Abstract
Identification of gene expression mechanisms began with works on embryonic induction. The same mechanism of cell-cell interactions also contributes to the process of oncogenesis. Damage to epithelial cells’ genetic apparatus turns them into precancerous stem cells that are not yet capable of tumor growth. They can be transformed into cancer stem cells and undergo further progression as a result of epigenetic effects of apocrine secretion by surrounding activated stromal cells (mostly myofibroblasts). These factors may activate the damaged genetic information. On the contrary, the level of malignancy can be decreased by adding culture medium from non-activated stromal cells. One must not exclude the possibility that in a number of cases genetically altered bone marrow may migrate to damaged or inflamed tissues and become there a source of stromal cells, as well as of parenchymal stem cells in a damaged organ, where they may give rise to changed epithelial (precancerous) stem cells or to activated stromal cells, thus leading to malignant tumor growth. Cancer treatment should also affect activated stromal cells. It may prevent emergence and progression of cancerous stem cells.
Keywords: Cancer stem cells, Cancer stroma, Cancer cells, Fibroblasts, Myofibroblasts, Bone-marrow derived cancer cells
Epigenetic Effects of the Tissue Environment on Cell Differentiation
The background to understanding the control role of some cells in development of others is deeply rooted in H. Schpemann’s work on embryonic induction. The fundamental concept is that a mature region of the embryo sends a signal to another region that determines the fate of the latter. The determining signal is produced by the cells of the inducing tissue. For example, neural tube and neural plate are induced from the ectoderm under the influence of the subjacent archenteron roof. Later, derivatives of the neural tube and neural plate induce the development of lens, cutaneous glands, etc. [1]. The phenomenon of embryonic induction appeared to be one of the basic laws of development and intercellular interaction. Various embryologists have expressed the opinion that carcinogenic induction is one of those factors that induces malignant transformation [2].
An inducer can only trigger cell differentiation if the cell has the relevant genetic information encoded in its DNA [3].
Genotype becomes phenotype through expression of the relevant genes, whereas triggering is epigenetic in nature [4], i.e., genome-encoded stem cell differentiation requires tight epigenetic control through micro-environmental factors that are external to these cells [5]. DNA-associated information is more stable than epigenetic information. The latter is more labile and can change over the course of cell differentiation under the influence of external factors in the course of cell differentiation [6]. The chemistry of epigenetic change includes alterations in DNA methylation and in chromatin structure. Hypomethylation of regulatory promoter region 5′ of the genes results in increased expression of such genes (e.g., in the case of oncogenes), whereas hypermethylation suppresses their functions (e.g., in the case of genes that suppress cancer progression). Alterations in chromatin structure are related to methylation of DNA cytosine residues and acetylation of the nucleosomal histones forming the structure around which the DNA is folded. Small RNA molecules (microRNA) also play a role in the gene expression algorithm [7, 8]. Predisposition to abnormal gene methylation is age-related; this is one of the factors contributing to the increased incidence of cancer with age. It is supposed that epigenetic status is to some extent related to diet and is substantially affected by smoking and alcohol consumption [9–11].
Epigenetic influence is to some extent regulated by various intercellular barriers, e.g., by the basal lamina. It serves as a regulating barrier for the transfer of inducer macromolecules from cells located beneath the membrane to those above it. As has been demonstrated in the case of developing duodenum, this membrane is still fragmented during early embryogenesis. It is this discontinuity of the basal lamina that makes epithelial-mesenchymal interactions possible, since it allows maximum inducer access to the maturing epithelium [12].
Studies on prostate development have made it possible to formulate some basic principles regarding mesenchymal-epithelial interactions. First, it was demonstrated that prostate development requires reciprocal mesenchymal-epithelial interactions. Second, mesenchyme induces and determines epithelial development and differentiation. Third, certain types of epithelium can only develop in the presence of a specific mesenchyme. Fourth, mesenchymal-epithelial interactions are reciprocal. Urogenital sinus mesenchyme induces the development of adult prostatic epithelium [13].
It should mentioned that certain authors use the term “mesenchyme” erroneously when discussing semi-differentiated elements of connective tissue. Mesenchyme as such does not exist in a differentiated organism [14]. The term “mesenchyme” found below is merely a repetition of the terminology used by the authors cited.
Mature colonic mucosa is a good example of the role, which intercellular interactions play in cell development in a mature organism. This mucosa contains pericryptal fibroblasts, or myofibroblasts originating from fibroblasts [15], which exhibit some smooth muscle morphological features. They regulate the growth and differentiation of adjacent epithelial cells [16]. It has been suggested that there is a paracrine interaction between pericryptal fibroblasts and colonic epithelium [17].
Modern approaches to the problem of cell induction make it possible to state that the inducing signal triggers certain genes within cells of the induced tissue, thus determining function and morphology. Molecular factors that act as inducers include fibroblast growth factor, transforming growth factor—β (TGF-β) [18], hepatocyte growth factor [19], insulin-like growth factor 1 [20] and certain other proteins, in addition to heparan sulphate proteoglycan and laminin originating from the basal lamina [21, 22]. According to some studies, the paracrine factor released by cell-inducers is an isoform of cyclooxygenase (COX-2) [23, 24]. A similar function is also attributed to chloride intracellular channel 4 (CLIC4) [25].
Conclusion
So, it is possible to assume that histogenesis is a result of reciprocal interactions of internal (genetic determination) and external regulating (epigenetic) factors.
DNA Damage as the First Stage of Malignant Transformation
Tumor development is a multi-stage process involving the accumulation of various damage events. The first group of such damages are damages to the genome: mutations of various types, point mutations, amplifications and rearrangements of proto-oncogenes leading to their transformation into oncogenes. Damage to or switching off of genes that are responsible for apoptosis in cells with damaged DNA is another prerequisite for malignant transformation. In particular, it may occur if an allelic gene is lost, for example as a result of deletion. The remaining allele may have a point mutation [26, 27]. It is speculated that malignant transformation may also require mutations in certain other groups of genes. These include the malfunction of genes that are responsible for DNA repair [28] and the accumulation of recessive mutations at a critical “cancer initiator locus” [29]. Over-expression of the c-Myc gene imposes a “mutator” phenotype. This gene makes cells susceptible to various mutations, including oncogenic mutations [30]. Certain mutations, e.g., those in the BRCA genes, are inheritable [31].
Oncogenic viruses can induce chromosomal instability that can in turn result in oncogenic mutations [32]. Cells may undergo oncogenic virus mediated fusion (somatic hybridization). The resulting cell would be tetraploid and characterized by chromosomal instability. As a result, such cells are affected to a greater degree by mutations, including mutations in genes that suppress malignant growth [33, 34]. Viruses can also have their own versions of oncogenes. Thus, for example, the E6 protein of HPV-16 inactivates p53 in squamous cell carcinomas [35]. There are two options for cells that have been infected: they may be eliminated through apoptosis or continue their existence as chronically infected cells. In the latter case, this chronic infection creates the potential for malignant transformation [36].
Conclusion
Hence, DNA alterations are the first and indispensable component of the malignant transformation process.
Epigenetic Influence of Activated Stroma Triggers Malignant Transformation of Precancerous Cells
The second group of damages leading to the emergence of cancer cells involves the epigenetic oncogenic influence of the adjacent tissues [37]. There are two different types of DNA alterations leading to the emergence of two different forms of colon cancer [38]: on one hand, there may be chromosome instability or loss of heterozygosity resulting in aneuploidy and in loss of alleles [39], and, on the other hand, microsatellite instability may result in mutations in cells with an almost diploid karyotype and relatively rare allele loss [32]. Inflammation is also one of the epigenetic factors of malignant transformation [40].
“Cancerization fields” are another example of the role played by the epigenetic factors. These fields were first described by Slaughter [41]. He found abnormal hyperplastic epithelial cells around squamous tumors of the mouth; however, these cells did not demonstrate phenotypic traits typical of cancer cells. Since then, cancerization fields have been described in various other organs: lung, esophagus, breast, colon and several others. Biomolecular analysis has shown that cancerization field cells are genetically altered in the same way as cancer cells; nevertheless, their morphology and behaviour differ from those of cancer cells [42]. Other studies have confirmed the existence of morphologically non-malignant cancerization field epithelial cells with genetic alterations (e.g., mutations in the p53 gene) [43]. These studies have demonstrated that genetic changes are not sufficient for the emergence of a cancer cell [44]. Tumor initiation and progression of such genetically altered cells is promoted by their microenvironment [45], through epigenetic changes which promote expression of the altered genotype to produce a malignant phenotype [46]. Some data is now available on the existence of fields where epigenetically altered cells without oncogenic genetic alterations are located. Such fields have been found in Helicobacter pylori infected stomach and in some other organs: esophagus, liver, colon, lungs, kidneys. However, malignant transformation is not the only process associated with epigenetic alterations [47]. This means that epigenetic changes in the way the genetic information is read only become oncogenically relevant if the cell genome has oncogenic changes.
Each stage in cancer progression is characterized by the existence of a corresponding cancer stem cell. In other words, cancer initiation and progression can be represented as a sequence of cancer stem cells characterized by successively increasing malignancy [48]. Tissue stem cells are critical for tissue homeostasis regulation and regeneration of damaged tissue. Bone marrow derived stem cells often migrate to damaged or inflamed tissues and become a source of stromal stem cells [49–52], as well as parenchymal stem cells in a damaged organ, to which they are recruited [53–56], and may undergo malignant transformation [48]. It is also important that cancer cells may induce cancer stem cell transformation in non-stem cells if the parenchyma is damaged [57]
Cancer cells can develop from a stem cell of any type; however, most malignant cells are derived from genetically altered tissue stem cells.
It is widely accepted that the earlier the differentiation stage of a cell that has undergone malignant transformation, the more heterogeneous will be the resulting tumor [58]. However, the phenotypic heterogeneity of cancer cells in advanced stages of the disease can be in part explained by the fact that the parenchymal cells of a tumor may undergo an epithelial-mesenchymal transition (EMT) and acquire stem cell characteristics. This process can generate cancer stem cells, from which new clones then derive.
Precancerous stem cells constitute the very beginning of the malignant transformation process. They have the potential for transformation into either a normal tissue cell or into a malignant cell, or they can enter the quiescent phase G0 [59]. It has been observed that whereas low fibroblast saturation density in cell cultures is associated with resistance to cancer, high fibroblast saturation density is typical of individuals in families with hereditable forms of cancer [60]. In the bodies of immunodeficient mice, unlike in healthy animals, a precancerous stem cell always gives rise to a tumor [61].
Precancerous stem cells have been found in mammary tissue. The transition from a precancerous stem cell to a cancer stem cell does not require genetic alterations; changes in the expression of certain genes as a result of epigenetic influences is sufficient for this transition [62]. Cancer stem cells can both self-renew and produce the cells that constitute the bulk of the tumor. Mitosis frequency in the latter reflects the degree of tumor malignancy [63]. Thus, precancerous stem cells emerge after accumulation of all the required mutations; whereas it is the impact of epigenetic factors that determines their fate as cancer cells or as dormant stem cells.
Further cancer progression is associated with the emergence of migrating cancer stem cells, characterized by their smaller size and invasive growth. This phenomenon is known as epithelial-mesenchymal transition [64]. The reverse process, mesenchymal-epithelial transition, takes place when a metastatic deposit is established. Through this process, a cancer cell regains its stationary state and thus gives rise to the organization of primary tumor tissue, an in situ metastatic carcinoma [65]. Thus, epithelial-mesenchymal transition gives rise to a migrating cancer stem cell, whereas transformation of the latter into a stationary stem cell requires mesenchymal-epithelial transition. The biological characteristics of metastatic stem cells differ from those of stem cells in the primary tumor. Metastatic cancer stem cells from the primary cancer can metastasize to various organs. On the other hand, cancer stem cells originating from metastatic sites only metastasize to a limited number of organs. Thus, stem cells from the primary prostate cancer metastasize into liver, lungs, and brain, whereas stem cells from the metastatic sites can only give rise to bone metastases [58]. This means that a stationary stem cell from the primary cancer is not identical to a stationary stem cell from the metastatic site. Nor is a migrating stem cell from the primary cancer the same as a migrating stem cell from the metastatic site. The population of cancer stem cells is highly heterogeneous. This heterogeneity can be established using various immunohistochemical markers: CD133, CD44, CD166, CD49b [66]. The malignant progression of a stationary cancer stem cell, i.e., its transformation into an invasive and then into a migrating cancer stem cell, occurs under the influence of stromal cells [25, 67, 68].
Myofibroblasts produce paracrine factors that epigenetically affect cancer stem cells [69–73]. This effect only occurs if the altered epithelial cell has undergone the required genetic alterations [74]. Myofibroblasts release a paracrine factor that activates Wnt signaling in target cells. The Wnt signaling pathway determines the expression of certain genes; as a result, it can activate malignant pathways [75]. Endothelial cells are also capable of influencing cancer cells through paracrine regulation [76].
Changes in stromal cells are the most common cause for the disruption of stromal-epithelial interactions, required for malignant transformation. Studies on the role of stroma and subjacent connective tissue in the malignant transformation of epithelium have demonstrated that the emergence, growth and fate of cancer cells strongly depend on the condition of the underlying connective tissue [77]. The application of coal tar to the skin of mice induces changes in both the epithelium and in the underlying connective tissue. In the same study, it has been demonstrated that tar applications also have a systemic effect [78]. Mice whose skin was treated with 20-methylcholanthrene for 12 weeks developed skin cancer in the control group; however, carcinogen-treated epidermis did not yield tumors when transplanted to the denuded dermis of the untreated body side [79, 80]. This data is a convincing argument in favour of the role connective tissue plays in carcinogenesis.
Electron microscopy of throat and skin epithelium and underlying connective tissue in precancerous condition revealed gaps in the basal lamina under the altered epithelium, allowing epithelial cell cytoplasmic projections to extend into the connective tissue [81].
Studies on the condition of basal lamina in cancer or precancer conditions have clearly demonstrated that if epithelial dysplasia is present, the underlying basal lamina appears more distinct. At the non-invasive stage of cancer, swelling, looseness and indistinct contours of the basal membrane may be observed. The first stages of invasive growth are associated with the destruction of all basal lamina components. Newly developed basal lamina may only be observed in the most differentiated regions of the neoplasm. This means that changes in basal lamina develop under the influence of cancer cells [82].
Precancerous conditions of the uterine cervix are characterized by changes in elastic fibres caused by abnormal fibrillogenesis. Newly formed fibres do not reach maturity and are easily destroyed. This means that connective tissue reactivity is already aberrant at the early stages of neoplastic growth [83].
Morphological studies on human colon carcinogenesis have demonstrated that, for human colon adenocarcinomas, cytological changes in the epithelium followed by disorganization of tissue structure are the first signs of malignant cell transformation. Subsequently, it is possible to detect invasion and metastases. In experiments with chemically induced tumors, changes have been found in both epithelium and in the connective tissue, where collagen fiber swelling, an increase in the number of elastic fibers, fibroblast proliferation, and lymphocyte and mast cell infiltration of the stroma have been observed. The latter are presumed to have a protective function. Destruction of basal lamina between stroma and epithelium is the first stage of skin, mammary and colon cancer progression, irrespective of the specific cancer induction method. Electron microscopy studies have clearly confirmed the existence of a strong link between changes in epithelium-connective border area and carcinogenesis [84–86].
Aberrant crypts in colon mucosa are the earliest identifiable lesions in colon cancer progression. Their formation is the result of genetic and epigenetic damage [87, 88]. These aberrant crypts are the earliest, pre-neoplastic stages of a malignancy. The nuclei of some epithelial cells are enlarged. It has been found that epithelial cells from aberrant crypts have gene mutations, which are the earliest stage of cancer progression [89]. Stromal cells are also altered [90]. When an inflammatory process begins in the colon, the number of inflammatory cells (neutrophils and endothelium) in the stroma increases. With progression of chronic disease, the level of monocytes (macrophages) and lymphocytes increases and the structure of the endothelium and fibroblasts changes. Dysplastic changes in cryptal epithelium show a positive correlation with the decrease in the number of pericryptal fibroblasts. This can be a sign of progression of the pre-cancerous state of cryptal epithelium [91]. The interaction between stroma and epithelium can either result in a protective outcome or in malignant transformation [65]. So, epithelial stem cell malignant transformation is initiated by certain mutations. Later changes are regulated by stromal signals, i.e., to a certain extent, malignant change depends on the cell’s location in the crypt, differences in stromal environment being a possible explanation [92].
Various polyps are potentially pre-cancerous intestinal neoplasms. Their histological structure already differs from that of normal mucosa at the early stages of their development. The genotype of their cells shows several mutations and epigenetic damage [86]. A study of 123 sporadic colorectal polyps and 41 sporadic colorectal invasive carcinomas revealed genetic instability in both epithelial and stromal cells [93].
The same laboratory had previously published the results of their study on microsatellite instability and p53 mutations in cancer and stromal cells from 40 sporadic cancer specimens [94]. According to this report, p53 gene mutations in stromal cells are associated with microsatellite instability. This data confirms the hypothesis that genetic changes (p53) alone can only lead to cancerization field formation, whereas transformation of these cells into cancer cells requires signals from altered stromal cells [95].
In another paper, the authors demonstrated presence of the TGFBR1*6A mutation in both epithelial and stromal cells adjacent to intestinal tumors, whereas lymphocytes from those locations did not have this mutation. The authors believe that this mutation in stromal cells promotes the initiation of intestinal cancer if the epithelial cell genotype is damaged. According to the authors, this article is the first report of decreased TGF-β activity in stroma as one of the stages in colon cancer progression in humans [96].
Of the many types of stromal cells, activated fibroblasts [78] and myofibroblasts [75] are noted for their influence on epithelial cells. They can be activated by inflammation, by adjacent cancer cells, wounds, ageing, etc. [97]. Activated stromal cells release into the blood a substance (a trigger for certain genes) that stimulates cell proliferation. This reaction has a protective role in a wounded organ; however, it also promotes cancer progression [98]. This means that cancer progression is to a great extent determined by the influence of the activated stroma surrounding precancerous stem cells. In the absence of a triggering signal from the altered stroma, a genetically altered precancerous cell may not reveal its malignant potential. Stroma from the regions adjacent to the mammary tumor show clear genetic and epigenetic changes [99, 100]; however, this fact cannot be considered as strong evidence in support of the role of stroma in malignant progression, since such changes could develop in response to the nearby tumor. However, there is also data providing more solid support for the opinion that changes in stroma develop independently of cancer: fibroblasts isolated from the healthy relatives of patients with familial breast disease exhibited a phenotype that was similar to that of tumor-associated fibroblasts [101].
Conclusion
This means that activated stromal cells act as an inducer of malignant transformation in the epithelium, which has genetic preconditions for such a transformation [102–104].
Other authors share this opinion that cancer initiation and progression is a process that is determined by genetic alterations in an epithelial stem cell and by epigenetic changes mediated by its microenvironment [25, 105–107]. It has been found in animal studies that malignant transformation of mammary cells occurs if fibroblasts have been previously exposed to a carcinogen. Extracellular matrix that has been treated in such a way also has this capability [108].
Normal Fibroblasts Prevent Malignant Transformation
It is interesting to note that the malignant phenotype can be reversed in culture by integrin-blocking antibodies (Integrins are cell surface receptors that regulate cell shape, motility, and the cell cycle) [108].
Normal fibroblasts are also capable of inhibiting or preventing tumor formation [107]. Stationary cancer stem cells from a metastasizing tumor undergo epithelial-mesenchymal transition under the influence of stromal signals. Transformation of cancer stem cells of a cancer in situ into invasive cancer stem cells occurs via epithelial-mesenchymal transition under the influence of epigenetic signals from activated stroma. In target organs, signals from non-activated stroma induce the reverse (mesenchymal-epithelial) transition of invasive cells. These cells develop into carcinomas in situ. Once the stroma becomes activated by the tumor, its signals increase invasive ability of the tumor cells. [109].
Stroma influences the development of oral squamous epithelial stem cells. Altered stroma triggers carcinogenesis by inducing the development of precancerous stem cells [110].
The same epigenetic triggering influence of activated stroma (fibroblasts) on precancerous cells has been observed in the initiation and progression of prostate cancer [111–113]. On the contrary, healthy stroma containing smooth muscle cells impedes the malignant progression of prostate adenocarcinoma in rats. Stroma from rats with advanced prostate cancer does not have any effect on genetically normal prostate cells; at the same time, it promotes cancer progression in genetically altered cells [114].
The state of fibroblasts was found to alter cancer cell gene expression in experiments, when cancer cells and fibroblasts were cocultured [115, 116]. Endodermally derived epithelium and myofibroblasts of mesodermal origin interact epigenetically. The actual inducers may be affecting miRNA, various growth factors and so on [116, 117].
It should be mentioned that the malignant transformation of colon epithelial stem cells does not prevent them from undergoing differentiation in various directions. Such cells retain antigenic markers typical for their cell lineage. These markers include secretory components (columnar cells), mucin antigen (goblet cells), chromogranin (enteroendocrine cells), and lysozyme (Paneth cells). Immunohistochemical analysis of these markers makes it possible to classify such tumors for diagnostic, therapeutic or prognostic purposes [118].
Conclusion
Several papers have now provided scarce, but compelling data on the ability of normal fibroblasts to decrease malignancy of tumor cells or even to induce their reverse transformation. It can be surmised that cultural medium of embryonic fibroblasts may contain some active components capable of producing such an effect.
Conclusion
The role of stroma in cancer initiation and progression has been confirmed by many experiments using cell cultures, in studies on animals and by examination of clinical specimens [119–129]. It is, therefore, an established opinion that carcinoma is an epithelial stem cell disease. It is caused by the accumulated mutations, which transform the cell into a precancerous stem cell, and by the influence of activated adjacent stroma [130], which sends altered epigenetic signals through the basal lamina, if the integrity of the latter is disrupted. Under this stromal influence the cell develops malignancy traits [35], i.e., it becomes a stationary and then a metastatic (migrating) cancer stem cell.
Tumors include bone marrow derived stem cells that can produce both epithelial and stromal stem cells [48]. The stromal cells of tumors are also pathologically altered. Such changes may precede the tumor or may result from the proximity of the stroma to tumor cells. Therefore, cancer is a structure that consists of various tissues; as a result of their interactions, those tissues undergo changes in the process of cancer progression [131].
To a great extent, a cancer can progress and survive because the cells of which it is comprised are organized as a harmoniously collaborating metabolic domain [132]. We believe that one should not exclude a variant of tumor formation from a mutant bone marrow derived stem cell that can give rise to both precancerous and activated (altered) stromal cells in the affected organ. The further interactions of these cells initiate the tumor.
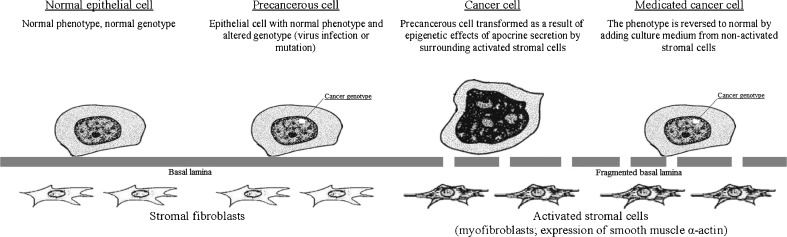
The above data on the dual nature of the malignant pathology—genetic changes that transform epithelial cells into precancerous stem cells, and epigenetic changes that are caused by the influence of altered (activated) stroma and result in implementation of the malignant program and cancer progression—have stimulated studies on epigenetic differentiation therapy that is directed against various cancer stem cells [133–138] (see Fig. 1).
Fig. 1.
Epithelial-stromal interactions and influence of normal fibroblast cell culture on the cancer cells
Acknowledgments
I am grateful to Dr. David E. Burstein for editing this paper and to Dr. Valentina V. Molchanova for her critical notes. I greatly appreciate the support of Dr. Allan Schiller and Dr. Edmond Sabo.
Footnotes
In loving memory of associate member of The USSR Academy of Medical Sciences Doctor of Biological Sciences, Prof. A.G.Knorre.
References
- 1.Spemann H, Mangold H. Induction of embryonic primordia by implantation of organizers from a different species. 1923. Int J Dev Biol. 2001;45(1):13–38. [PubMed] [Google Scholar]
- 2.Holtfreter J (1951) Some aspects of embryonic induction. Growth (Suppl 10):117–152 [PubMed]
- 3.Jacobson AG. Inductive processes in embryonic development. Science. 1966;152(3718):25–34. doi: 10.1126/science.152.3718.25. [DOI] [PubMed] [Google Scholar]
- 4.Riccardi VM. Cell—cell interaction as an epigenetic determinant in the expression of mutant neural crest cells. Birth Defects Original Article Series. 1979;15(8):89–98. [PubMed] [Google Scholar]
- 5.Cvekl A, Mitton KP. Epigenetic regulatory mechanisms in vertebrate eye development and disease. Heredity. 2010;105(1):135–151. doi: 10.1038/hdy.2010.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteller M. Epigenetics in cancer. N Engl J Med. 2008;358(11):1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 7.Klenov MS, Gvozdev VA. Heterochromatin formation: role of short RNAs and DNA methylation. Biochem Biokhim. 2005;70(11):1187–1198. doi: 10.1007/s10541-005-0247-4. [DOI] [PubMed] [Google Scholar]
- 8.Ryazansky SS, Gvozdev VA. Small RNAs and cancerogenesis. Biochem Biokhim. 2008;73(5):514–527. doi: 10.1134/s0006297908050040. [DOI] [PubMed] [Google Scholar]
- 9.Feinberg AP, Ohlsson R, Henikoff S. The epigenetic progenitor origin of human cancer. Nat Rev Genet. 2006;7(1):21–33. doi: 10.1038/nrg1748. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed FE. Colorectal cancer epigenetics: the role of environmental factors and the search for molecular biomarkers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2007;25(2):101–154. doi: 10.1080/10590500701399184. [DOI] [PubMed] [Google Scholar]
- 11.Kanwal R, Gupta S. Epigenetics and cancer. J Appl Physiol. 2010;109(2):598–605. doi: 10.1152/japplphysiol.00066.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathan M, Hermos JA, Trier JS. Structural features of the epithelio-mesenchymal interface of rat duodenal mucosa during development. J Cell Biol. 1972;52(3):577–588. doi: 10.1083/jcb.52.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cunha GR. Mesenchymal-epithelial interactions: past, present, and future. Differ Res Biol Divers. 2008;76(6):578–586. doi: 10.1111/j.1432-0436.2008.00290.x. [DOI] [PubMed] [Google Scholar]
- 14.Knorre AG (1971) Some remarks about differentiation of mesenchyme and its tissue derivatives. In: Embryonic histogenesis. Medicina, Leningrad, pp 364–368
- 15.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 16.Sappino AP, Dietrich PY, Skalli O, Widgren S, Gabbiani G. Colonic pericryptal fibroblasts. Differentiation pattern in embryogenesis and phenotypic modulation in epithelial proliferative lesions. Virchows Archiv A Pathological Anat Histopathol. 1989;415(6):551–557. doi: 10.1007/BF00718649. [DOI] [PubMed] [Google Scholar]
- 17.Rockman SP, Demmler K, Roczo N, Cosgriff A, Phillips WA, Thomas RJ, Whitehead RH. Expression of interleukin-6, leukemia inhibitory factor and their receptors by colonic epithelium and pericryptal fibroblasts. J Gastroenterol Hepatol. 2001;16(9):991–1000. doi: 10.1046/j.1440-1746.2001.02588.x. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson AG, Sater AK. Features of embryonic induction. Development. 1988;104(3):341–359. doi: 10.1242/dev.104.3.341. [DOI] [PubMed] [Google Scholar]
- 19.Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55(4):1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayatollahi M, Soleimani M, Geramizadeh B, Imanieh MH. Insulin-like growth factor 1 (IGF-I) improves hepatic differentiation of human bone marrow-derived mesenchymal stem cells. Cell Biol Int. 2011;35(11):1169–1176. doi: 10.1042/CBI20110016. [DOI] [PubMed] [Google Scholar]
- 21.Bissell MJ, Ram TG. Regulation of functional cytodifferentiation and histogenesis in mammary epithelial cells: role of the extracellular matrix. Environ Heal Perspect. 1989;80:61–70. doi: 10.1289/ehp.898061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kedinger M, Lefebvre O, Duluc I, Freund JN, Simon-Assmann P. Cellular and molecular partners involved in gut morphogenesis and differentiation. Philos Trans R Soc London B Biol Sci. 1998;353(1370):847–856. doi: 10.1098/rstb.1998.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adegboyega PA, Ololade O, Saada J, Mifflin R, Di Mari JF, Powell DW. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin Cancer Res: an official journal of the American Association for Cancer Research. 2004;10(17):5870–5879. doi: 10.1158/1078-0432.CCR-0431-03. [DOI] [PubMed] [Google Scholar]
- 24.Konstantinopoulos PA, Vandoros GP, Karamouzis MV, Gkermpesi M, Sotiropoulou-Bonikou G, Papavassiliou AG. EGF-R is expressed and AP-1 and NF-kappaB are activated in stromal myofibroblasts surrounding colon adenocarcinomas paralleling expression of COX-2 and VEGF. Cell Oncol: the official journal of the International Society for Cellular Oncology. 2007;29(6):477–482. doi: 10.1155/2007/831416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schauer IG, Sood AK, Mok S, Liu J. Cancer-associated fibroblasts and their putative role in potentiating the initiation and development of epithelial ovarian cancer. Neoplasia. 2011;13(5):393–405. doi: 10.1593/neo.101720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 27.Fearon ER. A genetic basis for the multi-step pathway of colorectal tumorigenesis. Princess Takamatsu Symp. 1991;22:37–48. [PubMed] [Google Scholar]
- 28.Calvert PM, Frucht H. The genetics of colorectal cancer. Ann Intern Med. 2002;137(7):603–612. doi: 10.7326/0003-4819-137-7-200210010-00012. [DOI] [PubMed] [Google Scholar]
- 29.Kopelovich L. Heritable colorectal cancer and cancer genes: systemic expressions. Mol Carcinog. 1993;8(1):3–6. doi: 10.1002/mc.2940080104. [DOI] [PubMed] [Google Scholar]
- 30.Prochownik EV. c-Myc: linking transformation and genomic instability. Curr Mol Med. 2008;8(6):446–458. doi: 10.2174/156652408785747988. [DOI] [PubMed] [Google Scholar]
- 31.Bayrakli F, Akgun B, Soylemez B, Kaplan M, Gurelik M. Variation in the BRCA2 gene in a child with medulloblastoma and a family history of breast cancer. J Neurosurg Pediatr. 2011;8(5):476–478. doi: 10.3171/2011.8.PEDS11210. [DOI] [PubMed] [Google Scholar]
- 32.Cherry LM. The genetic etiology of familial and nonfamilial colorectal cancer. Proc (Baylor Univ Med Cent) 2011;24(2):139–141. doi: 10.1080/08998280.2011.11928702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mekler LB, Artamonova SI, Bodyagin DA, Drize OB, Mkheidze DM, Osechinskii IV, Solenov VN. Somatic hybridization and oncogenesis; (Mechanism of formation of malignant tumors and metastases by the action of antilymphocytic serum) Sov J Dev Biol. 1975;5(3):201–219. [PubMed] [Google Scholar]
- 34.Gao P, Zheng J. Oncogenic virus-mediated cell fusion: new insights into initiation and progression of oncogenic viruses-related cancers. Cancer Lett. 2011;303(1):1–8. doi: 10.1016/j.canlet.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 35.Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 36.Saha A, Kaul R, Murakami M, Robertson ES. Tumor viruses and cancer biology: modulating signaling pathways for therapeutic intervention. Cancer Biol Ther. 2010;10(10):961–978. doi: 10.4161/cbt.10.10.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 38.Piard F, Martin L, Chapusot C, Ponnelle T, Faivre J. Genetic pathways in colorectal cancer: interest for the pathologist. Ann Pathol. 2002;22(4):277–288. [PubMed] [Google Scholar]
- 39.Lee AJ, Endesfelder D, Rowan AJ, Walther A, Birkbak NJ, Futreal PA, Downward J, Szallasi Z, Tomlinson IP, Howell M, Kschischo M, Swanton C. Chromosomal instability confers intrinsic multidrug resistance. Cancer Res. 2011;71(5):1858–1870. doi: 10.1158/0008-5472.CAN-10-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6(5):963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 42.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63(8):1727–1730. [PubMed] [Google Scholar]
- 43.Hockel M, Dornhofer N. The hydra phenomenon of cancer: why tumors recur locally after microscopically complete resection. Cancer Res. 2005;65(8):2997–3002. doi: 10.1158/0008-5472.CAN-04-3868. [DOI] [PubMed] [Google Scholar]
- 44.Burdette WJ. Colorectal carcinogenesis. Cancer. 1974;34(3):872–877. doi: 10.1002/1097-0142(197409)34:3+<872::aid-cncr2820340714>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 45.Trujillo KA, Heaphy CM, Mai M, Vargas KM, Jones AC, Vo P, Butler KS, Joste NE, Bisoffi M, Griffith JK. Markers of fibrosis and epithelial to mesenchymal transition demonstrate field cancerization in histologically normal tissue adjacent to breast tumors. Int J Cancer. 2011;129(6):1310–1321. doi: 10.1002/ijc.25788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boumber Y, Issa JP. Epigenetics in cancer: what’s the future? Oncology (Williston Park) 2011;25(3):220–226. [PubMed] [Google Scholar]
- 47.Ushijima T. Epigenetic field for cancerization. J Biochem Mol Biol. 2007;40(2):142–150. doi: 10.5483/bmbrep.2007.40.2.142. [DOI] [PubMed] [Google Scholar]
- 48.Zou GM. Cancer initiating cells or cancer stem cells in the gastrointestinal tract and liver. J Cell Physiol. 2008;217(3):598–604. doi: 10.1002/jcp.21541. [DOI] [PubMed] [Google Scholar]
- 49.Cogle CR, Theise ND, Fu D, Ucar D, Lee S, Guthrie SM, Lonergan J, Rybka W, Krause DS, Scott EW. Bone marrow contributes to epithelial cancers in mice and humans as developmental mimicry. Stem Cells. 2007;25(8):1881–1887. doi: 10.1634/stemcells.2007-0163. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Fan X, Houghton J. Tumor microenvironment: the role of the tumor stroma in cancer. J Cell Biochem. 2007;101(4):805–815. doi: 10.1002/jcb.21159. [DOI] [PubMed] [Google Scholar]
- 51.Ishii S, Tsuji S, Tsujii M, Kanazawa Y, Nishida T, Iijima H, Yasumaru M, Irie T, Yamamoto K, Tsutsui S, Eguchi H, Kawano S, Hayashi N. Involvement of bone marrow-derived stromal cells in gastrointestinal cancer development and metastasis. J Gastroenterol Hepatol. 2008;23(Suppl 2):S242–S249. doi: 10.1111/j.1440-1746.2008.05446.x. [DOI] [PubMed] [Google Scholar]
- 52.Alison MR, Lim S, Houghton JM. Bone marrow-derived cells and epithelial tumours: more than just an inflammatory relationship. Curr Opin Oncol. 2009;21(1):77–82. doi: 10.1097/CCO.0b013e32831de4cf. [DOI] [PubMed] [Google Scholar]
- 53.Spyridonidis A, Schmitt-Graff A, Tomann T, Dwenger A, Follo M, Behringer D, Finke J. Epithelial tissue chimerism after human hematopoietic cell transplantation is a real phenomenon. Am J Pathol. 2004;164(4):1147–1155. doi: 10.1016/S0002-9440(10)63203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okumura T, Wang SS, Takaishi S, Tu SP, Ng V, Ericksen RE, Rustgi AK, Wang TC. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Investig: a journal of technical methods and pathology. 2009;89(12):1410–1422. doi: 10.1038/labinvest.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valcz G, Krenacs T, Sipos F, Wichmann B, Toth K, Leiszter K, Balogh Z, Csizmadia A, Hagymasi K, Muzes G, Masszi T, Molnar B, Tulassay Z. Appearing of bone marrow derived stem cells in healthy and regenerating colonic epithelium. Orv Hetil. 2009;150(40):1852–1857. doi: 10.1556/OH.2009.28719. [DOI] [PubMed] [Google Scholar]
- 56.Valcz G, Krenacs T, Sipos F, Leiszter K, Toth K, Balogh Z, Csizmadia A, Muzes G, Molnar B, Tulassay Z. The role of the bone marrow derived mesenchymal stem cells in colonic epithelial regeneration. Path Oncol Res: POR. 2011;17(1):11–16. doi: 10.1007/s12253-010-9262-x. [DOI] [PubMed] [Google Scholar]
- 57.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tu SM, Lin SH, Logothetis CJ. Stem-cell origin of metastasis and heterogeneity in solid tumours. Lancet Oncol. 2002;3(8):508–513. doi: 10.1016/s1470-2045(02)00820-3. [DOI] [PubMed] [Google Scholar]
- 59.Naumov GN, Bender E, Zurakowski D, Kang SY, Sampson D, Flynn E, Watnick RS, Straume O, Akslen LA, Folkman J, Almog N. A model of human tumor dormancy: an angiogenic switch from the nonangiogenic phenotype. J Natl Cancer Inst. 2006;98(5):316–325. doi: 10.1093/jnci/djj068. [DOI] [PubMed] [Google Scholar]
- 60.Rubin H. Fields and field cancerization: the preneoplastic origins of cancer: asymptomatic hyperplastic fields are precursors of neoplasia, and their progression to tumors can be tracked by saturation density in culture. BioEssays: news and reviews in molecular, cellular and developmental biology. 2011;33(3):224–231. doi: 10.1002/bies.201000067. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Shen R, Ye Y, Pu XA, Liu X, Duan W, Wen J, Zimmerer J, Wang Y, Liu Y, Lasky LC, Heerema NA, Perrotti D, Ozato K, Kuramochi-Miyagawa S, Nakano T, Yates AJ, Carson WE, 3rd, Lin H, Barsky SH, Gao JX. Precancerous stem cells have the potential for both benign and malignant differentiation. PLoS One. 2007;2(3):e293. doi: 10.1371/journal.pone.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Damonte P, Hodgson JG, Chen JQ, Young LJ, Cardiff RD, Borowsky AD. Mammary carcinoma behavior is programmed in the precancer stem cell. Breast Cancer Res: BCR. 2008;10(3):R50. doi: 10.1186/bcr2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dreesen O, Brivanlou AH. Signaling pathways in cancer and embryonic stem cells. Stem Cell Rev. 2007;3(1):7–17. doi: 10.1007/s12015-007-0004-8. [DOI] [PubMed] [Google Scholar]
- 64.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5(9):744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 65.Boman BM, Huang E. Human colon cancer stem cells: a new paradigm in gastrointestinal oncology. J Clin Oncol: official journal of the American Society of Clinical Oncology. 2008;26(17):2828–2838. doi: 10.1200/JCO.2008.17.6941. [DOI] [PubMed] [Google Scholar]
- 66.Botchkina IL, Rowehl RA, Rivadeneira DE, Karpeh MS, Jr, Crawford H, Dufour A, Ju J, Wang Y, Leyfman Y, Botchkina GI. Phenotypic subpopulations of metastatic colon cancer stem cells: genomic analysis. CANCER GENOMICS PROTEOMICS. 2009;6(1):19–29. [PubMed] [Google Scholar]
- 67.Vasiliev JM. The role of connective tissue proliferation in invasive growth of normal and malignant tissues: a review. Br J Cancer. 1958;12(4):524–536. doi: 10.1038/bjc.1958.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gout S, Huot J. Role of cancer microenvironment in metastasis: focus on colon cancer. Cancer Microenviron: official journal of the International Cancer Microenvironment Society. 2008;1(1):69–83. doi: 10.1007/s12307-008-0007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1 Pt 1):C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 70.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277(2 Pt 1):C183–C201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 71.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200(4):429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 72.De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 73.Polyak K, Haviv I, Campbell IG. Co-evolution of tumor cells and their microenvironment. Trends Genet: TIG. 2009;25(1):30–38. doi: 10.1016/j.tig.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Potter JD. Morphostats: a missing concept in cancer biology. Cancer Epidemiol Biomarkers Prev: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10(3):161–170. [PubMed] [Google Scholar]
- 75.Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, Tuynman JB, Todaro M, Merz C, Rodermond H, Sprick MR, Kemper K, Richel DJ, Stassi G, Medema JP. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat Cell Biol. 2010;12(5):468–476. doi: 10.1038/ncb2048. [DOI] [PubMed] [Google Scholar]
- 76.Franses JW, Baker AB, Chitalia VC, Edelman ER. Stromal endothelial cells directly influence cancer progression. Sci Transl Med. 2011;3(66):66ra65. doi: 10.1126/scitranslmed.3001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M. Stromal gene expression predicts clinical outcome in breast cancer. Nat Med. 2008;14(5):518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 78.Ulezko-Stroganova KP. Problem of cancer and active mesenchyme. Leningrad: All-Union Scientific Research Institute of Obstetrics and Gynecology; 1940. [Google Scholar]
- 79.Billingham RE, Orr JW, Woodhouse DL. Transplantation of skin components during chemical carcinogenesis with 20-methylcholanthrene. Br J Cancer. 1951;5(4):417–432. doi: 10.1038/bjc.1951.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Marchant J, Orr JW. Further attempts to analyse the role of epidermis and deeper tissues in experimental chemical carcinogenesis by transplantation and other method. Br J Cancer. 1953;7(3):329–341. doi: 10.1038/bjc.1953.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sugar J, Farago L. Ultrastructure of laryngeal precanceroses. Acta Otolaryngol. 1966;62(4):319–332. doi: 10.3109/00016486609119577. [DOI] [PubMed] [Google Scholar]
- 82.Murav’ev GN. Basement membranes in precancerous conditions and neoplasms of the uterine cervix. Vopr Onkol. 1979;25(9):27–32. [PubMed] [Google Scholar]
- 83.Murav’ev GN. Elastic fibers of the cervical stroma in precancer and cancer. Vopr Onkol. 1975;21(10):26–34. [PubMed] [Google Scholar]
- 84.Tarin D. Fine structure of murine mammary tumours: the relationship between epithelium and connective tissue in neoplasms induced by various agents. Br J Cancer. 1969;23(2):417–425. doi: 10.1038/bjc.1969.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bosman FT, de Bruine A, Flohil C, van der Wurff A, ten Kate J, Dinjens WW. Epithelial-stromal interactions in colon cancer. Int J Dev Biol. 1993;37(1):203–211. [PubMed] [Google Scholar]
- 86.Ponz de Leon M, Di Gregorio C. Pathology of colorectal cancer. Dig Liver Dis: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2001;33(4):372–388. doi: 10.1016/s1590-8658(01)80095-5. [DOI] [PubMed] [Google Scholar]
- 87.Cheng L, Lai MD. Aberrant crypt foci as microscopic precursors of colorectal cancer. World J Gastroenterol: WJG. 2003;9(12):2642–2649. doi: 10.3748/wjg.v9.i12.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grady WM. Epigenetic events in the colorectum and in colon cancer. Biochem Soc Trans. 2005;33(Pt 4):684–688. doi: 10.1042/BST0330684. [DOI] [PubMed] [Google Scholar]
- 89.Drut R. Colonic aberrant crypts in children with familial adenomatous polyposis. Int J Surg Pathol. 2007;15(3):258–261. doi: 10.1177/1066896907302119. [DOI] [PubMed] [Google Scholar]
- 90.Pretlow TP, O’Riordan MA, Pretlow TG, Stellato TA. Aberrant crypts in human colonic mucosa: putative preneoplastic lesions. J Cell Biochem Suppl. 1992;16G:55–62. doi: 10.1002/jcb.240501111. [DOI] [PubMed] [Google Scholar]
- 91.Radovic S, Selak I, Babic M, Bratovic I. Demonstration of perycriptal fibroblasts in inflammatory-regenerative and dysplastic epithelial lesions of the flat colonic mucosa. Adv Clin Pathol: the official journal of Adriatic Society of Pathology. 2001;5(4):139–145. [PubMed] [Google Scholar]
- 92.van den Brink GR, Offerhaus GJ. The morphogenetic code and colon cancer development. Cancer Cell. 2007;11(2):109–117. doi: 10.1016/j.ccr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 93.Ishiguro K, Yoshida T, Yagishita H, Numata Y, Okayasu T. Epithelial and stromal genetic instability contributes to genesis of colorectal adenomas. Gut. 2006;55(5):695–702. doi: 10.1136/gut.2005.079459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsumoto N, Yoshida T, Yamashita K, Numata Y, Okayasu I. Possible alternative carcinogenesis pathway featuring microsatellite instability in colorectal cancer stroma. Br J Cancer. 2003;89(4):707–712. doi: 10.1038/sj.bjc.6601141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Humphries A, Wright NA. Colonic crypt organization and tumorigenesis. Nat Rev Cancer. 2008;8(6):415–424. doi: 10.1038/nrc2392. [DOI] [PubMed] [Google Scholar]
- 96.Bian Y, Knobloch TJ, Sadim M, Kaklamani V, Raji A, Yang GY, Weghorst CM, Pasche B. Somatic acquisition of TGFBR1*6A by epithelial and stromal cells during head and neck and colon cancer development. Hum Mol Genet. 2007;16(24):3128–3135. doi: 10.1093/hmg/ddm274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Worthley DL, Giraud AS, Wang TC. Stromal fibroblasts in digestive cancer. Cancer Microenviron: official journal of the International Cancer Microenvironment Society. 2010;3(1):117–125. doi: 10.1007/s12307-009-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang HY, Sneddon JB, Alizadeh AA, Sood R, West RB, Montgomery K, Chi JT, van de Rijn M, Botstein D, Brown PO. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2(2):E7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wernert N, Hugel A, Locherbach C. Genetic alterations in the fibroblastic stroma of invasive colon and breast carcinomas. Verh Dtsch Ges Pathol. 1998;82:317–321. [PubMed] [Google Scholar]
- 100.Hu M, Yao J, Cai L, Bachman KE, van den Brule F, Velculescu V, Polyak K. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37(8):899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 101.Weaver VM, Gilbert P. Watch thy neighbor: cancer is a communal affair. J Cell Sci. 2004;117(Pt 8):1287–1290. doi: 10.1242/jcs.01137. [DOI] [PubMed] [Google Scholar]
- 102.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Micke P, Ostman A. Tumour-stroma interaction: cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer. 2004;45(Suppl 2):S163–S175. doi: 10.1016/j.lungcan.2004.07.977. [DOI] [PubMed] [Google Scholar]
- 104.Hu M, Polyak K. Microenvironmental regulation of cancer development. Curr Opin Genet Dev. 2008;18(1):27–34. doi: 10.1016/j.gde.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 106.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39(11):1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin HJ, Zuo T, Chao JR, Peng Z, Asamoto LK, Yamashita SS, Huang TH. Seed in soil, with an epigenetic view. Biochim Biophys Acta. 2009;1790(9):920–924. doi: 10.1016/j.bbagen.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137(1):231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Leber MF, Efferth T. Molecular principles of cancer invasion and metastasis (review) Int J Oncol. 2009;34(4):881–895. doi: 10.3892/ijo_00000214. [DOI] [PubMed] [Google Scholar]
- 110.Ge L, Meng W, Zhou H, Bhowmick N. Could stroma contribute to field cancerization? Med Hypotheses. 2010;75(1):26–31. doi: 10.1016/j.mehy.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 111.Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107(1):1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- 112.Wong YC, Wang XH, Ling MT. Prostate development and carcinogenesis. Int Rev Cytol. 2003;227:65–130. doi: 10.1016/s0074-7696(03)01008-8. [DOI] [PubMed] [Google Scholar]
- 113.Zhao H, Ramos CF, Brooks JD, Peehl DM. Distinctive gene expression of prostatic stromal cells cultured from diseased versus normal tissues. J Cell Physiol. 2007;210(1):111–121. doi: 10.1002/jcp.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hayward SW, Grossfeld GD, Tlsty TD, Cunha GR. Genetic and epigenetic influences in prostatic carcinogenesis (review) Int J Oncol. 1998;13(1):35–47. [PubMed] [Google Scholar]
- 115.Rozenchan PB, Carraro DM, Brentani H, de Carvalho Mota LD, Bastos EP, e Ferreira EN, Torres CH, Katayama ML, Roela RA, Lyra EC, Soares FA, Folgueira MA, Goes JC, Brentani MM. Reciprocal changes in gene expression profiles of cocultured breast epithelial cells and primary fibroblasts. International journal of cancer. Int J Cancer. 2009;125(12):2767–2777. doi: 10.1002/ijc.24646. [DOI] [PubMed] [Google Scholar]
- 116.DeCossee JJ, Gossens CL, Kuzma JF, Unsworth BR. Breast cancer: induction of differentitaion by embryonic tissue. Science. 1973;181(4104):1057–1058. doi: 10.1126/science.181.4104.1057. [DOI] [PubMed] [Google Scholar]
- 117.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2(3):203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 118.Ho SB, Itzkowitz SH, Friera AM, Jiang SH, Kim YS. Cell lineage markers in premalignant and malignant colonic mucosa. Gastroenterology. 1989;97(2):392–404. doi: 10.1016/0016-5085(89)90075-9. [DOI] [PubMed] [Google Scholar]
- 119.Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differ Res Biol Divers. 2002;70(9–10):486–497. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- 120.Zalatnai A. Molecular aspects of stromal-parenchymal interactions in malignant neoplasms. Curr Mol Med. 2006;6(6):685–693. doi: 10.2174/156652406778195053. [DOI] [PubMed] [Google Scholar]
- 121.Billottet C, Jouanneau J. Tumor-stroma interactions. Bull Cancer. 2008;95(1):51–56. doi: 10.1684/bdc.2008.0560. [DOI] [PubMed] [Google Scholar]
- 122.Provenzano PP, Inman DR, Eliceiri KW, Knittel JG, Yan L, Rueden CT, White JG, Keely PJ. Collagen density promotes mammary tumor initiation and progression. BMC Med. 2008;6:11. doi: 10.1186/1741-7015-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schmid SA, Dietrich A, Schulte S, Gaumann A, Kunz-Schughart LA. Fibroblastic reaction and vascular maturation in human colon cancers. Int J Radiat Biol. 2009;85(11):1013–1025. doi: 10.3109/09553000903258897. [DOI] [PubMed] [Google Scholar]
- 124.Augsten M, Hagglof C, Pena C, Ostman A. A digest on the role of the tumor microenvironment in gastrointestinal cancers. Cancer Microenviron: official journal of the International Cancer Microenvironment Society. 2010;3(1):167–176. doi: 10.1007/s12307-010-0040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron: official journal of the International Cancer Microenvironment Society. 2010;3(1):149–166. doi: 10.1007/s12307-010-0038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang X, Jin H. The epigenetic basis of the Warburg effect. Epigenetics: official journal of the DNA Methylation Society. 2010;5(7):566–568. doi: 10.4161/epi.5.7.12662. [DOI] [PubMed] [Google Scholar]
- 127.Tripathi M, Billet S, Bhowmick NA. Understanding the role of stromal fibroblasts in cancer progression. Cell Adhes Migr. 2012;6(3):231–235. doi: 10.4161/cam.20419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Cichon MA, Degnim AC, Visscher DW, Radisky DC. Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer. J Mammary Gland Biol Neoplasia. 2010;15(4):389–397. doi: 10.1007/s10911-010-9195-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Rev. 2012;31(1–2):195–208. doi: 10.1007/s10555-011-9340-x. [DOI] [PubMed] [Google Scholar]
- 130.Prendergast GC, Jaffee EM. Cancer immunologists and cancer biologists: why we didn’t talk then but need to now. Cancer Res. 2007;67(8):3500–3504. doi: 10.1158/0008-5472.CAN-06-4626. [DOI] [PubMed] [Google Scholar]
- 131.Tarin D. Tissue interactions in morphogenesis, morphostasis and carcinogenesis. J Theor Biol. 1972;34(1):61–72. doi: 10.1016/0022-5193(72)90054-9. [DOI] [PubMed] [Google Scholar]
- 132.Koukourakis MI, Giatromanolaki A, Harris AL, Sivridis E. Comparison of metabolic pathways between cancer cells and stromal cells in colorectal carcinomas: a metabolic survival role for tumor-associated stroma. Cancer Res. 2006;66(2):632–637. doi: 10.1158/0008-5472.CAN-05-3260. [DOI] [PubMed] [Google Scholar]
- 133.Egger G, Liang G, Aparicio A, Jones PA. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429(6990):457–463. doi: 10.1038/nature02625. [DOI] [PubMed] [Google Scholar]
- 134.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4(2):143–153. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 135.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol/Hematol. 2004;51(1):1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 136.Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7(2):139–147. doi: 10.1038/nrc2067. [DOI] [PubMed] [Google Scholar]
- 137.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445(7123):106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 138.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9(1):44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]