin α2, collagen 6, and α7 integrin [38-42]. Notably, patient mutations in SSPN and β1D integrin have not been identified [43-45].
There are many strategies currently being investigated for the treatment of muscular dystrophy. Several therapeutic approaches focus on adenoviral delivery of gene replacement therapy or exon skipping to produce a shortened, but functional dystrophin protein. This review will focus on therapeutic strategies to replace the DGC with compensatory adhesion complexes, UGC and α7β1 integrin. In particular, this review will discuss the potential of SSPN as a therapeutic target to upregulate both the UGC and α7β1 integrin. SSPN is a tetraspanin-like protein that was discovered as a core-component of the DGC [46]. Although SSPN has been shown to ameliorate the pathology of the mdx mouse model of DMD [47, 48], many questions remain to be answered before SSPN-related therapies are suitable for patients.
Restoring Myofiber Function with Compensatory Adhesion Complexes
The adhesion complexes responsible for stabilizing the myofiber membrane have distinct biochemical and functional properties, including sarcolemmal localization and ECM/actin connections in normal muscle. In muscle, the DGC is the most widely expressed adhesion complex, as it is found at all regions of the sarcolemma including the neuromuscular and myotendinous junctions (NMJs and MTJs) [3, 49-52]. The UGC, a homologous complex to the DGC, is restricted to NMJ and MTJ regions of the sarcolemma, where utrophin replaces dystrophin [53, 54]. Although utrophin and dystrophin are structurally homologous, they bind to F-actin through distinct sites [55, 56] and only dystrophin contains nNOS binding sites [57]. It is thought that muscle ischemia occurs in DMD because nNOS is not anchored to the sarcolemmal membrane [57]. The UGC also differs from the DGC in the glycosylation of α-DG, which is a critical determinant of the binding specificity for ECM ligands [47, 58-60]. α7β1 integrins, which are also highly enriched within NMJ and MTJ structures and expressed at low levels at non-junctional regions of the sarcolemma, are the predominant integrin heterodimers expressed in adult muscle [25-27]. Integrins bind F-actin through a complex of proteins including integrin-linked kinase (ILK), PINCH, and α/β-parvin [61-63]. The ILK, PINCH, and parvin protein complex has been implicated in facilitating cell signaling through Akt/PKB, GSK3β/β-catenin, JNK, and α-Pix/Rac1 pathways [61, 62, 64]. ILK interacts specifically with β1 integrin and deletion of ILK causes muscular dystrophy that resembles the α7 integrin deficient mouse model [65]. Laminin-211 binds α7β1 integrin through laminin-type G domains (LG) 1-3, whereas binding to α-DG is mediated through LG1-3 and 4-5 [66-70]. Given that many differences exist between the three adhesion complexes, the extent to which UGC- and integrin-based therapeutics for DMD fully replace DGC function remains an unanswered question.
The concept of the UGC and integrins acting as compensatory adhesion complexes and their potential as therapeutic targets to replace the DGC arose from studies in the mdx mouse model of DMD. Muscle pathology in mdx muscle is less severe than that observed in boys with DMD and the mdx mouse maintains a fairly normal lifespan [71]. It was hypothesized that increased abundance of the UGC and α7β1 integrin at extra-junctional regions of the sarcolemma in mdx muscle may partially compensate for the loss of the DGC, resulting in moderate dystrophic pathology without affecting lifespan, which is in stark contrast with the severe dystrophic pathology and premature lethality observed in the DMD population. To experimentally address this hypothesis, mdx:utrophin-null and mdx:α7 integrin-null double knockout mice were created. The additional loss of utrophin or α7 integrin in mdx mice exacerbated dystrophic symptoms to more closely resemble DMD. Furthermore, lifespan was shortened to 20- and 4-weeks, respectively for utrophin-deficient and α7 integrin-deficient mdx mice [72-75]. Significantly, over-expression of either utrophin or β1D integrin in mdx muscle prevented muscular dystrophy, demonstrating that the UGC and integrins are capable of replacing the DGC in the mouse model of DMD [76, 77]. Mental retardation and brain abnormalities have been detected in about 30% of patients with DMD [78]. Utrophin and its smaller isoforms are expressed in the blood vessels in all brain regions, walls of the lateral ventricle, cortex, subiculum, thalamus, brain stem nuclei, superior colliculus, and deep cerebellar nuclei [79]. Although utrophin stabilizes the sarcolemma in mdx mice, utrophin was not upregulated in a compensatory fashion in the brain of mdx mice [79]. Arginine butyrate alleviated mdx muscle disease through utrophin upregulation and increased all utrophin isoforms in the brain of mdx mice, but did not rescue cognitive deficits observed in behavioral assays including exploration, emotional reactivity, and spatial and fear memories [79]. These results suggest that utrophin cannot fully compensate for the loss of dystrophin in all tissues. The ability of α7β1 integrin to ameliorate cognitive deficits in mdx mice has not been tested.
Although the UGC and α7β1 integrin do not fully replace the DGC, they remain excellent candidates for protein replacement therapies. The packaging limit of adeno-associated virus (AAV) capsids prevents the delivery of the most ideal protein for the treatment of DMD, the entire dystrophin gene [80]. Additionally, recent evidence that the expression of AAV delivered mini-dystrophin is circumvented by an immune response to dystrophin in patient clinical trials [81], strengthens the importance of UGC and α7β1 integrin protein replacement therapies. A thorough investigation of the biochemical, structural, and functional differences between the UGC, α7β1 integrin, and the DGC may lead to the future development of combinatorial therapies designed to replace the DGC. The first goal is to determine if UGC- and α7β1 integrin-based therapies are safe and beneficial to patients with DMD. To date, only three therapies designed to replace the DGC with the UGC or integrins are near clinical trials. Two therapies designed to upregulate utrophin are currently in or near Phase 1 clinical trials: BMN195 (Summit plc) and biglycan (Trivorsan Pharmaceuticals). BMN195, a 5-(ethylsulfonyl)-2-(naphthalene-2-yl) benzodoxazole, was identified in a screen for small molecules, which upregulated utrophin mRNA 25% in human myoblasts, increased utrophin protein levels two-fold in DMD patient cells, and demonstrated efficacy in treatment of mdx mice [82]. Specifically, BMN195 reduced regeneration, inflammation, serum CK levels, and fibrosis, and prevented membrane damage due to eccentric contractions in treated mdx mice [82]. Although BMN195 was shown to be safe at all doses in a Phase 1 clinical trial by BioMarin Pharmaceuticals, it did not achieve plasma concentrations, even at the highest doses, required to increase utrophin expression1. Summit plc has since reformulated BMN195 to allow for better absorption and initiated Phase 1 clinical trials. The second utrophin upregulation therapy, biglycan, is an extracellular protein that is highly expressed, similar to utrophin, in regenerating and developing muscle [83, 84]. Injection of recombinant human biglycan protein improves muscle pathology in the mdx mouse by increasing cell surface expression of utrophin and γ-SG [85]. Furthermore, the inability of biglycan to ameliorate dystrophic pathology of the utrophin-deficient mdx mouse demonstrates the requirement of utrophin for biglycan therapies [85]. Biglycan is currently being developed for Phase 1 clinical trials by Trivorsan Pharmaceuticals. The only therapeutic designed to upregulate α7 integrin near Phase 1 clinical trials is laminin-111 protein. Laminin-111 is an ECM protein present in cardiac and skeletal muscles during embryonic development that is replaced by laminin-211 in adult muscle [69, 70]. Injection of Engelbreth-Holt-Swarm-derived purified natural mouse laminin-111 protein improves dystrophic pathology in the mdx and laminin α2-deficient dyW mouse models [86-89] through the upregulation of both utrophin and integrins at the sarcolemma. Prothelia is currently developing laminin-111 for clinical trials and a Phase 1 trial will occur upon the completion of preclinical trials. Laminin-111 has the potential for therapeutic use in merosin-deficient congenital muscular dystrophy (MDC1A), DMD, and LGMD2I (mutations in fukutin-related protein (FKRP)). The outcome of these clinical trials may illuminate whether utrophin and integrin therapeutics will alleviate the symptoms of DMD (Figure 1).
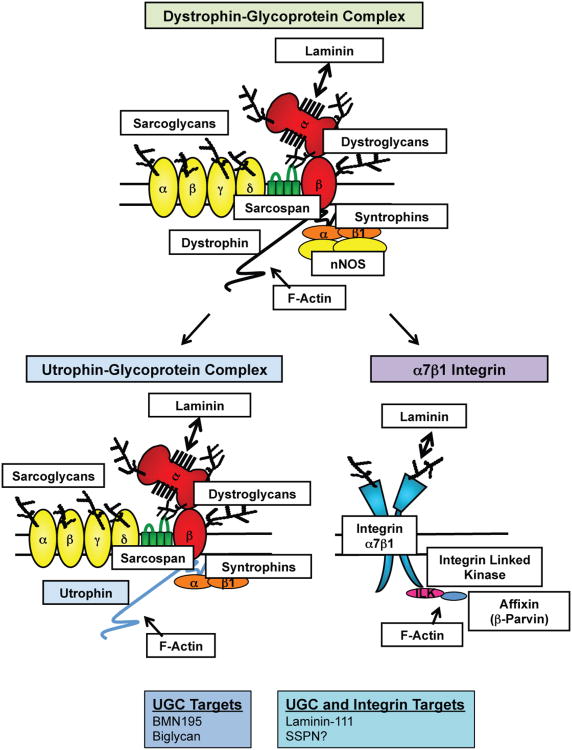
Figure 1. UGC- and α7β1 integrin-mediated replacement therapy for the DGC in DMD.
The DGC, UGC, and α7β1 integrins function to prevent contraction-induced damage of the sarcolemma by maintaining connections between the actin cytoskeleton and ECM. The DGC is composed of dystrophin, the dystroglycans (α- and β-DG), the sarcoglycans (α-, β-, γ-, and δ-SG), sarcospan (SSPN), and the syntrophins (α- and β-subunits). Neuronal nitric oxide synthase (nNOS) requires dystrophin and syntrophin to be anchored to the sarcolemmal membrane, where it is thought to function in preventing functional muscle ischemia. The UGC is homologous to the DGC, where utrophin replaces dystrophin. However, many differences exist between the UGC and DGC, including the glycosylation of α-DG, the domains in which actin binding occurs, and the lack of nNOS binding sites on utrophin. α7β1 integrin differs from the UGC/DGC in the globular domains by which laminin binds and the presence of adaptor proteins that facilitate actin binding, including ILK and β-parvin. BMN195 and biglycan are two utrophin upregulation therapeutics that are near/in clinical trials and laminin 111 is the only dual (utrophin and integrin) target therapy that is near clinical trials. This review proposes that AAV delivery of SSPN should be considered as an additional dual target therapy.
Although introduction of recombinant laminin-111 protein was initially discovered to increase α7β1 integrin protein levels, it has also been shown to increase the levels of utrophin in mdx muscle [89]. Given that neither the over-expression of utrophin or α7β1 integrin fully rescue dystrophic symptoms, it would be interesting to determine whether the upregulation of both adhesion complexes would be more beneficial than targeting a single complex alone. Experiments to directly test UGC and integrin association have not been performed and numerous murine genetic experiments are often complicated with compensatory actions of other proteins. Migration of the three adhesion complexes in the same sucrose gradient fractions after biochemical isolation of DG bound proteins using succinylated wheat germ agglutinin (sWGA) lectin raises the interesting question of whether integrins and the UGC/DGC physically interact at the sarcolemma [90, 91]. If laminin-111 is successful in Phase 1 trials, the potential benefit of targeting both the UGC and α7β1 integrin may be addressed in DMD patients. Similar to laminin-111, Adam12 and SSPN also secondarily upregulate both the UGC and integrins at extra-junctional regions of the sarcolemma when over-expressed in mdx mice [47, 48, 92] (Figure 1). Adam12 is an active metalloproteinase that is expressed during muscle development and regeneration. The mechanism by which it promotes cell adhesion through the stabilization of the UGC and α7β1 integrin is currently unknown [93-95].
Recent studies have implicated a role for SSPN in facilitating interactions between the UGC and α7β1 integrin. SSPN, a tetraspanin-like protein, functions with the SGs to stabilize the association of α-DG with β-DG in the DGC [19, 20, 46]. Over-expression of threefold levels of SSPN ameliorates mdx muscle by increasing the levels of the UGC, α7β1 integrin, and laminin-binding to α-DG [47, 48]. Interestingly, the increase in laminin-binding to α-DG was not observed with 1.5-fold levels of SSPN over-expression in mdx mice, demonstrating that there is a minimum threshold of SSPN expression required for restoring normal ECM binding to DG [47]. To determine if laminin-binding to α-DG is required for SSPN-mediated amelioration of dystrophic symptoms, threefold SSPN transgenic mice were crossed with LARGEmyd mice. LARGE, like-acetylglucosaminyltransferase, is a glycosyltransferase responsible for elongating O-mannose glycans in the mucin domain of α-DG and mutations in LARGE abolish laminin-binding to α-DG [96]. Over-expression of SSPN does not ameliorate muscular dystrophy in the LARGEmyd mouse model of hypoglycosylated α-DG (MDC1D) despite increasing levels of the UGC at the sarcolemma, demonstrating that functionally glycosylated α-DG capable of binding to laminin is a requisite for SSPN-mediated amelioration of dystrophic pathology [47]. Recent genetic studies demonstrated that the combined loss of SSPN and α7 integrin results in extensive muscle pathology and decreased specific force production possibly due to decreased protein levels of the DGC and UGC in 4.5 month old diaphragm muscle [90]. These studies suggest that SSPN and integrins genetically interact and affect protein abundance of the DGC/UGC, but do not establish a direct interaction between these adhesion complexes. Investigation of the dependence of SSPN-mediated amelioration of mdx mice on α7 integrin and utrophin would clarify whether both adhesion complexes are essential for SSPN-based therapeutics. Interestingly, α7 integrin over-expression in mdx:utrophin-null mice causes a 10% reduction in regeneration, a 3-fold increase in mean survival age, and reduced severity of joint contractures [97]. These results suggest that α7 integrin reduces the severity of muscular dystrophy in mdx mice partially independent of utrophin, although the muscle pathology was not significantly reduced. It is possible that utrophin is required for the full rescue effect of α7 integrin in mdx muscle or that higher than 2-fold levels of α7 integrin are needed to rescue mdx muscle independently of utrophin. The ability of utrophin alone to rescue mdx:α7 integrin-null muscle has not been tested. Thus, additional evidence is needed to establish a direct SSPN-mediated interaction between the UGC and α7β1 integrin at the sarcolemma while the benefit of upregulating both adhesion complexes for the treatment of DMD remains undetermined.
Glycosylation Of α-DG and Amelioration of Muscular Dystrophy
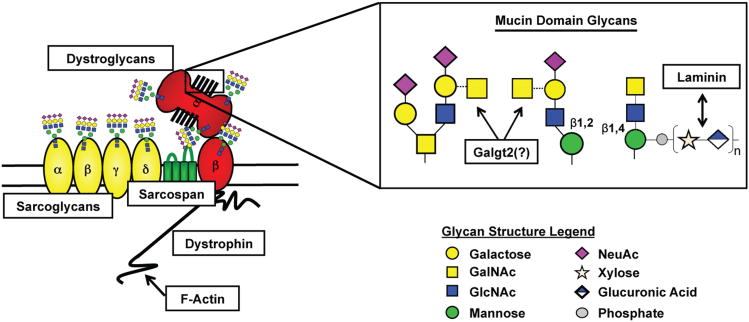
Appropriate glycosylation of sarcolemmal proteins is integral for proper muscle function. Over fifty percent of all known CMDs result from hypoglycosylation of α-DG and therefore are known as dystroglycanopathies [98]. Mutations in thirteen genes are known to cause aberrant glycosylation of α-DG. These gene products include: protein O-mannosyl transferase-1 and -2 (POMT1 and POMT2), protein O-linked mannose β-1,2-N-acetylglucosaminyltransferase (POMGnT1), LARGE, fukutin, FKRP, β-1,3-N-acetylgalactosaminyltransferase 2 (B3GALNT2), Isoprenoid Synthase Domain Containing (ISPD), Dolichyl-phosphate-mannosyltransferase subunits -2 and -3 (DPM2 and 3), Dolichol Kinase (DOLK), Glycosyltransferase-like domain containing (GTDC2), and Transmembrane protein 5 (TMEM5) [36, 37, 39, 99-113]. POMT1/2 act collaboratively to add the initiating mannose to serine or threonine residues of the mucin domain of α-DG [114, 115]. Classical mannose glycans are then elongated by the addition of a β-1,2-N-acetylglucosamine (β1,2GlcNAc) residue, while laminin-binding mannose glycans are elongated by the addition of a β-1,4-N-acetylglucosamine (β1,4GlcNAC) residue (Figure 2) [37, 99, 116]. It was recently proposed that B3GALNT2 may be the glycosyltransferase responsible for the addition of the GalNAc residue following β1,4GlcNAc addition to laminin-binding mannose glycans, however, this precise activity remains to be proven [108]. On laminin-binding glycan structures, LARGE creates the unique moiety required for binding of α-DG to laminin as well as other ECM components (Figure 2) [39, 117, 118]. Binding of α-DG to laminin has been shown to be dependent upon the xylosyl and glucuronyl transferase activities of LARGE [119]. Furthermore, knockout of LARGE activity causes a decrease in the molecular weight of α-DG [116]. Precise enzymatic functions of fukutin, FKRP, ISPD, GTDC2, and TMEM5 remain elusive; however, mutations in these genes lead to hypoglycosylation of α-DG and subsequent CMD phenotypes [100, 102-104, 107, 120]. DOLK and DPM-2/-3 are required for synthesis of the glycosylation precursors dolichol-phosphate and dolichol-phosphate-mannose respectively [121-123]; mutations in all three give rise to dystroglycanopathies as both glycosylation precursors are required for synthesis of N-glycans and O-mannosyl glycans [124, 125].
Figure 2. Glycosylation of the DGC.
Known and putative sites of glycosylation of DGC component proteins are depicted. Inset of laminin-binding glycan is provided along with potential site of Galgt2 modification. Colored symbols used to represent glycan structures are in accordance with the guidelines outlined by the Consortium of Functional Glycomics.
The dystroglycanopathies represent a diverse group of muscular dystrophies with broad phenotypic severities and wide ranging causative gene mutations. Walker-Warburg syndrome (WWS) cases result from the largest group of genetic mutations and can be attributed to mutations in POMT1, POMT2, LARGE, fukutin, FKRP, ISPD, β-1,3-N-acetylglucosaminyltransferase (B3GNT1), B3GALNT2, GTDC2, and TMEM5 [103, 107, 126, 127]. Patients presenting with muscle-eye-brain disease (MEB) represent a similarly heterogeneous population of phenotypic severities and genetic mutations. MEB has been reported to result from mutations in POMGnT1, fukutin and FKRP [37, 127, 128]. LGMD types 2K, N, M, I, and O result from mutations in POMT1, POMT2, fukutin, FKRP, and POMGnT1, respectively [28, 127-129]. CMD types 1C and 1D result from mutations in FKRP and LARGE, respectively [39, 130]. Furthermore, an insertion of a retrotransposon in the fukutin gene is known to cause Fukuyama congenital muscular dystrophy (FCMD) [102]. The genetic basis for the various dystroglycanopathies is varied and the resulting phenotypes are overlapping, which reinforces the need for a thorough understanding of the connection between specific genetic mutations and resulting biochemical dysfunctions that underlie or cause muscular dystrophy.
While DMD results from mutations in dystrophin, it is noteworthy that significant changes in glycosylation have been reported in the mouse model for DMD. The lectin Wisteria floribunda agglutinin (WFA) recognizes terminal β1,4 GalNAc residues of α-DG in skeletal muscle [60, 131] and binding is normally restricted to the NMJ and MTJ in wild-type myofibers [59]. However, WFA binds to the extrasynaptic sarcolemma in addition to the NMJ on mdx muscle cryosections [47, 58]. This redistribution of WFA staining to the extrasynaptic sarcolemma is concomitant with the redistribution of utrophin and the associated UGC in mdx mouse muscle.
Over-expression of both full length and truncated utrophin rescues the dystrophic phenotype in mdx mice [77, 132-135]. As WFA preferentially binds to α-DG in the UGC, increasing GalNAc modification of α-DG has been one approach to increase extrasynaptic utilization of utrophin as a potential therapy for DMD. The over-expression of terminal β-GalNAc glycosyltransferase galgt2 in wild-type mice has been shown to increase levels of UGC proteins, laminin-binding and reactivity of α-DG to WFA [59, 60]. Similar results were obtained from galgt2 over-expression in mdx mice, where rescue of laminin-binding and the dystrophic phenotype were additionally noted [59, 60]. Interestingly, transgenic over-expression of SSPN also increases the WFA reactivity of α-DG in mdx and LARGEmyd muscle while loss of SSPN reduces UGC levels and the reactivity of α-DG with WFA [47]. Importantly, no mitigation of dystrophic pathology or rescue of laminin-binding is observed in LARGEmyd mice over-expressing SSPN [47]. These studies are significant as they demonstrate that laminin-binding is required for SSPN-mediated amelioration of dystrophic pathology and that the glycans detected by increasing WFA reactivity are distinct from the structure created by LARGE activity. It has been proposed that SSPN over-expression mediates changes in glycosylation via increased galgt2 activity [47]; however, this mechanism requires further validation through the creation of galgt2-deficient mdx SSPN transgenic mice. Interestingly, over-expression of galgt2 has also been demonstrated to be effective in ameliorating the dystrophic phenotype in mouse models of CMD 1A (dyW) and α-SG-deficient LGMD 2D [136, 137]. It is noteworthy, however, that the over-expression of a neuronal homolog galgt1, which adds terminal β-GalNAc residues distinctly to ganglioside glycolipids, caused muscle pathology including decreased myofiber diameter and increased central nucleation in wild-type mice [138]. These stark differences resulting from the over-expression of either galgt2 or galgt1 demonstrate the tissue and acceptor substrate specificity of glycosylation and reinforce the importance of thoroughly evaluating the effects of manipulating glycosylation.
While genetic over-expression of a glycosyltransferase provides one potential therapeutic approach for manipulating glycosylation of sarcolemmal glycoproteins, pharmacologic approaches also provide potential therapeutics. Using high throughput screening, the small molecule lobeline was identified as a pharmacological treatment that altered C2C12 glycosylation in vitro [139]. Compounds from the Prestwick library of about 1200 FDA approved small molecules were added to myoblasts in differentiation media and changes in glycosylation were measured after two days of treatment [139]. Specifically, binding of terminal β-GalNAc modifications as detected by WFA binding increased in C2C12 cells as well as isolated wild-type and mdx myoblasts following treatment with lobeline during differentiation [139]. Lobeline treatment increased abundance of UGC proteins and laminin-binding in wild-type and mdx primary cell cultures [139]; these results are similar to changes resulting from galgt2 over-expression. It is noteworthy that the increase in WFA binding following lobeline treatment was dependent upon complex N-glycans and not O-mannose glycans such as those required for laminin-binding [139]. Furthermore, deoxymannojirimycin (DMNJ) inhibition of complex N-glycans necessary for WFA binding caused a decrease in laminin-binding as detected by laminin overlay [139]. While lobeline was first FDA approved for smoking cessation, in vitro studies have shown that lobeline may potentially act as a protein folding chaperone or nicotinic antagonist through the dopamine or vesicular monoamine transporter [140-143]. In the C2C12 studies, the molecular mechanism by which lobeline increased WFA binding in vitro was not determined. While the ability of lobeline to alter muscle glycosylation in vivo remains to be demonstrated, these studies represent strong proof of principle for the pharmacologic manipulation of sarcolemmal protein glycosylation as a potential therapeutic.
Protein Aggregation and Amelioration in Muscular Dystrophies
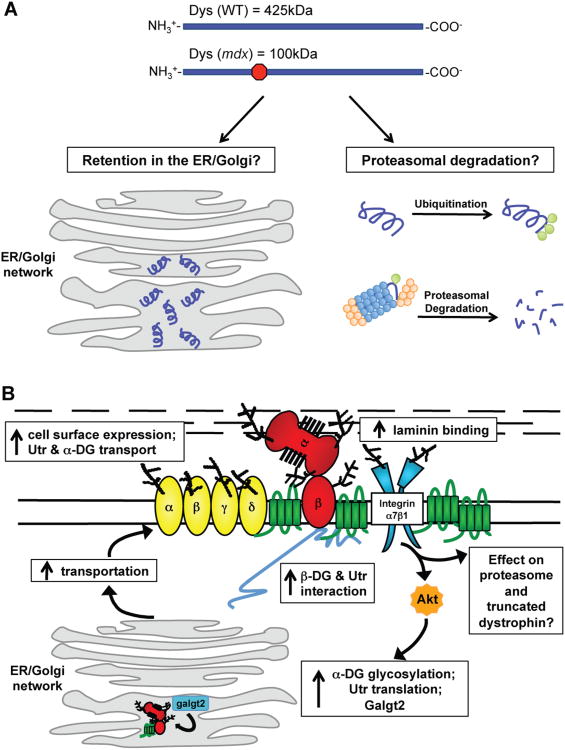
The mdx mouse contains a point mutation in exon 23 [144], resulting in a premature termination codon that is predicted to result in a 115 kDa protein [145]. It has previously been shown that dystrophin mRNA levels are decreased in skeletal muscle, cardiac muscle, and brains of three mdx mouse strains [146]. However, dystrophin mRNA levels vary widely, depending on the causative mutation in human DMD and Becker muscular dystrophy (BMD) patients [147]. It was previously thought that the 115 kDa dystrophin protein is rapidly degraded after synthesis in mdx muscle, but studies suggest that truncated dystrophin may be expressed at the sarcolemma [148-150] or retained in the ER/Golgi [47].
A 70-80 kDa dystrophin reactive to a C-terminal antibody was found in a group of Japanese Spitz dogs displaying progressive Duchenne-like muscular dystrophy [149]. These dogs displayed exercise intolerance, an abnormal gait, and pain upon handling of muscles beginning at 10 to 12 weeks of age. The symptoms worsened over time, suggesting that the 70-80 kDa dystrophin was unable to restore sarcolemmal stability. In a separate study, immunohistochemical assays revealed expression of truncated dystrophin in myotubes isolated from three 12-week old aborted fetuses at risk for DMD, suggesting that mutant dystrophin is synthesized in human DMD patients [148]. Recently, the truncated form of dystrophin was detected within the ER/Golgi compartments of mdx mice [47], suggesting that the 115 kDa dystrophin may be retained in the ER/Golgi compartments.
The mutation in mdx mice leads to the loss of the β-DG binding domain, leaving only the actin binding domains in the dystrophin fragment [145]. The N-terminus of dystrophin binds directly to F-actin in the cytoplasm of the myofiber, while the C-terminus of dystrophin contains cysteine-rich domains that bind the C-terminus of β-DG [4, 151]. Recent studies utilizing truncated dystrophin have provided more insight into which segments of the protein are required for sarcolemmal stability. One study utilized rAAV6-microdystrophin to evaluate the role of the actin-binding domains in sarcolemmal stabilization [152]. Four months after injection into the tibialis anterior, muscles treated with micro-dystrophins lacking actin-binding domains displayed decreased specific force and a decreased ability to protect against contraction-induced injury [152], indicating the importance of an intact actin-binding domain in maintaining sarcolemmal stability.
Other studies have analyzed different isoforms of dystrophin. The dystrophin isoform Dp116 is expressed in Schwann cells within the peripheral nervous system [153]. This isoform lacks the actin-binding domains while retaining the complete dystroglycan-binding domain [153]. Although expression of Dp116 in mdx:utrophin-null mice increased muscle mass, life span, and maximal force, the specific force and histopathology did not improve [153]. It is hypothesized that Dp116, or the dystroglycan binding domain, attempts to stabilize the DGC at the sarcolemma through other interactions with the cytoskeleton, although specifics have yet to be elucidated [153]. The expression of a non-muscle isoform of dystrophin, Dp71, which lacks N-terminal actin-binding domains and spectrin-like repeats, restores the DGC in muscle, suggesting that the β-DG binding domain is required for assembly of the DGC [154]. Thus, it would be interesting to examine other mice with C-terminal mutations in dystrophin. Such experiments would assess the ability of truncated dystrophin to be incorporated and expressed with the DGC at the sarcolemma and restore functionality of dystrophic muscle. This provides an exciting opportunity to examine ways to express fragmented dystrophin at the sarcolemma to improve dystrophic pathology. Other studies involving protein retention within cellular compartments in different muscular dystrophies may provide insights into the therapeutic translation of these findings.
Studies in FCMD, which is caused by mutations in fukutin [102], have provided insight into the importance of protein processing within the ER/Golgi. The exact role of fukutin remains unclear, but mutations in fukutin lead to abnormal glycosylation of α-DG [155], a reduction in laminin-binding activity [155], and the mislocalization and retention of fukutin in the ER [156]. A recent study analyzed thirteen missense fukutin constructs in C2C12 cells and discovered that four were mislocalized to the ER. To understand whether mutant fukutin still leads to the production of functionally glycosylated α-DG, site-directed mutagenesis was used to generate mutants for transfection into fukutin-null mouse embryonic stem cells [156]. The expression of the four missense fukutin mutants restored α-DG reactivity against the IIH6C4 antibody and laminin-binding to α-DG, suggesting α-DG is functionally glycosylated with the expression of mutant fukutin [156]. Using treatments to improve protein folding, including curcumin, a molecule isolated from the spice turmeric, and low temperature culturing conditions, ER retention of fukutin was corrected in the four mutants shown to be mislocalized in the ER. In order to elucidate the reasons for aberrant fukutin trafficking, brefeldin A was used to examine anterograde transport and nocodazole was used to examine retrograde transport [156]. After incubation with brefeldin A, the fukutin mutants remain accumulated in the ER [156]. The results suggest that while mutant fukutin proteins are processed in the ER, they are unable to be transported to the Golgi through the anterograde pathway, providing valuable insight into how the protein trafficking process is affected upon the mislocalization of a mutant protein. Thus, it is possible that truncated dystrophin may be retained within the ER/Golgi compartments and is not transported to the cell surface in mdx muscle. It will be important for future studies to utilize a similar strategy to elucidate the effects of 115 kDa dystrophin on retrograde and anterograde transport in mdx cells. Additionally, treatment with pharmacological agents, such as curcumin or exposure to low temperature, may potentially alleviate accumulation of truncated dystrophin in the ER/Golgi compartments and improve the transport of DGC and UGC components to the sarcolemmal membrane.
Curcumin was found to target the NF-κB pathway [157] and has been used to treat dystrophic muscle. The activation of NF-κB, which is involved in the modulation of immune responses and regulation of myogenesis, is increased in DMD patient muscle [158]. Intraperitoneal administration of curcumin inhibits NF-κB activation and reduces dystrophic pathology in mdx mice [159]. However, a different study reports that curcumin treatment is unable to inhibit the NF-κB pathway and does not improve specific force in the diaphragm muscle of mdx mice [160]. Future studies examining the effect of curcumin on the accumulation of truncated dystrophin in the ER/Golgi will be important in determining what role, if any, it plays in pathogenesis.
Protein mislocalization has also been implicated in LGMDs 2C-F pathologies caused by mutations in the SG genes [161]. The severity of pathology in LGMDs 2C-F and cellular fate of the affected SG varies according to the mutation [161]. According to data compiled from the Leiden University mutation database, the most common mutations are α-p.R77C, β-p.S114F, and γ-p.C283Y, which cause a mild to severe phenotype [161]. These data led to an examination of the noted point mutations and their effect on intracellular fate and expression of the SGs at the cell surface. Following treatment with kifunensine, an α-mannosidase I inhibitor that prevents ER-associated degradation [162], the expression of sarcoglycans with mild mutations thought to cause the least amount of structural modification was restored at the cell surface. Future studies manipulating ER quality control in wild-type and mdx cells might provide more information on how changes in the ER can affect DGC and UGC expression at the cell surface.
Heat Shock Proteins and Proteasome Inhibition in the Treatment of Muscular Dystrophy
Heat shock proteins (Hsp) are induced to combat cellular stress when organisms are under environmental strains such as heat, or during disease and infection [163]. Analysis of skeletal muscle from young DMD patients revealed an induction of Hsp72 and Hsp65 in hypercontracted fibers and Hsp90 in regenerating muscle [164]. Hsp72 is also increased at the mRNA level following electrical stimulation for tetanic contractions in isolated single skeletal muscle fibers from Xenopus laevis [165] and after high-intensity exercise in normal Wistar rats [166], suggesting a role for Hsp72 in protection against muscle stress. Treatment of mdx and utrophin-deficient mdx mice with BGP-15, a pharmacologic inducer of Hsp72, showed that an increase in Hsp72 expression improved SERCA function and dystrophic pathology, decreased kyphosis, and ultimately extended lifespan [167]. Targeting Hsp72 to improve protein folding and quality control during cellular stress and disease progression provides a possible avenue to improve muscle function while prolonging the lifespan of patients with muscular dystrophy.
Ubiquitin is an important component in proteasome regulation and can direct proteins towards degradation [168]. Ubiquitin was also found to be elevated in hypercontracted, regenerating, and necrotic myofibers in the skeletal muscle of young DMD patients [164]. The increase in ubiquitin protein may suggest misregulation of the ubiquitin-proteasome pathway as well as an increase in protein degradation in DMD patients, which may contribute to the pathogenesis of DMD. Recent studies attempting to ameliorate dystrophic pathology using proteasome inhibitors have produced mixed results. Velcade and MLN273, two FDA-approved proteasome inhibitors, were injected into the gastrocnemius muscle of mdx mice [169]. A truncated 97 kDa dystrophin product, as well as an increase in α-DG, β-DG, and α-SG, were detected using immunoblotting and immunohistochemistry 24 hours post treatment [169]. Similarly, another proteasome inhibitor, MG-132, rescued the membrane localization of dystrophin, α-DG, β-DG, and α-SG [170]. However, physiological functionality was not examined and experiments such as grip strength and force production were not performed. This information is critical for the pursuit of such pharmacological agents as treatments for DMD (Fig. 3A).
Figure 3. Effects of SSPN over-expression in mdx mice on the cell surface protein expression and protein processing and possible outcomes of truncated dystrophin within the cell.
(A) Wild-type mice express full-length dystrophin, which is 425 kDa. The mdx mutation leads to a premature stop codon that results in a truncated dystrophin of 100 kDa. Dystrophin is depicted in purple, the proteasome is blue and orange, and ubiquitin is green. Truncated dystrophin can be retained in the ER/Golgi. Alternatively, truncated dystrophin is ubiquitinated and sent to the proteasomal degradation pathway. (B) The over-expression of SSPN in mdx muscle leads to molecular events resulting in the restoration of laminin-binding and rescue of mdx pathology. SSPN activates Akt, which leads to an increase in utrophin and integrins. Galgt2, one enzyme responsible for GalNAc modification of α-DG, is also increased in isolated ER/Golgi membranes. SSPN also improves utrophin-DG transportation to the sarcolemma while simultaneously restoring laminin-binding and membrane stability. SSPN's effect on the trafficking of truncated dystrophin and proteasomal degradation is still unknown. DGs (red), SGs (yellow), SSPN (green), integrins (blue), and Akt (orange) are shown. Utrophin (Utr) is depicted in gray.
Although some drugs such as Velcade and MLN273 have shown promise for the attenuation of dystrophic pathology in mdx mice, other studies have described conflicting results. In a separate study, long-term administration of a MG-132 failed to restore dystrophin expression in mdx muscle and ultimately increased susceptibility to contraction-induced damage [171]. The effects of proteasome inhibitors on grip strength, force production, and DGC protein expression need to be analyzed more rigorously to better determine their efficacy in improving muscle function and dystrophic pathology.
A Possible Function for Sarcospan as a Chaperone Protein
New data suggests a role for SSPN in protein trafficking to the sarcolemma. A recent study isolated ER/Golgi membranes from mdx muscle and immunoblots revealed an increase in α-DG and utrophin compared to wild-type muscle [47]. This suggests a possible compensatory mechanism is at play, whereby the system attempts to utilize utrophin upon the loss of dystrophin (Fig. 3B). Interestingly, SSPN transgenic mdx mice revealed decreased levels of WFA reactive α-DG and utrophin in ER/Golgi preparations, while immunofluorescence assays showed an increased abundance of these proteins at the sarcolemma [47]. SSPN over-expression increased WFA binding 1.8-fold per α-DG molecule as detected by WFA overlay [47]. Increased extrasynaptic binding of WFA may be the result of general chaperone trafficking of glycoprotein complexes by SSPN. However, the increase in WFA-reactive glycosylation per α-DG protein demonstrates a role for SSPN in specific changes in glycosylation of α-DG. This modification in α-DG glycosylation may result from downstream transcriptional effects of increased Akt signaling [47] or could additionally be a more direct result of chaperone activities of SSPN in trafficking. SSPN might act directly as a chaperone to stabilize α-DG during ER/Golgi trafficking and addition of glycans resulting in increased glycan modification per α-DG molecule. Importantly, over-expression of SSPN drives changes in glycosylation of α-DG, which aid in the amelioration of mdx pathology [47]. These studies highlight a promising role for SSPN as a therapeutic and it would be interesting to examine SSPN over-expression in dystroglycanopathy mouse models where direct manipulation of glycosylation ameliorates dystrophic pathology [136, 137]. SSPN may possess chaperone-like functions, and the over-expression of SSPN may improve overall protein folding and quality control as well as transport to the cell surface.
How to Achieve Forced over-Expression of SSPN
Membrane proteins are synthesized by ribosomes on the ER and disulfide bonds are synthesized and rearranged in the ER lumen [172]. Since SSPN is an integral membrane protein with disulfide bonds requiring processing through the ER, it most likely cannot be administered systemically. The small size of SSPN makes it an excellent gene to be delivered through AAV. AAV delivery of α-SG in α-SG-deficient patients and γ-SG in γ-SG-deficient patients resulted in no adverse events, demonstrating that intramuscular AAV delivery is likely to be safe in adult patients [173, 174]. The only patients that did not express SG following AAV delivery had pre-existing immunity to the AAV serotype used, demonstrating the need for pre-screening of AAV serotypes [173, 174]. However, there are many challenges to be overcome before systemic delivery of AAV is feasible. Systemic delivery of therapeutics will be required for amelioration of fatal dystrophic symptoms in the diaphragm and heart muscles. Surprisingly, a T-cell mediated immune reaction against dystrophin prevented the expression of mini-dystrophin following AAV delivery in DMD patients [81]. The precise reason for the varied T-cell immune responses (against mini-dystrophin and against self revertant dystrophin) in the 6 trial patients is unknown. These results warrant caution in future AAV mini/micro-dystrophin and exon skipping trials. Importantly, immune responses should not be a problem in secondary approaches to upregulate utrophin or α7β1 integrin for protein replacement therapeutics. A drug screen for compounds that upregulate SSPN offers an additional approach to AAV-mediated SSPN therapy. A similar approach led to the discovery of BMN195 for utrophin upregulation and is currently in Phase 1 clinical trials.
Unanswered Questions for SSPN-Based Therapeutics
Over-expression of SSPN under the human skeletal actin promoter results in a 60% reduction in regeneration in the mdx mouse model of DMD by replacing the DGC with the UGC and α7β1 integrin [47, 48]. As with most therapeutic targets, there are many questions that remain to be answered for SSPN-based therapeutics. For SSPN to become a viable therapeutic for the treatment of DMD, the following questions remain to be addressed:
Does SSPN delivery with AAV prevent dystrophic pathology in the mdx mouse model? This is an important question that needs to be answered, as it will determine the delivery system used for SSPN-based therapeutics. It would also be useful to further determine the feasibility of AAV delivery of SSPN in the golden retriever model of DMD (GRMD), as the dog model provides a more realistic clinical model for systemic delivery.
Does SSPN eliminate/reduce dystrophic pathology in the heart and diaphragm? The human skeletal actin promoter is not highly expressed in the diaphragm or heart muscles. Since death occurs from respiratory and/or cardiac failure in DMD patients, an ideal therapeutic target should prevent dystrophic pathology in the heart and diaphragm. This question can be addressed with systemic delivery of AAV6-SSPN in the mdx mouse model. AAV6 has been shown to infect the heart and the diaphragm [175, 176].
Can SSPN reverse or prevent dystrophic pathology after the onset of dystrophy? The human skeletal actin promoter is turned on early in muscle development. SSPN is likely preventing the onset of dystrophic pathology rather than reversing pathology. Boys with DMD are often diagnosed with the disease well after the onset of dystrophic pathology, so effective treatments should reverse or halt pathology already in progress. To determine if SSPN is effective after the onset of disease, a murine inducible transgenic system could be engineered to turn SSPN expression on later in life. Intramuscular or systemic injection of AAV-SSPN after the onset of pathology in mdx mice will also address this question.
Is widespread expression of SSPN safe? The promoter used in murine studies of SSPN over-expression in the mdx mouse model restricted SSPN expression to striated skeletal muscle [177]. An effective treatment in patients with DMD would require systemic delivery of SSPN in order to target the heart and diaphragm. It is important to determine whether long-term systemic delivery of SSPN is safe. This question can also be addressed with systemic delivery of AAV-SSPN in mdx mice. Since AAV infection of particular tissues depends on the AAV serotype, it would be important to test SSPN delivery with the serotypes approved for use in patients.
Does SSPN upregulate the UGC and integrins in human myoblasts? Many recent treatment strategies have been tested in DMD myoblasts, including BMN195 and dantrolene [82, 178]. The ability to demonstrate that a therapy acts through similar targets in human cells offers a nice proof-of-principle for the approach being tested.
Which patient populations will benefit from SSPN-based therapeutics? It is important when developing therapies for muscular dystrophy to determine how many different muscle diseases will benefit from the treatment. This will allow for the design and recruitment of appropriate patient populations for clinical trials. SSPN is effective in ameliorating pathology in the mdx model of DMD [47, 48]. SSPN did not reduce dystrophic pathology in the LARGEmyd model of hypoglycosylation of α-DG (MDC1D) [47]. It would be interesting to determine if SSPN can ameliorate various limb-girdle and congenital muscular dystrophies.
Conclusions
In the mdx model of DMD, the SSPN transgene ameliorates dystrophic pathology by increasing the UGC and α7β1 integrin extra-synaptic sarcolemma, the synapse specific GalNAc glycosylation of α-DG, and the transport of utrophin and α-DG from the ER/Golgi to the cell surface [47, 48, 179]. These studies raise important questions about the mechanisms by which SSPN over-expression accomplishes these observed effects. Biochemical evidence that SSPN is a component of both the DGC and UGC complexes and genetic analysis of mice lacking both SSPN and α7 integrin have led to the proposal that SSPN stabilizes the UGC and integrins through direct interactions at the sarcolemma [46, 47, 90, 179]. More experiments are needed to address the requirement of utrophin and α7 integrin for SSPN-mediated amelioration of mdx muscle, as well as the possibility that the UGC and α7β1 integrin function synergistically at the sarcolemma. Additionally, SSPN may act either directly or indirectly as a chaperone protein to facilitate the efficient assembly and export of the UGC to the cell surface [47]; however, more studies are needed to elucidate the role of SSPN in the ER/Golgi. Over-expression of constitutively active Akt signaling has been shown to be beneficial in mdx muscle by increasing the UGC and integrins at the sarcolemma, reducing membrane damage, and improving the force generating capacity of muscle [180, 181]. SSPN-mediated amelioration of mdx muscle results in a similar increase in active Akt signaling and downstream muscle growth pathways [47]. Many therapeutic approaches for DMD concentrate on a single target. Although experimental strategies for the treatment of DMD have been developed for over 25 years, steroids remain the only approved drugs to slow the progression of the disease. Thus, the best treatment strategy remains an open question. SSPN is unique because it incorporates several beneficial therapeutic targets into a single protein that is small and easily packaged in AAV delivery systems. SSPN is also ubiquitously expressed in other tissues throughout the body so systemic delivery and immune response should not be an issue [46]. We are currently experimentally addressing the unanswered questions for SSPN therapeutics.
Acknowledgments
We thank A.W. Kwok, J. Lee, and J. Oh for critically reading this manuscript. This work was supported by grants from the Genetic Mechanisms Pre-doctoral Training Fellowship USPHS National Research Service Award GM07104, the Edith Hyde Fellowship, the Eureka Pre-doctoral Training Fellowship, and the Ruth L. Kirschstein National Service Award T32AR059033 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases to J.L.M.; Muscular Dystrophy Association RG 135449 and NIH P30 AR057230 to L.G.B; and NIH/NIAMS (R01 AR048179) to R.C-W.
Abbreviations
- AAV
adeno-associated virus
- AR-LGMD
autosomal recessive limb-girdle muscular dystrophy
- B3GALNT2
β-1,3-N-acetylgalactosaminyltransferase 2
- B3GNT1
β-1,3-N-acetylglucosaminyltransferase
- BMD
Becker muscular dystrophy
- CMD
congenital muscular dystrophy
- DMNJ
deoxymannojirimycin
- DG
dystroglycan
- DGC
dystrophin-glycoprotein complex
- DMD
Duchenne muscular dystrophy
- DOLK
Dolichol Kinase
- DPM2/3
Dolichyl-phosphate-mannosyltransferase subunits -2 and -3
- ECM
extracellular matrix
- ER
endoplasmic reticulum
- F-actin
filamentous actin
- FCMD
Fukuyama congenital muscular dystrophy
- FKRP
fukutin-related protein
- GRMD
golden retriever muscular dystrophy
- GTDC2
Glycosyltransferase-like domain containing
- Hsp
heat shock protein
- ILK
integrin-linked kinase
- ISPD
isoprenoid synthase domain containing
- LARGE
like-acetylglucosaminyltransferase
- MDC1A
merosin-deficient congenital muscular dystrophy 1A
- MEB
muscle-eye-brain disease
- MTJ
myotendinous junction
- NMJ
neuromuscular junction
- nNOS
neuronal nitric oxide synthase
- POMT
protein O-mannosyl transferase
- POMGnT1
protein O-linked mannose β-1,2-acetylglucosaminyltransferase
- SG
sarcoglycan
- SSPN
sarcospan
- sWGA
succinylated wheat germ agglutinin
- TMEM5
Transmembrane protein 5
- UGC
utrophin-glycoprotein complex
- Utr
utrophin
- WFA
Wisteria floribunda agglutinin
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- 1.Ervasti JM, Ohlendieck K, Kahl SD, Gaver MG, Campbell KP. Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature. 1990;345:315–319. doi: 10.1038/345315a0. [DOI] [PubMed] [Google Scholar]
- 2.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 4.Levine BA, Moir AJ, Patchell VB, Perry SV. The interaction of actin with dystrophin. FEBS Lett. 1990;263:159–162. doi: 10.1016/0014-5793(90)80728-2. [DOI] [PubMed] [Google Scholar]
- 5.Campbell KP, Kahl SD. Association of dystrophin and an integral membrane glycoprotein. Nature. 1989;338:259–262. doi: 10.1038/338259a0. [DOI] [PubMed] [Google Scholar]
- 6.Moorwood C. Syncoilin, an intermediate filament-like protein linked to the dystrophin associated protein complex in skeletal muscle. Cell Mol Life Sci. 2008;65:2957–2963. doi: 10.1007/s00018-008-8306-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal Nitric oxide synthase. Circ Res. 2003;92:554–560. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- 10.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506(3):817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin EA, Barresi R, Byrne BJ, Tsimerinov EI, Scott BL, Walker AE, Gurudevan SV, Anene F, Elashoff RM, Thomas GD, Victor RG. Tadalafil alleviates muscle ischemia in patients with becker muscular dystrophy. Sci Transl Med. 2012;4:162r. doi: 10.1126/scitranslmed.3004327. a155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell. 1991;66:1121–1131. doi: 10.1016/0092-8674(91)90035-w. [DOI] [PubMed] [Google Scholar]
- 14.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355:696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 15.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol. 1993;122:809–823. doi: 10.1083/jcb.122.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ervasti JM, Kahl SD, Campbell KP. Purification of dystrophin from skeletal muscle. J Biol Chem. 1991;266:9161–9165. [PubMed] [Google Scholar]
- 17.Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol. 1993;5:82–87. doi: 10.1016/s0955-0674(05)80012-2. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida M, Ozawa E. Glycoprotein complex anchoring dystrophin to sarcolemma. J Biochem. 1990;108:748–752. doi: 10.1093/oxfordjournals.jbchem.a123276. [DOI] [PubMed] [Google Scholar]
- 19.Crosbie RH, Lebakken CS, Holt KH, Venzke DP, Straub V, Lee JC, Grady RM, Chamberlain JS, Sanes JR, Campbell KP. Membrane targeting and stabilization of sarcospan is mediated by the sarcoglycan subcomplex. J Cell Biol. 1999;145:153–165. doi: 10.1083/jcb.145.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crosbie RH, Lim LE, Moore SA, Hirano M, Hays AP, Maybaum SW, Collin H, Dovico SA, Stolle CA, Fardeau M, et al. Molecular and genetic characterization of sarcospan: insights into sarcoglycan-sarcospan interactions. Hum Mol Genet. 2000;9:2019–2027. doi: 10.1093/hmg/9.13.2019. [DOI] [PubMed] [Google Scholar]
- 21.Holt KH, Campbell KP. Assembly of the sarcoglycan complex Insights for muscular dystrophy. J Biol Chem. 1998;273:34667–34670. doi: 10.1074/jbc.273.52.34667. [DOI] [PubMed] [Google Scholar]
- 22.Weller B, Karpati G, Carpenter S. Dystrophin-deficient mdx muscle fibers are preferentially vulnerable to necrosis induced by experimental lengthening contractions. J Neurol Sci. 1990;100:9–13. doi: 10.1016/0022-510x(90)90005-8. [DOI] [PubMed] [Google Scholar]
- 23.Pearce M, Blake DJ, Tinsley JM, Byth BC, Campbell L, Monaco AP, Davies KE. The utrophin and dystrophin genes share similarities in genomic structure. Hum Mol Genet. 1993;2:1765–1772. doi: 10.1093/hmg/2.11.1765. [DOI] [PubMed] [Google Scholar]
- 24.Tinsley JM, Blake DJ, Roche A, Fairbrother U, Riss J, Byth BC, Knight AE, Kendrick-Jones J, Suthers GK, Love DR, et al. Primary structure of dystrophin-related protein. Nature. 1992;360:591–593. doi: 10.1038/360591a0. [DOI] [PubMed] [Google Scholar]
- 25.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 26.Song WK, Wang W, Foster RF, Bielser DA, Kaufman SJ. H36-alpha 7 is a novel integrin alpha chain that is developmentally regulated during skeletal myogenesis. J Cell Biol. 1992;117:643–657. doi: 10.1083/jcb.117.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von der Mark H, Durr J, Sonnenberg A, von der Mark K, Deutzmann R, Goodman SL. Skeletal myoblasts utilize a novel beta 1-series integrin and not alpha 6 beta 1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991;266:23593–23601. [PubMed] [Google Scholar]
- 28.Balci B, Uyanik G, Dincer P, Gross C, Willer T, Talim B, Haliloglu G, Kale G, Hehr U, Winkler J, Topaloglu H. An autosomal recessive limb girdle muscular dystrophy (LGMD2) with mild mental retardation is allelic to Walker-Warburg syndrome (WWS) caused by a mutation in the POMT1 gene. Neuromuscul Disord. 2005;15:271–275. doi: 10.1016/j.nmd.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Bonnemann CG, Modi R, Noguchi S, Mizuno Y, Yoshida M, Gussoni E, McNally EM, Duggan DJ, Angelini C, Hoffman EP. Beta-sarcoglycan (A3b) mutations cause autosomal recessive muscular dystrophy with loss of the sarcoglycan complex. Nat Genet. 1995;11:266–273. doi: 10.1038/ng1195-266. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura K, Tome FM, Collin H, Azibi K, Chaouch M, Kaplan JC, Fardeau M, Campbell KP. Deficiency of the 50K dystrophin-associated glycoprotein in severe childhood autosomal recessive muscular dystrophy. Nature. 1992;359:320–322. doi: 10.1038/359320a0. [DOI] [PubMed] [Google Scholar]
- 31.McNally EM, Bonnemann CG, Kunkel LM, Bhattacharya SK. Deficiency of adhalin in a patient with muscular dystrophy and cardiomyopathy. N Engl J Med. 1996;334:1610–1611. doi: 10.1056/NEJM199606133342417. [DOI] [PubMed] [Google Scholar]
- 32.McNally EM, Passos-Bueno MR, Bonnemann CG, Vainzof M, de Sa Moreira E, Lidov HG, Othmane KB, Denton PH, Vance JM, Zatz M, Kunkel LM. Mild and severe muscular dystrophy caused by a single gamma-sarcoglycan mutation. Am J Hum Genet. 1996;59:1040–1047. [PMC free article] [PubMed] [Google Scholar]
- 33.McNally EM, Duggan D, Gorospe JR, Bonnemann CG, Fanin M, Pegoraro E, Lidov HG, Noguchi S, Ozawa E, Finkel RS, et al. Mutations that disrupt the carboxyl-terminus of gamma-sarcoglycan cause muscular dystrophy. Hum Mol Genet. 1996;5:1841–1847. doi: 10.1093/hmg/5.11.1841. [DOI] [PubMed] [Google Scholar]
- 34.Nigro V, de Sa Moreira E, Piluso G, Vainzof M, Belsito A, Politano L, Puca AA, Passos-Bueno MR, Zatz M. Autosomal recessive limb-girdle muscular dystrophy, LGMD2F, is caused by a mutation in the delta-sarcoglycan gene. Nat Genet. 1996;14:195–198. doi: 10.1038/ng1096-195. [DOI] [PubMed] [Google Scholar]
- 35.Noguchi S, McNally EM, Ben Othmane K, Hagiwara Y, Mizuno Y, Yoshida M, Yamamoto H, Bonnemann CG, Gussoni E, Denton PH, et al. Mutations in the dystrophin-associated protein gamma-sarcoglycan in chromosome 13 muscular dystrophy. Science. 1995;270:819–822. doi: 10.1126/science.270.5237.819. [DOI] [PubMed] [Google Scholar]
- 36.van Reeuwijk J, Janssen M, van den Elzen C, Beltran-Valero de Bernabe D, Sabatelli P, Merlini L, Boon M, Scheffer H, Brockington M, Muntoni F, et al. POMT2 mutations cause alpha-dystroglycan hypoglycosylation and Walker-Warburg syndrome. J Med Genet. 2005;42:907–912. doi: 10.1136/jmg.2005.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yoshida A, Kobayashi K, Manya H, Taniguchi K, Kano H, Mizuno M, Inazu T, Mitsuhashi H, Takahashi S, Takeuchi M, et al. Muscular dystrophy and neuronal migration disorder caused by mutations in a glycosyltransferase, POMGnT1. Dev Cell. 2001;1:717–724. doi: 10.1016/s1534-5807(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, et al. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19:94–97. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 39.Longman C, Brockington M, Torelli S, Jimenez-Mallebrera C, Kennedy C, Khalil N, Feng L, Saran RK, Voit T, Merlini L, et al. Mutations in the human LARGE gene cause MDC1D, a novel form of congenital muscular dystrophy with severe mental retardation and abnormal glycosylation of alpha-dystroglycan. Hum Mol Genet. 2003;12:2853–2861. doi: 10.1093/hmg/ddg307. [DOI] [PubMed] [Google Scholar]
- 40.Jones KJ, Morgan G, Johnston H, Tobias V, Ouvrier RA, Wilkinson I, North KN. The expanding phenotype of laminin alpha2 chain (merosin) abnormalities: case series and review. J Med Genet. 2001;38:649–657. doi: 10.1136/jmg.38.10.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mercuri E, Yuva Y, Brown SC, Brockington M, Kinali M, Jungbluth H, Feng L, Sewry CA, Muntoni F. Collagen VI involvement in Ullrich syndrome: a clinical, genetic, and immunohistochemical study. Neurology. 2002;58:1354–1359. doi: 10.1212/wnl.58.9.1354. [DOI] [PubMed] [Google Scholar]
- 42.Tome FM, Evangelista T, Leclerc A, Sunada Y, Manole E, Estournet B, Barois A, Campbell KP, Fardeau M. Congenital muscular dystrophy with merosin deficiency. C R Acad Sci III. 1994;317:351–357. [PubMed] [Google Scholar]
- 43.Durbeej M, Campbell KP. Muscular dystrophies involving the dystrophin-glycoprotein complex: an overview of current mouse models. Curr Opin Genet Dev. 2002;12:349–361. doi: 10.1016/s0959-437x(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 44.Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- 45.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 46.Crosbie RH, Heighway J, Venzke DP, Lee JC, Campbell KP. Sarcospan, the 25-kDa transmembrane component of the dystrophin-glycoprotein complex. J Biol Chem. 1997;272:31221–31224. doi: 10.1074/jbc.272.50.31221. [DOI] [PubMed] [Google Scholar]
- 47.Marshall JL, Holmberg J, Chou E, Ocampo AC, Oh J, Lee J, Peter AK, Martin PT, Crosbie-Watson RH. Sarcospan-dependent Akt activation is required for utrophin expression and muscle regeneration. J Cell Biol. 2012;197:1009–1027. doi: 10.1083/jcb.201110032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peter AK, Marshall JL, Crosbie RH. Sarcospan reduces dystrophic pathology: stabilization of the utrophin-glycoprotein complex. J Cell Biol. 2008;183:419–427. doi: 10.1083/jcb.200808027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffman EP, Knudson CM, Campbell KP, Kunkel LM. Subcellular fractionation of dystrophin to the triads of skeletal muscle. Nature. 1987;330:754–758. doi: 10.1038/330754a0. [DOI] [PubMed] [Google Scholar]
- 50.Zubrzycka-Gaarn EE, Bulman DE, Karpati G, Burghes AH, Belfall B, Klamut HJ, Talbot J, Hodges RS, Ray PN, Worton RG. The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle. Nature. 1988;333:466–469. doi: 10.1038/333466a0. [DOI] [PubMed] [Google Scholar]
- 51.Miike T, Miyatake M, Zhao J, Yoshioka K, Uchino M. Immunohistochemical dystrophin reaction in synaptic regions. Brain Dev. 1989;11:344–346. doi: 10.1016/s0387-7604(89)80067-1. [DOI] [PubMed] [Google Scholar]
- 52.Samitt CE, Bonilla E. Immunocytochemical study of dystrophin at the myotendinous junction. Muscle Nerve. 1990;13:493–500. doi: 10.1002/mus.880130605. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J, Yoshioka K, Miyatake M, Miike T. Dystrophin and a dystrophin-related protein in intrafusal muscle fibers, and neuromuscular and myotendinous junctions. Acta Neuropathol. 1992;84:141–146. doi: 10.1007/BF00311386. [DOI] [PubMed] [Google Scholar]
- 54.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992;360:588–591. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- 55.Prochniewicz E, Henderson D, Ervasti JM, Thomas DD. Dystrophin and utrophin have distinct effects on the structural dynamics of actin. Proc Natl Acad Sci U S A. 2009;106:7822–7827. doi: 10.1073/pnas.0812007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rybakova IN, Humston JL, Sonnemann KJ, Ervasti JM. Dystrophin and utrophin bind actin through distinct modes of contact. J Biol Chem. 2006;281:9996–10001. doi: 10.1074/jbc.M513121200. [DOI] [PubMed] [Google Scholar]
- 57.Li D, Bareja A, Judge L, Yue Y, Lai Y, Fairclough R, Davies KE, Chamberlain JS, Duan D. Sarcolemmal nNOS anchoring reveals a qualitative difference between dystrophin and utrophin. J Cell Sci. 2010;123:2008–2013. doi: 10.1242/jcs.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Durko M, Allen C, Nalbantoglu J, Karpati G. CT-GalNAc transferase overexpression in adult mice is associated with extrasynaptic utrophin in skeletal muscle fibres. J Muscle Res Cell Motil. 2010;31:181–193. doi: 10.1007/s10974-010-9222-9. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci U S A. 2002;99:5616–5621. doi: 10.1073/pnas.082613599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia B, Hoyte K, Kammesheidt A, Deerinck T, Ellisman M, Martin PT. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev Biol. 2002;242:58–73. doi: 10.1006/dbio.2001.0530. [DOI] [PubMed] [Google Scholar]
- 61.Legate KR, Montanez E, Kudlacek O, Fassler R:ILK, PINCH, parvin the tIPP of integrin signalling. Nat Rev Mol Cell Biol. 2006;7:20–31. doi: 10.1038/nrm1789. [DOI] [PubMed] [Google Scholar]
- 62.Hannigan G, Troussard AA, Dedhar S. Integrin-linked kinase: a cancer therapeutic target unique among its ILK. Nat Rev Cancer. 2005;5:51–63. doi: 10.1038/nrc1524. [DOI] [PubMed] [Google Scholar]
- 63.Wu C. PINCH, N(i)ck and the ILK: network wiring at cell-matrix adhesions. Trends Cell Biol. 2005;15:460–466. doi: 10.1016/j.tcb.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 64.McDonald PC, Fielding AB, Dedhar S. Integrin-linked kinase--essential roles in physiology and cancer biology. J Cell Sci. 2008;121:3121–3132. doi: 10.1242/jcs.017996. [DOI] [PubMed] [Google Scholar]
- 65.Gheyara AL, Vallejo-Illarramendi A, Zang K, Mei L, St-Arnaud R, Dedhar S, Reichardt LF. Deletion of integrin-linked kinase from skeletal muscles of mice resembles muscular dystrophy due to alpha 7 beta 1-integrin deficiency. Am J Pathol. 2007;171:1966–1977. doi: 10.2353/ajpath.2007.070555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smirnov SP, McDearmon EL, Li S, Ervasti JM, Tryggvason K, Yurchenco PD. Contributions of the LG modules and furin processing to laminin-2 functions. J Biol Chem. 2002;277:18928–18937. doi: 10.1074/jbc.M201880200. [DOI] [PubMed] [Google Scholar]
- 67.Talts JF, Mann K, Yamada Y, Timpl R. Structural analysis and proteolytic processing of recombinant G domain of mouse laminin alpha2 chain. FEBS Lett. 1998;426:71–76. doi: 10.1016/s0014-5793(98)00312-3. [DOI] [PubMed] [Google Scholar]
- 68.Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18:863–870. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmberg J, Durbeej M. Laminin-211 in skeletal muscle function. Cell Adh Migr. 2013;7:111–121. doi: 10.4161/cam.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gawlik KI, Durbeej M. Skeletal muscle laminin and MDC1A: pathogenesis and treatment strategies. Skelet Muscle. 2011;1:9. doi: 10.1186/2044-5040-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanabe Y, Esaki K, Nomura T. Skeletal muscle pathology in X chromosome-linked muscular dystrophy (mdx) mouse. Acta Neuropathol. 1986;69:91–95. doi: 10.1007/BF00687043. [DOI] [PubMed] [Google Scholar]
- 72.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 73.Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L, Watt DJ, Dickson JG, Tinsley JM, Davies KE. Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell. 1997;90:717–727. doi: 10.1016/s0092-8674(00)80532-2. [DOI] [PubMed] [Google Scholar]
- 74.Rooney JE, Welser JV, Dechert MA, Flintoff-Dye NL, Kaufman SJ, Burkin DJ. Severe muscular dystrophy in mice that lack dystrophin and alpha7 integrin. J Cell Sci. 2006;119:2185–2195. doi: 10.1242/jcs.02952. [DOI] [PubMed] [Google Scholar]
- 75.Guo C, Willem M, Werner A, Raivich G, Emerson M, Neyses L, Mayer U. Absence of alpha 7 integrin in dystrophin-deficient mice causes a myopathy similar to Duchenne muscular dystrophy. Hum Mol Genet. 2006;15:989–998. doi: 10.1093/hmg/ddl018. [DOI] [PubMed] [Google Scholar]
- 76.Liu J, Milner DJ, Boppart MD, Ross RS, Kaufman SJ. beta1D chain increases alpha7beta1 integrin and laminin and protects against sarcolemmal damage in mdx mice. Hum Mol Genet. 2012;21:1592–1603. doi: 10.1093/hmg/ddr596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–1444. doi: 10.1038/4033. [DOI] [PubMed] [Google Scholar]
- 78.Poysky J. Behavior patterns in Duchenne muscular dystrophy: report on the Parent Project Muscular Dystrophy behavior workshop 8-9 of December 2006, Philadelphia, USA. Neuromuscul Disord. 2007;17:986–994. doi: 10.1016/j.nmd.2007.06.465. [DOI] [PubMed] [Google Scholar]
- 79.Perronnet C, Chagneau C, Le Blanc P, Samson-Desvignes N, Mornet D, Laroche S, De La Porte S, Vaillend C. Upregulation of brain utrophin does not rescue behavioral alterations in dystrophin-deficient mice. Hum Mol Genet. 2012;21:2263–2276. doi: 10.1093/hmg/dds047. [DOI] [PubMed] [Google Scholar]
- 80.Goyenvalle A, Seto JT, Davies KE, Chamberlain J. Therapeutic approaches to muscular dystrophy. Hum Mol Genet. 2011;20:R69–78. doi: 10.1093/hmg/ddr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tinsley JM, Fairclough RJ, Storer R, Wilkes FJ, Potter AC, Squire SE, Powell DS, Cozzoli A, Capogrosso RF, Lambert A, et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Casar JC, McKechnie BA, Fallon JR, Young MF, Brandan E. Transient up-regulation of biglycan during skeletal muscle regeneration: delayed fiber growth along with decorin increase in biglycan-deficient mice. Dev Biol. 2004;268:358–371. doi: 10.1016/j.ydbio.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 84.Lechner BE, Lim JH, Mercado ML, Fallon JR. Developmental regulation of biglycan expression in muscle and tendon. Muscle Nerve. 2006;34:347–355. doi: 10.1002/mus.20596. [DOI] [PubMed] [Google Scholar]
- 85.Amenta AR, Yilmaz A, Bogdanovich S, McKechnie BA, Abedi M, Khurana TS, Fallon JR. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A. 2011;108:762–767. doi: 10.1073/pnas.1013067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goudenege S, Lamarre Y, Dumont N, Rousseau J, Frenette J, Skuk D, Tremblay JP. Laminin-111: a potential therapeutic agent for Duchenne muscular dystrophy. Mol Ther. 2010;18:2155–2163. doi: 10.1038/mt.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rooney JE, Gurpur PB, Yablonka-Reuveni Z, Burkin DJ. Laminin-111 restores regenerative capacity in a mouse model for alpha7 integrin congenital myopathy. Am J Pathol. 2009;174:256–264. doi: 10.2353/ajpath.2009.080522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rooney JE, Knapp JR, Hodges BL, Wuebbles RD, Burkin DJ. Laminin-111 protein therapy reduces muscle pathology and improves viability of a mouse model of merosin-deficient congenital muscular dystrophy. Am J Pathol. 2012;180:1593–1602. doi: 10.1016/j.ajpath.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rooney JE, Gurpur PB, Burkin DJ. Laminin-111 protein therapy prevents muscle disease in the mdx mouse model for Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2009;106:7991–7996. doi: 10.1073/pnas.0811599106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marshall JL, Chou E, Oh J, Kwok A, Burkin DJ, Crosbie-Watson RH. Dystrophin and utrophin expression require sarcospan: loss of alpha7 integrin exacerbates a newly discovered muscle phenotype in sarcospan-null mice. Hum Mol Genet. 2012;21:4378–4393. doi: 10.1093/hmg/dds271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han R, Kanagawa M, Yoshida-Moriguchi T, Rader EP, Ng RA, Michele DE, Muirhead DE, Kunz S, Moore SA, Iannaccone ST, et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of alpha-dystroglycan. Proc Natl Acad Sci U S A. 2009;106:12573–12579. doi: 10.1073/pnas.0906545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moghadaszadeh B, Albrechtsen R, Guo LT, Zaik M, Kawaguchi N, Borup RH, Kronqvist P, Schroder HD, Davies KE, Voit T, et al. Compensation for dystrophin-deficiency: ADAM12 overexpression in skeletal muscle results in increased alpha 7 integrin, utrophin and associated glycoproteins. Hum Mol Genet. 2003;12:2467–2479. doi: 10.1093/hmg/ddg264. [DOI] [PubMed] [Google Scholar]
- 93.Galliano MF, Huet C, Frygelius J, Polgren A, Wewer UM, Engvall E. Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion. J Biol Chem. 2000;275:13933–13939. doi: 10.1074/jbc.275.18.13933. [DOI] [PubMed] [Google Scholar]
- 94.Kronqvist P, Kawaguchi N, Albrechtsen R, Xu X, Schroder HD, Moghadaszadeh B, Nielsen FC, Frohlich C, Engvall E, Wewer UM. ADAM12 alleviates the skeletal muscle pathology in mdx dystrophic mice. Am J Pathol. 2002;161:1535–1540. doi: 10.1016/S0002-9440(10)64431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yagami-Hiromasa T, Sato T, Kurisaki T, Kamijo K, Nabeshima Y, Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- 96.Grewal PK, Hewitt JE. Mutation of Large, which encodes a putative glycosyltransferase, in an animal model of muscular dystrophy. Biochim Biophys Acta. 2002;1573:216–224. doi: 10.1016/s0304-4165(02)00387-2. [DOI] [PubMed] [Google Scholar]
- 97.Burkin DJ, Wallace GQ, Nicol KJ, Kaufman DJ, Kaufman SJ. Enhanced expression of the alpha 7 beta 1 integrin reduces muscular dystrophy and restores viability in dystrophic mice. J Cell Biol. 2001;152:1207–1218. doi: 10.1083/jcb.152.6.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mercuri E, Muntoni F. The ever-expanding spectrum of congenital muscular dystrophies. Ann Neurol. 2012;72:9–17. doi: 10.1002/ana.23548. [DOI] [PubMed] [Google Scholar]
- 99.Beltran-Valero de Bernabe D, Currier S, Steinbrecher A, Celli J, van Beusekom E, van der Zwaag B, Kayserili H, Merlini L, Chitayat D, Dobyns WB, et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am J Hum Genet. 2002;71:1033–1043. doi: 10.1086/342975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Beltran-Valero de Bernabe D, Voit T, Longman C, Steinbrecher A, Straub V, Yuva Y, Herrmann R, Sperner J, Korenke C, Diesen C, et al. Mutations in the FKRP gene can cause muscle-eye-brain disease and Walker-Warburg syndrome. J Med Genet. 2004;41:e61. doi: 10.1136/jmg.2003.013870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brockington M, Blake DJ, Prandini P, Brown SC, Torelli S, Benson MA, Ponting CP, Estournet B, Romero NB, Mercuri E, et al. Mutations in the fukutin-related protein gene (FKRP) cause a form of congenital muscular dystrophy with secondary laminin alpha2 deficiency and abnormal glycosylation of alpha-dystroglycan. Am J Hum Genet. 2001;69:1198–1209. doi: 10.1086/324412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kobayashi K, Nakahori Y, Miyake M, Matsumura K, Kondo-Iida E, Nomura Y, Segawa M, Yoshioka M, Saito K, Osawa M, et al. An ancient retrotransposal insertion causes Fukuyama-type congenital muscular dystrophy. Nature. 1998;394:388–392. doi: 10.1038/28653. [DOI] [PubMed] [Google Scholar]
- 103.Roscioli T, Kamsteeg EJ, Buysse K, Maystadt I, van Reeuwijk J, van den Elzen C, van Beusekom E, Riemersma M, Pfundt R, Vissers LE, et al. Mutations in ISPD cause Walker-Warburg syndrome and defective glycosylation of alpha-dystroglycan. Nat Genet. 2012;44:581–585. doi: 10.1038/ng.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Silan F, Yoshioka M, Kobayashi K, Simsek E, Tunc M, Alper M, Cam M, Guven A, Fukuda Y, Kinoshita M, et al. A new mutation of the fukutin gene in a non-Japanese patient. Ann Neurol. 2003;53:392–396. doi: 10.1002/ana.10491. [DOI] [PubMed] [Google Scholar]
- 105.van Reeuwijk J, Brunner HG, van Bokhoven H. Glyc-O-genetics of Walker-Warburg syndrome. Clin Genet. 2005;67:281–289. doi: 10.1111/j.1399-0004.2004.00368.x. [DOI] [PubMed] [Google Scholar]
- 106.van Reeuwijk J, Grewal PK, Salih MA, Beltran-Valero de Bernabe D, McLaughlan JM, Michielse CB, Herrmann R, Hewitt JE, Steinbrecher A, Seidahmed MZ, et al. Intragenic deletion in the LARGE gene causes Walker-Warburg syndrome. Hum Genet. 2007;121:685–690. doi: 10.1007/s00439-007-0362-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DB, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T, et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nat Genet. 2012;44:575–580. doi: 10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stevens E, Carss KJ, Cirak S, Foley AR, Torelli S, Willer T, Tambunan DE, Yau S, Brodd L, Sewry CA, et al. Mutations in B3GALNT2 Cause Congenital Muscular Dystrophy and Hypoglycosylation of alpha-Dystroglycan. Am J Hum Genet. 2013 doi: 10.1016/j.ajhg.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Barone R, Aiello C, Race V, Morava E, Foulquier F, Riemersma M, Passarelli C, Concolino D, Carella M, Santorelli F, et al. DPM2-CDG: a muscular dystrophy-dystroglycanopathy syndrome with severe epilepsy. Ann Neurol. 2012;72:550–558. doi: 10.1002/ana.23632. [DOI] [PubMed] [Google Scholar]
- 110.Lefeber DJ, Schonberger J, Morava E, Guillard M, Huyben KM, Verrijp K, Grafakou O, Evangeliou A, Preijers FW, Manta P, et al. Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am J Hum Genet. 2009;85:76–86. doi: 10.1016/j.ajhg.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lefeber DJ, de Brouwer AP, Morava E, Riemersma M, Schuurs-Hoeijmakers JH, Absmanner B, Verrijp K, van den Akker WM, Huijben K, Steenbergen G, et al. Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet. 2011;7:e1002427. doi: 10.1371/journal.pgen.1002427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manzini MC, Tambunan DE, Hill RS, Yu TW, Maynard TM, Heinzen EL, Shianna KV, Stevens CR, Partlow JN, Barry BJ, et al. Exome sequencing and functional validation in zebrafish identify GTDC2 mutations as a cause of Walker-Warburg syndrome. Am J Hum Genet. 2012;91:541–547. doi: 10.1016/j.ajhg.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vuillaumier-Barrot S, Bouchet-Seraphin C, Chelbi M, Devisme L, Quentin S, Gazal S, Laquerriere A, Fallet-Bianco C, Loget P, Odent S, et al. Identification of mutations in TMEM5 and ISPD as a cause of severe cobblestone lissencephaly. Am J Hum Genet. 2012;91:1135–1143. doi: 10.1016/j.ajhg.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jurado LA, Coloma A, Cruces J. Identification of a human homolog of the Drosophila rotated abdomen gene (POMT1) encoding a putative protein O-mannosyl-transferase, and assignment to human chromosome 9q34.1. Genomics. 1999;58:171–180. doi: 10.1006/geno.1999.5819. [DOI] [PubMed] [Google Scholar]
- 115.Willer T, Amselgruber W, Deutzmann R, Strahl S. Characterization of POMT2, a novel member of the PMT protein O-mannosyltransferase family specifically localized to the acrosome of mammalian spermatids. Glycobiology. 2002;12:771–783. doi: 10.1093/glycob/cwf086. [DOI] [PubMed] [Google Scholar]
- 116.Yoshida-Moriguchi T, Yu L, Stalnaker SH, Davis S, Kunz S, Madson M, Oldstone MB, Schachter H, Wells L, Campbell KP. O-mannosyl phosphorylation of alpha-dystroglycan is required for laminin binding. Science. 2010;327:88–92. doi: 10.1126/science.1180512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brockington M, Torelli S, Prandini P, Boito C, Dolatshad NF, Longman C, Brown SC, Muntoni F. Localization and functional analysis of the LARGE family of glycosyltransferases: significance for muscular dystrophy. Hum Mol Genet. 2005;14:657–665. doi: 10.1093/hmg/ddi062. [DOI] [PubMed] [Google Scholar]
- 118.Kanagawa M, Saito F, Kunz S, Yoshida-Moriguchi T, Barresi R, Kobayashi YM, Muschler J, Dumanski JP, Michele DE, Oldstone MB, Campbell KP. Molecular recognition by LARGE is essential for expression of functional dystroglycan. Cell. 2004;117:953–964. doi: 10.1016/j.cell.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 119.Inamori K, Yoshida-Moriguchi T, Hara Y, Anderson ME, Yu L, Campbell KP. Dystroglycan function requires xylosyl- and glucuronyltransferase activities of LARGE. Science. 2012;335:93–96. doi: 10.1126/science.1214115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brockington M, Yuva Y, Prandini P, Brown SC, Torelli S, Benson MA, Herrmann R, Anderson LV, Bashir R, Burgunder JM, et al. Mutations in the fukutin-related protein gene (FKRP) identify limb girdle muscular dystrophy 2I as a milder allelic variant of congenital muscular dystrophy MDC1C. Hum Mol Genet. 2001(10):2851–2859. doi: 10.1093/hmg/10.25.2851. [DOI] [PubMed] [Google Scholar]
- 121.Shridas P, Waechter CJ. Human dolichol kinase, a polytopic endoplasmic reticulum membrane protein with a cytoplasmically oriented CTP-binding site. J Biol Chem. 2006;281:31696–31704. doi: 10.1074/jbc.M604087200. [DOI] [PubMed] [Google Scholar]
- 122.Maeda Y, Tanaka S, Hino J, Kangawa K, Kinoshita T. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. EMBO J. 2000;19:2475–2482. doi: 10.1093/emboj/19.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maeda Y, Tomita S, Watanabe R, Ohishi K, Kinoshita T. DPM2 regulates biosynthesis of dolichol phosphate-mannose in mammalian cells: correct subcellular localization and stabilization of DPM1, and binding of dolichol phosphate. EMBO J. 1998;17:4920–4929. doi: 10.1093/emboj/17.17.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Burda P, Aebi M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999;1426:239–257. doi: 10.1016/s0304-4165(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 125.Manya H, Chiba A, Yoshida A, Wang X, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc Natl Acad Sci U S A. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buysse K, Riemersma M, Powell G, van Reeuwijk J, Chitayat D, Roscioli T, Kamsteeg EJ, van den Elzen C, van Beusekom E, Blaser S, et al. Missense mutations in beta-1,3-N-acetylglucosaminyltransferase 1 (B3GNT1) cause Walker-Warburg syndrome. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dobson CM, Hempel SJ, Stalnaker SH, Stuart R, Wells L. O-Mannosylation and human disease. Cell Mol Life Sci. 2012 doi: 10.1007/s00018-012-1193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yis U, Uyanik G, Heck PB, Smitka M, Nobel H, Ebinger F, Dirik E, Feng L, Kurul SH, Brocke K, et al. Fukutin mutations in non-Japanese patients with congenital muscular dystrophy: less severe mutations predominate in patients with a non-Walker-Warburg phenotype. Neuromuscul Disord. 2011;21:20–30. doi: 10.1016/j.nmd.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 129.Hehr U, Uyanik G, Gross C, Walter MC, Bohring A, Cohen M, Oehl-Jaschkowitz B, Bird LM, Shamdeen GM, Bogdahn U, et al. Novel POMGnT1 mutations define broader phenotypic spectrum of muscle-eye-brain disease. Neurogenetics. 2007;8:279–288. doi: 10.1007/s10048-007-0096-y. [DOI] [PubMed] [Google Scholar]
- 130.Brockington S, Clarke A. The relative influence of temperature and food on the metabolism of a marine invertebrate. J Exp Mar Bio Ecol. 2001;258:87–99. doi: 10.1016/s0022-0981(00)00347-6. [DOI] [PubMed] [Google Scholar]
- 131.Smith PL, Lowe JB. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J Biol Chem. 1994;269:15162–15171. [PubMed] [Google Scholar]
- 132.Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, Davies K, Gillis JM. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat Med. 1997;3:1216–1221. doi: 10.1038/nm1197-1216. [DOI] [PubMed] [Google Scholar]
- 133.Gilbert R, Nalbantoglu J, Petrof BJ, Ebihara S, Guibinga GH, Tinsley JM, Kamen A, Massie B, Davies KE, Karpati G. Adenovirus-mediated utrophin gene transfer mitigates the dystrophic phenotype of mdx mouse muscles. Hum Gene Ther. 1999;10:1299–1310. doi: 10.1089/10430349950017987. [DOI] [PubMed] [Google Scholar]
- 134.Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet. 1998;19:79–82. doi: 10.1038/ng0598-79. [DOI] [PubMed] [Google Scholar]
- 135.Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE. Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- 136.Xu R, Chandrasekharan K, Yoon JH, Camboni M, Martin PT. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol. 2007(171):181–199. doi: 10.2353/ajpath.2007.060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xu R, DeVries S, Camboni M, Martin PT. Overexpression of Galgt2 reduces dystrophic pathology in the skeletal muscles of alpha sarcoglycan-deficient mice. Am J Pathol. 2009;175:235–247. doi: 10.2353/ajpath.2009.080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Singhal N, Xu R, Martin PT. Distinct contributions of Galgt1 and Galgt2 to carbohydrate expression and function at the mouse neuromuscular junction. Mol Cell Neurosci. 2012;51:112–126. doi: 10.1016/j.mcn.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cabrera PV, Pang M, Marshall JL, Kung R, Nelson SF, Stalnaker SH, Wells L, Crosbie-Watson RH, Baum LG. High throughput screening for compounds that alter muscle cell glycosylation identifies new role for N-glycans in regulating sarcolemmal protein abundance and laminin binding. J Biol Chem. 2012;287:22759–22770. doi: 10.1074/jbc.M111.334581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Santha E, Sperlagh B, Zelles T, Zsilla G, Toth PT, Lendvai B, Baranyi M, Vizi ES. Multiple cellular mechanisms mediate the effect of lobeline on the release of norepinephrine. J Pharmacol Exp Ther. 2000;294:302–307. [PubMed] [Google Scholar]
- 141.Dwoskin LP, Crooks PA. A novel mechanism of action and potential use for lobeline as a treatment for psychostimulant abuse. Biochem Pharmacol. 2002;63:89–98. doi: 10.1016/s0006-2952(01)00899-1. [DOI] [PubMed] [Google Scholar]
- 142.Wilhelm CJ, Johnson RA, Eshleman AJ, Janowsky A. Lobeline effects on tonic and methamphetamine-induced dopamine release. Biochem Pharmacol. 2008;75:1411–1415. doi: 10.1016/j.bcp.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee WC, Kang D, Causevic E, Herdt AR, Eckman EA, Eckman CB. Molecular characterization of mutations that cause globoid cell leukodystrophy and pharmacological rescue using small molecule chemical chaperones. J Neurosci. 2010;30:5489–5497. doi: 10.1523/JNEUROSCI.6383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bulfield G, Siller WG, Wight PA, Moore KJ. X chromosome-linked muscular dystrophy (mdx) in the mouse. Proc Natl Acad Sci U S A. 1984;81:1189–1192. doi: 10.1073/pnas.81.4.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- 146.Chamberlain JS, Pearlman JA, Muzny DM, Gibbs RA, Ranier JE, Caskey CT, Reeves AA. Expression of the murine Duchenne muscular dystrophy gene in muscle and brain. Science. 1988;239:1416–1418. doi: 10.1126/science.3347839. [DOI] [PubMed] [Google Scholar]
- 147.Chelly J, Gilgenkrantz H, Lambert M, Hamard G, Chafey P, Recan D, Katz P, de la Chapelle A, Koenig M, Ginjaar IB, et al. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990;63:1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- 148.Ginjaar IB, Bakker E, van Paassen MM, den Dunnen JT, Wessels A, Zubrzycka-Gaarn EE, Moorman AF, van Ommen GJ. Immunohistochemical studies show truncated dystrophins in the myotubes of three fetuses at risk for Duchenne muscular dystrophy. J Med Genet. 1991;28:505–510. doi: 10.1136/jmg.28.8.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jones BR, Brennan S, Mooney CT, Callanan JJ, McAllister H, Guo LT, Martin PT, Engvall E, Shelton GD. Muscular dystrophy with truncated dystrophin in a family of Japanese Spitz dogs. J Neurol Sci. 2004;217:143–149. doi: 10.1016/j.jns.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 150.Helliwell TR, Ellis JM, Mountford RC, Appleton RE, Morris GE. A truncated dystrophin lacking the C-terminal domains is localized at the muscle membrane. Am J Hum Genet. 1992;50:508–514. [PMC free article] [PubMed] [Google Scholar]
- 151.Jung D, Yang B, Meyer J, Chamberlain JS, Campbell KP. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem. 1995;270:27305–27310. doi: 10.1074/jbc.270.45.27305. [DOI] [PubMed] [Google Scholar]
- 152.Banks GB, Gregorevic P, Allen JM, Finn EE, Chamberlain JS. Functional capacity of dystrophins carrying deletions in the N-terminal actin-binding domain. Hum Mol Genet. 2007;16:2105–2113. doi: 10.1093/hmg/ddm158. [DOI] [PubMed] [Google Scholar]
- 153.Imamura M, Araishi K, Noguchi S, Ozawa E. A sarcoglycan-dystroglycan complex anchors Dp116 and utrophin in the peripheral nervous system. Hum Mol Genet. 2000;9:3091–3100. doi: 10.1093/hmg/9.20.3091. [DOI] [PubMed] [Google Scholar]
- 154.Cox GA, Sunada Y, Campbell KP, Chamberlain JS. Dp71 can restore the dystrophin-associated glycoprotein complex in muscle but fails to prevent dystrophy. Nat Genet. 1994;8:333–339. doi: 10.1038/ng1294-333. [DOI] [PubMed] [Google Scholar]
- 155.Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- 156.Tachikawa M, Kanagawa M, Yu CC, Kobayashi K, Toda T. Mislocalization of fukutin protein by disease-causing missense mutations can be rescued with treatments directed at folding amelioration. J Biol Chem. 2012;287:8398–8406. doi: 10.1074/jbc.M111.300905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 158.Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- 159.Pan Y, Chen C, Shen Y, Zhu CH, Wang G, Wang XC, Chen HQ, Zhu MS. Curcumin alleviates dystrophic muscle pathology in mdx mice. Mol Cells. 2008;25:531–537. [PubMed] [Google Scholar]
- 160.Durham WJ, Arbogast S, Gerken E, Li YP, Reid MB. Progressive nuclear factor-kappaB activation resistant to inhibition by contraction and curcumin in mdx mice. Muscle Nerve. 2006;34:298–303. doi: 10.1002/mus.20579. [DOI] [PubMed] [Google Scholar]
- 161.Soheili T, Gicquel E, Poupiot J, N'Guyen L, Le Roy F, Bartoli M, Richard I. Rescue of sarcoglycan mutations by inhibition of endoplasmic reticulum quality control is associated with minimal structural modifications. Hum Mutat. 2012;33:429–439. doi: 10.1002/humu.21659. [DOI] [PubMed] [Google Scholar]
- 162.Wang F, Song W, Brancati G, Segatori L. Inhibition of endoplasmic reticulum-associated degradation rescues native folding in loss of function protein misfolding diseases. J Biol Chem. 2011;286:43454–43464. doi: 10.1074/jbc.M111.274332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Schlesinger MJ. Heat shock proteins. J Biol Chem. 1990;265:12111–12114. [PubMed] [Google Scholar]
- 164.Bornman L, Polla BS, Lotz BP, Gericke GS. Expression of heat-shock/stress proteins in Duchenne muscular dystrophy. Muscle Nerve. 1995;18:23–31. doi: 10.1002/mus.880180105. [DOI] [PubMed] [Google Scholar]
- 165.Stary CM, Walsh BJ, Knapp AE, Brafman D, Hogan MC. Elevation in heat shock protein 72 mRNA following contractions in isolated single skeletal muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2008;295:R642–648. doi: 10.1152/ajpregu.00852.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Carmeli E, Beiker R, Maor M, Kodesh E. Increased iNOS, MMP-2, and HSP-72 in skeletal muscle following high-intensity exercise training. J Basic Clin Physiol Pharmacol. 2010;21:127–146. doi: 10.1515/jbcpp.2010.21.2.127. [DOI] [PubMed] [Google Scholar]
- 167.Gehrig SM, van der Poel C, Sayer TA, Schertzer JD, Henstridge DC, Church JE, Lamon S, Russell AP, Davies KE, Febbraio MA, Lynch GS. Hsp72 preserves muscle function and slows progression of severe muscular dystrophy. Nature. 2012;484:394–398. doi: 10.1038/nature10980. [DOI] [PubMed] [Google Scholar]
- 168.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 169.Bonuccelli G, Sotgia F, Capozza F, Gazzerro E, Minetti C, Lisanti MP. Localized treatment with a novel FDA-approved proteasome inhibitor blocks the degradation of dystrophin and dystrophin-associated proteins in mdx mice. Cell Cycle. 2007;6:1242–1248. doi: 10.4161/cc.6.10.4182. [DOI] [PubMed] [Google Scholar]
- 170.Bonuccelli G, Sotgia F, Schubert W, Park DS, Frank PG, Woodman SE, Insabato L, Cammer M, Minetti C, Lisanti MP. Proteasome inhibitor (MG-132) treatment of mdx mice rescues the expression and membrane localization of dystrophin and dystrophin-associated proteins. Am J Pathol. 2003;163:1663–1675. doi: 10.1016/S0002-9440(10)63523-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Selsby J, Morris C, Morris L, Sweeney L. A proteasome inhibitor fails to attenuate dystrophic pathology in mdx mice. PLoS Curr. 2012;4:e4. doi: 10.1371/4f84a944d8930. f84a944d8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Braakman I, Bulleid NJ. Protein folding and modification in the mammalian endoplasmic reticulum. Annu Rev Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 173.Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010(68):629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mendell JR, Rodino-Klapac L, Sahenk Z, Malik V, Kaspar BK, Walker CM, Clark KR. Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett. 2012;527:90–99. doi: 10.1016/j.neulet.2012.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL, Miller AD, Chamberlain JS. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 176.Zincarelli C, Soltys S, Rengo G, Koch WJ, Rabinowitz JE. Comparative cardiac gene delivery of adeno-associated virus serotypes 1-9 reveals that AAV6 mediates the most efficient transduction in mouse heart. Clin Transl Sci. 2010;3:81–89. doi: 10.1111/j.1752-8062.2010.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Brennan KJ, Hardeman EC. Quantitative analysis of the human alpha-skeletal actin gene in transgenic mice. J Biol Chem. 1993;268:719–725. [PubMed] [Google Scholar]
- 178.Kendall GC, Mokhonova EI, Moran M, Sejbuk NE, Wang DW, Silva O, Wang RT, Martinez L, Lu QL, Damoiseaux R, et al. Dantrolene enhances antisense-mediated exon skipping in human and mouse models of Duchenne muscular dystrophy. Sci Transl Med. 2012;4:164r. doi: 10.1126/scitranslmed.3005054. a160. [DOI] [PubMed] [Google Scholar]
- 179.Marshall JL, Watson RH. Sarcospan: a small protein with large potential for Duchenne muscular dystrophy. Skelet Muscle. 2013;3:1. doi: 10.1186/2044-5040-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kim MH, Kay DI, Rudra RT, Chen BM, Hsu N, Izumiya Y, Martinez L, Spencer MJ, Walsh K, Grinnell AD, Crosbie RH. Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Hum Mol Genet. 2011;20:1324–1338. doi: 10.1093/hmg/ddr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peter AK, Ko CY, Kim MH, Hsu N, Ouchi N, Rhie S, Izumiya Y, Zeng L, Walsh K, Crosbie RH. Myogenic Akt signaling upregulates the utrophin-glycoprotein complex and promotes sarcolemma stability in muscular dystrophy. Hum Mol Genet. 2009;18:318–327. doi: 10.1093/hmg/ddn358. [DOI] [PMC free article] [PubMed] [Google Scholar]