Abstract
U19/EAF2 is regulated by androgens in the prostate and capable of regulating transcriptional elongation of RNA Pol II via interaction with the ELL family proteins. Inactivation of U19/EAF2 induces tumorigenesis in multiple organs; however the mechanism of U19/EAF2 tumor suppression remains unclear. To elucidate potential mechanisms of U19/EAF2 action, we performed cDNA microarray analysis and identified 164 mRNA transcripts regulated by U19/EAF2 in the mouse ventral prostate. Bioinformatics analysis indicated that U19/EAF2 knockout activates the RAS-BRAF-ERK signaling pathway, which is known to play important roles in carcinogenesis. qPCR verified increased expression of BRAF mRNA, and immunostaining and Western blot analysis demonstrated increased expression of p-ERK at the protein level suggested U19/EAF2 knockout activates this important pathway. These findings indicate that loss of EAF2 up-regulates transcription of RAS cascade genes including Grb2, PI3K, and BRAF, leading to elevated p-ERK levels, which may represent a major functional role of U19/EAF2 in the prostate. Furthermore, these observations suggest that U19/EAF2 is a key player in crosstalk between androgen receptor and the RAS-BRAF-ERK signaling pathway.
Electronic supplementary material
The online version of this article (doi:10.1007/s12307-013-0132-4) contains supplementary material, which is available to authorized users.
Keywords: EAF2, Prostate cancer, ERK
Introduction
Androgens play a key role in prostate development, maturation, homeostasis, and pathogenesis including benign prostatic hyperplasia (BPH) and prostate cancer [1–4]. Androgen action is mediated through the androgen receptor (AR), a ligand-regulated transcription factor that regulates the expression of many androgen-responsive genes [5, 6]. Defining the functions of various androgen-responsive genes will provide insights into the mechanisms of androgen action in the prostate.
Androgen action in the prostate, particularly in the regulation of prostate homeostasis, is likely to involve crosstalk with various growth signaling, including the EGF, IGF, TGF-β, FGF, and VEGF signaling pathways [7, 8]. Many experiments demonstrating crosstalk between AR and various signaling pathways have been carried out in prostate cancer cells. The importance of crosstalk in the normal prostate is less clear. Defining AR crosstalk with important signaling pathways in the mouse prostate will provide new insights into the mechanisms by which androgens regulate prostate growth, regression, and homeostasis under physiological conditions.
One important growth signal pathway is the RAS-BRAF-MEK-ERK cascade, which is frequently activated in carcinogenesis [9]. Activation of ERK via phosphorylation is a critical step in tumor development and maintenance. The RAS-ERK pathway is exquisitely sensitive to small changes in the levels of or activity of BRAF and BRAF may be the primary target of oncogenic RAS [10]. The BRAF gene encodes a protein belonging to the raf/mil family of serine/threonine protein kinases and plays a role in regulating the MAP kinase/ERKs signaling pathway, which affects cell division, differentiation, and secretion. In humans, activating oncogenic mutations in this gene have also been associated with development of the cardio-facio-cutaneous (CFC) syndrome [11] and various cancers, most frequently in melanoma, papillary thyroid cancer and colon cancer [12–16]. Elevated ERK signaling has been found in prostate cancer patients [7, 17–19]. Sorafenib, an inhibitor of human BRAF protein [20], is in Phase II clinical trial in androgen-independent prostate cancer [21].
Our previous studies showed that androgen-responsive gene (Up-regulated gene 19/ELL associated factor 2) U19/EAF2 encodes a potential tumor suppressor. U19/EAF2 expression is frequently down-regulated in advanced human prostate cancer specimens as well as in established prostate cancer cell lines [22]. U19/EAF2, along with its homolog EAF1, has been reported to regulate transcriptional elongation of RNA polymerae II via interaction with the ELL (11 lysine-rich leukemia) family proteins [23–25]. Overexpression of U19/EAF2 induces apoptosis and suppresses prostate xenograft tumor growth [22]. Inactivation of U19/EAF2 in a murine knockout model leads to high rates of lung adenocarcinoma, B cell lymphoma, hepatocellular carcinoma, and prostate intraepithelial neoplasia (PIN) [26]. The U19/EAF2 KO prostate exhibited epithelial hyperplasia and dysplasia, suggesting that U19/EAF2 contributes to the suppression of prostate tumors. The mechanisms of U19/EAF2 acting as a novel tumor suppressor in multiple mouse tissues are not clear.
Our previous studies showed that U19/EAF2 knockout prostate exhibited elevated cell proliferation, suggesting a possible link between U19/EAF2 with growth control signaling pathways [26]. Identification of signaling pathways regulated by U19/EAF2 in the mouse prostate model would suggest potential mechanisms of U19/EAF2 action and provide further insights into androgen action in the prostate.
Using cDNA microarray, this study identified 49 up-regulated and 115 down-regulated genes in the U19/EAF2 knockout prostate as compared to wild-type controls. Bioinformatics analysis of these differentially regulated genes revealed multiple pathways that are regulated by U19/EAF2 in the prostate. Loss of EAF2 in the murine prostate induced an up-regulation of RAS cascade genes including Grb2, PI3K, and BRAF, leading to elevated p-ERK levels, which may represent a major mechanism by which U19/EAF2 down-regulation induces prostate tumorigenesis.
Materials and Methods
Animals
We previously generated U19/EAF2 heterozygous mice (U19/EAF2+/−) using HM1 embryonic stem cells [26]. Briefly, heterozygous mice were thereafter backcrossed to the C57BL/6J strain (The Jackson Laboratory, Bar Harbor, ME) for more than 12 generations to generate U19/EAF2 knockout (KO) mice with a pure C57BL/6J background under approval by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Genotyping was determined by PCR analysis of mouse tail genomic DNA as described previously [26].
Tissue Preparation and Microarray Hybridization
Ventral prostate lobes (VP) from 3 month old wild-type (WT) and U19/EAF2 KO virgin male mice were microdissected from the anterior (AP), dorsal (DP), and lateral (DL) lobes in phosphate-buffered saline with the aid of a dissecting Carl Zeiss Stemi 2000 Steromicroscope (Zeiss) and immediately snap-frozen in liquid nitrogen. Five lobes of each type from the same mouse strain were pooled in order to minimize individual differences. Expression profiling experiments were performed by the Microarray Laboratory at University of Pittsburgh. Microarray hybridization was performed with a pre-equilibrated Mouse Genome 430 2.0 Genechip array (Affymetrix Inc., Santa Clara, CA). In addition, to compensate for the extremely small sample setup (n = 1, with pooling of five animals), we performed 6 independent biological replicates of individual ventral lobes from wild type mice and accessed their gene expression using the Illumina Mouse-48 K Expression BeadChip (Illumina, San Diego, CA). All datasets have been deposited in the NCBI public database GEO with the following accession number: GSE34511.
Bioinformatics Data Analysis
Probe intensities too low to be considered as present were filtered out using the long established Affymetrix default methods. Probes presenting detection p-values smaller than 0.01 were further considered in knockout (KO) vs wild type (WT) gene expression comparison. Differential expression statistical significance was obtained with the HTself2 method [27] which uses self-self experiments to extrapolate p-values for small sample sized experiments. Small modifications to the HTself2 methods were introduced to make it suitable for single-color microarray data. In order to facilitate reuse and detailed methodological reproducibility and understanding, the analysis script written in the R statistical language (R Development Core Team, 2011) is freely available with no usage restriction at: http://labpib.fmrp.usp.br/~rvencio/htself2/. The whole filtered average log intensity range was used to establish the null probability density distribution considering virtual self-self experiments generated from every possible combination among 6 wild type mice expression data similarly as in previous works [28–30]. A Bonferroni-corrected p-value of 0.01 was used as statistical significance threshold, which was found to correspond to a 2.5-fold change cutoff. Functional enrichment analysis was performed with DAVID (http://david.abcc.ncifcrf.gov/) as described in Huang et al. [31]. Functional and ontology enrichment analysis was performed using the DAVID web-based tool [32] and Ingenuity Pathways Analysis (IPA) 5.0 (Ingenuity Systems).
Gene Expression Validation
In addition to the samples used in microarray analysis, the ventral prostates and livers of an independent set of wild-type or U19/EAF2 knockout male mice (n = 3) at 3 months of age were used for total RNA isolation using Trizol® Reagent (Invitrogen, USA). Animal tissues were homogenized with a Kontes pellet pestle for 30 s twice (Fisher Scientific, Fair View, NJ). qPCR verified expression scored by cDNA arrays of ventral prostate tissue and expression levels in liver tissue (Platinum SYBR®Green qPCR SuperMix-UDG, Invitrogen, USA). PCR amplification was carried out using Applied Biosystems StepOne™Plus™ Real-Time PCR Systems (Applied Biosystems CA, USA). PCR amplification of various genes was normalized to the average Ct value of the housekeeping gene GAPDH. Primer sequences are listed in Table 1. GAPDH was chosen as an internal control because there was no difference in GAPDH expression between wild-type and U19/EAF2 knockout prostate in the microarray data. Also GAPDH has been used as a normalization control in prostate research [33]. Each experimental sample was assayed in triplicate.
Table 1.
The list of genes and primer sequences used for RT-PCR analysis
| ID | Symbol | Entrez gene name | Direction | Sequence |
|---|---|---|---|---|
| 1422651_at | Adipoq | adiponectin, C1Q and collagen domain containing | Forward | 5′- TGTTCCTCTTAATCCTGCCCA |
| Reverse | 5′- CCA ACC TGC ACA AGT TCC CTT | |||
| 1428944_at | Ube1l2 | ubiquitin-like modifier activating enzyme 6 | Forward | 5′- GAG CCT GCG GTG CAA GTA A |
| Reverse | 5′- CCA CTC CAA GAC CAC CCA TAC | |||
| 1425032_at | Abpb | androgen binding protein beta | Forward | 5′- GCA TGT GCT CCT TTT GTC GG |
| Reverse | 5′- TGG TTC CTC ATT GAA GCA ATC C | |||
| 1419554_at | CD47 | CD47 antigen | Forward | 5′- TGC GGT TCA GCT CAA CTA CTG |
| Reverse | 5′- GCT TTG CGC CTC CAC ATT AC | |||
| 1449676_at | Rab2 | RAB2A, member RAS oncogene family | Forward | 5′- GCG ACA CAG GTG TTG GTA AAT |
| Reverse | 5′- CAT CAA TCG TTA TCA TCC GAG CA | |||
| 1449028_at | Rhou | ras homolog gene family, member U | Forward | 5′- GGC TAC CCC ACC GAG TAC AT |
| Reverse | 5′- GGG GCC TCA GCT TGT CAA A | |||
| 1435831_at | UPK IB | uroplakin 1B | Forward | 5′- CAC TGT TCG TTG CTT CCA GG |
| Reverse | 5′- GCT TCG AGA AGT GGG TAA AGA CT | |||
| 1450849_at | hnRNP | heterogeneous nuclear ribonucleoprotein U | Forward | ATG AGT TCT TCG CCT GTT AAT GT |
| Reverse | 5′- CCT GGA GTC GAT CCA TGA GA | |||
| 1449405_at | Tensin | tensin 1 | Forward | 5′- GTT TCT CCA AGC ATA CAG CCA |
| Reverse | 5′- GTT CAG AGA GGT TGA ATA GCA GG | |||
| 1460302_at | THBS1 | thrombospondin 1 | Forward | 5′- GGG GAG ATA ACG GTG TGT TTG |
| Reverse | 5′- CGG GGA TCA GGT TGG CAT | |||
| 1448556_at | PRLR | prolactin receptor | Forward | 5′- GGG GAG ATA ACG GTG TGT TTG |
| Reverse | 5′- CGG GGA TCA GGT TGG CAT | |||
| M32599 | GAPDH | glyceraldehyde-3-phosphate dehydrogenase | Forward | 5′-AGG TCG GTG TGA ACG GAT TTG |
| Reverse | 5′-GTA GAC CAT GTA GTT GAG GTC A |
Western Blot of Mouse Tissue
Protein lysates were obtained from freshly isolated individual mouse liver and prostate tissues. Animal tissues were homogenized by a hand homogenizer, Kontes® Pellet Pestle®/Cordless Motor (Fisher, USA) in RIPA lysis buffer (50 mM Tris–HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1 % (v/v) NP-40, 0.1 % SDS, 0.25 % sodium deoxycholate, 1 mM sodium orthovanadate, 1 mM PMSF, 1:100 dilution of protease inhibitor cocktail (P8340, Sigma-Aldrich, St. Louis, MO). Protein concentration was determined by BCA Protein Assay (Thermo Scientific, Rockford, IL). The whole cell lysate (WCL) (45 ug/lane) were boiled in SDS sample buffer, separated on a NEXT GEL™ 10 % (Amresco) under reducing conditions, and then transferred onto a nitrocellulose membrane. Blotted proteins were probed with antibodies as follows: Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody (1:1000, #9101), p44/42 MAPK (Erk1/2) antibody (1:1000, #9102, Cell Signaling Technology) and followed with HRP (Horseradish peroxidase) labeled secondary antibodies (Santa Cruz Biotechnology). Signals were visualized using chemiluminescence (ECL™ Western Blotting Detection Reagents®, GE Healthcare) and were exposed to X-ray film (Fuji film).
Immunohistochemical Analyses
Tissue specimens from 3 month-old male mice were frozen in OCT (Tissue Tek, Sakura Finetechnical USA, Torrance, CA) immediately after dissection and stored at −80 °C. Serial 5 μm sections were fixed in cold acetone and processed for immunohistochemistry. Sections were incubated with rabbit polyclonal Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody (1:1000, #9101, Cell Signaling Technology) or rabbit polyclonal p44/42 MAPK (Erk1/2) antibody (1:1000, #9102, Cell Signaling Technology) at room temperature for 1 h. Antigen was localized using HRP-conjugated anti- rabbit IgG (Santa Cruz Biotechnology) as the secondary antibody and diaminobenzidine tetrahydrochloride as the chromagen. Negative control experiments were performed using goat or rat IgG as primary antibody. The sections were lightly counterstained with hematoxylin.
Statistical Analysis
All values represent the mean ± standard error of the mean (SEM). Statistical significance between paired data was assessed by unpaired Student’s t test and any p-value less than 0.05 was considered statistically significant.
Results
Microarray Analysis of Genes Differentially Expressed in the Ventral Prostate of U19/EAF2 Knockout Mouse
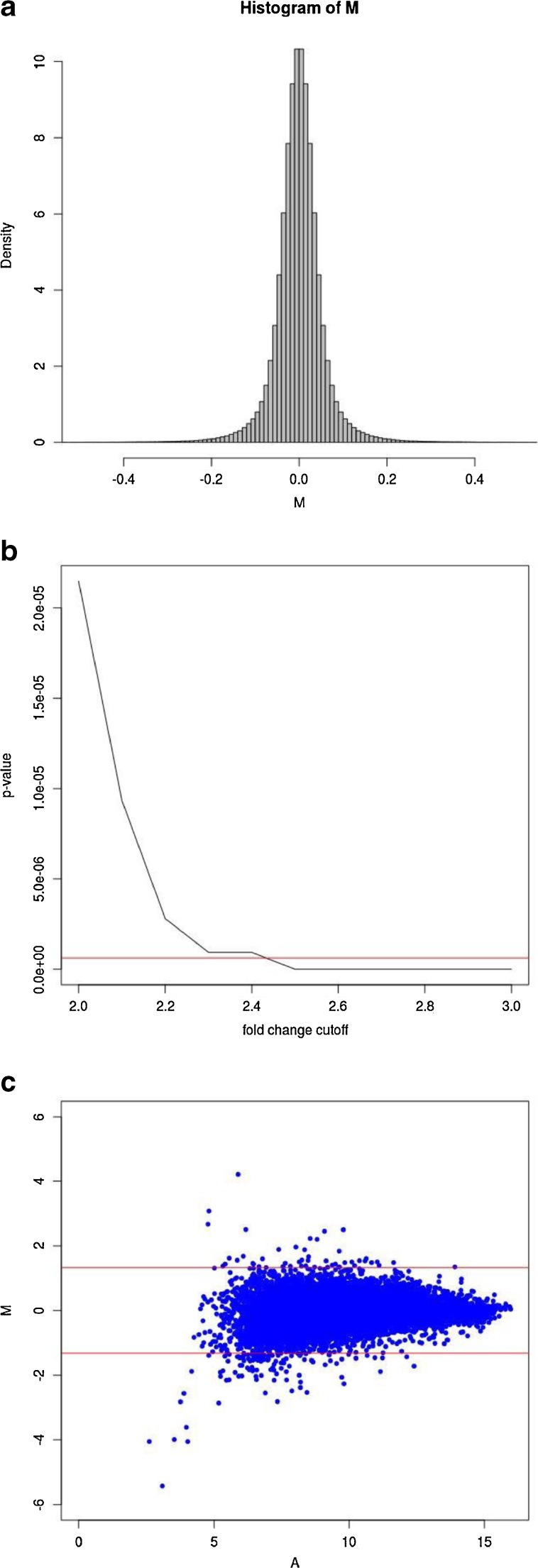
To identify target genes of U19/EAF2, we performed cDNA microarray analysis of the ventral prostate from wild-type and U19/EAF2 knockout mice at 3 months of age. On a C57BL/6J background, U19/EAF2 knockout mice displayed no histological abnormality in the prostate at this age (Ai et al., in revision). Thus, all changes are unlikely to be secondary to pathological changes in the knockout prostate. Due to an extremely small sample setup (n = 1, pooled from five animals), appropriate bioinformatics strategies were necessary. Methods to deal with such special situations exist and we used the HTself2 method [27] with small modifications. The idea behind this method is to guide fold-change cutoff selection, an arbitrary, but necessary, step to define the differentially expressed genes, with experimental data that estimates the natural inter-mice genetic noise in gene expression measurements. In order to estimate this background noise we performed six independent biological replicates of wild type (WT) mice and assessed their gene expression. Although the statistical method was originally designed for ratio-based two-color microarray, we adapted our results to take advantage of it by simply creating virtual experiments taking all possible combinations from the 6 WT expression profiles (WT1 vs WT2, WT1 vs WT3, …, WT6 vs WT5) similarly as carried out in Pascal et al. [26–28]. The probability density distribution of the log fold-change obtained with this strategy can then be used to derive a statistically consistent cutoff criteria for differential expression finding in the knockout vs wild type experiment (KO/WT) (Fig. 1a). In small sample scenarios it is important to be overly conservative, therefore, the significance figures (p-values) found with the modified Cortez et al. [23] method were also corrected for multiple testing issues with the highly stringent Bonferroni correction [34] (Fig. 1b). We found that a 2.5-fold change cutoff corresponded to Bonferroni-corrected p-values of less than 0.01 so this threshold was applied to define up-regulated (KO/WT > 2.5) and down-regulated (KO/WT < 1/2.5) genes (Fig. 1c, Table 2).
Fig. 1.
HTself2 analysis of microarray data. a. Self-self empirical probability density distribution. Virtual log ratios (M) where derived from all possible pairwise combinations among 6 independent wild type experiments analyzing gene expression in the ventral prostate at age 3 month. Fold-changes greater than 2-fold (M > 1 or M < −1) have a very small probability of happening (<0.0001) due by chance alone considering the intrinsic inter-animal genetic noise. b. Interchange between statistical significance and fold-chance cutoffs. Statistical significance thresholds (p-value) can be mapped directly to equivalent fold-change cutoffs to define differentially expressed genes in the knockout vs wild type experiment. The horizontal red line represents a traditional 0.01 significance cutoff after multiple testing correction by the stringent Bonferroni method. A 2.5-fold change cutoff criteria would be equivalent to a corrected p-value smaller than 0.01. c. MA-plot showing the knockout (KO) vs wild type (WT) experiment. Virtual log ratios (M) remain stable around zero (KO/WT = 1) along all average log intensity (A) scale. Horizontal red lines represent fold-change cutoffs (KO/WT > 2.5-fold or KO/WT < 1/2.5) presenting multiple testing adjusted p-values < 0.01 and, therefore, define the differentially expressed genes
Table 2.
Differentially expressed genes in wild-type and U19/EAF2 knockout ventral prostate
| ID | WT intensity | KO intensity | log2(KO/WT) | Fold-change KO vs WT | Diff expr | Gene name |
|---|---|---|---|---|---|---|
| 1416497_at | 1364.2 | 3584.2 | 1.39 | 2.6 | KO > WT | protein disulfide isomerase associated 4 |
| 1417225_at | 913.2 | 2773.2 | 1.6 | 3 | KO > WT | ADP-ribosylation factor-like 6 interacting protein 5 |
| 1417594_at | 333.9 | 967.7 | 1.54 | 2.9 | KO > WT | G kinase anchoring protein 1 |
| 1417867_at | 170.9 | 629.2 | 1.88 | 3.7 | KO > WT | complement factor D (adipsin) |
| 1418666_at | 33.9 | 99.6 | 1.55 | 2.9 | KO > WT | pentraxin related gene |
| 1419554_at | 372.1 | 939.8 | 1.34 | 2.5 | KO > WT | CD47 antigen (Rh-related antigen, integrin-associated signal transducer) |
| 1419946_s_at | 408.9 | 1083.9 | 1.41 | 2.7 | KO > WT | predicted gene 5865; RAB2A, member RAS oncogene family |
| 1420037_at | 780.4 | 2270.4 | 1.54 | 2.9 | KO > WT | ATP synthase, H+ transporting, mitochondrial F1 complex, alpha subunit, isoform 1 |
| 1420125_at | 30.3 | 172 | 2.51 | 5.7 | KO > WT | T-cell leukemia translocation altered gene |
| 1421163_a_at | 371.3 | 2100.2 | 2.5 | 5.7 | KO > WT | nuclear factor I/A |
| 1421636_at | 37.8 | 120.8 | 1.68 | 3.2 | KO > WT | TBC1 domain family, member 8B |
| 1422142_at | 141.5 | 365.9 | 1.37 | 2.6 | KO > WT | nephrosis 1 homolog, nephrin (human) |
| 1422651_at | 13.8 | 255.5 | 4.21 | 19 | KO > WT | adiponectin, C1Q and collagen domain containing |
| 1423420_at | 25 | 64.8 | 1.37 | 2.6 | KO > WT | adrenergic receptor, beta 1 |
| 1424101_at | 503.7 | 1278.3 | 1.34 | 2.5 | KO > WT | heterogeneous nuclear ribonucleoprotein L |
| 1425032_at | 653.3 | 1851.5 | 1.5 | 2.8 | KO > WT | androgen binding protein beta |
| 1428944_at | 421.4 | 1505.4 | 1.84 | 3.6 | KO > WT | ubiquitin-like modifier activating enzyme 6 |
| 1431638_at | 53.7 | 145.4 | 1.44 | 2.7 | KO > WT | RIKEN cDNA 4930592A05 gene |
| 1432372_a_at | 177.7 | 523.4 | 1.56 | 2.9 | KO > WT | sepiapterin reductase |
| 1433078_at | 9.7 | 81.7 | 3.07 | 8.4 | KO > WT | RIKEN cDNA 5830435N06 gene |
| 1434987_at | 175.1 | 819 | 2.23 | 4.7 | KO > WT | aldehyde dehydrogenase 2, mitochondrial |
| 1434988_x_at | 204.5 | 647.5 | 1.66 | 3.2 | KO > WT | aldehyde dehydrogenase 2, mitochondrial |
| 1435679_at | 265.9 | 673.9 | 1.34 | 2.5 | KO > WT | optineurin |
| 1438120_x_at | 728.3 | 1907.3 | 1.39 | 2.6 | KO > WT | interleukin-1 receptor-associated kinase 1 |
| 1438219_at | 87.9 | 325.6 | 1.89 | 3.7 | KO > WT | similar to hCG45299; purine rich element binding protein A |
| 1438309_at | 209.5 | 613 | 1.55 | 2.9 | KO > WT | activin A receptor, type IC |
| 1438562_a_at | 1250 | 3547.9 | 1.51 | 2.8 | KO > WT | protein tyrosine phosphatase, non-receptor type 2 |
| 1439224_at | 187.2 | 485.4 | 1.37 | 2.6 | KO > WT | ankyrin repeat and IBR domain containing 1 |
| 1439260_a_at | 494.3 | 1256.2 | 1.35 | 2.5 | KO > WT | ectonucleotide pyrophosphatase/phosphodiesterase 3 |
| 1439263_at | 63.6 | 163.1 | 1.36 | 2.6 | KO > WT | hypothetical LOC14210 |
| 1441830_x_at | 10.9 | 69.3 | 2.67 | 6.4 | KO > WT | A kinase (PRKA) anchor protein 10 |
| 1446291_at | 25.9 | 70.1 | 1.44 | 2.7 | KO > WT | RIKEN cDNA 9330175H22 gene |
| 1447996_at | 548.2 | 1532.2 | 1.48 | 2.8 | KO > WT | expressed sequence AI848149 |
| 1448068_at | 120.3 | 331.6 | 1.46 | 2.8 | KO > WT | sterol O-acyltransferase 1 |
| 1448503_at | 9654.6 | 24560 | 1.35 | 2.5 | KO > WT | similar to myeloid cell leukemia sequence 1; myeloid cell leukemia sequence 1 |
| 1448761_a_at | 438.7 | 1188.7 | 1.44 | 2.7 | KO > WT | coatomer protein complex, subunit gamma 2 |
| 1449114_at | 77.5 | 194.4 | 1.33 | 2.5 | KO > WT | serine/threonine kinase 3 (Ste20, yeast homolog) |
| 1449434_at | 233.9 | 1278.2 | 2.45 | 5.5 | KO > WT | carbonic anhydrase 3 |
| 1449681_at | 170.2 | 483 | 1.5 | 2.8 | KO > WT | hepatoma-derived growth factor |
| 1450232_at | 26.9 | 82.6 | 1.62 | 3.1 | KO > WT | X-linked inhibitor of apoptosis |
| 1451285_at | 905.5 | 2620.4 | 1.53 | 2.9 | KO > WT | fusion, derived from t(12;16) malignant liposarcoma (human) |
| 1452124_at | 1915.1 | 5358.9 | 1.48 | 2.8 | KO > WT | ankyrin 3, epithelial |
| 1454278_at | 209.1 | 545.3 | 1.38 | 2.6 | KO > WT | RIKEN cDNA A430105D02 gene |
| 1454634_at | 64.1 | 194.1 | 1.6 | 3 | KO > WT | fucokinase |
| 1455381_at | 210.2 | 965.8 | 2.2 | 4.6 | KO > WT | hypothetical protein LOC100045622; RIKEN cDNA 4921513D23 gene |
| 1456505_at | 1089.1 | 3046.2 | 1.48 | 2.8 | KO > WT | Braf transforming gene |
| 1456843_at | 133 | 518.9 | 1.96 | 3.9 | KO > WT | Yamaguchi sarcoma viral (v-yes) oncogene homolog 1 |
| 1456961_at | 117.9 | 302.4 | 1.36 | 2.6 | KO > WT | enhancer of yellow 2 homolog (Drosophila); predicted gene 16373 |
| 1459882_at | 52.3 | 143.9 | 1.46 | 2.8 | KO > WT | ASF1 anti-silencing function 1 homolog A (S. cerevisiae) |
| 1425191_at | 210.2 | 79.1 | −1.41 | 2.7 | WT > KO | occludin/ELL domain containing 1 |
| 1443220_at | 315.3 | 81.1 | −1.96 | 3.9 | WT > KO | reticulon 3 |
| 1442846_at | 572.3 | 142.7 | −2 | 4 | WT > KO | pre B-cell leukemia transcription factor 1; region containing RIKEN cDNA 2310056B04 gene; pre B-cell leukemia transcription factor 1 |
| 1459309_at | 45.4 | 17.3 | −1.39 | 2.6 | WT > KO | RUN and FYVE domain containing 3 |
| 1455956_x_at | 189.3 | 72.6 | −1.38 | 2.6 | WT > KO | cyclin D2 |
| 1443077_at | 401.9 | 121.9 | −1.72 | 3.3 | WT > KO | hypothetical protein LOC100043982; RIKEN cDNA 1700081L11 gene |
| 1432134_at | 56 | 1.3 | −5.43 | 43 | WT > KO | methyltransferase like 4, pseudogene 1 |
| 1446196_at | 34.7 | 9.4 | −1.88 | 3.7 | WT > KO | predicted gene 7996; high mobility group AT-hook 2 |
| 1434497_at | 166.2 | 59.1 | −1.49 | 2.8 | WT > KO | RIKEN cDNA 4933431E20 gene |
| 1446149_at | 260.6 | 73.2 | −1.83 | 3.6 | WT > KO | protein phosphatase 3, catalytic subunit, beta isoform |
| 1435061_at | 705.3 | 234.1 | −1.59 | 3 | WT > KO | nudix (nucleoside diphosphate linked moiety X)-type motif 11; nudix (nucleoside diphosphate linked moiety X)-type motif 10 |
| 1427217_at | 69 | 25.9 | −1.41 | 2.7 | WT > KO | zinc finger protein 455 |
| 1435415_x_at | 117.5 | 36.3 | −1.69 | 3.2 | WT > KO | MARCKS-like 1; predicted gene 9106 |
| 1441724_at | 283.7 | 113.3 | −1.32 | 2.5 | WT > KO | stromal antigen 1 |
| 1459398_at | 231.8 | 88.3 | −1.39 | 2.6 | WT > KO | pellino 1 |
| 1433288_at | 76.7 | 18.7 | −2.04 | 4.1 | WT > KO | RIKEN cDNA 1520401O13 gene |
| 1435831_at | 1969 | 408.8 | −2.27 | 4.8 | WT > KO | uroplakin 1B |
| 1455464_x_at | 1735.9 | 415.6 | −2.06 | 4.2 | WT > KO | uroplakin 1B |
| 1428492_at | 155.5 | 46.1 | −1.75 | 3.4 | WT > KO | GLI pathogenesis-related 2 |
| 1433944_at | 111.9 | 40.4 | −1.47 | 2.8 | WT > KO | HECT domain containing 2 |
| 1457348_at | 1313.1 | 421.5 | −1.64 | 3.1 | WT > KO | ADP-ribosylation factor 4 |
| 1440314_at | 474.4 | 187.7 | −1.34 | 2.5 | WT > KO | thyroid hormone receptor interactor 12 |
| 1444125_at | 434.2 | 61.7 | −2.82 | 7 | WT > KO | BEN domain containing 6 |
| 1455164_at | 560 | 100.1 | −2.48 | 5.6 | WT > KO | CDC42 GTPase-activating protein |
| 1450849_at | 7353.3 | 2719.9 | −1.43 | 2.7 | WT > KO | heterogeneous nuclear ribonucleoprotein U |
| 1456834_at | 102.5 | 37.2 | −1.46 | 2.8 | WT > KO | ring finger protein 144B |
| 1450457_at | 282.9 | 78.7 | −1.85 | 3.6 | WT > KO | similar to Casitas B-lineage lymphoma; Casitas B-lineage lymphoma |
| 1444951_at | 552.5 | 192.7 | −1.52 | 2.9 | WT > KO | DENN/MADD domain containing 1B |
| 1456078_x_at | 267 | 84.4 | −1.66 | 3.2 | WT > KO | tubulin, beta 2c, psuedogene 1; tubulin, beta 2C; tubulin, beta 2c, pseudogene 2 |
| 1458666_at | 200 | 45.5 | −2.14 | 4.4 | WT > KO | Rho GTPase activating protein 1; predicted gene 8514 |
| 1417055_at | 4404.3 | 1187.6 | −1.89 | 3.7 | WT > KO | RIKEN cDNA 0610009D07 gene |
| 1455986_at | 73 | 19.4 | −1.91 | 3.8 | WT > KO | zinc finger, DHHC domain containing 17 |
| 1460066_at | 993.6 | 310.5 | −1.68 | 3.2 | WT > KO | expressed sequence C80889 |
| 1434730_at | 122 | 47.3 | −1.37 | 2.6 | WT > KO | expressed sequence AI854517 |
| 1452284_at | 155.3 | 54.9 | −1.5 | 2.8 | WT > KO | protein tyrosine phosphatase, receptor type Z, polypeptide 1 |
| 1460336_at | 635.5 | 136.9 | −2.21 | 4.6 | WT > KO | peroxisome proliferative activated receptor, gamma, coactivator 1 alpha |
| 1439902_at | 66.5 | 4 | −4.06 | 17 | WT > KO | complement component 5a receptor 1 |
| 1444692_at | 98.9 | 22.4 | −2.14 | 4.4 | WT > KO | EST AI316844 |
| 1457618_at | 337.2 | 109.6 | −1.62 | 3.1 | WT > KO | DEAD (Asp-Glu-Ala-Asp) box polypeptide 6 |
| 1452027_a_at | 129.4 | 46.1 | −1.49 | 2.8 | WT > KO | transformation related protein 63 |
| 1430464_at | 131.5 | 32.3 | −2.03 | 4.1 | WT > KO | RIKEN cDNA 9430021M05 gene |
| 1430472_at | 36.1 | 5.1 | −2.82 | 7.1 | WT > KO | armadillo repeat containing 1 |
| 1442918_at | 190.5 | 46.5 | −2.03 | 4.1 | WT > KO | neuron navigator 3 |
| 1424051_at | 471.4 | 178.8 | −1.4 | 2.6 | WT > KO | collagen, type IV, alpha 2 |
| 1460427_a_at | 261.3 | 103.7 | −1.33 | 2.5 | WT > KO | a disintegrin and metallopeptidase domain 28 |
| 1436968_x_at | 990.6 | 381.6 | −1.38 | 2.6 | WT > KO | kelch-like 24 (Drosophila) |
| 1457343_at | 315.3 | 102.7 | −1.62 | 3.1 | WT > KO | adaptor-related protein complex AP-4, sigma 1 |
| 1447402_at | 168.9 | 58.1 | −1.54 | 2.9 | WT > KO | ataxin 1 |
| 1445066_at | 121.8 | 29.8 | −2.03 | 4.1 | WT > KO | tetratricopeptide repeat domain 7B |
| 1441579_at | 36 | 6.1 | −2.56 | 5.9 | WT > KO | doublesex and mab-3 related transcription factor like family A1 |
| 1435529_at | 217.8 | 75.3 | −1.53 | 2.9 | WT > KO | predicted gene 14446 |
| 1437311_at | 1432.7 | 504.2 | −1.51 | 2.8 | WT > KO | small nucleolar RNA host gene 11 (non-protein coding) |
| 1449318_at | 181.9 | 50.7 | −1.84 | 3.6 | WT > KO | similar to Tubulin, gamma 2; tubulin, gamma 2 |
| 1436981_a_at | 9813.1 | 2978.1 | −1.72 | 3.3 | WT > KO | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide; predicted gene 4202 |
| 1448359_a_at | 161.8 | 63 | −1.36 | 2.6 | WT > KO | HIG1 domain family, member 1A |
| 1458700_at | 170.8 | 60.9 | −1.49 | 2.8 | WT > KO | leucine rich repeat containing 8 family, member C |
| 1447905_x_at | 2642.3 | 1006.4 | −1.39 | 2.6 | WT > KO | nucleoporin 62 |
| 1442867_at | 335.6 | 97.1 | −1.79 | 3.5 | WT > KO | multiple EGF-like-domains 11 |
| 1421868_a_at | 146.1 | 56.3 | −1.38 | 2.6 | WT > KO | pancreatic lipase |
| 1443441_x_at | 110.9 | 42.9 | −1.37 | 2.6 | WT > KO | predicted gene 5141 |
| 1439847_s_at | 149.5 | 41.9 | −1.84 | 3.6 | WT > KO | Kruppel-like factor 12 |
| 1441764_at | 144.8 | 57 | −1.35 | 2.5 | WT > KO | PR domain containing 10 |
| 1459104_at | 175.5 | 69.6 | −1.33 | 2.5 | WT > KO | budding uninhibited by benzimidazoles 3 homolog (S. cerevisiae) |
| 1446736_at | 184.9 | 62.2 | −1.57 | 3 | WT > KO | MyoD family inhibitor domain containing |
| 1440716_at | 106.7 | 28.3 | −1.91 | 3.8 | WT > KO | RIKEN cDNA 6430604M11 gene |
| 1453593_at | 183.5 | 70.2 | −1.39 | 2.6 | WT > KO | vestigial like 3 (Drosophila) |
| 1446421_at | 151.7 | 57.6 | −1.4 | 2.6 | WT > KO | schwannomin interacting protein 1 |
| 1433183_at | 24.9 | 1.5 | −4.05 | 17 | WT > KO | RIKEN cDNA 6720477C19 gene |
| 1426095_a_at | 270.4 | 103 | −1.39 | 2.6 | WT > KO | tumor necrosis factor receptor superfamily, member 22 |
| 1431079_at | 172.2 | 64.3 | −1.42 | 2.7 | WT > KO | C1q and tumor necrosis factor related protein 2 |
| 1424507_at | 77 | 21.1 | −1.87 | 3.6 | WT > KO | Ras and Rab interactor 1 |
| 1421603_a_at | 395.1 | 106.3 | −1.89 | 3.7 | WT > KO | carcinoembryonic antigen-related cell adhesion molecule 1; carcinoembryonic antigen-related cell adhesion molecule 2 |
| 1451870_a_at | 117.7 | 40.1 | −1.55 | 2.9 | WT > KO | bromodomain containing 4 |
| 1431306_at | 97.5 | 13.4 | −2.86 | 7.3 | WT > KO | 5′-nucleotidase, cytosolic III |
| 1436713_s_at | 78.8 | 26.6 | −1.57 | 3 | WT > KO | maternally expressed 3 |
| 1441701_at | 326.4 | 107.2 | −1.61 | 3 | WT > KO | zinc finger protein 148 |
| 1443447_at | 133.9 | 52.5 | −1.35 | 2.6 | WT > KO | 5-azacytidine induced gene 2 |
| 1453371_at | 438.4 | 174.5 | −1.33 | 2.5 | WT > KO | regulation of nuclear pre-mRNA domain containing 2 |
| 1429882_at | 1621.2 | 632.3 | −1.36 | 2.6 | WT > KO | RIKEN cDNA 6820431F20 gene |
| 1445530_at | 46.1 | 2.9 | −3.99 | 16 | WT > KO | transmembrane protein 194B |
| 1444279_at | 668.1 | 229.4 | −1.54 | 2.9 | WT > KO | HECT, UBA and WWE domain containing 1 |
| 1458832_at | 176 | 68.7 | −1.36 | 2.6 | WT > KO | growth hormone receptor |
| 1452517_at | 1086.2 | 369.7 | −1.55 | 2.9 | WT > KO | pleckstrin homology domain containing, family H (with MyTH4 domain) member 1 |
| 1425847_a_at | 148.2 | 51.1 | −1.54 | 2.9 | WT > KO | RIKEN cDNA B230120H23 gene |
| 1438662_at | 93.1 | 20.9 | −2.16 | 4.5 | WT > KO | adherens junction associated protein 1 |
| 1425934_a_at | 671.3 | 128.8 | −2.38 | 5.2 | WT > KO | UDP-Gal:betaGlcNAc beta 1,4-galactosyltransferase, polypeptide 4 |
| 1422140_at | 163 | 46.2 | −1.82 | 3.5 | WT > KO | similar to putative G-protein coupled receptor; predicted gene 2635; component of Sp100-rs; predicted gene 2666; predicted gene 7582; predicted gene 7592; predicted gene 7609 |
| 1443239_at | 138.6 | 47 | −1.56 | 2.9 | WT > KO | microtubule-associated protein 2 |
| 1421916_at | 381 | 144 | −1.4 | 2.6 | WT > KO | platelet derived growth factor receptor, alpha polypeptide |
| 1419971_s_at | 4010.9 | 1451.2 | −1.47 | 2.8 | WT > KO | solute carrier family 35, member A5 |
| 1419972_at | 434.2 | 98 | −2.15 | 4.4 | WT > KO | solute carrier family 35, member A5 |
| 1448346_at | 3281.1 | 1193.2 | −1.46 | 2.7 | WT > KO | cofilin 1, non-muscle; similar to Cofilin-1 (Cofilin, non-muscle isoform); predicted gene 6180 |
| 1419021_at | 55.1 | 4.5 | −3.61 | 12 | WT > KO | mcf.2 transforming sequence |
| 1434958_at | 95.8 | 35.5 | −1.43 | 2.7 | WT > KO | sacsin |
| 1433382_at | 217.5 | 77.5 | −1.49 | 2.8 | WT > KO | energy homeostasis associated |
| 1446512_at | 214 | 85.2 | −1.33 | 2.5 | WT > KO | predicted gene 5909; zinc finger CCCH-type containing 15 |
| 1432298_at | 196 | 68.6 | −1.51 | 2.9 | WT > KO | RIKEN cDNA 4921508M14 gene |
| 1446095_at | 217.3 | 53.9 | −2.01 | 4 | WT > KO | antisense Igf2r RNA |
| 1445453_at | 270.3 | 91 | −1.57 | 3 | WT > KO | helicase with zinc finger domain |
| 1452494_s_at | 75.6 | 29.5 | −1.36 | 2.6 | WT > KO | solute carrier organic anion transporter family, member 1b2 |
| 1442833_at | 837.4 | 144.8 | −2.53 | 5.8 | WT > KO | DNA segment, Chr 15, ERATO Doi 30, expressed |
| 1447049_at | 112.7 | 34.8 | −1.7 | 3.2 | WT > KO | IQ motif and WD repeats 1 |
| 1441283_at | 325.7 | 112 | −1.54 | 2.9 | WT > KO | cyclin Y; similar to cyclin fold protein 1 |
| 1434067_at | 267.8 | 78.7 | −1.77 | 3.4 | WT > KO | expressed sequence AI662270 |
| 1434068_s_at | 290.4 | 49.6 | −2.55 | 5.9 | WT > KO | expressed sequence AI662270 |
| 1422313_a_at | 540.1 | 213.1 | −1.34 | 2.5 | WT > KO | insulin-like growth factor binding protein 5 |
| 1449405_at | 2035.8 | 731.6 | −1.48 | 2.8 | WT > KO | tensin 1 |
| 1425555_at | 164.7 | 45 | −1.87 | 3.7 | WT > KO | CDC2-related kinase, arginine/serine-rich |
| 1420073_s_at | 176.4 | 64.8 | −1.44 | 2.7 | WT > KO | gb:C78041 EST |
| 1443347_at | 99.8 | 37 | −1.43 | 2.7 | WT > KO | gb:BG063775 EST |
| 1444498_at | 147.3 | 52.9 | −1.48 | 2.8 | WT > KO | gb:BM116117 EST |
| 1444591_at | 316.8 | 71.1 | −2.16 | 4.5 | WT > KO | gb:AI429418 EST |
| 1445902_at | 139.1 | 40.7 | −1.77 | 3.4 | WT > KO | gb:BG076325 EST |
| 1457122_at | 342.7 | 122.1 | −1.49 | 2.8 | WT > KO | gb:BB209527 EST |
| 1458257_at | 109.4 | 40.7 | −1.43 | 2.7 | WT > KO | gb:BM196689 EST |
Several genes identified as down-regulated in the U19/EAF2 murine prostate have been reported to play roles in the extracellular matrix of the tumor microenvironment, notably a 2.5-fold decrease in a disintegrin and metallopeptidase domain 28 (ADAM28), a 7.3-fold decrease in bromodomain containg 4 (BRD4), a 2.7-fold decrease in occludin (OCLN) and a 2.6-fold decrease in collagen IV (COL4A2). ADAM28 can bind to the integrin α4β1, suggesting a potential adhesive and proteolytic role inflammatory and immune processes [35]. The bromodomain containing 4 (BRD4) gene has been shown to regulate the expression extra-cellular matrix-related genes and inhibit metastasis in breast cancer [36]. Occludin is a tight junction protein that has been associated with loss of polarity in prostate cancer cells [37]. Collagen IV is the major component of the basement membrane and the C-terminal portion of the protein, known as canstatin, is an inhibitor of angiogenesis and tumor growth [38]. Disrupted EAF2 regulation of these genes could promote changes in the extracellular matrix thereby contributing to prostate tumor development and progression.
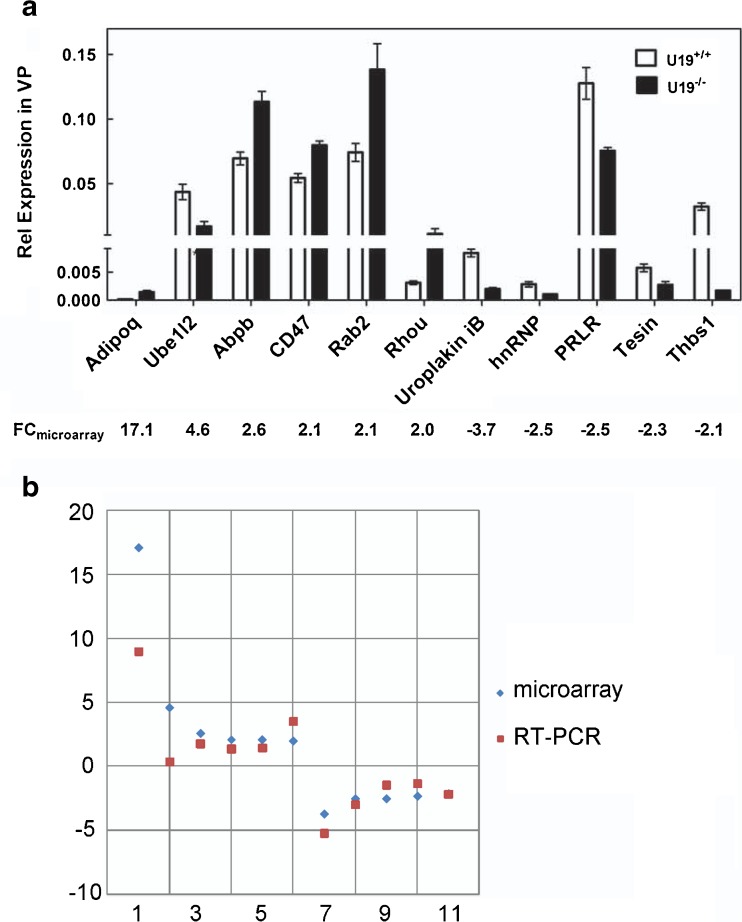
qPCR in a different set of WT and KO mice (n = 3) at 3 months of age was used to validate that genes identified by cDNA microarray were indeed up- or down-regulated by U19/EAF2 knockout in the mouse prostate. The following genes: Adipoq (* p = 0.006), Ube1l2 (* p = 0.01), Abpb (* p = 0.01), CD47 (* p = 0.006), Rab2 (* p = 0.04), Rhou (* p = 0.05), Uroplakin iB (* p = 0.01), hnRNP (* p = 0.02), PRLR (* p = 0.05), Tesin (* p = 0.03), and Thbs1 (* p = 0.0004) were chosen randomly or because they encode products that are important in tumorigenesis for qPCR analysis, and p values were determined by comparing the U19/EAF2 knockout with wild-type (Fig. 2a). Fold changes from the microarray platforms were plotted versus the corresponding fold changes from qPCR (Fig. 2b), and Pearson correlation coefficient between microarray and qPCR results was 0.92, indicating high concordance among these independent biological replicates.
Fig. 2.
Concordance of microarray and qPCR gene expression analyses. a Bar chart of qPCR data results and corresponding array signals. Data were normalized to GAPDH. b Log-fold changes from the microarray platforms are plotted versus the corresponding fold changes from qPCR. R = 0.92. Genes 1–11 represent Adipoq, Ube1l2, Abpb, CD47, Rab2, Rhou, Uroplakin iB, hnRNP, Tesin, Thbs1, and PRLR, respectively
Gene Ontology and KEGG Pathway Analysis of U19/EAF2 Downstream Genes
The known functional classification of differentially expressed genes was analyzed for significant enrichment with respect to various functional categories using the DAVID annotation tool [32] (http://david.abcc.ncifcrf.gov/), which examines all the functions represented by each gene in a list of genes and identifies groups that are functionally related. The over-represented ontology-based classification groups form the basis for identifying functional processes represented in the change of state induced by U19/EAF2 loss. The major biological themes among the up-regulated and down-regulated genes are shown in (Additional File 1). The enrichment of functional categories phosphoprotein, acetylation, phosphate metabolic process, phosphorus metabolic process and phosphorylation was prominent in both up-regulated and down-regulated genes in U19/EAF2 ko vs WT. Notable differences found included enrichment of nucleotide binding, cytoplasm, ATP-binding, death, and apoptosis in up-regulated genes; and alternative splicing, splice variant, nucleus, positive regulation of transcription and gene expression and intracellular signaling cascade in down-regulated genes. One notable gene in the phosphoprotein category was the down-regulated gene Ras and Rab interactor 1 (RIN1). RIN1 is a known RAS effector that has been shown to mediate actin remodeling associated with adhesion and migration of epithelial cells [39]. In this study, knockdown of RIN1 in MCF10A cells enhanced cell migration significantly. Reduced expression of RIN1 in the EAF2 knockout model may also contribute to the disruption of the RAS signaling pathway.
Up-Regulation of RAS-BRAF Signaling and Phosphorylated ERK in Response to U19/EAF2 Loss
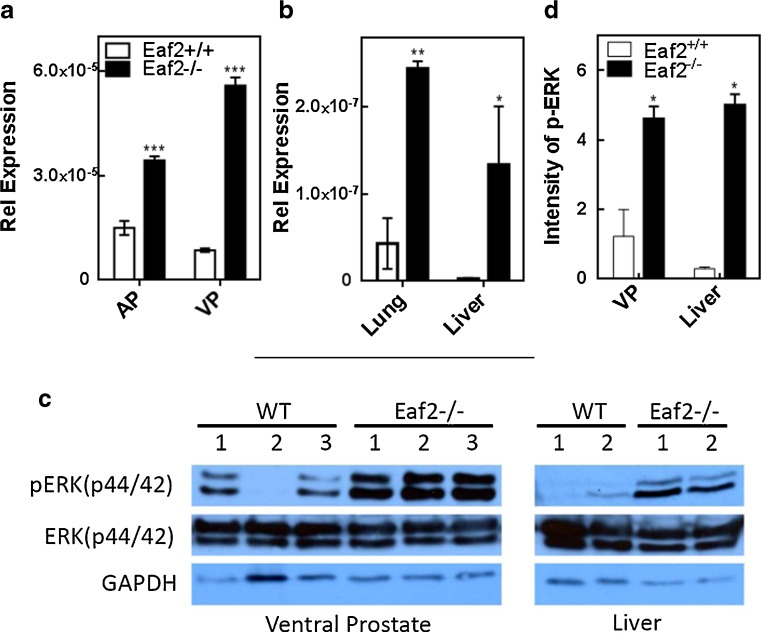
Since U19/EAF2 is a tumor suppressor, we were particularly interested in identifying its downstream genes relevant to carcinogenesis. KEGG pathways identified using DAVID in differentially expressed genes are listed in Table 3. BRAF was listed in three of the identified pathways: MAPK signaling pathway, focal adhesion and pathways in cancer. BRAF (1456505_at) encodes a protein belonging to the raf/mil family of serine/threonine protein kinases and plays a role in regulating the MAPK/ERKs signaling pathway, which affect cell division, differentiation, and secretion. Since BRAF is a key regulator in the RAS and p-ERK signaling pathway and elevated ERK pathway is associated with prostate cancer progression [7, 17–19], we verified BRAF mRNA up-regulation by U19/EAF2 knockout in 3-month old mouse prostate by qPCR (Fig. 3a, *** p < 0.001). We also tested the effect of U19/EAF2 knockout on BRAF expression in the liver and lung because these two organs developed malignant tumors in U19/EAF2 knockout mice [26]. As expected, BRAF mRNA levels in U19/EAF2 knockout lung (** p < 0.01) and liver (* p < 0.05) (Fig. 3b) were significantly increased compared to wild-type. Elevated p-ERK signal is associated with increased RAS-BRAF cascade signaling. Activation of pathway intermediates such as RAS and BRAF as RAS and BRAF lead to activation of ERK signaling [40–44]. Western Blot analysis showed that total ERK protein levels were not changed in the knockout organs but phosphorylated ERK levels were increased in the ventral prostates and livers of the knockout mice (Fig. 3c), validating the elevated p-ERK level in the knockout prostate and liver (Fig. 3d). Figure 4 summarizes the effects of U19/EAF2 loss on the p-ERK signaling pathway identified by microarray and verified by qPCR and western blot analyses.
Table 3.
KEGG pathways identified by DAVID analysis of differentially expressed genes in the U19/EAF2 knockout ventral prostate
| Rank | KEGG pathway name | Genes | % | P-value |
|---|---|---|---|---|
| 1 | Ubiquitin mediated proteolysis | HUWE1, XIAP, CBL, TRIP12, UBA6 | 3.3 | 0.02 |
| 2 | MAPK signaling pathway | BRAF, ACVRIC, PDGFRA, PPP3CB, STK3, ZAK | 3.9 | 0.04 |
| 3 | Focal adhesion | BRAF, XIAB, COL4A2, CCND2, PDGFRA | 3.3 | 0.06 |
| 4 | Cell cycle | BUB3, CCND2, STAG1, GM4202 | 2.6 | 0.07 |
| 5 | Pathways in cancer | BRAF, XIAP, ALVRIC, COL4A2, PDGFRA, CBL | 3.9 | 0.09 |
Fig. 3.
Effect of U19/EAF2 knockout on the expression of BRAF mRNA and p-ERK in the mouse model. a. qPCR analysis of the BRAF mRNA levels in anterior prostate (AP) and ventral prostate (VP) and b. lung and liver of U19/EAF2 knockout and wild-type control mice.. Data were normalized to GAPDH, (n = 3). c. Western blot analysis of phosphorylated ERK, pERK(p44/42), in U19/EAF2 knockout and wild-type mouse tissues. Images shown are representative blots for ERK, pERK and GAPDH from ventral prostates and liver lysates of individual U19/EAF2 knockout and wild-type control mice. d. Intensity of p-ERK in /EAF2 knockout and wild-type mouse tissues. Band densities in the Western blots were analyzed with IMAGEJ (National Institutes of Health) and normalized against GAPDH. Data represent the mean ± SEM; * p < 0.05. ** p < 0.01, *** p < 0.001 versus WT
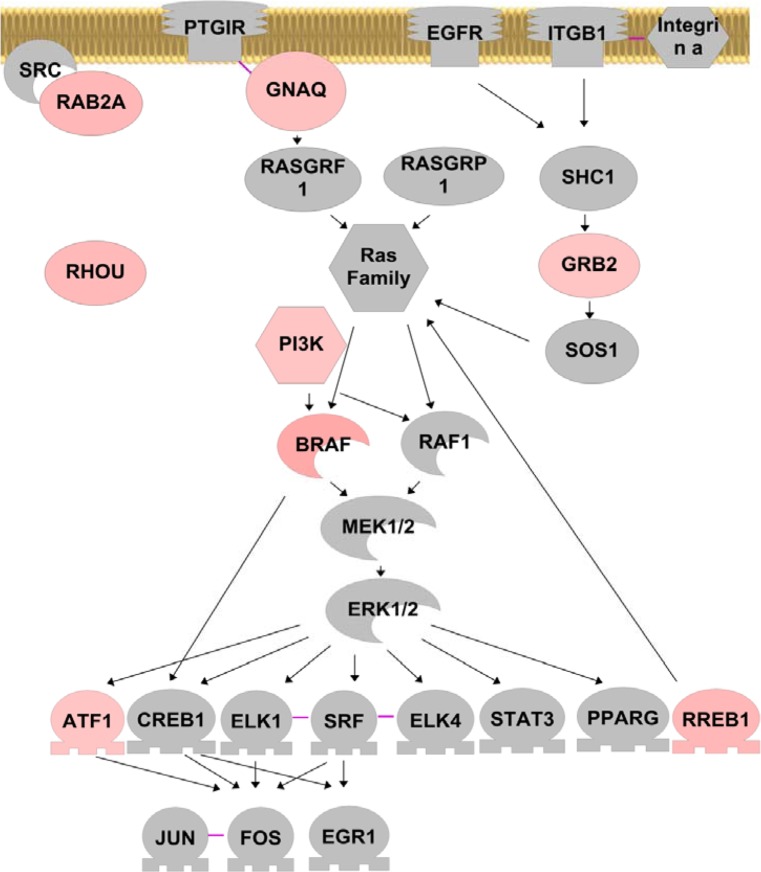
Fig. 4.
Effect of U19/EAF2 knockout on ERK pathway in the murine prostate. Genes up-regulated upon U19/EAF2 knockout are colored in peach. Color intensity is used to indicate the degree of up-regulation, with light peach color for less up-regulation and darker peach color for more up-regulation. Solid arrows indicate positive regulation; solid purple lines indicate protein binding
Discussion
Androgens play an important role in maintaining prostate homeostasis [45, 46]. Disruption of prostatic homeostasis may lead to abnormal prostate growth and/or carcinogenesis. However, the mechanisms of androgen-regulated prostatic homeostasis are poorly understood. U19/EAF2 represents one important androgen-responsive gene involved in prostate homeostasis, because U19/EAF2 knockout prostate exhibited elevated cell proliferation [26]. U19/EAF2 also plays an important role in the regulation of TSP1 [47]. TSP-1 expression is inhibited in a large number of tumors, including breast and androgen-dependent prostate carcinomas in which there is an inverse correlation between TSP1 expression and microvessel density [48–50]. In addition to the increased incidence in mPIN lesions, the EAF2 knockout mouse prostate is also characterized by increased microvessel density and stromal inflammation [51]. These studies suggest that EAF2 loss impacts not only epithelial cell proliferation but also the surrounding stroma and vasculature. Identification of pathways regulated by U19/EAF2 using cDNA microarray provides mechanistic insights into U19/EAF2 action as well as androgen action in the prostate microenvironment.
Bioinformatics analysis using DAVID software revealed multiple pathways associated with and potential disease relevance of genes that are differentially regulated in U19/EAF2 knockout prostate (Additional File 1, Table 3). Many of the U19/EAF2 downstream genes are involved in mRNA transcription and mRNA transcription regulation, suggesting that U19/EAF2 can regulate mRNA transcription directly as a transcription factor and/or a transcription elongation factor as well as indirectly through up- or down-regulating genes capable of modulating transcription. U19/EAF2 modulation of mRNA transcription is supported by the identification of more than 100 differentially-regulated mRNA transcripts. Identification of genes involved in protein phosphorylation and modification as well as proteolysis suggests a role for U19/EAF2 in the regulation of various signaling pathways at the protein levels, which was substantiated by the elevated phosphorylation of ERK in the U19/EAF2 knockout prostate (Fig. 3). Interestingly, U19/EAF2 knockout also affects genes involved in the metabolism of nucleoside, nucleotide, and nucleic acid, suggesting a potential role for U19/EAF2 in the regulation of metabolism. The above observations provided evidence for U19/EAF2 regulation of biological processes at levels of mRNA, protein and metabolism.
According to KEGG pathway analysis, the biological processes affected by U19/EAF2 knockout are most relevant to cancer, reproductive system diseases, cell death, and cell cycle (Additional File 1, Table 3). This finding is consistent with the observation that U19/EAF2 knockout mouse exhibited tumor development in multiple organs, defects in reproductive systems including testes and the prostate, elevated cell death in testes, and elevated cell proliferation in the prostate [26, 52]. Toxicity analysis revealed the relevance of U19/EAF2 downstream genes with diseases in the liver and heart multiple times, which again agrees with the phenotypes observed in the U19/EAF2 knockout mice, particularly hepatocellular carcinoma, elevated angiogenesis in the liver and heart enlargement. The identification of U19/EAF2 downstream genes will provide a strong foundation to explore the molecular mechanisms underlining various phenotypes caused by U19/EAF2 knockout.
As a potential transcription factor/transcription elongation factor regulated by androgens, U19/EAF2 may mediate androgen-induced expression of some androgen-responsive genes. A search for androgen responsive genes [53] identified 2 androgen-responsive genes differentially expressed in U19/EAF2 knockout prostate. X-linked inhibitor of apoptosis (XIAP), which was identified by KEGG pathway analysis in ubiquitin mediated proteolysis, focal adhesion and pathways in cancer, was up-regulated 3.1-fold in U19/EAF2 knockout prostate. XIAP is down-regulated by androgens in normal prostate epithelial cells [54]. Serine/threonine kinase 3 (STK3), was up-regulated 2.5-fold in U19/EAF2 knockout prostate. STK3 is a MAPK signaling pathway gene that is up-regulated in response to androgens in human breast cancer cells [55]. These observations suggest a role for U19/EAF2 in mediating androgen regulation of gene expression in the prostate. Since U19/EAF2 is a putative DNA-binding transcription factor, its regulation of downstream genes may involve direct binding of U19/EAF2 to the promoter/enhance regions of these targeted genes. However, it is also possible that U19/EAF2 can enhance transcription elongation. Further studies will be required to determine the mechanism by which U19/EAF2 controls the expression of its downstream genes.
Identification of the RAS-BRAF-MEK-ERK cascade as a downstream target of U19/EAF2 in the prostate provides new insights into the mechanism associate with tumorigenesis in U19/EAF2 knockout mice (Fig. 4). Multiple genes, GRB2, PI3K, RHOU, RAB2, BRAF and RREB-1, were up-regulated in U19/EAF2 knockout prostate, suggesting coordinated regulation of this important pathway by U19/EAF2. Elevated phosphorylation of ERK in the U19/EAF2 knockout prostate confirmed the activation of RAS-BRAF-MEK-ERK signaling upon U19/EAF2 deletion. The activation of this pathway was not limited to the prostate, because ERK phosphorylation was also significantly enhanced in the liver of U19/EAF2 knockout mice (Fig. 3). Since abnormal activation of RAS-BRAF-MEK-ERK is often associated with and functionally important in tumorigenesis, activation of this pathway is likely a key step leading to tumorigenesis in U19/EAF2 knockout mice.
In summary, this study revealed multiple pathways regulated by U19/EAF2 in the prostate and identified RAS-BRAF-MEK-ERK pathway as a downstream target of U19/EAF2. Bioinformatic analysis of U19/EAF2 downstream genes showed the functional relevance of U19/EAF2 regulated pathways with cancer, liver diseases, cardiac abnormality, and defects in reproductive system, which was observed in U19/EAF2 knockout mice [26, 52]. These observations argue that these U19/EAF2 downstream pathways are likely mediating U19/EAF2 function in vivo, with the RAS-BRAF-MEK-ERK signaling as a potential key mediator of U19/EAF2 knockout induced tumorigenesis.
Electronic supplementary material
Functional categories enriched in differentially expressed genes in U19/EAF2 knockout ventral prostate. (XLS 51 kb)
Acknowledgement
This study was supported in part by National Institutes of Health Grants R37 DK51193, R01 CA 108675, and P50 CA90386 and the Tippins Foundation (LEP). This project used the UPCI Animal Facility and was supported in part by award P30CA047904. We also thank Junkui Ai and Liquan Cai for critical reading.
Disclosure Summary
The authors have nothing to disclose.
References
- 1.Kozlowski JM, Ellis WJ, Grayhack JT. Advanced prostatic carcinoma. Early versus late endocrine therapy. Urol Clin N Am. 1991;18(1):15–24. [PubMed] [Google Scholar]
- 2.Montie J, Pienta K. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. [Review] Urology. 1994;43(6):892–899. doi: 10.1016/0090-4295(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 3.O’Leary MP, Roehrborn CG, Black L (2007) Dutasteride significantly improves quality of life measures in patients with enlarged prostate. Prostate Cancer Prostatic Dis [DOI] [PubMed]
- 4.Griffiths K, Eaton C, Harper M, Peeling B, Davies P. Steroid hormones and the pathogenesis of benign prostatic hyperplasia. [Review] Eur Urol. 1991;20(Suppl 1):68–77. doi: 10.1159/000471750. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Z, Wong C, Sar M, Wilson E. The androgen receptor: an overview. [Review] Recent Prog Horm Res. 1994;49:249–274. doi: 10.1016/b978-0-12-571149-4.50017-9. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Tufts R, Haleem R, Cai X. Genes regulated by androgen in the rat ventral prostate. Proc Natl Acad Sci USA. 1997;94:12999–13004. doi: 10.1073/pnas.94.24.12999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu ML, Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr Relat Cancer. 2008;15(4):841–849. doi: 10.1677/ERC-08-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culig Z. Androgen receptor cross-talk with cell signalling pathways. Growth Factors. 2004;22(3):179–184. doi: 10.1080/08977190412331279908. [DOI] [PubMed] [Google Scholar]
- 9.Maurer G, Tarkowski B, Baccarini M. Raf kinases in cancer-roles and therapeutic opportunities. Oncogene. 2011;30(32):3477–3488. doi: 10.1038/onc.2011.160. [DOI] [PubMed] [Google Scholar]
- 10.Marais R, Light Y, Paterson HF, Mason CS, Marshall CJ. Differential regulation of Raf-1, A-Raf, and B-Raf by oncogenic ras and tyrosine kinases. J Biol Chem. 1997;272(7):4378–4383. doi: 10.1074/jbc.272.7.4378. [DOI] [PubMed] [Google Scholar]
- 11.Niihori T, Aoki Y, Narumi Y, Neri G, Cave H, Verloes A, Okamoto N, Hennekam RC, Gillessen-Kaesbach G, Wieczorek D, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38(3):294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 12.Tabernero J, Dienstmann R. BRAF as a target for cancer therapy. Anti-Cancer Agent Me. 2011;11(3):285–295. doi: 10.2174/187152011795347469. [DOI] [PubMed] [Google Scholar]
- 13.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 14.Halilovic E, Solit DB. Therapeutic strategies for inhibiting oncogenic BRAF signaling. Curr Opin Pharmacol. 2008;8(4):419–426. doi: 10.1016/j.coph.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Pratilas CA, Xing F, Solit DB (2011) Targeting oncogenic BRAF in human cancer. Curr Top Microbiol Immunol [DOI] [PMC free article] [PubMed]
- 16.Ball DW, Jin N, Rosen DM, Dackiw A, Sidransky D, Xing M, Nelkin BD. Selective growth inhibition in BRAF mutant thyroid cancer by the mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244. J Clin Endocrinol Metab. 2007;92(12):4712–4718. doi: 10.1210/jc.2007-1184. [DOI] [PubMed] [Google Scholar]
- 17.Gioeli D, Mandell JW, Petroni GR, Frierson HF, Jr, Weber MJ. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res. 1999;59(2):279–284. [PubMed] [Google Scholar]
- 18.Gioeli D. Signal transduction in prostate cancer progression. Clin Sci (Lond) 2005;108(4):293–308. doi: 10.1042/CS20040329. [DOI] [PubMed] [Google Scholar]
- 19.Garraway LA, Thomas RK, Baker AC, DeBiasi RM, Winckler W, LaFramboise T, Lin WM, Wang M, Feng W, Zander T, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39(3):347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 20.Barford D, Wan PTC, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM, Jones CM, Marshall CJ, Springer CJ, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116(6):855–867. doi: 10.1016/S0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 21.Dahut WL, Scripture C, Posadas E, Jain L, Gulley JL, Arlen PM, Wright JJ, Yu Y, Cao L, Steinberg SM, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14(1):209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, Zhang Q, Jiang F, Pins M, Kozlowski JM, Wang Z. Suppression of prostate tumor growth by U19, a novel testosterone-regulated apoptosis inducer. Cancer Res. 2003;63(15):4698–4704. [PubMed] [Google Scholar]
- 23.Simone F, Luo RT, Polak PE, Kaberlein JJ, Thirman MJ. ELL-associated factor 2 (EAF2), a functional homolog of EAF1 with alternative ELL binding properties. Blood. 2003;101(6):2355–2362. doi: 10.1182/blood-2002-06-1664. [DOI] [PubMed] [Google Scholar]
- 24.Xiao W, Jiang F, Wang Z. ELL binding regulates U19/Eaf2 intracellular localization, stability, and transactivation. Prostate. 2006;66(1):1–12. doi: 10.1002/pros.20309. [DOI] [PubMed] [Google Scholar]
- 25.Shilatifard A, Duan DR, Haque D, Florence C, Schubach WH, Conaway JW, Conaway RC. ELL2, a new member of an ELL family of RNA polymerase II elongation factors. Proc Natl Acad Sci U S A. 1997;94(8):3639–3643. doi: 10.1073/pnas.94.8.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao W, Zhang Q, Habermacher G, Yang X, Zhang AY, Cai X, Hahn J, Liu J, Pins M, Doglio L, et al. U19/Eaf2 knockout causes lung adenocarcinoma, B-cell lymphoma, hepatocellular carcinoma and prostatic intraepithelial neoplasia. Oncogene. 2008;27(11):1536–1544. doi: 10.1038/sj.onc.1210786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortez DA, Tonon AP, Colepicolo P, Vencio RZ. Combining P values to improve classification of differential gene expression in the HTself software. Genetics and Molecular Research: GMR. 2011;10(4):3586–3595. doi: 10.4238/2011.December.5.5. [DOI] [PubMed] [Google Scholar]
- 28.Pascal LE, Vencio RZ, Page LS, Liebeskind ES, Shadle CP, Troisch P, Marzolf B, True LD, Hood LE, Liu AY. Gene expression relationship between prostate cancer cells of Gleason 3, 4 and normal epithelial cells as revealed by cell type-specific transcriptomes. BMC Cancer. 2009;9:452. doi: 10.1186/1471-2407-9-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pascal LE, Goo YA, Vencio RZ, Page LS, Chambers AA, Liebeskind ES, Takayama TK, True LD, Liu AY. Gene expression down-regulation in CD90+ prostate tumor-associated stromal cells involves potential organ-specific genes. BMC Cancer. 2009;9:317. doi: 10.1186/1471-2407-9-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pascal LE, Vencio RZ, Vessella RL, Ware CB, Vencio EF, Denyer G, Liu AY. Lineage relationship of prostate cancer cell types based on gene expression. BMC Medical Genomics. 2011;4:46. doi: 10.1186/1755-8794-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.da Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 32.Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003;4(5):P3. doi: 10.1186/gb-2003-4-5-p3. [DOI] [PubMed] [Google Scholar]
- 33.Ai J, Wang Y, Dar JA, Liu J, Liu L, Nelson JB, Wang Z. HDAC6 regulates androgen receptor hypersensitivity and nuclear localization via modulating Hsp90 acetylation in castration-resistant prostate cancer. Mol Endocrinol. 2009;23(12):1963–1972. doi: 10.1210/me.2009-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Q, Pavey ES, Carter RE. Bonferroni-based correction factor for multiple, correlated endpoints. Pharm Stat. 2012;11(4):300–309. doi: 10.1002/pst.1514. [DOI] [PubMed] [Google Scholar]
- 35.Bridges LC, Tani PH, Hanson KR, Roberts CM, Judkins MB, Bowditch RD. The lymphocyte metalloprotease MDC-L (ADAM 28) is a ligand for the integrin alpha4beta1. J Biol Chem. 2002;277(5):3784–3792. doi: 10.1074/jbc.M109538200. [DOI] [PubMed] [Google Scholar]
- 36.Crawford NP, Alsarraj J, Lukes L, Walker RC, Officewala JS, Yang HH, Lee MP, Ozato K, Hunter KW. Bromodomain 4 activation predicts breast cancer survival. Proc Natl Acad Sci U S A. 2008;105(17):6380–6385. doi: 10.1073/pnas.0710331105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Busch C, Hanssen TA, Wagener C. B OB: Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum Pathol. 2002;33(3):290–298. doi: 10.1053/hupa.2002.32218. [DOI] [PubMed] [Google Scholar]
- 38.van der Rest M, Garrone R. Collagen family of proteins. FASEB J: Off Publ Fed Am Soc Exp Biol. 1991;5(13):2814–2823. [PubMed] [Google Scholar]
- 39.Hu H, Bliss JM, Wang Y, Colicelli J. RIN1 is an ABL tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Current biology: CB. 2005;15(9):815–823. doi: 10.1016/j.cub.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 40.Lewis TS, Shapiro PS, Ahn NG. Signal transduction through MAP kinase cascades. Adv Cancer Res. 1998;74:49–139. doi: 10.1016/S0065-230X(08)60765-4. [DOI] [PubMed] [Google Scholar]
- 41.Cobb MH, Goldsmith EJ. How map kinases are regulated. J Biol Chem. 1995;270(25):14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 42.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5(5):341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 43.Allen LF, Sebolt-Leopold J, Meyer MB. Cl-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK) Semin Oncol. 2003;30(5):105–116. doi: 10.1053/j.seminoncol.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 44.Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, Rosen N. (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci U S A. 2009;106(11):4519–4524. doi: 10.1073/pnas.0900780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruchovsky N, Lesser B, Doorn EV, Craven S. Hormonal effects on cell proliferation in rat prostate. Vitam Horm. 1975;33:61–102. doi: 10.1016/S0083-6729(08)60951-6. [DOI] [PubMed] [Google Scholar]
- 46.Isaacs J, Furuya Y, Berges R. The role of androgen in the regulation of programmed cell death/apoptosis in normal and malignant prostatic tissue. [Review] Sem Cancer Biol. 1994;5(5):391–400. [PubMed] [Google Scholar]
- 47.Su F, Pascal LE, Xiao W, Wang Z. Tumor suppressor U19/EAF2 regulates thrombospondin-1 expression via p53. Oncogene. 2010;29(3):421–431. doi: 10.1038/onc.2009.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colombel M, Filleur S, Fournier P, Merle C, Guglielmi J, Courtin A, Degeorges A, Serre CM, Bouvier R, Clezardin P, et al. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res. 2005;65(1):300–308. [PubMed] [Google Scholar]
- 49.Fontana A, Filleur S, Guglielmi J, Frappart L, Bruno-Bossio G, Boissier S, Cabon F, Clezardin P. Human breast tumors override the antiangiogenic effect of stromal thrombospondin-1 in vivo. Int J Cancer J Int Du Cancer. 2005;116(5):686–691. doi: 10.1002/ijc.20584. [DOI] [PubMed] [Google Scholar]
- 50.Kwak C, Jin RJ, Lee C, Park MS, Lee SE. Thrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasia. BJU Int. 2002;89(3):303–309. doi: 10.1046/j.1464-4096.2001.01417.x. [DOI] [PubMed] [Google Scholar]
- 51.Pascal LE, Ai J, Rigatti LH, Lipton AK, Xiao W, Gnarra JR, Wang Z. EAF2 loss enhances angiogenic effects of Von Hippel-Lindau heterozygosity on the murine liver and prostate. Angiogenesis. 2011;14(3):331–343. doi: 10.1007/s10456-011-9217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiao W, Ai J, Habermacher G, Volpert O, Yang X, Zhang AY, Hahn J, Cai X, Wang Z. U19/Eaf2 binds to and stabilizes von hippel-lindau protein. Cancer Res. 2009;69(6):2599–2606. doi: 10.1158/0008-5472.CAN-08-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang M, Ma Y, Chen C, Fu X, Yang S, Li X, Yu G, Mao Y, Xie Y, Li Y. Androgen-responsive gene database: integrated knowledge on androgen-responsive genes. Mol Endocrinol. 2009;23(11):1927–1933. doi: 10.1210/me.2009-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long RM, Morrissey C, Walsh S, Hamilton HJ, Farrell N, O’Neill A, Fitzpatrick JM, Watson WR. Alterations in the expression of inhibitors of apoptosis during differentiation of prostate epithelial cells. BJU Int. 2007;100(2):445–449. doi: 10.1111/j.1464-410X.2007.06932.x. [DOI] [PubMed] [Google Scholar]
- 55.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, Gerald WL. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Functional categories enriched in differentially expressed genes in U19/EAF2 knockout ventral prostate. (XLS 51 kb)