Abstract
A series of overexpression studies have shown that lumican suppresses angiogenesis in tumors produced from pancreatic adenocarcinoma, fibrosarcoma, and melanoma tumor cells. Despite lumican’s anti-angiogenic activity, a clear correlation of differential expression of lumican in various cancers and cancer malignancy has failed to emerge. Therefore, we hypothesized that either 1.) endogenously expressed lumican is not anti-angiogenic or alternatively that 2.) lumican exhibits angiostatic activity only in limited microenvironments. Previously, lumican was shown to suppress tumor growth and angiogenesis in subcutaneously injected PanO2 pancreatic adenocarcinoma cells. Therefore, to determine if endogenously expressed lumican is anti-angiogenic we subcutaneously injected PanO2 cells into wild-type and lumican knockout mice and compared tumor growth and vascular densities of the resulting tumors. We found that tumors grown in lumican knockout animals were larger and contained significantly elevated vascular densities compared to those grown in wild-type mice. Interestingly however lumican knockout animals did not exhibit enhanced angiogenesis in aortic ring assays, matrigel plugs, or healing wound biopsies raising the possibility that lumican suppresses angiogenesis only in tumor microenvironments. To test this possibility, we sought a tumor model wherein lumican did not exhibit anti-angiogenic activity. Utilizing the 4T1 breast cancer model, we found that lumican suppressed 4T1 tumor growth and lung metastasis, but not angiogenesis. In conclusion, these results show that the angiostatic activity of lumican is dependent on currently undefined microenvironmental cues and therefore helps to understand why differential expression of lumican does not consistently correlate with human tumor malignancy.
Keywords: Lumican, Angiogenesis, Cancer, Microenvironment
Introduction
The cellular microenvironment provides critical input as cells attempt to achieve and maintain cellular homeostasis. In particular, extracellular matrix (ECM) proteins in the microenvironment govern a variety of processes such as proliferation, migration, apoptosis, and response to cellular signaling stimuli [27]. While ECM provides critical input to cells and tissues, cells also remodel their local microenvironment to reinforce cellular activities and programs. Because of this interplay between cells and ECM, altered cellular microenvironments can have profound impacts on various disease states. In particular, the development and growth of cancers involves pathological remodeling of ECM into a reactive tumor stroma that supports cellular programs of malignancy and drives the growth, neovascularization, invasion, and metastasis of tumors [14].
While reactive tumor stroma generally promotes tumor aggressiveness, not all components of the tumor stroma work in favor of the tumor [16]. Instead, there have also been identified several ECM molecules that possess distinct anti-tumor, anti-angiogenic, and anti-metastatic activities. Identification and characterization of these anti-cancer ECM proteins represents an important therapeutic avenue towards suppressing cancer in humans.
Lumican is a member of small leucine rich proteoglycan (SLRPs) family of extracellular matrix (ECM) proteins. The structural role of lumican is attributed toward its ability to promote fibrillogenesis and stabilization of collagen fibers [3, 17]. Beyond lumican’s structural role, lumican is also a matricellular protein that has been implicated in cancer although a single correlation between differential lumican expression and cancer malignancy has not been identified. For instance, lumican expression in colorectal cancers enhanced cancer cell migration and invasion [18] and correlated with poor prognosis for patients with colon cancer [20]. Similarly, lumican expression in pancreatic cancer cells enhanced cancer cell proliferation [25] and stromal lumican expression in pancreatic cancer correlated with decreased survival in pancreatic cancer patients. In contrast to these examples that highlight lumican’s ability to promote cancer malignancy, lumican has also been shown to suppress malignancy in several cancer types. For example, lumican has been well documented to suppress melanoma cell migration, invasion, and metastasis [4–6, 23]. Similarly, lumican expression is negatively correlated with metastatic prostate cancer [9, 11] and lumican was shown to suppress metastatic prostate cancer cell migration and invasion both in vitro and in vivo [8]. In addition to these examples, lumican is also differentially expressed in tumors of the breast [10, 12] although the impact of lumican in breast cancer has not been directly investigated. Collectively, the available data clearly implicate lumican in cancer although the precise role of lumican in various cancers remains elusive.
Finally, lumican has also been documented as an inhibitor of tumor angiogenesis [2, 6, 15, 24] although the range of lumican’s angiostatic activity in various circumstances has not been fully explored. Therefore, the goal of the current study was to examine lumican’s anti-angiogenic activity under various pathological and non-pathological circumstances. Our results confirm the anti-angiogenic activity of lumican, and demonstrate that lumican is not anti-angiogenic under all circumstances. Rather, we find that lumican suppresses angiogenesis in some, but not all cancer models and that lumican does not suppress angiogenesis in non-pathological settings including healing wounds, cultures of aortic rings, and matrigel plugs. Collectively, these results are tightly correlated with previous results showing that lumican exhibits cancer/tissue specific activities and extend these observations to include tissue specific affects on angiogenesis. The major challenge in future investigations will therefore be to determine the mechanistic basis for lumican’s tissue specific activities.
Materials and Methods
Wound Healing Model
Control and lumican knockout Male C57BL/6 mice were anaesthetized by intraperitoneal injection of ketamine hydrochloride (2 mg/g body weight) and xylazine (0.4 mg/g body weight). Shaved skin was sterilized using an alcohol swab and a biopsy punch was applied to create a circular full thickness skin wound about 6 mm in diameter below the shoulder blades. Tissues were collected from sacrificed mice 3, 7, 14, and 21 days after wounding and stained with anti-CD34 antibodies.
Mouse Breeding and Genotyping
Lumican −/− mice in C57BL/6 background were generously provided by Dr. Winston Kao (University of Cincinnati College of Medicine). Heterozygous (Lum −/+) male and female mice were crossed to produce homozygous knockout (Lum −/−) mice. PCR based genotyping was performed on DNA isolated from ear tissue using DirectPCR Lysis Reagent (Viagen Biotech, CA). PCR was performed using specific primers targeting wild type vs. mutant Lumican alleles. The wild type allele was amplified with forward 5′ TACTTCAAGCGCTTCACTGG and reverse 5′ CGAGACTAGTGAGACGTGCT oligos and amplified a 190 bp fragment. The knockout allele was detected with forward oligo 5′ CAAGTTCATTGACCTCCAGG and the same reverse oligo used for WT allele amplification and a 390 bp fragment exactly as previously described [19]. Wild type and mutant PCR reactions were performed in a single reaction using following conditions: 1 μl template DNA (extracted from ear sample), 100 nM primers, 1X standard buffer, 320 μM dNTPs, and 66 U/ml Taq Polymerase (New England Bio Labs Inc.), and total volume 25 μl. Thermocycle conditions used for each reactions are as follows: 94 °C for 2 min (1X); [94 °C for 45 s; 64 °C for 30 s; 72 °C for 30 s] (35X); 72 °C for 5 min (1X); and 4 °C.
Immunohistochemistry
Isolated tumors were fixed in 4 % paraformaldehyde for 1 h then placed in 70 % ethanol before paraffin embedding, sectioning, and staining with CD-31 antibodies to analyze microvascular densities. Tumor histology was performed in the Clarian Pathology Laboratory at Indiana University (Indianapolis, IN). Quantification of vascular density was performed by tracing CD-31 staining patterns onto white paper with black ink and scanning the resulting copy to obtain total vascular area using Image J software (NCBI).
In-vivo Tumor Growth and Metastasis Studies
Freshly cultured Pancreatic Adenocarcinoma (PanO2) cells were resuspended in sterile 1X PBS and 1 × 106 cells per 100 μl were injected subcutaneously between the shoulder blades of approximately 10-week-old Lumican WT, HET, and KO C57BL/6 mice (three mice per condition). For 4T1, 4 × 103 cells per 100 μl were injected directly into breast fat-pads of syngeneic BALB/c mice. Tumors in mice were monitored on a daily basis and primary tumors were measured externally using calipers between days 7–15 in an interval of 2 days. Metastasis to lungs was monitored by dissecting both lungs from tumor bearing mice, mincing the tissue with a razor, and chemically digesting the minced lung tissue for 2 h with collagenase solution. Large undigested pieces of lung were allowed to settle, then 10 × 106 cells were plated into 10 cm dishes in the presence of 6-thioguanine to kill normal cells and select for resistant 4T1 cells present in lung. After approximately 10 days in culture, colonies of metastatic cells were stained with crystal violet and overall staining density was determined with Image-J software.
In-vitro Angiogenic Outgrowth Assay (Aortic Ring Assay)
Aoritc ring assays were performed essentially as previously described [21] with the following exceptions. Aortas (~1 cm in length) were removed from 5 week old C57Bl/6 wild type, Lum −/+, or Lum −/− mice. The aortic sections were washed in 1X PBS and cut into small rings of equal sizes and implanted into fibrin gels. Fibrin solutions were prepared by mixing 1.5 mg/ml fibrinogen with serum free EGM2 media (Lonza Inc.), and gelled by adding 0.06 U/ml Thrombin to 0.5 ml fibrinogen solution in 24-well plates into which the aortic rings were immediately implanted. After fibrin gels formed at room temperature for 20 min, 1 ml EGM2 complete media (Lonza Inc.) was added to the top of each culture. The plates were incubated at 37 °C in a 5 % CO2 incubator. Aortic rings were observed daily for signs of angiogenic sprouting and recorded. Individual sprout lengths were measured after 9 days of culture.
Matrigel Plug Assay
Matrigel implantation was performed on 6-week-old C57BL/6 WT, HET, or KO mice. Briefly, mice were injected subcutaneously in the ventral groin area with Matrigel (700 μl/injection; BD Biosciences, Bedford, MA) supplemented with bovine bFGF (300 ng/mL; R&D Systems, Minneapolis, MN). Ten days post-implantation, the mice were sacrificed and the plugs were harvested. Three mice were used per experimental condition and the experiments were repeated thrice in its entirety.
Cell Culture and Lumican Overexpressing Cell Lines
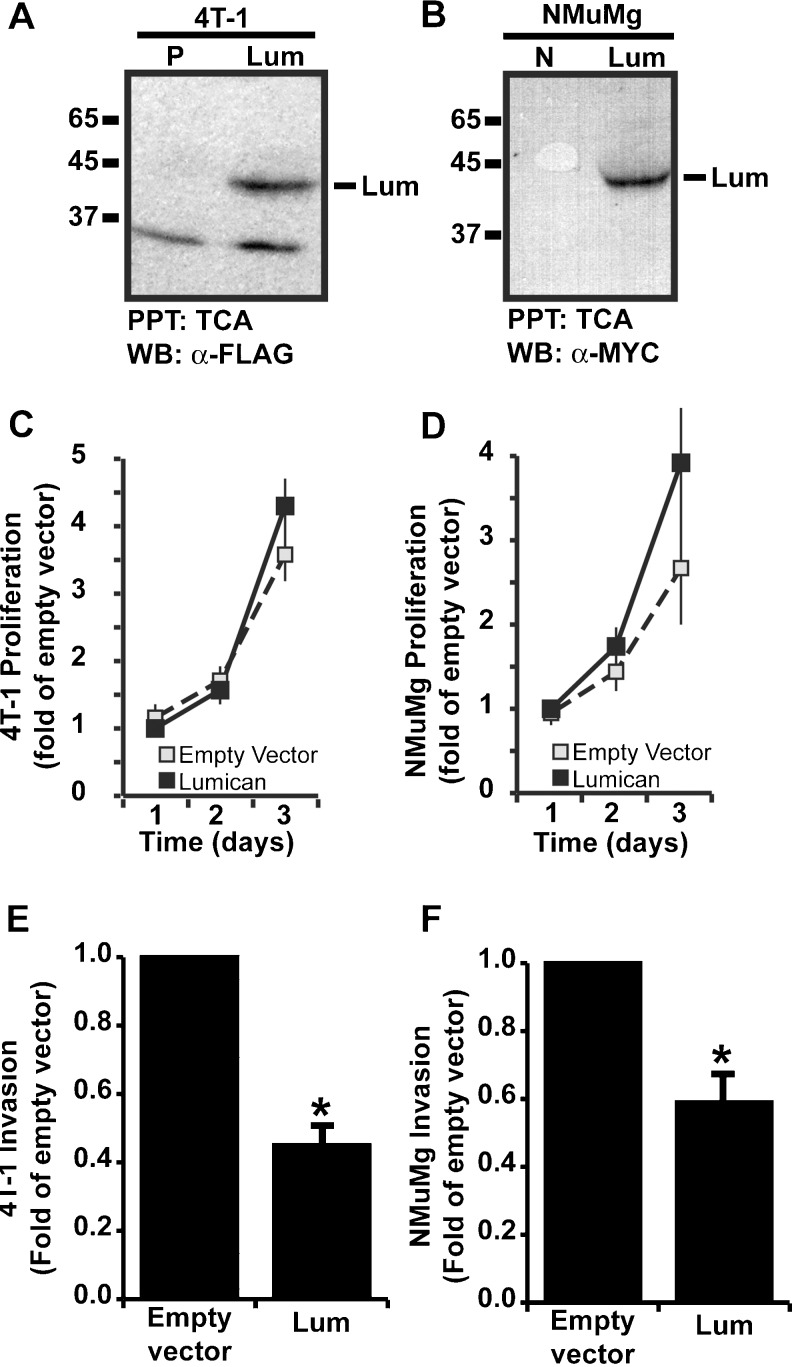
4T-1 and NMuMg breast cancer cell lines were maintained in either DMEM (4T-1) or DMEM supplemented with 10 μg/ml insulin (NMuMg). The lumican overexpressing 4T-1 cell line was created by cloning murine lumican cDNA into a retroviral vector to produce stable overexpressing cells. To accomplish this, oligos were designed such that an ATTB1 site was appended to the 5′ end, while the FLAG epitope tag and ATTB2 sequences were appended to the 3′ end through two rounds of sequential PCR with the following oligos. First forward lumican oligo: AAAGCAGGCTTCACC TTAATACGACTCACTATAGGG. Second forward lumican oligo: GGGGACAAGTTTGT ACAAAAAAGCAGGCTTCACCATG. First reverse lumican oligo: TACTTATCGTCGTCATCCTTGTAATCGTTAAC GGTGATTTCATT. Second reverse lumican oligo: GGGGACCACTTTGTACAAGAAAGCTGGGTCCT ACTTATCGTCGTCATC. The PCR product was cloned by BP clonase into an entry vector and subsequently cloned with LR clonase into a custom made pMSCV retroviral vector featuring the ATTR1-CMr-ccdB-ATTR2 cassette blunt cloned into the HPA1 sites of pMSCV-PURO. Clones were selected by ampicillin resistance, tested for inserts by restriction digest with EcoR1 and Xho1, and sequenced (Functional Genomics). The retroviral vector used to produce lumican overexpressing NMuMg cells was previously described [24].
Empty vector control and lumican retroviral supernatants were produced in EcoPac II retroviral packaging cells and infected into 4T-1 and NMuMg as described previously [7, 24]. Infected cells were isolated by either puromycin selection (4T-1 cells, 1 μg/ml), or G418 selection (NMuMg cells, 50 μg/ml) to yield stable polyclonal populations of control or lumican-overexpressing cells. Lumican expression was confirmed by TCA/DOC precipitation of conditioned media and western blotting with either anti-FLAG M2 monoclonal antibodies (4T-1 cells), or 9E10 anti-Myc antibodies (NMuMg cells).
Results
Endogenously Expressed Lumican Suppresses Tumor Angiogenesis
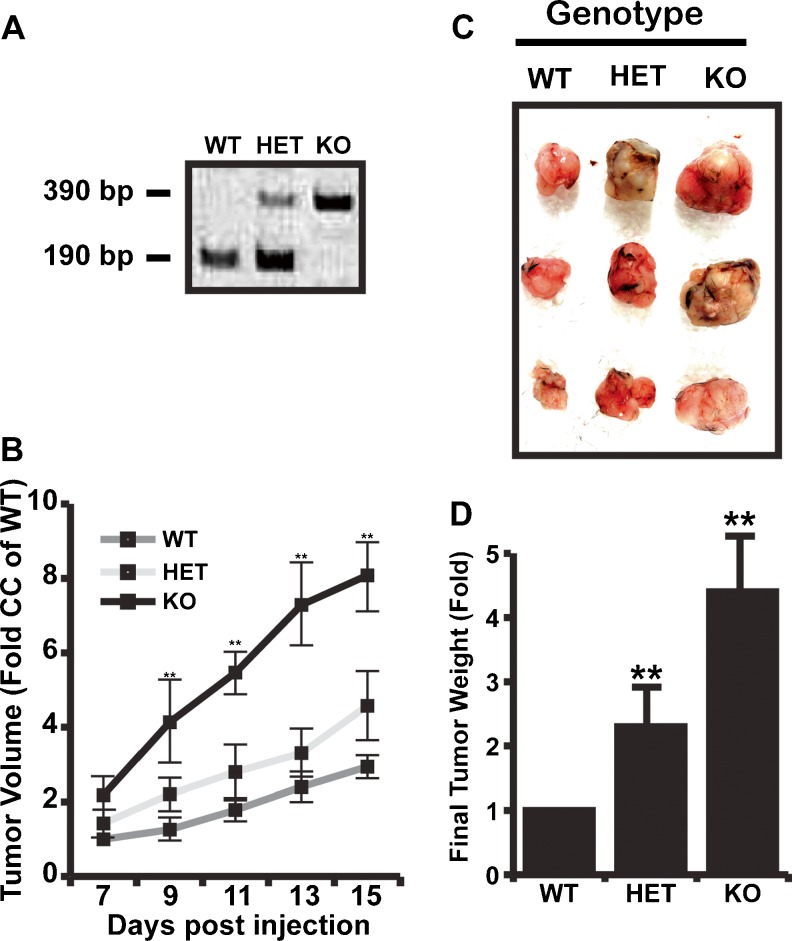
Previously, lumican was shown to decrease tumor angiogenesis in pancreatic adenocarcinoma (PanO2) cells, MCA102 fibrosarcoma cells, and B16F10 melanoma cells [6, 24]. However, these studies relied on ectopic expression from implanted tumors to drive lumican expression and failed to account for host expression of lumican. Therefore, we compared the growth and angiogenesis of tumors formed by injection of pancreatic adenocarcinoma (PanO2) cells in wild-type (WT), heterozygous (HET), and lumican knockout (KO) mice. Importantly, the PanO2 cell line was previously used to illustrate the anti-angiogenic activity of overexpressed lumican in vivo [24] and made an ideal cell line with which to examine host lumican contributions to tumor growth and angiogenesis. Wild-type C57BL6 mice, or HET and KO littermates were identified by PCR (Fig. 1a). PanO2 tumor growth in lumican KO mice was significantly accelerated compared to tumor growth in WT mice (Fig. 1b). Tumor growth in lumican HET mice strongly tended toward accelerated growth although this increase in growth rate failed to reach statistical significance. At dissection, tumors recovered from KO and HET mice were approximately 4.4 and 2.2 fold larger respectively than tumors recovered from WT mice (Fig. 1c).
Fig. 1.
Lumican is an endogenous inhibitor of tumor growth. a PCR based genotyping. PCR amplified 190 bp Lumican product in wild type (WT) mouse and 390 bp mutant DNA product in Lumican knockout (KO) mouse. PCR amplified both 190 bp Lumican product and 390 bp mutant DNA product in heterozygous (HET) mouse. b 1 × 106 Pancreatic Adenocarcinoma (PanO2) cells were injected subcutaneously in triplicate into WT, HET, and KO mice. One week after injection, tumors were detectable and tumor size was measured every other day. c Representative tumors from a single experiment isolated from mice after 15 days. d Bar graph representing final tumor weights from WT, HET, and KO mice. Each data point represents Mean ± SD. * indicate P < 0.05, Student T-test, N = 3
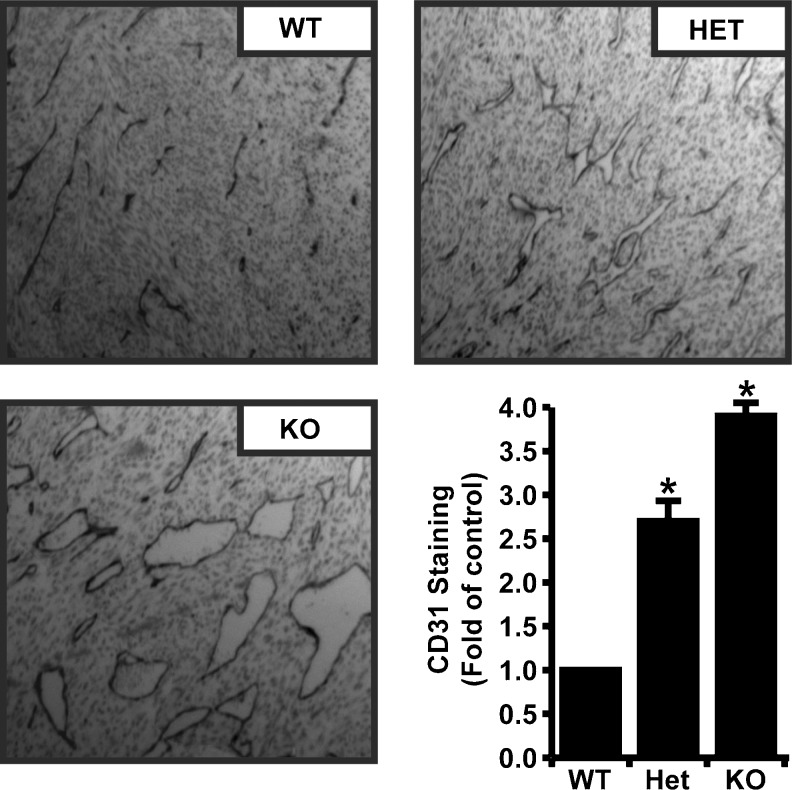
To determine if host expressed lumican also suppressed PanO2 tumor angiogenesis, tumors were dissected from WT, HET, and KO mice and processed for immunohistochemistry with anti-CD31 antibodies to detect vascular elements. As shown in Fig. 2, both lumican KO and HET tumors exhibited a striking increase in vascular density. Interestingly, vessels in lumican KO and HET tumors tended to be of larger caliber than their WT counterparts. Densitometry with ImageJ software revealed 4.0 and 2.7 fold more CD31 staining in lumican KO and HET tumors respectively. Collectively, these results illustrated that host-derived lumican suppresses tumor growth and angiogenesis in a similar fashion to ectopically expressed lumican and therefore indicated that lumican is an endogenous inhibitor of PanO2 tumor angiogenesis.
Fig. 2.
Endogenous Lumican has anti-angiogenic activity. PanO2 tumor isolates from mice were sectioned, fixed, and stained with anti-CD-31 antibody to detect microvascular density in tumors. Shown are representative immunostaining results from a single experiment that was performed three times in its entirety. CD-31 staining was quantified by densitometry with Image J software and is depicted as the fold increase compared to control mice. Each data point represents the mean ± SE of three independent experiments. * indicate P < 0.05, Student T-test
Lumican Does Not Suppress Angiogenesis under All Circumstances
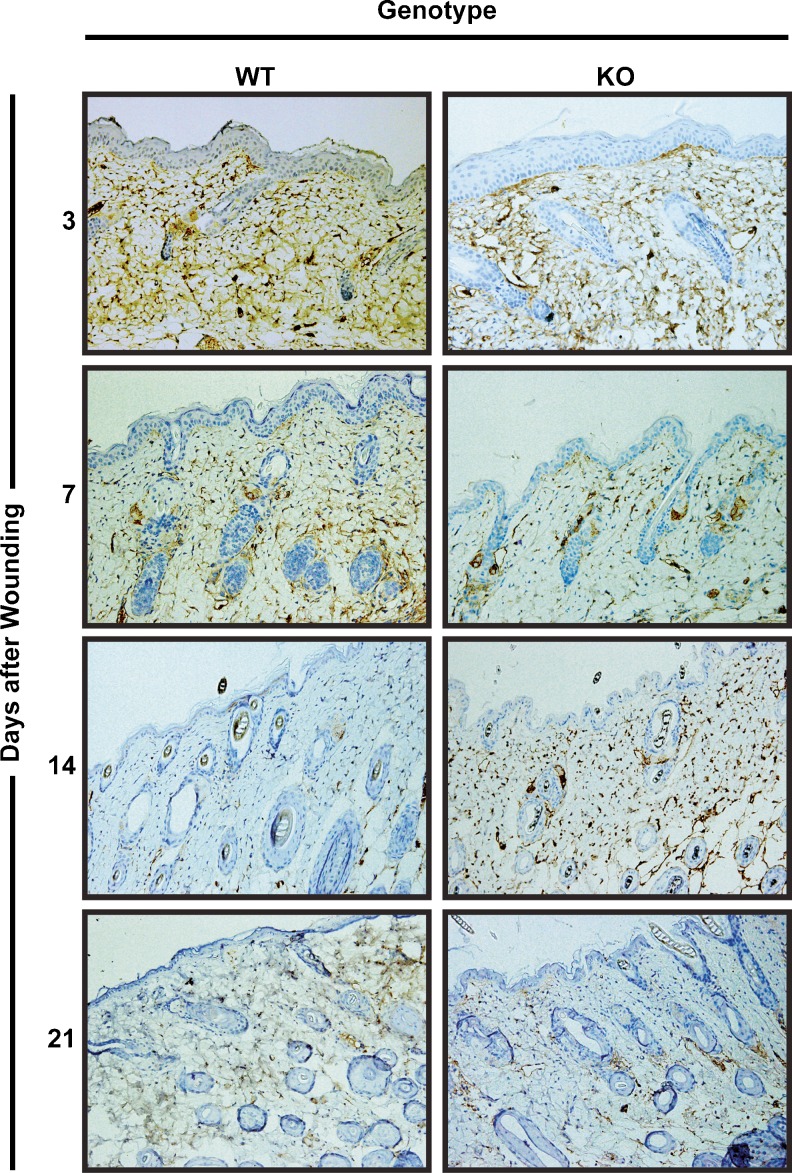
Based on our results showing that endogenous lumican suppressed PanO2 tumor angiogenesis, we were interested to determine if endogenous lumican would also inhibit angiogenesis in non-pathological settings. In particular, new blood vessels are a crucial component of granulation tissue where they help drive tissue healing. Therefore, we compared vascular density in healing wounds from control and lumican KO mice. Cutaneous wounds were made by punch biopsy and the resulting healing wounds were collected 3, 7, 14, and 21 days after initial wounding. Wound samples were stained with anti-CD34 antibodies to visualize neovascularization in the healing wounds. As shown in Fig. 3, similar densities of CD34 staining were detected at all time points in WT and KO mice suggesting that lumican did not significantly affect angiogenesis in healing tissue.
Fig. 3.
Endogenous lumican does not suppress angiogenesis in healing wounds. Skin punches from wild-type (WT) and lumican knockout (KO) mice were removed to produce wounds. Healing wounds were collected from mice 3, 7, 14, or 21 days after wounding and vascular density was compared by immunohistochemistry with anti-CD34 antibodies
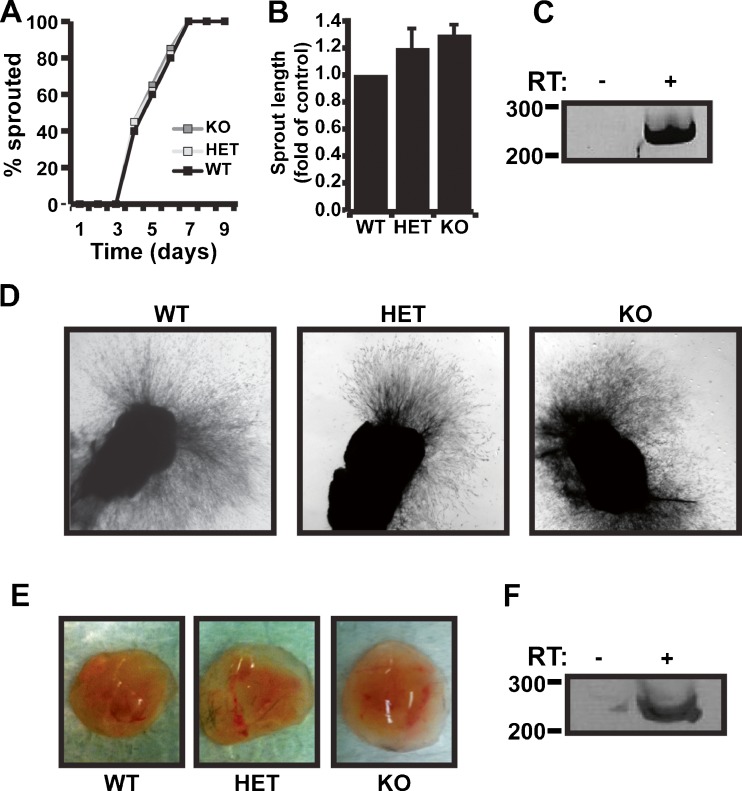
In addition, we also compared angiogenic sprouting from WT, HET, and KO aortic rings. Aortas were isolated from WT, HET, and lumican KO mice, embedded into fibrin gels, and monitored over a 10 day period for signs of angiogenic sprouting. As shown in Fig. 4a, the initiation of angiogenic sprouting was indistinguishable between WT, HET, and KO rings. In addition, the final length of aortic outgrowths was not significantly longer in HET and KO cultures compared to WT outgrowths (Fig. 4b). Importantly, RT-PCR confirmed the presence of lumican in WT aortic outgrowth indicating that the failure to observe a decrease in angiogenic sprouting was not due to the absence of lumican expression (Fig. 4c). Finally, we also examined the effect of lumican deletion on Matrigel plug angiogenesis. Our previous results demonstrated that recombinantly produced and purified lumican suppressed angiogenesis in matrigel plugs [2]. Therefore, this system provided a unique opportunity to directly compare exogenous versus endogenous lumican angiostatic activity. Solutions of matrigel containing 300 μg/ml of bFGF were subcutaneously injected into WT, HET, or lumican KO mice and 10 days later were dissected and compared for evidence of vascularization/blood content. As shown in Fig. 4e, matrigel plugs dissected from WT, HET, and KO mice all contained similar amounts of blood suggesting angiogenesis was not altered in the absence of lumican. Importantly RT-PCR positively detected lumican mRNA in control matrigel plugs indicating that lumican was present in the matrigel plugs (Fig. 4f). Collectively, the failure of lumican deletion to affect angiogenesis in healing wounds, aortic ring assays, or matrigel plugs strongly suggested that endogenously expressed lumican is not angiostatic in these microenvironments and instead, that lumican is anti-angiogenic only under certain circumstances.
Fig. 4.
Endogenous lumican does not suppress aortic sprouting or vascularization of Matrigel plugs. a Aortas were isolated from wild-type (WT), heterozygous lumican knockout (Het), or homozygous lumican knockout (KO) mice and sectioned then, implanted into fibrin gels. Aortic cultures were inspected daily for angiogenic sprouting. The time required to initiate angiogenic sprouting is depicted as the percent number of sprouting rings over a 9-day window until 100 % of rings had sprouted. Data presented is the average of four individual experiments each consisting of at least 5 aortic rings for each genotype. b The final length of angiogenic sprouts was measured (pixel length) and average length compared to WT rings is depicted. c Lumican mRNA expression was confirmed in WT culture of aortic rings. A negative reverse transcription (-RT) control was used to control for specific lumican mRNA amplification. d Representative images of WT, Het, and KO aortic ring cultures after 9 days in culture. e Solutions of Matrigel containing 300 ng/ml of bFGF were injected subcutaneously into WT, Het, and lumican KO mice. After 10 days, the resulting Matrigel plugs were dissected and blood content was compared as a sign of vascularization. f RT-PCR was used as in C to confirm expression of lumican in control Matrigel plugs
Lumican Suppresses Breast Cancer Growth and Metastasis, but Not Angiogenesis
Lumican has been shown to suppress angiogenesis in tumors formed from a variety of cancer cell lines including PanO2 pancreatic adenocarcinoma cells, MCA102 fibrosarcoma cells, and B16 melanoma cells, however endogenous lumican was unable to suppress angiogenesis in healing wounds, sprouting aortic rings, and matrigel plugs. These observations suggested that lumican is angiostatic in some but not all microenvironments. Interestingly, these results also suggested that the anti-angiogenic activity of lumican may be restricted to tumor microenvironments and not operant in non-pathogenic microenvironments. To test this hypothesis, we sought to find a tumor model wherein lumican does not exhibit angiostatic activity. Prior work has implicated elevated lumican expression with both increased and decreased breast cancer malignancy [12, 22], but the direct effects of lumican on breast tumor cells and breast cancer have not been assayed. Therefore, we sought to examine the effect of lumican on breast cancer growth, vascularization, and malignancy. We created lumican overexpressing breast cancer cell lines by transducing NMuMg normal murine mammary gland, and 4T1 breast cancer cell lines with retroviral particles encoding either lumican or empty vector control sequences. Overexpression of lumican in the resulting cell lines was confirmed by TCA/DOC precipitation of conditioned media followed by western blotting with either anti-Myc 9E10 (NMuMg) or anti-FLAG M2 (4T1) antibodies to detect C-terminally tagged lumican transgene expression. As shown in Fig. 5a and b, transgene expression of lumican protein was evident in overexpressing cell lines but not in empty vector control cells. To determine if lumican had a direct effect on breast cancer cells, we compared proliferation rates and invasive activity in control and lumican overexpressing cells. As shown in Fig. 5c and d, lumican did not significantly affect cell proliferation in either 4T1 or NMuMg cell lines but did significantly decrease cell invasion through Matrigel coated Boyden chambers in both cell lines (Fig. 5e, f).
Fig. 5.
Lumican decreases invasion but not proliferation of breast cancer cells. a–b 4T1 and NMuMg breast cancer cells were transduced with either empty puromycin (P) or neomycin (N) resistance vectors or lumican expressing retroviral vectors. Overexpression of lumican was confirmed by western blot analysis of TCA/DOC precipitated conditioned media with anti-FLAG antibodies (4T1) or anti-MYC 9E10 antibodies (NMuMg). In addition to the lumican core protein, anti-FLAG antibodies also detected a ~35 kDa protein of unknown identity. c–d Empty vector control and lumican overexpressing 4T1 or NMuMg cell lines were cultured on Matrigel coated Boyden chambers and induced to invade towards a 2 % FBS gradient for 48 h. Invading cells were stained with crystal violet and quantified by densitometry. Data presented is the average fold decrease +/− SE of four independent experiments. * indicates p < .05, student’s t-test. e–f WST1 reagent was used to compare proliferation rates of empty vector control and lumican overexpressing 4T1 and NMuMg cells. Proliferation was measured daily for 3 days. Data is depicted as average fold increase (+/− SE) of four independent experiments compared to empty vector control on day 1
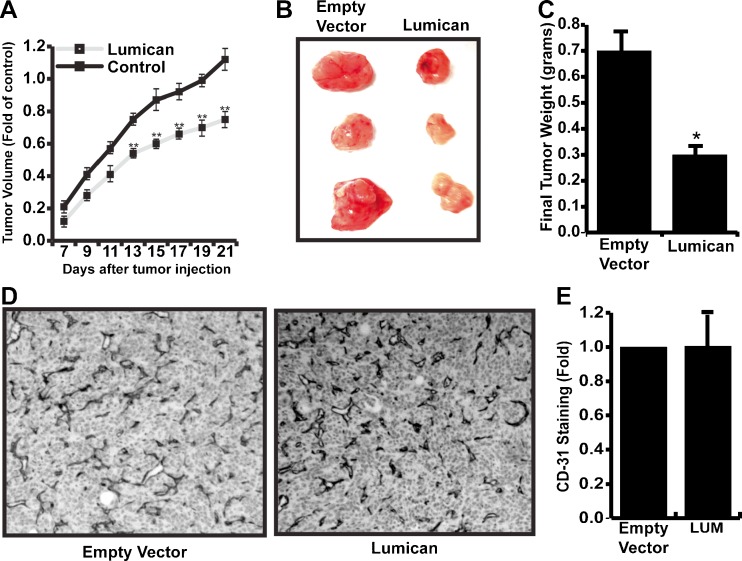
Having shown that lumican suppresses breast cancer cell invasion in vitro, it was important to determine if lumican suppresses tumor growth, angiogenesis, and invasion/metastasis in vivo. To accomplish this, control and 4T1-lumican cells were orthotopically injected into the fatpad of syngeneic BALB/c mice. After 7 days, tumors were measurable and tumor volume was recorded every other day for 14 days. As shown in Fig. 6a, lumican overexpressing 4T1 cells formed tumors significantly slower than their control counterparts. Moreover, lumican expression decreased the final tumor mass by approximately 60 % compared to control tumors (Fig. 6b, c). To determine if reduced tumor mass was associated with reduced tumor angiogenesis, control and 4T1-lumican tumors were sectioned and stained with anti-CD31 antibodies. Surprisingly, we were unable to detect any difference in the vascular density between control and 4T1-lumican tumors (Fig. 6d, e).
Fig. 6.
Lumican suppresses growth but not angiogenesis in 4T1 breast cancers. a Equal numbers of empty vector control and lumican overexpressing 4T1 breast cancer cells were orthotopically injected in triplicate into the fat-pad of syngenic mice. Tumors were evident after 7 days and tumor volume was measured every other day for 14 days. Tumor volume is depicted as average fold increase (+/− SE) of three independent experiments compared to control tumors on day 7. b representative pictures of tumors dissected from a single experiment. c Final mass of dissected tumors was compared and is depicted as the average decrease (+/− SE) of three independent experiments compared to final control tumor mass. * indicates p < .05, student’s t-test. d Dissected tumors were sectioned and vascular density was monitored by immunohistochemistry with anti-CD31 antibodies. e Densitometry by Image J software was used to quantify CD31 staining in tumor sections
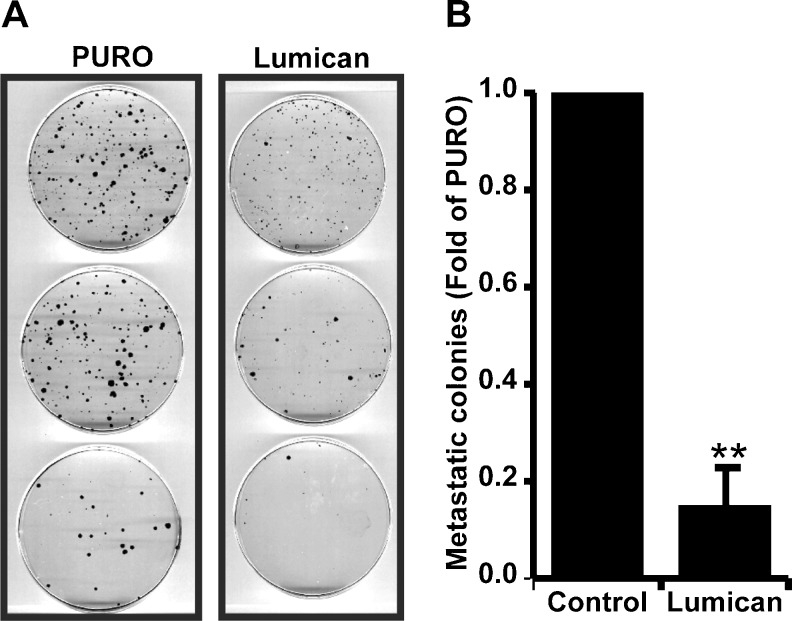
Given our in vitro data showing that lumican suppressed 4T1 and NMuMg invasion, it was important to determine if lumican suppressed 4T1 invasion/metastasis in vivo. To accomplish this, lungs were collected from control or lumican overexpressing tumors and rendered to single cell suspensions by digestion with Collagenase solution. Equal numbers of cells were subsequently cultured on tissue culture dishes in the presence of 6-thioguanine to select for 4T1 cells/colonies. As shown in Fig. 7, lungs from lumican overexpressing cells generated significantly fewer 4T1 colonies compared to lungs from control animals.
Fig. 7.
Lumican suppresses 4T1 breast cancer metastasis to Lungs. a Images showing metastatic colonies of 4T1 breast cancer cells in lungs from mice injected with control or Lumican overexpressing (Lumican) 4T1 breast cancer cells. b Represents a quantification of metastatic colonies in lungs from mice injected with either control or Lumican overexpressing (Lumican) 4T1 cancer cells. Each data point represents Mean ± SD. ** indicate P < 0.05, Student t-test, N = 3
Collectively, these results showed that lumican blocks breast cancer growth and metastasis to lung independently of angiogenesis suppression. Interestingly, this was the first example wherein lumican failed to suppress tumor angiogenesis and suggested that lumican may not be a universal inhibitor of angiogenesis but rather may inhibit angiogenesis only under restricted circumstances.
Discussion
We and others have begun to unravel the function of lumican in experimental models of cancer and angiogenesis, however, there remain significant questions regarding lumican function in these processes. One persisting question has to do with the fact that although lumican has until now been shown to consistently suppresses angiogenesis [2, 6, 24], lumican is nonetheless commonly overexpressed in cancers but does not consistently correlate with decreased malignancy as would be predicted for an angiogenesis inhibitor. Our current results help clarify this paradox by showing that lumican’s anti-angiogenic activity manifests only under restricted circumstances. In combination with our previous results [24], we have found that both overexpressed and endogenously expressed lumican suppressed angiogenesis in PanO2 tumors while overexpressed lumican failed to suppress angiogenesis in 4T1 breast cancer tumors. Therefore, we believe that the failure of lumican to consistently correlate with decreased cancer malignancy is due to the differential ability of lumican to suppress angiogenesis in various tumor types. The mechanistic basis for this remains unexplored, but several hypotheses present themselves. First, previous reports have shown that lumican is proteolytically cleaved by MMP14 [13] raising the possibility that tumors with high MMP14 activity may be resistant to the anti-angiogenic activity of lumican. Our data however did not support this hypothesis since PanO2 tumors but not 4T1 tumors expressed high levels of MMP14 mRNA (data not shown). An second hypothesis is that heterogenous endothelium within different tissues or tumor types [1] may have differential sensitivity to lumican. However, our data also do not support this hypothesis since recombinant lumican, but not endogenously expressed lumican suppressed angiogenesis in matrigel plugs [2], and Fig. 4). Based on this observation, a final hypothesis is that non-glycosylated lumican core protein such as recombinantly produced protein blocks angiogenesis while endogenously expressed and glycosylated lumican is unable to suppress angiogenesis. If correct, it will be critical in future studies to compare lumican glycosylation in various tumor cell types. Future studies will be directed at testing this hypothesis.
Despite the fact that lumican did not suppress 4T1 tumor angiogenesis, lumican overexpression did potently suppress 4T1 tumor growth and metastasis. This result indicates that in addition to lumican’s anti-angiogenic activity, this matricellular molecule also directly impacts tumor cell physiology. Thus, the direct effect on tumor cell invasion and migration coupled with the anti-angiogenic activity of lumican indicates that lumican is a multi-functional matricellular protein that like many other matricellular molecules exhibits contextual and cell specific activities.
Finally, we also investigated the anti-angiogenic activity of lumican in non-tumor models of angiogenesis and were unable to detect a significant anti-angiogenic activity in wound healing assays, aortic ring assays, or matrigel plug implantations. These results are also consistent with our previous results showing that wound healing is delayed in lumican knockout mice, not accelerated as would be expected if lumican were anti-angiogenic in granulation tissue [26]. Moreover, as is the case for tumors, these results seem to indicate that lumican is only anti-angiogenic under restricted circumstances. More provocatively, these results also suggest that the anti-angiogenic activity of endogenously expressed lumican functions only within a restricted subset of tumor microenvironments, therefore, raising the possibility that lumican may represent an attractive therapeutic avenue to specifically inhibit angiogenesis in some tumors while sparing angiogenesis in other tissues such as healing wounds.
Collectively, these new results broaden our understanding of lumican’s role in vascular biology and raise new questions regarding the molecular mechanism by which lumican suppresses angiogenesis.
Acknowledgments
We are thankful to the NIH (Grant # 3R15CA133829-01A1S1 to ARA), and the INBRE program NIH Grant # P20 RR016454 (National Center for Research Resources) and # P20 GM103408 (National Institute of General Medical Sciences) for funding support. Thank you to Dr. Ken Cornell (Boise State University) for critical reading of the manuscript and excellent suggestions.
References
- 1.Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med. 2012;2:a006429. doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albig AR, et al. Transcriptome analysis of endothelial cell gene expression induced by growth on matrigel matrices: identification and characterization of MAGP-2 and lumican as novel regulators of angiogenesis. Angiogenesis. 2007;10:197–216. doi: 10.1007/s10456-007-9075-z. [DOI] [PubMed] [Google Scholar]
- 3.Austin BA, et al. Altered collagen fibril formation in the sclera of lumican-deficient mice. Invest Ophthalmol Vis Sci. 2002;43:1695–1701. [PubMed] [Google Scholar]
- 4.Brezillon S, et al. Expression of lumican, a small leucine-rich proteoglycan with antitumour activity, in human malignant melanoma. Clin Exp Dermatol. 2007;32:405–416. doi: 10.1111/j.1365-2230.2007.02437.x. [DOI] [PubMed] [Google Scholar]
- 5.Brezillon S, et al. Lumican core protein inhibits melanoma cell migration via alterations of focal adhesion complexes. Cancer Lett. 2009;283:92–100. doi: 10.1016/j.canlet.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 6.Brezillon S, et al. Lumican inhibits B16F1 melanoma cell lung metastasis. J Physiol Pharmacol. 2009;60(Suppl 4):15–22. [PubMed] [Google Scholar]
- 7.Brown LJ, et al. Lipocalin-7 is a Matricellular regulator of angiogenesis. PLoS One. 2010;5:e13905. doi: 10.1371/journal.pone.0013905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coulson-Thomas VJ, et al. Lumican expression, localization and antitumor activity in prostate cancer. Exp Cell Res. 2013;319:967–981. doi: 10.1016/j.yexcr.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhanasekaran SM, et al. Delineation of prognostic biomarkers in prostate cancer. Nature. 2001;412:822–826. doi: 10.1038/35090585. [DOI] [PubMed] [Google Scholar]
- 10.Eshchenko TY, et al. Expression of different proteoglycans in human breast tumors. Biochemistry (Mosc) 2007;72:1016–1020. doi: 10.1134/S0006297907090143. [DOI] [PubMed] [Google Scholar]
- 11.Lapointe J, et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci U S A. 2004;101:811–816. doi: 10.1073/pnas.0304146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leygue E, et al. Expression of lumican in human breast carcinoma. Cancer Res. 1998;58:1348–1352. [PubMed] [Google Scholar]
- 13.Li Y, et al. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004;64:7058–7064. doi: 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- 14.Lu P, et al. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niewiarowska J, et al. Lumican inhibits angiogenesis by interfering with alpha2beta1 receptor activity and downregulating MMP-14 expression. Thromb Res. 2011;128:452–457. doi: 10.1016/j.thromres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Proia DA, Kuperwasser C. Stroma: tumor agonist or antagonist. Cell Cycle. 2005;4:1022–1025. doi: 10.4161/cc.4.8.1903. [DOI] [PubMed] [Google Scholar]
- 17.Rada JA, et al. Regulation of corneal collagen fibrillogenesis in vitro by corneal proteoglycan (lumican and decorin) core proteins. Exp Eye Res. 1993;56:635–648. doi: 10.1006/exer.1993.1081. [DOI] [PubMed] [Google Scholar]
- 18.Radwanska A, et al. Overexpression of lumican affects the migration of human colon cancer cells through up-regulation of gelsolin and filamentous actin reorganization. Exp Cell Res. 2012;318:2312–2323. doi: 10.1016/j.yexcr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Saika S, et al. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seya T, et al. Lumican expression in advanced colorectal cancer with nodal metastasis correlates with poor prognosis. Oncol Rep. 2006;16:1225–1230. [PubMed] [Google Scholar]
- 21.Sharma B, Albig AR (2013) Matrix Gla protein reinforces angiogenic resolution. Microvasc Res 85:24–33 [DOI] [PMC free article] [PubMed]
- 22.Troup S, et al. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 23.Vuillermoz B, et al. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res. 2004;296:294–306. doi: 10.1016/j.yexcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Williams KE, Fulford LA, Albig AR. Lumican reduces tumor growth via induction of fas-mediated endothelial cell apoptosis. Cancer Microenviron. 2010;4:115–126. doi: 10.1007/s12307-010-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto T, et al. Secreted 70 kDa lumican stimulates growth and inhibits invasion of human pancreatic cancer. Cancer Lett. 2012;320:31–39. doi: 10.1016/j.canlet.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Yeh JT, et al. Impaired skin wound healing in lumican-null mice. Br J Dermatol. 2010;163:1174–1180. doi: 10.1111/j.1365-2133.2010.10008.x. [DOI] [PubMed] [Google Scholar]
- 27.Zagris N. Extracellular matrix in development of the early embryo. Micron. 2001;32:427–438. doi: 10.1016/S0968-4328(00)00011-1. [DOI] [PubMed] [Google Scholar]