Abstract
To study the mechanism of interaction of tumor cells with bone marrow cells continuous wave electron paramagnetic resonance (EPR) experiments at 9 GHz including a spin trapping of superoxide were carried out. The common features of the EPR spectra in healthy and tumor affected tissues of donors and rats as well as their difference are presented and discussed. It is proposed that labile iron pool plays a significant role in mechanisms of tumor invasion. We hope that the observed EPR features could be used to study the mechanisms of invasion and progression of tumor in different organs.
Keywords: Electron spin resonance spectroscopy, Medical oncology, Bone marrow, Iron metabolism disorders
Introduction
Though the evolution of cancer is unique in each individual patient, all cancers have certain similar features and mechanisms that control growth and progression. A lot of efforts are done to investigate them by different analytical methods. Electron paramagnetic/spin resonance (EPR/ESR) is widely used for the biomedical applications including cancer related research since 1950s [1–8]. EPR is well known as the most sensitive tool for the detection, identification and quantification of the naturally existing or artificially created radicals (intentionally incorporated, generated during the chemical reactions, ageing, under the light, UV, X-ray or gamma irradiation, etc.). All the modern advanced EPR approaches such as an EPR imaging, high-field EPR, double resonance techniques and their pulsed variations [6, 9, 10] are still based on the conventional 9 GHz EPR measurements. Different companies all over the world produce low-cost table EPR spectrometers for their using in the bio-chemical and clinical laboratories.
Concerning cancer related research EPR was mainly focused on the investigations of tumor itself. In our opinion, the capabilities of the method for the scientific purposes and for its clinical applications are still not fully exploited. No EPR investigations of bone marrow (BM) tissues are done so far to our knowledge. We present the main output of our EPR research of the tumor affected BM in rats and humans.
BM is considered as an essential lymphoid organ with substantial impact on tumor cell dissemination and tumor–immune responses. Stromal cells of BM are widely recognized to collaborate with cancerous cells to create a tumor-permissive microenvironment capable of providing continuous support for tumor growth, progression, angiogenesis, invasion, and metastasis. Reactive oxygen species (including superoxide) generated by cancer cells and cells in cancer microenvironments can guide and maintain the growth and differentiation of local and distant cancers and their interactions with host microenvironments [7, 11].
Materials and Methods
Experimental group of 20 of the mongrel female rats with a body mass of 100 ± 9 g rats was implanted in-vivo by the Guérin carcinoma (GC or T8) cells under the control of the R.E. Kavetsky Institute Animal Ethics Committee. Ten animals served as a control group.
Stromal and parenchyma cells of BM from the femur and tibia were acquired by flushing their diaphysis with the phosphate buffer solution followed by the disaggregation of the obtained suspension [12].
Human material was gathered from 18 female patients (45-71 y.o.) with the breast cancer at the disease stages T2-3, N1, N2 and M0 and from 11 female patients (41-68 y.o.) with polytraumas. The last group served as a control group. The patients gave their consent to use the tissues obtained by surgery and biopsy for the research purposes. BM samples were acquired by the puncture of the various sections of the breastbone (sternum) and the iliac crest. The obtained samples were frozen and stored in the special 0.5 ml containers in liquid nitrogen (77 K).
EPR measurements were conducted on RE-1307 EPR spectrometer (USSR) operating in continuous-wave (cw) mode at frequency of 9.03 GHz (X-band) by using conventional modulation technique. The obtained results were statistically compared using non-parametric Mann–Whitney U test. The values presented as Mean(SD) were considered to be statistically significant at the level of P < 0.05.
Oxidation of the 1-hydroxy-2,2,6,6-tetramethyl-4-oxo-piperidine hydrochloride (TEMPONE-H) to the stable nitroxyl radical TEMPONE was used to trap superoxide [13]. For this 200 μL of the BM cells suspension containing of about 103 BM cells was mixed at room temperature with 20 μL of the spin-trapping solution (2 mg of the powder of TEMPONE-H dissolved in 10 mL of the phosphate buffer with pH = 7.4). Then EPR spectra of the nitroxyl radical in the resulting sample were acquired at room temperature with the magnetic field sweep rate of 100 G/120 s and modulation amplitude of 0.2 G Concentration rate of growth of the nitroxyl radical in the EPR mesh which corresponds to the generation rate of superoxide anion was measured with a 2-min intervals via the change of the amplitude of the second hyperfine component of the EPR spectrum during 6 min after the introduction of the spin trap into the cell suspension. The measured relative values were re-calculated to the total number of the viable cells counted in a Goryaev chamber (hemocytometer) to extract the absolute quantities.
Results and Discussion
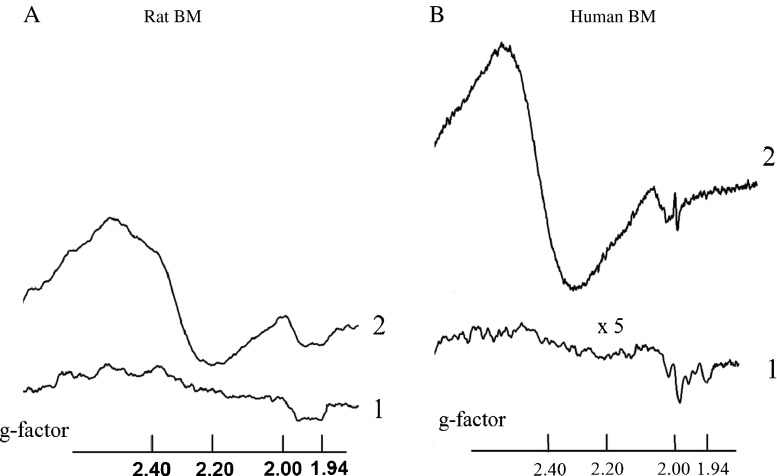
Figure 1 presents the typical EPR spectra of animal and human BMs detected at T = 77 K. Due to the fast spin–lattice relaxation times these EPR spectra are not detected at room temperature (RT).
Fig. 1.
a left panel EPR spectra (re-calculated to the values of g-factors) of samples of (1) BM of the intact animals; (2) BM of the Guérin carcinoma grafted rats. b, right panel EPR spectra the samples obtained from the human donors: (1) BM of patients with polytraumas; (2) BM of patients with breast cancer. The curve (1) is detected at five times higher receiver gain. All spectra are registered at T = 77 К
The response of the BM cells to the invasion of tumor cells and their metabolites manifests in the increased production of superoxide radicals. Superoxide generation rates for the rats and human BMs measured in the spin-trap containing mixture at RT are presented in Table 1. In the tumor affected tissues it is measured to be about eight times higher than that in a norm.
Table 1.
Generation rates of superoxide radicals at RT in healthy and tumor affected BMs (nmol/103 cells⋅min)
| BM of the Guérin carcinoma grafted rats | 3.50(7) | BM of patients with breast cancer | 2.95(16) |
| BM of intact animals | 0.45(3) | BM of patients with polytraumas | 0.38(9) |
Correlation between the integral intensities of the EPR spectra and superoxide generation rates confirms that the obtained EPR signals in the materials studied are mainly originated from the superoxide-generating cells in the bone marrow: fibroblasts, osteoblasts, endothelial cells, adipose tissue as well as from neutrophils and macrophages. The both human and rat derived BMs have very similar features in the obtained EPR spectra.
The EPR signal with g ≈ 2.00 is usually ascribed to the free radical centers practically completely localized in mitochondria—semiquinones of flavoproteides found in the inner membrane of mitochondria and coenzyme Q semiquinones (ubisemiquinones) [1–5].
Signal at g ≈ 1.94 is related to the activity of the iron-sulfur cluster N2 of NADH: ubiquinone oxidoreductase (complex I) which is responsible for the proton pumping in this largest and is the most complicated enzyme in the electron transport chain (ETC) of mitochondria [1–5]. In the tumor affected human BM this signal practically disappears while no great changes are observed in the rat tissues. It might be a manifestation of a difference between the rat and human stromal cells observed in [12] which nature is not yet explained. We hope the obtained EPR feature could help to unravel the origin(s) of that difference.
The most pronounced and the most intriguing in nature is an intensive broad signal at g ≈ 2.20–2.60 that appears in the tumor affected BMs. The signal with the similar spectroscopic parameters is to be reported arising “sometimes” in “a part” of the pathologically changed tissues [1, 4, 8]. The last trend is to connect the origin of at least part of this broad signal with the ferritin molecules [8]. But how does this signal arise in the tumor microenviroment is still an open question.
Pulatova with her co-workers connected this signal obtained in some samples of the mouse and dogs blood at the latest stages of the acute radiation syndrome (in both in-vivo and in-vitro experiments) with the activity of a “free” iron pool (pp.101–106 and Fig. 2.31 in Ref. [4]). Speaking present-day language—with a pool of loosely bound iron, which is termed the labile iron pool (LIP) [14, 15]. Released due to the destructions of different proteins and iron-containing compartments, a “free” iron binds with the diverse low-molecular weight substances as phosphate, nucleotides, hydroxyl, amino and sulfhydryl groups, etc. which are in excess are existing in tumor and tumor affected tissues. A “free” iron concentration is increasing at the earlier and at the latest disease stages. Superposition of EPR signals from all these iron complexes results in a broad intensive signal. Therefore, this accumulated signal could probably serve to measure LIP level and to estimate a grade of tumor invasion.
Cells with higher LIP levels invariably exhibit higher levels of reactive oxygen species. It leads in its turn to the damage of other microenvironment cells. We hope, therefore, that the obtained EPR features could be further used to track the spatial tumor progression.
Conclusion
Tumor iron metabolism and mitochondrial dysfunction are considered as the major players in tumorigenesis [15]. Tumor cells undergoing profound changes in their own intrinsic metabolism affect the microenvironment. The main impact on the BM microenviroment is through the metabolic disorder and increased production of superoxide radicals in stromal cells. In our opinion, based on the presented EPR measurements, a “free” iron could serve as a tool for such kind of influence. These tumor metabolites can reprogram the mitochondrial metabolism and mitochondrial activity of NADPH oxidase of normal cells towards to the increased generation of superoxide radicals.
We hope that the observed EPR features could be fruitfully used to study the mechanisms of invasion and progression of tumor in different organs and motivate the scientific community for their advanced studies by using more elaborated EPR and other magnetic resonance techniques. Study of the mechanisms of tumor invasion and progression in BM by EPR is a matter of the ongoing research. Concerning clinical applications, we propose that the method could be used to measure a “free” iron level (or at least the paramagnetic part of that which thought to be the most reactive and cannot be measured by other analytical tools) and, therefore, to estimate the tumor malignant potential in lesions formation.
Acknowledgments
A part of this work is done in the framework of the Cooperation Agreement between R.E. Kavetsky Institute (Ukraine) and Kazan Federal University (Russia). We appreciate a support of Dr. Sergei Orlinskii and Dr. Sergei Nikitin (Kazan) for the incarnation of this Agreement inspired by Prof. N.I. Silkin.
References
- 1.Mallard JR, Kent M. Electron spin resonance in biological tissues. Phys Med Biol. 1969;14:373–396. doi: 10.1088/0031-9155/14/3/201. [DOI] [PubMed] [Google Scholar]
- 2.Emanuel NM, Kavetskii RE, Tarusov BN, Sidorik EP. Cancer biophysics. Kyiv: Naukova Dumka; 1976. [Google Scholar]
- 3.Azhipa JI. Medical-biological aspects in electron paramagnetic resonance method applications. Moscow: Nauka; 1983. [Google Scholar]
- 4.Pulatova MK, Rikhireva GT, Kuropteva ZV. Electron spin resonance in molecular radiobiology. Moscow: Energoatomizdat; 1989. [Google Scholar]
- 5.Saifutdinov RG, Larina LI, Vakul’skaya TI, Voronkov MG. Electron paramagnetic resonance in biochemistry and medicine. New York: Springer; 2001. [Google Scholar]
- 6.Eaton SS, Eaton GR, Berliner LJ. Biomedical EPR—Part A: Free radicals, metals. Springer: Medicine and Physiology; 2005. [Google Scholar]
- 7.Burlaka AP, Sidorik EP. Radical forms of oxygen and nitrogen oxide in the tumors process. Kyiv: Naukova Dumka; 2006. [Google Scholar]
- 8.Yurtaeva SV, Efimov VN, Silkin NI, Rodionov AA, Burmistrov MV, Panov AV, Moroshek AA. Magnetic resonance of ferritin crystalline particles in tumor tissue. Appl Magn Reson. 2012;42:299–311. doi: 10.1007/s00723-012-0312-2. [DOI] [Google Scholar]
- 9.Swartz HM, Khan N, Buckey J, Comi R, Gould L, Grinberg O, Hartford A, et al. Clinical applications of EPR: overview and perspectives. NMR Biomed. 2004;17:335–351. doi: 10.1002/nbm.911. [DOI] [PubMed] [Google Scholar]
- 10.Gafurov MR, Yavkin BV, Biktagirov TB, Mamin GV, Orlinskii SB, et al. Atherosclerotic plaque and hydroxyapatite nanostructures studied by high-frequency EPR. Magn Reson Solids. 2013;15:13102. [Google Scholar]
- 11.D’Autréaux B, Toledano MB. ROS as signaling molecules: mechanisms that generate specificity in ROS homeostasis. Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 12.Javazon EH, Colter DC, Schwarz EJ, Prockop DJ. Rat marrow stromal cells are more sensitive to plating density and expand more rapidly from single-cell-derived colonies than human marrow stromal cells. Stem Cells. 2001;19:219–225. doi: 10.1634/stemcells.19-3-219. [DOI] [PubMed] [Google Scholar]
- 13.Janzen EG. Spin-trapping. Acc Chem Res. 1971;4:31–40. doi: 10.1021/ar50037a005. [DOI] [Google Scholar]
- 14.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott RL, Head JF. Cancer: tumor iron metabolism, mitochondrial dysfunction and tumor immunosuppression; “A tight partnership—was Warburg correct?”. J Cancer Ther. 2012;3:278–311. doi: 10.4236/jct.2012.34039. [DOI] [Google Scholar]