Abstract
In type 1 diabetes (T1D), a break in central and peripheral tolerance results in antigen-specific T cells destroying insulin-producing, pancreatic beta cells. Herein, we discuss the critical sub-population of dendritic cells responsible for mediating both the cross-presentation of islet antigen to CD8+ T cells and the direct presentation of beta cell antigen to CD4+ T cells. These cells, termed merocytic dendritic cells (mcDC), are more numerous in non-obese diabetic (NOD), and antigen-loaded mcDC rescue CD8+ T cells from peripheral anergy and deletion, and stimulate islet-reactive CD4+ T cells. When purified from the pancreatic lymph nodes of overtly diabetic NOD mice, mcDC can break peripheral T cell tolerance to beta cell antigens in vivo and induce rapid onset T cell-mediated T1D in young NOD mouse. Thus, the mcDC subset appears to represent the long-sought critical antigen-presenting cell responsible for breaking peripheral tolerance to beta cell antigen in vivo.
Keywords: Type 1 diabetes, NOD mice, Merocytic dendritic cells, Tolerance
Type 1 diabetes mellitus (T1D) is the most prevalent pediatric autoimmune disease
In T1D, T cell-mediated destruction of insulin-producing pancreatic beta cells leads to acute-onset hyperglycemia. It is the most common autoimmune disease of children and has an overall incidence approaching 1 in 250 in the general United States population [1]; its incidence is, unfortunately, rising (at 3% a year, and even faster in very young, [2, 3]). Both genetic and environmental factors influence T1D development, with current data suggesting a concordance rate between 35 and 50% among monozygotic twins [4]. The NOD mouse is widely used to investigate the etiology, immunology and genetics of T1D (reviewed in [5, 6]). NOD studies have established that diabetogenic CD4+ and CD8+ T cells are central to the disease process and orchestrate the destruction of pancreatic beta cells [7–12]. Presently, the only widely approved treatment is daily, insulin-replacement therapy. While exogenous insulin is a life-saving therapy, it is not a cure; T1D patients still face a plethora of short- and long-term challenges, including higher levels of psycho-social problems [13] and secondary complications, such as heart disease, stroke, neuropathy, nephropathy and retinopathy that cause increased daily hardships and diminished quantity and quality of life [14]. Thus, a better understanding of immunopathogenesis and etiology of T1D is vitally important and clinically significant to improved both child and adult health in the United States.
The non-obese diabetic (NOD) mouse is the “gold standard” of rodent T1D research
The NOD mouse is an excellent model that is extensively used to elucidate key molecular and cellular mechanisms in the immunopathogenesis of T1D. The NOD mouse is a valued model for studying T1D in animals for a number of key reasons: (1) it exhibits spontaneous T1D that is clearly autoimmune in character, and genetically and environmentally regulated; (2) it exhibits substantial disease similarity with human T1D; (3) it is an inbred small animal model that is relatively inexpensive to house and maintain; (4) it is amenable to study using the gene manipulation techniques of the modern laboratory; and (5) it has a wealth of available strain- and species-specific reagents to aid in its analysis. Thus, the NOD mouse has become indispensable in our quest to understand the basic etiology and immunopathogenesis of T1D. Unfortunately, despite substantial recent progress, the basic etiology remains elusive.
The role for CD4+ and CD8+ T cells in T1D
Studies in the NOD mouse have shown that both CD4+ and CD8+ T cell respond to pancreatic beta cell self-antigens (self-Ag) and that both cell types can kill pancreatic beta cells [7, 9–12, 15–20]; in addition, both subsets are required for the effective transfer of diabetes into NOD mice and the genetic absence of either subsets results in no insulitis or diabetes in NOD mice [21–25].
The immunopathogenesis of T1D
The initial pathological manifestation of T1D (often referred to as “checkpoint 1”) in both mice and humans is insulitis; this occult infiltration of the pancreatic islets of Langerhans by leukocytes leads to localized inflammation followed by selective destruction of insulin-producing beta cells, when the infiltration is complete and extensive and exclusive beta cell apoptosis is observed [26, 27]. Moreover, insulitis is controlled by the tenor of the autoimmune response, with pronounced differences in localized immunoregulation, cellularity and cytokine production during the transition from a benign to pernicious state [28].
The destructive phase of insulitis demarcates the second stage of the disease, often termed “checkpoint 2” (reviewed in [26]). Briefly, during this phase, CD8+ T cells mediate the direct, Ag-driven killing of beta cells via cognate recognition of beta cell Ag presented by MHC class I on the surface of the beta cells, while CD4+ T cells kill via indirect means, through the localized liberation of cytokines that are themselves directly toxic to beta cells or stimulated the activation and maturation of resident macrophage, that can, in turn, kill beta cells by nitric oxide or other free radials [29]. When the majority of beta cells undergo apoptotic cell death, overt diabetes ensues [27, 29–32].
The BDC2.5 T cell receptor (TCR) transgenic mice have provided useful information on the genesis and pathogenesis of CD4+ T cells in T1D
Studies on the role of Aire-driven intrathymic expression of beta cell Ag in the medullary epithelial cells clearly show a significant role for central (deletion) tolerance to islet self Ags during thymocyte development [33–36]. Yet this is not the sole tolerance mechanism. For example, many self-Ag specific T cells do escape the thymus. A prominent example is seen in the BDC2.5 TCR transgenic mice (BDC2.5/NOD) where the developing T cells express the rearranged TCR-α and TCR-β chains from the diabetogenic CD4+ T cell clone, BDC2.5, that was originally isolated by Haskins and colleagues from an overtly diabetic female NOD mouse [20, 37, 38], and responds to the pancreatic beta cell antigen, Chromogranin A [39]. Developing thymocytes from this transgenic mouse express the transgene encoded TCR-α and TCR-β chains are selected on MHC class II (I-Ag7) on the NOD thymic stromal cells, escape negative selected during development, and mature into fully functional CD4+ T cells [20]. The BDC2.5 TCR-αβ are most strongly expressed in young (<6-week old) NOD mice, as revealed by an anti-clonotypic mAb, αBDC2.5 [40]. It is during this early period that the mouse displays a rapid development of insulitis, but lags in the development of diabetes [40]. The lag in diabetes is thought to result from endogenous TCR-α chains rearrangements that produce an alternative partner for the transgenic TCR-β chain to provide for a more diverse TCR repertoire, and this repertoire contains not only other CD4+ and CD8+ T cells but also Foxp3+ Treg cells and CD1d-tetramer-reactive NKT cells as shown in Fig. 1 (and ref [41]). These data, along with the waning BDC2.5 TCR expression with age, suggest that these diverse T cells act to regulate the diabetogenic T cells that developed earlier and that drive the initial insulitis observed. When crossed onto NOD.scid (Prkdcscid) mutant or Rag1-, Rag2- or TCRa-deficient mice, the resulting BDC2.5/NOD mice, unable to express alternative endogenously encoded TCR-α, produce T cells that express a monoclonal BDC2.5 TCR-αβ repertoire and exhibit both accelerated insulitis and diabetes [27, 42, 43], such that these mice do not survive to breeding age. Similar studies have been performed with other CD4+ and CD8+ TCR transgenic mice, with largely the same results [21, 44–47]. Thus, taken together, these data suggest that while central tolerance to islet Ag is vitally important in reducing auto-reactive T cells, a significant proportion of self-Ag T cells are still exported from the thymus and must be controlled by active mechanisms of peripheral tolerance. Unfortunately, in the NOD mouse, unlike most other strains, peripheral tolerance is routinely breached and most NOD mice eventually develop a T cell-dependent T1D. Thus, it is critical to understand what cellular and molecular actors conspire to break peripheral T cell tolerance in the diabetes-prone NOD mouse.
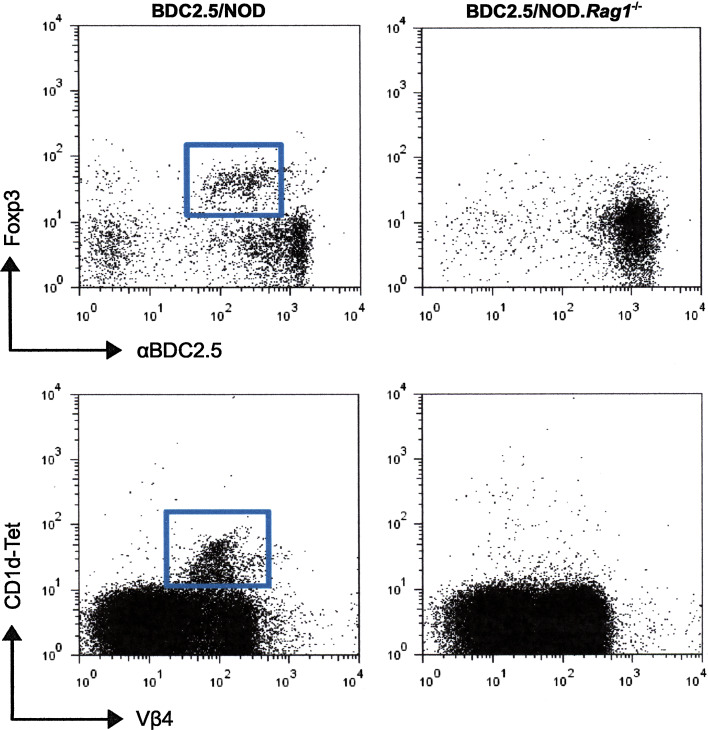
Fig. 1.
BDC2.5/NOD mice contain both Treg cells and NKT cells while BDC2.5/NOD.Rag1−/− mice do not. The transgenic TCR-β chain excludes the uses of endogenous TCR-β, all T cells are Vβ4+. However, in the NOD background functional endogenous TCR-α can form, pair with the transgenic TCR-β and be expressed on the cell surface. These cells appear as clonotypic low (α BDC2.5), but Vβ4+.Some of these express intracellular Foxp3 and have regulatory function and some express a TCR-α that allows them to recognize glycolipid antigens presented by CD1d and therefore react with the CD1d-tetramer used to identify NKT cells. Live, size gated splenic CD4+ T cells from 12-week-old mice were analyzed. 50–100 × 105 events were collected
Natural beta cell turnover and remodeling liberates Ag recognized by auto-reactive CD4+ and CD8+ T cells
As mice grow and mature, they are exposed to physiological and environmental stress that alters homeostatic insulin requirements. As a result, the numbers of and insulin output by beta cells is under constant flux [48]. Islets have a defined lifespan and normal turnover is a natural consequence [49, 50]. In addition, several studies have suggested that a wave of apoptotic beta cell turnover, coincident with weaning, is seen in 2–3-week-old rats and mice as they transition from a diet largely of mother’s milk to a more complex diet of solid food [51–53]. It has been suggested that this wave of islet cell remodeling affords the normal antigen-presenting cell of the immune system with a bolus of self Ag for (cross)-presentation to auto-reactive CD4+ and CD8+ T cells (reviewed in [29]). Thus, beta cell antigens are constantly available for antigen presentation in vivo, either by normal insulin de-graduation or apoptosis.
Peripheral tolerance to beta cells is broken by dendritic cell presentation of antigen from apoptotic beta cells
T1D is controlled by cognate interactions between islet-Ag-containing dendritic cells (DCs) that surveil and drain from the pancreas and naïve islet-specific T cells in the pancreatic lymph nodes (PLN, [54]). CD4+ T cells are activated in the PLN by DC presenting islet Ag via the I-Ag7 class II MHC, while CD8+ T cells are activated by cross-presentation of islet Ag by MHC class I on DC [55, 56]. Thus, in the end, the breaking of peripheral tolerance for both CD4+ and CD8+ T cells is mediated by Ag-loaded DCs that drain the pancreas. This process is somewhat paradoxical; while DCs have long been appreciated as crucial APC during times of inflammation, the clearance of apoptotic cells by DCs is generally considered to be non-inflammatory, even tolerogenic [57]. Yet, under certain situations, acquisition of antigen from apoptotic cells by DCs can be pro-inflammatory and can lead to the priming of self-reactive T cells [58]; this appears to be the case for apoptotic pancreatic beta cell Ags in T1D-prone individuals or animals. What is not fully understood is which DC subset is responsible for breaking peripheral tolerance to islet Ag and how this process unfolds mechanistically.
Dendritic cell are developmentally diverse subsets with distinct functions
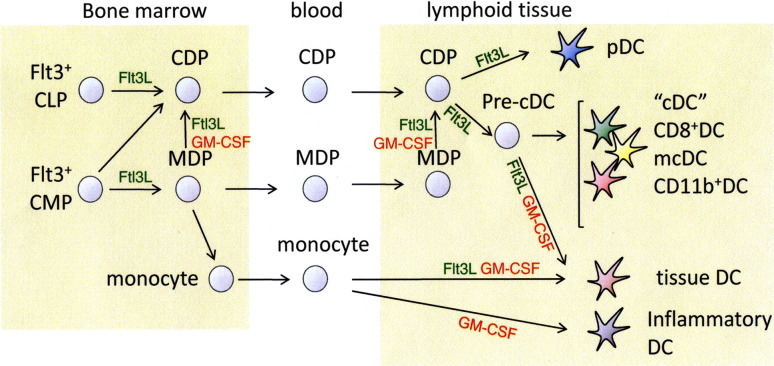
Dendritic cells are sparsely distributed bone marrow-derived, phenotypically and functionally heterogeneous leukocytes. DCs are thought to arise from Flt3+ common lymphoid precursors (CLP) and common myeloid precursors (CMP) in the bone marrow. CLP generate common dendritic cell precursors (CDP) that give rise to plasmacytoid DC (pDC) and—via an intermediate pre-cDC stage the “conventional” DC (cDC). Flt3+ CMP on the other hand can generate CDP and “macrophage” DC precursors (MDP). The MDP can further differentiate into macrophages, cDC and monocytes that give rise to tissue DC in and inflammatory DC (Fig. 2 and in review [59, 60]).
Fig. 2.
Overview of DC differentiation pathways in mice. Flt3, FLT3 fms-like tyrosine kinase 3, FLT3L FLT3 ligand, GM-CSF granulocyte macrophage colony-stimulating factor, CLP common lymphoid precursors, CMP common myeloid precursors, MDP macrophage DC precursor, pDC plasmacytoid dendritic cells, cDC conventional dendritic cells, mcDC merocytic DC
Conventional DC (cDC) are generally divided based on their expression of the surface molecules CD8a, CD4, CD11b, CD205 and 33D1 [60, 61]. The two major subsets in mouse spleen and lymph nodes are the CD11c+CD8a+CD11b− DC that are localized primarily to the T cell area and marginal zone, and the CD11c+CD11b+ CD8a− DC that can be found in the red pulp and bridging channels. CD205 expression is found in the CD8a+ DC while 33D1, CD172, and CD4 expression is contained within the CD11b+ population. However, these classifications are a simplified subdivision, as in vivo studies have revealed the existence of several other cDC subsets (such as merocytoc DC, natural killer DC, IKDC, [62–64]). Moreover, these subsets rely on the more physiologically relevant grouping of cDC around function rather than a simple collection of cell surface markers. This is especially relevant for merocytic dendritic cells which as yet lacks an exclusive surface marker but has a unique functional phenotype (see below).
The presentation of cell-associated protein antigens differs between dendritic cells subsets
Most human and mouse DC subsets can activate CD4+ and CD8+ T cells when pulsed with cognate peptides, as this requires no antigen processing and limited secondary signaling [65]. While most human and mouse DC subsets can capture protein and cell-associated antigens, not all subsets are able to process and present the Ag fragments (epitopes) to naïve CD4+ T and CD8+ T cells [56, 65, 66]. In particular, cross-presentation of cell-associated antigens to CD8+ T cells is thought to be limited to one or two DC subsets, as specialized antigen handling is required to shuttle exogenously acquired antigen into the endogenous antigen presentation system associated with class I MHC [67]. Moreover, only a few DC subsets can present cell-associated antigenic epitopes to both CD4+ and CD8+ T cell epitopes. Additionally, studies in both humans and mice show that detection and subsequent clearance of apoptotic cells leads to a tolerogenic state in most DC subsets. Phagocytosis of apoptotic material has, in fact, been shown to prevent DC maturation and inhibit pro-inflammatory cytokines production. In addition, the uptake of apoptotic cells by various lineages of phagocytes has been shown to induce specific immunoregulatory factors, including IL-10, TGFβ and prostaglandin E2, that dampen adaptive immune responses [68–71].
CD8DC
CD8DCs are considered the classic cross-presenting DC and, for a long time, the only mouse DC population with the ability to cross-present cell-associated antigens to CD8+ T cells [72]. CD8DCs display more efficient phagocytic uptake of dead cells and loading of antigenic- peptides into MHC class I than many other DC populations [73, 74]. In addition, CD8DCs are able to produce high levels of bioactive interleukin-12 (IL-12p70) that helps in their induction of Th1, Tc1 and NK responses. However, their capacity to present antigens in MHC class II to CD4+ T cells under conditions of limiting antigen is relatively poor (reviewed in [72]). Finally, CD8 only serves as a useful DC subset marker in mice, as it is absent from DCs in many other species and notably absent from human DCs. This has led to the false impression that this DC subset is absent from the human DC system, their likely human counterpart are CD141+ DC [75–77].
CD11bDC
Presentation of exogenous antigen by CD11bDCs is considered to be geared towards CD4+T cells. Although CD11bDCs phagocytose apoptotic cells quickly, they are poor presenters of cell-associated antigens in both MHC class I and II. Moreover, in the absence of other inflammatory stimuli, CD11bDC produce anti-inflammatory cytokines, including IL-10 that suppresses T cell priming. In the presence of inflammatory stimuli (including TLR/NLR stimuli or inflammatory cytokines) CD11bDCs do prime CD4+T cells to cell-associated antigens. This priming, however, is relatively poor when compared with soluble antigens and often fails to induce memory T cell responses.
Plasmacytoid DC (pDC)
The pDC subset is a major non-conventional dendritic cell population, that are CD11loB220+PDCA-1+. The pDC is a relatively rare population of DC in lymphoid tissues and the blood. pDC are predominantly known for their unique ability to secrete large amounts of type I interferons in response to pathogen-derived products. Recently the consensus has emerged that pDC can activate naïve T cells as well as memory cells. However, compared to other DC populations, pDC are considered poor presenters of exogenous antigens, recent studies indicate that they can efficiently present endogenous antigens, especially in the context of infection [78–80]. They may be particularly potent at activating natural killer (NK) T cells in vivo.
Merocytic DC (mcDC)
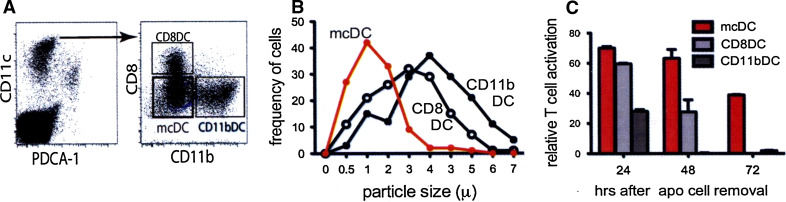
Merocytic DC, have only recently been identified within the CD11c+CD11b−/loCD8α−PDCA-1− DC subset based on their capacity to potently (cross-) prime both CD4+ and CD8+ T cells to cell-associated antigens, Fig. 3a and in ref. [58, 81, 82]. Most of the current knowledge on mcDCs and their function comes from recent studies where mcDCs were used for autologous tumor vaccine presentation in mouse models of cancer. These experiments in tumor bearing mice have shown that mcDC can prime naïve T cells—both CD4+ and CD8+—in a tumor suppressive environment as well reinvigorate T cells rendered anergic by the tumor [82]. Moreover, T cells primed by mcDC displayed a greater capacity for primary expansion, cytokine production, and memory formation on a per cell basis than those primed by other DC subsets. Given its potential in preclinical tumor models, the identification of the human equivalent has become a topic of intense research.
Fig. 3.
Biological and functional characteristics of mcDC. a identification of mcDC within the CD11chigh population. Upon gating for CD11chigh and exclusion of pDC by PDCA-1, mcDC are found in the CD8−CD11b−/low population. b Particle size of phagocytosed apoptotic material. Indicated DC populations were incubated with Violet Trace-labeled apoptotic cells and particle size was determined 20 h later using Amnis Imagestream. c Prolonged Ag presentation by mcDC. Indicated DC populations were incubated with OVA-expressing MHC class I deficient irradiated tumor cells. After 20 h, DC were purified and an OVA-specific CD8+T cell hybridoma was added after an additional 24, 48, or 72 h. mcDCs were the only DCs that showed activation of the T cell hybridoma. Viability of the different DC populations was comparable indicating that mcDC have a distinct capacity for persistent Ag presentation
The mcDCs potent priming potential resides in no small measure in its other critical phenotypic characteristics—antigen storage in non-acidic cytosolic compartments concomitant with prolonged antigen presentation and the type I IFN production upon uptake of apoptotic cells. Rather than engulfing the entire apoptotic body, mcDC seem to actively “nibble” on the corpses. The particles (meros = particle in Greek) obtained through this pathway are significantly smaller than apoptotic material taken in by CD8DCs and CD11bDCs, Fig. 3b. Importantly, the particles are stored in non-acidic organelles—“merosomes”—in the cytosol for many days and function as an antigen depot [82]. This endows the mcDCs with the capacity for prolonged Ag presentation, which increases the window during which rare antigen-specific T cells can encounter and interact with their cognate antigen, Fig. 3c. In addition, increasing the length of antigenic stimulation has been shown to positively affect T cell expansion, acquisition of effector functions, and memory development [83–86].
The mcDCs are further characterized by their unique capacity to produce proinflammatory type I IFN, instead of IL-12p70 or the immunoregulatory IL-10, after uptake of dying cells [58, 82]. Type I IFN can provide an autocrine adjuvant effect on mcDCs through the up-regulation of MHC I/II and co-stimulatory molecules and a paracrine adjuvant effect on T cells through alterations of pro-and anti-apoptotic molecule expression [87–89]. This is supported by our observations that mcDCs lacking a functional type I IFN receptor (Ifnar−/−) showed significantly reduced priming and absence of wild-type T cell memory upon presentation of autologous tumor vaccines in vivo [82]. In addition, Ifnar−/− CD8 T cells showed poor priming and failure to develop into memory cells upon priming by WT mcDCs [82].
The capacity of mcDC to prime both CD4+ and CD8+ T cells is critically important in the clearance of tumors. We and others have shown that CD4+ T cell help during the priming of CD8+ T cells is required for optimal CD8+ T cell activation, primary expansion, acquisition of effector function, and the development of memory [90–92]. This is most clearly appreciated in models where increasing cognate CD4+ T cell help through transfer of (transgenic) CD4+ T cells or pre-immunization augments the induction of CD8+ T cell responses [93, 94]. In addition, ample evidence indicates that CD4+ T cell help plays an ancillary role in the maintenance, reactivation, and expansion of existing memory CD8+ T cells [95].
There is a specific role for dendritic cells in the initiation and progression of T1D
A number of studies suggest distinct and vital roles for DCs in promoting or inhibiting T1D in the NOD mouse, including studies showing that DC are the first leukocytes to infiltrate islets during the initial phases of insulitis, only followed secondarily by T cells [96]. Pancreatic islet resident DC resemble those in the dermis and include both CD8+CD103+ and CD11b+ DCs, although the relative number of CD8+CD103+ DCs is much lower that CD11bDCs and rarely exceeds 20% of the total tissue DC pool [97]. In addition, none of the islet resident DCs express langerin [98]. DCs are essential for the retention of lymphocytes in peri-insulitis lesions prior to the onset of progressive insulitis [99, 100], while Turley et al., have proposed that diabetogenic T cells activation is triggered via antigen presented on CD11b+CD11c+DC [54]. This is largely in agreement with our published data that the presentation of islet antigen to BDC2.5 T cells in vivo requires cDC, and that the ablation of cDC, using CD11c promoter driven expression of diphtheria toxin receptor (DTR) on all DC subsets followed by diphtheria toxin (DT) treatment, results in no T cell activation, no insulitis and no diabetes [101].
Conventional DCs restore antigen presentation, insulitis, and diabetes in DT-treated CD11c-DTR mice
The temporal ablation of DC abrogated the development of diabetes, allowing us to determine the DC subset responsible for breaking peripheral tolerance to islet cell antigen in vivo. Following DT treatment of CD11c-DTR/NOD mice, treated mice received either cDC or pDC from wild type (WT, non-DTR) NOD mice. When these mice then received sort-purified naïve BDC2.5 T cells diabetes developed only in the mice reconstituted with cDC but not pDC subsets, suggested that cDC but not pDC were critical for in vivo antigen presentation and subsequent diabetes, and parallel our in vitro results [101]. However, pDC do play a vital role in the negative regulation of effector T cell function in vivo [101]. In this regard, pDC appear to act on locally in the pancreas to dampen the inflammatory response via the production of indoleamine 2,3-dioxygenase (IDO) [101].
The NOD mouse has elevated levels of mcDCs
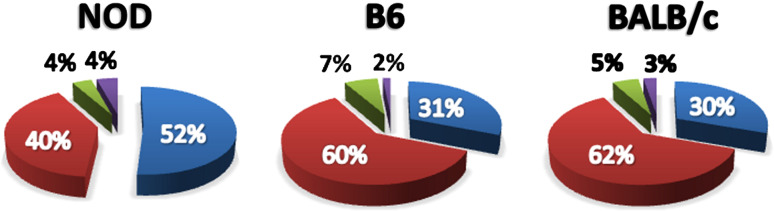
The population of cDC that restored insulitis and diabetes to DC-ablated NOD mice included several distinct DC subsets, including CD8DCs, CD11bDCs and mcDCs, thus, it was vital to determine if one or more of these subsets were responsible for the observed restoration of T cell activation and diabetes. When the frequency and absolute numbers of these subsets were measured in the spleen and PLN of NOD and age-matched C57BL/6 (B6) and BALB/c mice, it became clear that the frequency of mcDC was increased in NOD mice compared to the non-disease-prone B6 and BALB/c, Fig. 4. Moreover, the mcDC population in spleen and PLN mice increased in NOD with age while mcDC populations did not change over time in B6 and BALB/c mice [102]. The increase in frequency of mcDC in the NOD mice came largely at the expense of myeloid DCs (CD11bDC).
Fig. 4.
Increased frequency and function of merocytic DC in NOD mice with age. NOD mcDC increase with age when compared to both B6 and BALB/c mice. Subset analysis was performed at 4, 6, 8, 12, and 20 weeks of age. A comparison at 12 weeks of age is shown. Each subset was enumerated as a percent of total DC. Blue mcDC, Red CD11bDC, Green CD8DC and Purple pDC
NOD mcDC have augmented type 1 interferon production after exposure to irradiated cells
One hallmark of the mcDC subset is its ability to produce type 1 IFN upon activation by either an encounter with irradiated/dying cells and/or by toll receptor signaling [58, 82]. The production of type 1 IFN by mcDCs is thought to explain in part their ability to break peripheral tolerance to antigen in vivo [58]. Interestingly, the production of type 1 IFN by NOD mcDC from PLN and spleen was likewise enhanced after exposure to irradiated cells when compared to BALB/c mcDC of the same age [102]. Thus, the heightened numbers of and IFN production by NOD mcDC were noteworthy, particularly so, in that they coincided with the development and progression of insulitis in NOD mice, and reinforce the recent published data suggest that type 1 IFN is critical for the initiation of T1D in the NOD mouse [103].
NOD mcDCs efficiently acquire and present beta cell antigen from irradiated islet cells
The defining attributes of mcDC are their unique abilities to acquire, store and present antigen from apoptotic cells [58, 82]. Unlike other cDC, mcDCs acquire antigen by merocytosis, or “nibbling” small fragments of antigen from dead or dying cells, rather than by engulfment of the entire dying cell [58]. The antigenic fragments acquired by merocytosis are retained by mcDC as visually distinct punctate cytoplasmic vesicles. We found that in the NOD mice, mcDC readily acquired from apoptotic islet cells. After a simple overnight co-culture with CFSE-marked irradiated islet cells, the NOD mcDCs exhibited clearly visible merocytic vesicles containing islet antigen. The mcDCs retained their antigen load in relatively neutral pH endosomes, while the CD11bDC had only widely distributed CFSE staining indicative of classical phagocytosis, and low pH fluorescence quenching associated with antigen degradation. Moreover, in some cases had visible cytoplasmic-localized apoptotic bodies (Fig. 5). On average the mcDC vesicles contained roughly a two-fold greater CFSE signal than those from CD11bDC [102].
Fig. 5.
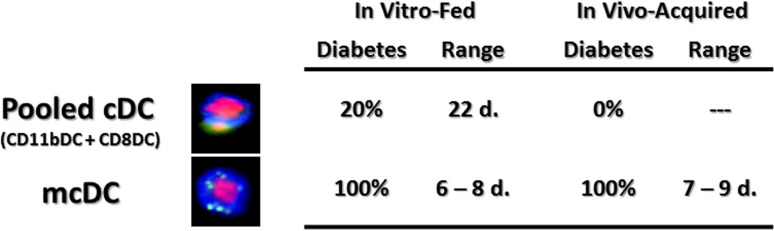
mcDC have a larger subset of small, high-intensity particle of irradiated islet cells than CD11b+DC. Representative composite images from pooled cDC (CD11bDC and CD8DC) and mcDC fed irradiated CFSE-labeled islet cells. A pool of CD11bDC, CD8DC and mcDC from NOD were exposed to irradiated islet cells in vitro and then purified and transferred (5 × 105) to 4-week-old NOD recipients. Recipients that received mcDC but not the other cDC rapidly developed T1D. Rapid onset of T1D in NOD mice upon transfer of mcDC isolated from the PLN of overtly diabetic female NOD mice. DC were isolated from the PLN of ~40 diabetic NOD females, sort-purified and transferred (2 × 105) into 4-week-old female NOD mice
Islet cell-derived Ag is efficiently presented to naïve CD4+ and CD8+ T cells by merocytic DC
The imaging flow cytometry clearly showed that mcDC have a distinct and readily identifiable intracellular pattern of antigen acquisition and storage. However, these data did not establish if mcDC were more efficient at presenting self-antigen than CD11bDC on a per cell basis. This was however, the case. We found that mcDC co-cultured with irradiated beta cells were 9- to tenfold better than CD11bDC or other cDC subsets at presenting exogenously acquired beta cell Ag to CD4+ and CD8+ T cells [102].
Antigen-exposed NOD mcDC break tolerance and transfer diabetes to young, pre-diabetic NOD mice
When NOD DC subsets were exposed to irradiated islet cells overnight, purified and transferred into 28-day old NOD recipients, all of the NOD recipients of antigen-loaded mcDC became diabetic within the first 14 days, while recipients that received a pooled fraction of antigen-exposed CD11bDCs and CD8DCs did not, Fig. 5 and [102]. This accelerated transfer of diabetes also holds true for NOD mice that received mcDCs that acquired their islet antigen under native in vivo conditions, as mcDCs from the draining PLN of overtly diabetic NOD mice likewise transferred diabetes to young disease-free recipient NOD mice, Fig. 5. The tempo and disease incidence were similar to those seen when in vitro-loaded mcDC were used (Fig. 5). None of the NOD recipients that either received the non-mcDC pool or were left untreated progressed on to overt diabetes within the assay period (Fig. 5). Importantly then, these data suggested that the mcDC subset (1) acquire islet antigen naturally in vivo, (2) were competent antigen-presenting cells when transferred to young disease-free recipients, (3) were capable of promoting the breaking of self-tolerance to islet cell antigen, and (4) were capable of hastening diabetes development in vivo.
Merocytic dendritic cells represent a novel therapeutic avenue for breaking T cell tolerance and enhancing vaccination
The data in the NOD mice and tumor vaccination suggest that mcDC may represent a new target for immune intervention. While there are a number of as yet unanswered questions on the development and function of mcDCs in NOD mice, they are an inviting target for therapeutic intervention at various phases of disease. It is currently not known if the distinct dysfunction of mcDC in the NOD mouse results from intrinsic defects in DC development, maturation or environmental factors, or a combination of these factors. Further research to elucidate the mechanisms that drive mcDC functions will be instrumental in the design of mcDC specific therapeutic targets. However, the ability to target these cells will likely profoundly affect the activation of rogue diabetogenic CD4+ and CD8+ T cells.
The targeting of DC is not a new concept in diabetes. However, most studies use an “add back” approach where the transfer of in vitro manipulated DCs is used to (1) alter cytokine production by the pathogenic T cells, (2) actively induce tolerance/anergy or delete pathogenic T cells, and (3) induce/increase the frequency of T cells with counter-regulatory functions. These approaches all interfere with the T cells after priming has occurred and do not necessarily prevent or reduce the priming of new pathogenic T cells by mcDC, Fig. 6. Moreover, our tumor studies indicate that mcDC are able to reinvigorate anergic or tolerized T cells, making them an impediment in strategies aimed at tolerance induction. The elimination of mcDC, or interference with their function, could be powerful tool to slow pre-diabetes and new onset diabetes, and provide a favorable environment for additional treatments whether they are geared towards the re-education of the immune-response or the grafting of islet cells. Increasing our understanding of the mcDC in animal models as well as the identification of the human mcDC equivalent would provide new targets for interventional therapy that could significantly enhance current immunotherapeutic approaches.
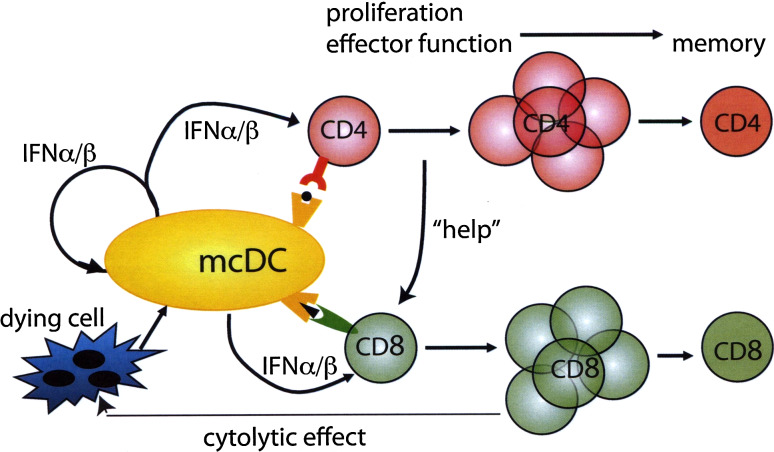
Fig. 6.
A model for mcDC-mediated priming to apoptotic cell-associated antigens. Uptake of dying cells by mcDC results in type I IFN induction and antigen presentation in MHC class I and II. Type I IFN has an autocrine adjuvant effect on the mcDC and a paracrine adjuvant effect on the CD4+ and CD8+ T cells leading to increased proliferation, effector function, and development of long-term memory. The increased CD4+T cell response provides “help” in the priming of the CD8+T cells that—upon acquisition of cytolytic effector functions—target more cells for destruction
Abbreviations
- T1D
Type 1 diabetes
- Ag
Antigen(s)
- TCR
T cell receptor
- APC
Antigen-presenting cell
- DC
Dendritic cell(s)
- FLT3
Fms-like tyrosine kinase 3
- FLT3L
FLT3 ligand
- mcDC
Merocytic dendritic cells
- IKDC
Natural killer dendritic cells
- Th1/Tc1
T helper cell type 1/T cytotoxic cell type 1
- pDC
Plasmacytoid dendritic cells
- cDC
Conventional dendritic cells
- PLN
Pancreatic lymph nodes
- CLP
Common lymphoid precursors
- CMP
Common myeloid precursors
- NOD
Non-obese diabetic
References
- 1.Atkinson MA. ADA Outstanding Scientific Achievement Lecture 2004. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can’t we prevent or reverse this disease? Diabetes. 2005;54:1253–1263. doi: 10.2337/diabetes.54.5.1253. [DOI] [PubMed] [Google Scholar]
- 2.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 3.Vehik K, Hamman RF, Lezotte D, Norris JM, Klingensmith G, Bloch C, Rewers M, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30:503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- 4.Cordell HJ, Todd JA. Multifactorial inheritance in type 1 diabetes. Trends Genet. 1995;11:499–504. doi: 10.1016/S0168-9525(00)89160-X. [DOI] [PubMed] [Google Scholar]
- 5.Cordell HJ, Todd JA, Lathrop GM. Mapping multiple linked quantitative trait loci in non-obese diabetic mice using a stepwise regression strategy. Genet Res. 1998;71:51–64. doi: 10.1017/S0016672398003152. [DOI] [PubMed] [Google Scholar]
- 6.Ridgway WM. The non obese diabetic (NOD) mouse: a unique model for understanding the interaction between genetics and T cell responses. Rev Endocr Metab Disord. 2003;4:263–269. doi: 10.1023/A:1025104429334. [DOI] [PubMed] [Google Scholar]
- 7.Wicker LS, Miller BJ, Mullen Y. Transfer of autoimmune diabetes mellitus with splenocytes from nonobese diabetic (NOD) mice. Diabetes. 1986;35:855–860. doi: 10.2337/diabetes.35.8.855. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Bendelac A, Bach JF, Carnaud C. Syngeneic T cell transfer of diabetes into NOD newborn mice: in situ studies of the autoimmune steps leading to insulin-producing cell destruction. Eur J Immunol. 1989;19:1947–1951. doi: 10.1002/eji.1830191028. [DOI] [PubMed] [Google Scholar]
- 9.Bendelac A, Carnaud C, Boitard C, Bach JF. Syngeneic transfer of autoimmune diabetes from diabetic NOD mice to healthy neonates. Requirement for both L3T4+ and Lyt-2+ T cells. J Exp Med. 1987;166:823–832. doi: 10.1084/jem.166.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz J, Benoist C, Mathis D. Major histocompatibility complex class I molecules are required for the development of insulitis in non-obese diabetic mice. Eur J Immunol. 1993;23:3358–3360. doi: 10.1002/eji.1830231244. [DOI] [PubMed] [Google Scholar]
- 11.Wicker LS, Leiter EH, Todd JA, Renjilian RJ, Peterson E, Fischer PA, Podolin PL, Zijlstra M, Jaenisch R, Peterson LB. Beta 2-microglobulin-deficient NOD mice do not develop insulitis or diabetes. Diabetes. 1994;43:500–504. doi: 10.2337/diabetes.43.3.500. [DOI] [PubMed] [Google Scholar]
- 12.Serreze DV, Leiter EH, Christianson GJ, Greiner D, Roopenian DC. Major histocompatibility complex class I-deficient NOD-B2mnull mice are diabetes and insulitis resistant. Diabetes. 1994;43:505–509. doi: 10.2337/diabetes.43.3.505. [DOI] [PubMed] [Google Scholar]
- 13.Wändell PE. Quality of life of patients with diabetes mellitus: an overview of research in primary health care in the Nordic countries. Scand J Prim Health Care. 2005;23:68–74. doi: 10.1080/02813430510015296. [DOI] [PubMed] [Google Scholar]
- 14.Anonoymous Guidelines. National Institute of Clinical Excellence. Diabet Med. 2005;22(Suppl 1):5–6. doi: 10.1111/j.1464-5491.2005.1531c.x. [DOI] [PubMed] [Google Scholar]
- 15.Yagi H, Matsumoto M, Kunimoto K, Kawaguchi J, Makino S, Harada M. Analysis of the roles of CD4+ and CD8+ T cells in autoimmune diabetes of NOD mice using transfer to NOD athymic nude mice. Eur J Immunol. 1992;22:2387–2393. doi: 10.1002/eji.1830220931. [DOI] [PubMed] [Google Scholar]
- 16.Christianson SW, Shultz LD, Leiter EH. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes. 1993;42:44–55. doi: 10.2337/diabetes.42.1.44. [DOI] [PubMed] [Google Scholar]
- 17.Hayward AR, Shriber M, Cooke A, Waldmann H. Prevention of diabetes but not insulitis in NOD mice injected with antibody to CD4. J Autoimmun. 1993;6:301–310. doi: 10.1006/jaut.1993.1026. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Gonzalez A, Benoist C, Mathis D. The role of CD8+ T cells in the initiation of insulin-dependent diabetes mellitus. Eur J Immunol. 1996;26:1762–1769. doi: 10.1002/eji.1830260815. [DOI] [PubMed] [Google Scholar]
- 19.Serreze DV, Gallichan WS, Snider DP, Croitoru K, Rosenthal KL, Leiter EH, Christianson GJ, Dudley ME, Roopenian DC. MHC class I-mediated antigen presentation and induction of CD8+ cytotoxic T-cell responses in autoimmune diabetes-prone NOD mice. Diabetes. 1996;45:902–908. doi: 10.2337/diabetes.45.7.902. [DOI] [PubMed] [Google Scholar]
- 20.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-E. [DOI] [PubMed] [Google Scholar]
- 21.Tsai S, Shameli A, Santamaria P. CD8+ T cells in type 1 diabetes. Adv Immunol. 2008;100:79–124. doi: 10.1016/S0065-2776(08)00804-3. [DOI] [PubMed] [Google Scholar]
- 22.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–263. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 23.Dilts SM, Solvason N, Lafferty KJ. The role of CD4 and CD8 T cells in the development of autoimmune diabetes. J Autoimmun. 1999;13:285–290. doi: 10.1006/jaut.1999.0323. [DOI] [PubMed] [Google Scholar]
- 24.Wong FS, Janeway CAJ. The role of CD4 and CD8 T cells in type I diabetes in the NOD mouse. Res Immunol. 1997;148:327–332. doi: 10.1016/S0923-2494(97)87242-2. [DOI] [PubMed] [Google Scholar]
- 25.Haskins K, Wegmann D. Diabetogenic T-cell clones. Diabetes. 1996;45:1299–1305. doi: 10.2337/diabetes.45.10.1299. [DOI] [PubMed] [Google Scholar]
- 26.André I, Gonzalez A, Wang B, Katz J, Benoist C, Mathis D. Checkpoints in the progression of autoimmune disease: lessons from diabetes models. Proc Natl Acad Sci USA. 1996;93:2260–2263. doi: 10.1073/pnas.93.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurrer MO, Pakala SV, Hanson HL, Katz JD. Beta cell apoptosis in T cell-mediated autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:213–218. doi: 10.1073/pnas.94.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pakala SV, Chivetta M, Kelly CB, Katz JD. In autoimmune diabetes the transition from benign to pernicious insulitis requires an islet cell response to tumor necrosis factor alpha. J Exp Med. 1999;189:1053–1062. doi: 10.1084/jem.189.7.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien BA, Harmon BV, Cameron DP, Allan DJ. Apoptosis is the mode of beta-cell death responsible for the development of IDDM in the nonobese diabetic (NOD) mouse. Diabetes. 1997;46:750–757. doi: 10.2337/diabetes.46.5.750. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien BA, Huang Y, Geng X, Dutz JP, Finegood DT. Phagocytosis of apoptotic cells by macrophages from NOD mice is reduced. Diabetes. 2002;51:2481–2488. doi: 10.2337/diabetes.51.8.2481. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26:104–115. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 34.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Mathis D, Benoist C. A decade of AIRE. Nat Rev Immunol. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 36.Mathis D, Benoist C. Aire. Annu Rev Immunol. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 37.Haskins K, Portas M, Bradley B, Wegmann D, Lafferty K. T-lymphocyte clone specific for pancreatic islet antigen. Diabetes. 1988;37:1444–1448. doi: 10.2337/diabetes.37.10.1444. [DOI] [PubMed] [Google Scholar]
- 38.Bradley BJ, Wang YY, Lafferty KJ, Haskins K. In vivo activity of an islet-reactive T-cell clone. J Autoimmun. 1990;3:449–456. doi: 10.1016/S0896-8411(05)80012-5. [DOI] [PubMed] [Google Scholar]
- 39.Stadinski BD, Delong T, Reisdorph N, Reisdorph R, Powell RL, Armstrong M, Piganelli JD, Barbour G, Bradley B, Crawford F, Marrack P, Mahata SK, Kappler JW, Haskins K. Chromogranin A is an autoantigen in type 1 diabetes. Nat Immunol. 2010;11:225–231. doi: 10.1038/ni.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanagawa O, Shimizu J, Vaupel BA. Thymic and postthymic regulation of diabetogenic CD8 T cell development in TCR transgenic nonobese diabetic (NOD) mice. J Immunol. 2000;164:5466–5473. doi: 10.4049/jimmunol.164.10.5466. [DOI] [PubMed] [Google Scholar]
- 41.Cain JA, Smith JA, Ondr JK, Wang B, Katz JD. NKT cells and IFN-gamma establish the regulatory environment for the control of diabetogenic T cells in the nonobese diabetic mouse. J Immunol. 2006;176:1645–1654. doi: 10.4049/jimmunol.176.3.1645. [DOI] [PubMed] [Google Scholar]
- 42.Lühder F, Katz J, Benoist C, Mathis D. Major histocompatibility complex class II molecules can protect from diabetes by positively selecting T cells with additional specificities. J Exp Med. 1998;187:379–387. doi: 10.1084/jem.187.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Höglund P, Mintern J, Waltzinger C, Heath W, Benoist C, Mathis D. Initiation of autoimmune diabetes by developmentally regulated presentation of islet cell antigens in the pancreatic lymph nodes. J Exp Med. 1999;189:331–339. doi: 10.1084/jem.189.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santamaria P, Utsugi T, Park BJ, Averill N, Kawazu S, Yoon JW. Beta-cell-cytotoxic CD8+ T cells from nonobese diabetic mice use highly homologous T cell receptor alpha-chain CDR3 sequences. J Immunol. 1995;154:2494–2503. [PubMed] [Google Scholar]
- 45.Santamaria P. The long and winding road to understanding and conquering type 1 diabetes. Immunity. 2010;32:437–445. doi: 10.1016/j.immuni.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 46.DiLorenzo TP, Graser RT, Ono T, Christianson GJ, Chapman HD, Roopenian DC, Nathenson SG, Serreze DV. Major histocompatibility complex class I-restricted T cells are required for all but the end stages of diabetes development in nonobese diabetic mice and use a prevalent T cell receptor alpha chain gene rearrangement. Proc Natl Acad Sci USA. 1998;95:12538–12543. doi: 10.1073/pnas.95.21.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haskins K. T-cell receptor transgenic (TCR-Tg) mice from two diabetogenic CD4+ islet-antigen-specific T-cell clones. J Autoimmun. 2004;22:107–109. doi: 10.1016/j.jaut.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117:2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennand K, Huangfu D, Melton D. All beta cells contribute equally to islet growth and maintenance. PLoS Biol. 2007;5:e163. doi: 10.1371/journal.pbio.0050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 51.Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995;44:249–256. doi: 10.2337/diabetes.44.3.249. [DOI] [PubMed] [Google Scholar]
- 52.Scaglia L, Cahill CJ, Finegood DT, Bonner-Weir S. Apoptosis participates in the remodeling of the endocrine pancreas in the neonatal rat. Endocrinology. 1997;138:1736–1741. doi: 10.1210/en.138.4.1736. [DOI] [PubMed] [Google Scholar]
- 53.Petrik J, Arany E, McDonald TJ, Hill DJ. Apoptosis in the pancreatic islet cells of the neonatal rat is associated with a reduced expression of insulin-like growth factor II that may act as a survival factor. Endocrinology. 1998;139:2994–3004. doi: 10.1210/en.139.6.2994. [DOI] [PubMed] [Google Scholar]
- 54.Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological beta cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J Exp Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belz GT, Carbone FR, Heath WR. Cross-presentation of antigens by dendritic cells. Crit Rev Immunol. 2002;22:439–448. [PubMed] [Google Scholar]
- 56.Belz GT, Shortman K, Bevan MJ, Heath WR. CD8alpha+ dendritic cells selectively present MHC class I-restricted noncytolytic viral and intracellular bacterial antigens in vivo. J Immunol. 2005;175:196–200. doi: 10.4049/jimmunol.175.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roos A, Xu W, Castellano G, Nauta AJ, Garred P, Daha MR, van Kooten C. Mini-review: a pivotal role for innate immunity in the clearance of apoptotic cells. Eur J Immunol. 2004;34:921–929. doi: 10.1002/eji.200424904. [DOI] [PubMed] [Google Scholar]
- 58.Janssen E, Tabeta K, Barnes MJ, Rutschmann S, McBride S, Bahjat KS, Schoenberger SP, Theofilopoulos AN, Beutler B, Hoebe K. Efficient T cell activation via a Toll-interleukin 1 receptor-independent pathway. Immunity. 2006;24:787–799. doi: 10.1016/j.immuni.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 60.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 61.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 62.Bedoui S, Prato S, Mintern J, Gebhardt T, Zhan Y, Lew AM, Heath WR, Villadangos JA, Segura E. Characterization of an immediate splenic precursor of CD8+ dendritic cells capable of inducing antiviral T cell responses. J Immunol. 2009;182:4200–4207. doi: 10.4049/jimmunol.0802286. [DOI] [PubMed] [Google Scholar]
- 63.Qiu C, Miyake Y, Kaise H, Kitamura H, Ohara O, Tanaka M. Novel subset of CD8{alpha}+ dendritic cells localized in the marginal zone is responsible for tolerance to cell-associated antigens. J Immunol. 2009;182:4127–4136. doi: 10.4049/jimmunol.0803364. [DOI] [PubMed] [Google Scholar]
- 64.Bonmort M, Dalod M, Mignot G, Ullrich E, Chaput N, Zitvogel L. Killer dendritic cells: IKDC and the others. Curr Opin Immunol. 2008;20:558–565. doi: 10.1016/j.coi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 65.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 66.Allan RS, Waithman J, Bedoui S, Jones CM, Villadangos JA, Zhan Y, Lew AM, Shortman K, Heath WR, Carbone FR. Migratory dendritic cells transfer antigen to a lymph node-resident dendritic cell population for efficient CTL priming. Immunity. 2006;25:153–162. doi: 10.1016/j.immuni.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 67.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 68.Williams CA, Harry RA, McLeod JD. Apoptotic cells induce dendritic cell-mediated suppression via interferon-gamma-induced IDO. Immunology. 2008;124:89–101. doi: 10.1111/j.1365-2567.2007.02743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peng YF, Elkon KB. Peripheral CD8 T-cell responses to apoptotic cell proteins and peptides. Crit Rev Immunol. 2007;27:357–365. doi: 10.1615/critrevimmunol.v27.i4.50. [DOI] [PubMed] [Google Scholar]
- 70.Ferguson TA, Kazama H. Signals from dying cells: tolerance induction by the dendritic cell. Immunol Res. 2005;32:99–108. doi: 10.1385/IR:32:1-3:099. [DOI] [PubMed] [Google Scholar]
- 71.Saas P, Bonnefoy F, Kury-Paulin S, Kleinclauss F, Perruche S. Mediators involved in the immunomodulatory effects of apoptotic cells. Transplantation. 2007;84:S31–S34. doi: 10.1097/01.tp.0000269113.59857.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 73.Schulz O, Reis e Sousa C. Cross-presentation of cell-associated antigens by CD8alpha+ dendritic cells is attributable to their ability to internalize dead cells. Immunology. 2002;107:183–189. doi: 10.1046/j.1365-2567.2002.01513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iyoda T, Shimoyama S, Liu K, Omatsu Y, Akiyama Y, Maeda Y, Takahara K, Steinman RM, Inaba K. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J Exp Med. 2002;195:1289–1302. doi: 10.1084/jem.20020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Crozat K, Guiton R, Contreras V, Feuillet V, Dutertre C, Ventre E, Vu Manh T, Baranek T, Storset AK, Marvel J, Boudinot P, Hosmalin A, Schwartz-Cornil I, Dalod M. The XC chemokine receptor 1 is a conserved selective marker of mammalian cells homologous to mouse CD8alpha+ dendritic cells. J Exp Med. 2010;207:1283–1292. doi: 10.1084/jem.20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jongbloed SL, Kassianos AJ, McDonald KJ, Clark GJ, Ju X, Angel CE, Chen CJ, Dunbar PR, Wadley RB, Jeet V, Vulink AJE, Hart DNJ, Radford KJ. Human CD141+(BDCA-3) + dendritic cells (DCs) represent a unique myeloid DC subset that cross-presents necrotic cell antigens. J Exp Med. 2010;207:1247–1260. doi: 10.1084/jem.20092140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bachem A, Güttler S, Hartung E, Ebstein F, Schaefer M, Tannert A, Salama A, Movassaghi K, Opitz C, Mages HW, Henn V, Kloetzel P, Gurka S, Kroczek RA. Superior antigen cross-presentation and XCR1 expression define human CD11c+ CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207:1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hoeffel G, Ripoche A, Matheoud D, Nascimbeni M, Escriou N, Lebon P, Heshmati F, Guillet J, Gannagé M, Caillat-Zucman S, Casartelli N, Schwartz O, De la Salle H, Hanau D, Hosmalin A, Marañón C. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 79.Colonna M, Cella M. Crosspresentation: plasmacytoid dendritic cells are in the business. Immunity. 2007;27:419–421. doi: 10.1016/j.immuni.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 80.Irla M, Küpfer N, Suter T, Lissilaa R, Benkhoucha M, Skupsky J, Lalive PH, Fontana A, Reith W, Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hennies CM, Reboulet RA, Garcia Z, Nierkens S, Wolkers MC, Janssen EM. Selective expansion of merocytic dendritic cells and CD8DCs confers anti-tumour effect of Fms-like tyrosine kinase 3-ligand treatment in vivo. Clin Exp Immunol. 2011;163:381–391. doi: 10.1111/j.1365-2249.2010.04305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Reboulet RA, Hennies CM, Garcia Z, Nierkens S, Janssen EM. Prolonged antigen storage endows merocytic dendritic cells with enhanced capacity to prime anti-tumor responses in tumor-bearing mice. J Immunol. 2010;185:3337–3347. doi: 10.4049/jimmunol.1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steptoe RJ, Ritchie JM, Harrison LC. Transfer of hematopoietic stem cells encoding autoantigen prevents autoimmune diabetes. J Clin Invest. 2003;111:1357–1363. doi: 10.1172/JCI15995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spierings DCJ, Lemmens EE, Grewal K, Schoenberger SP, Green DR. Duration of CTL activation regulates IL-2 production required for autonomous clonal expansion. Eur J Immunol. 2006;36:1707–1717. doi: 10.1002/eji.200635929. [DOI] [PubMed] [Google Scholar]
- 85.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naïve cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Belz GT, Kallies A. Effector and memory CD8+ T cell differentiation: toward a molecular understanding of fate determination. Curr Opin Immunol. 2010;22:279–285. doi: 10.1016/j.coi.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 87.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J Exp Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Le Bon A, Durand V, Kamphuis E, Thompson C, Bulfone-Paus S, Rossmann C, Kalinke U, Tough DF. Direct stimulation of T cells by type I IFN enhances the CD8 + T cell response during cross-priming. J Immunol. 2006;176:4682–4689. doi: 10.4049/jimmunol.176.8.4682. [DOI] [PubMed] [Google Scholar]
- 89.Huber JP, David Farrar J. Regulation of effector and memory T-cell functions by type I interferon. Immunology. 2011;132:466–474. doi: 10.1111/j.1365-2567.2011.03412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 92.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 93.Hamilton-Williams EE, Lang A, Benke D, Davey GM, Wiesmüller K, Kurts C. Cutting edge: TLR ligands are not sufficient to break cross-tolerance to self-antigens. J Immunol. 2005;174:1159–1163. doi: 10.4049/jimmunol.174.3.1159. [DOI] [PubMed] [Google Scholar]
- 94.Krawczyk CM, Shen H, Pearce EJ. Memory CD4 T cells enhance primary CD8 T-cell responses. Infect Immun. 2007;75:3556–3560. doi: 10.1128/IAI.00086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Williams MA, Holmes BJ, Sun JC, Bevan MJ. Developing and maintaining protective CD8+ memory T cells. Immunol Rev. 2006;211:146–153. doi: 10.1111/j.0105-2896.2006.00389.x. [DOI] [PubMed] [Google Scholar]
- 96.Rosmalen JG, Leenen PJ, Katz JD, Voerman JS, Drexhage HA. Dendritic cells in the autoimmune insulitis in NOD mouse models of diabetes. Adv Exp Med Biol. 1997;417:291–294. doi: 10.1007/978-1-4757-9966-8_47. [DOI] [PubMed] [Google Scholar]
- 97.Jansen A, Homo-Delarche F, Hooijkaas H, Leenen PJ, Dardenne M, Drexhage HA. Immunohistochemical characterization of monocytes-macrophages and dendritic cells involved in the initiation of the insulitis and beta-cell destruction in NOD mice. Diabetes. 1994;43:667–675. doi: 10.2337/diabetes.43.5.667. [DOI] [PubMed] [Google Scholar]
- 98.de Jong MAWP, Geijtenbeek TBH. Langerhans cells in innate defense against pathogens. Trends Immunol. 2010;31:452–459. doi: 10.1016/j.it.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 99.Nikolic T, Bouma G, Drexhage HA, Leenen PJM. Diabetes-prone NOD mice show an expanded subpopulation of mature circulating monocytes, which preferentially develop into macrophage-like cells in vitro. J Leukoc Biol. 2005;78:70–79. doi: 10.1189/jlb.1104662. [DOI] [PubMed] [Google Scholar]
- 100.Nikolic T, Geutskens SB, van Rooijen N, Drexhage HA, Leenen PJ M. Dendritic cells and macrophages are essential for the retention of lymphocytes in (peri)-insulitis of the nonobese diabetic mouse: a phagocyte depletion study. Lab Invest. 2005;85:487–501. doi: 10.1038/labinvest.3700238. [DOI] [PubMed] [Google Scholar]
- 101.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 102.Katz JD, Ondr JK, Opoka RJ, Garcia Z, Janssen EM. Cutting edge: merocytic dendritic cells break T cell tolerance to beta cell antigens in nonobese diabetic mouse diabetes. J Immunol. 2010;185:1999–2003. doi: 10.4049/jimmunol.1001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]