Abstract
The conserved multifunctional protein Gle1 regulates gene expression at multiple steps: nuclear messenger (m)RNA export, translation initiation, and translation termination. A GLE1 mutation (FinMajor) is causally linked to human lethal congenital contracture syndrome-1 (LCCS1); however, the resulting perturbations on Gle1 molecular function were unknown. FinMajor results in a Proline-Phenylalanine-Glutamine peptide insertion within the uncharacterized Gle1 coiled-coil domain. Here we find that Gle1 self-associates both in vitro and in living cells via the coiled-coil domain. Electron microscopy reveals high molecular mass Gle1 oligomers form ∼26 nm in diameter disk-shaped particles. With the Gle1-FinMajor protein, these particles are malformed. Moreover, functional assays document a specific requirement for proper Gle1 oligomerization during mRNA export but not for Gle1’s roles in translation. These results identify a novel mechanistic step in Gle1’s mRNA export function at nuclear pore complexes, and directly implicate altered export in LCCS1 disease pathology.
INTRODUCTION
Dysregulation of messenger (m)RNA metabolism has emerged as a significant factor in human disease pathologies, with proper control of mRNA transcription, processing, nuclear export, translation and turnover being critical to cellular homeostasis, signaling, division, and differentiation (Cooper et al., 2009; Hurt and Silver, 2008; Renoux and Todd, 2012). Gle1 is an essential multi-functional protein, conserved from yeasts to humans, that plays a direct role in both mRNA export and translation (Bolger et al., 2008; Murphy and Wente, 1996; Watkins et al., 1998). Mutations in the human (h) GLE1 gene are responsible for the autosomal recessive lethal congenital contracture syndrome-1 (LCCS1) disease (Nousiainen et al., 2008). LCCS1 is a severe in utero form of a heterogeneous group of disorders, termed arthrogryposis multiplex congenita (AMC), that occur in 1 of 3000 human births worldwide (Hall, 1985). LCCS1 disease pathology is characterized by lack of anterior horn motor neurons and severe atrophy of ventral spinal cord, along with joint and jaw deformities (Herva et al., 1985). Recent work indicates the pathological basis of this disease is attributed to a reduction in Gle1 activity causing the apoptosis of proliferative organ precursors during early development (Jao et al., 2012). However, in LCCS1, the primary molecular defects in hGle1 cellular roles are unknown.
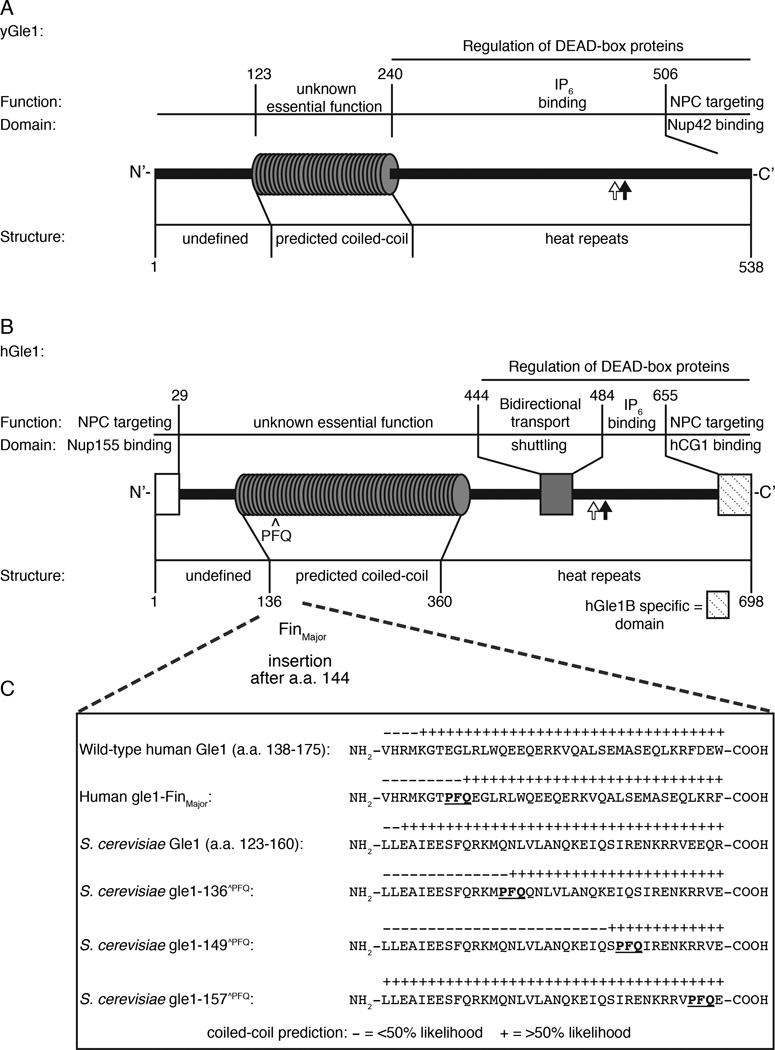
Previous studies have revealed multiple aspects of Gle1 structure and function in the budding yeast (y) Saccharomyces cerevisiae and human cells (Figure 1A, 1B). The C-terminal domains have extensive conservation, with 27% identical and 27% similar residues found between the regions by reported sequence alignments (from residues 250–538 for yGle1 and 360–659 for hGle1) (Watkins et al., 1998). Further, both have significant spans in their N-terminal regions that are predicted to form coiled-coil structures (Watkins et al., 1998). For mRNA export, hGle1 docks at the nuclear pore complex (NPC) through interactions with the NPC proteins hNup155 and hCG1 (yNup42 for yGle1) (Kendirgi et al., 2005; Murphy and Wente, 1996; Rayala et al., 2004; Strahm et al., 1999; Stutz et al., 1997). hGle1 is also dynamic and its shuttling between the nucleoplasm and cytoplasm is essential for efficient mRNA export in human cells (Kendirgi et al., 2003). Although humans have a single copy of the hGLE1 gene, there are at least two alternatively spliced isoforms (hGle1A and hGle1B) (Kendirgi et al., 2003). Whereas hGle1B has distinct steady state localization at the NPC, the hGle1A isoform lacks the C-terminal hCG1-binding domain and is predominantly cytoplasmic. Thus, there are potentially distinct subcellular pools of hGle1A and hGle1B that might reflect multiple roles in gene expression.
Figure 1. Conserved structural and functional elements of Gle1 from S. cerevisiae and humans.
(A) Diagram depicting functional and structural domains in S. cerevisiae (y)Gle1. Black arrow represents the position of the y-gle1-4 mutation (G382R) (Watkins et al., 1998). White arrow marks the location of conserved IP6-coordinating residues K377 and K378 (Alcazar-Roman et al., 2010). (B) Diagram depicting functional and structural domains in human (h)Gle1B (adapted from Kendirgi et al., 2005). “PFQ” denotes location of the FinMajor insertion after amino acid 144 in the predicted coiled-coil domain (Nousiainen et al., 2008). Black arrow represents the homologous position (Q548) of the residue mutated in y-gle1-4 (Watkins et al., 1998). White arrow marks the location of conserved IP6-coordinating residues K526 and K527 (Alcazar-Roman et al., 2010). (C) Paircoil2-generated structure predictions of Gle1 polypeptide sequences, showing the structural effect of h-gle1-FinMajor and y-gle1 engineered PFQ insertions. (+) indicates >50% probability of coiled-coil structure. PFQ insertions locations are designated by bold underlined typeface. See Table S1 and S2 for Paircoil2 scores and p-values.
During mRNA export and translation, yGle1 regulates the RNA-dependent ATPase activities of specific DEAD-box proteins (DBPs); thus, controlling the action of these DBPs in nucleotide-dependent unwinding of RNA duplexes and/or remodeling of the mRNA-particle (mRNP) protein composition (Alcazar-Roman et al., 2006; Bolger et al., 2008; Bolger and Wente, 2011; Weirich et al., 2006). Efficient yGle1 function at the NPC requires inositol hexakisphosphate (IP6,) binding (Alcazar-Roman et al., 2010; York et al., 1999), and together yGle1-IP6 triggers Dbp5-dependent mRNP remodeling events required for directional export through NPCs (Tran et al., 2007). Conserved residues in both yGle1 and hGle1 are critical for IP6 binding and Dbp5 activation (Figure 1A–B) (Alcazar-Roman et al., 2010; Montpetit et al., 2011). In translation termination, yGle1-IP6 directly interacts with Sup45 (eRF1) and is thought to activate Dbp5 for RNP remodeling to promote Sup35 (eRF3) association (Bolger et al., 2008). During translation initiation, yGle1 and hGle1 interact with eIF3 proteins, and yGle1 is known to modulate a different DBP, Ded1, for efficient start site recognition (Bolger et al., 2008; Bolger and Wente, 2011). Thus, Gle1 serves as a multifunctional effector of distinct steps in the gene expression pathway.
The major LCCS1 causative mutation in hGLE1 is designated FinMajor, and is a single nucleotide substitution that alters a splice site acceptor in the third intron (Nousiainen et al., 2008). This results in a three amino acid residue insertion (proline-phenylalanine-glutamine, PFQ) in the N-terminal coiled-coil domain of hGle1. LCCS1 patients are typically homozygous for the FinMajor mutation, whereas heterozygotes show no reported phenotype (Nousiainen et al., 2008). As noted above, the C-terminal domain of hGle1 is linked to DBP regulation, nucleocytoplasmic shuttling, and IP6 binding (Alcazar-Roman et al., 2010; Kendirgi et al., 2003; Montpetit et al., 2011; Weirich et al., 2006). The N-terminal coiled-coil domain is also essential in vivo (Watkins et al., 1998); however, putative protein interaction partners for the coiled-coil domain have not been defined. It is also unclear whether the coiled-coil domain is involved in mRNA export and/or translation or how it is functionally perturbed in human LCCS1 disease.
Here we investigated the function of the coiled-coil domain, and in doing so defined the underlying mechanism for LCCS1 at the molecular level. We show that the coiled-coil domain is critical for Gle1 self-association. Moreover, both hGle1 oligomerization and mRNA export functions are perturbed with the FinMajor protein. For yGle1, the coiled-coil domain is specifically required for mRNA export and not translation. These data reveal a novel step in the mRNA export pathway and provide direct evidence for a defect in hGle1 regulation of mRNA export at the NPC as the molecular mechanism causing the human LCCS1 disease.
RESULTS
Gle1 self-associates in vitro via its coiled-coil domain
To date no protein interaction partners for the Gle1 coiled-coil domain have been reported. Since coiled-coil domains are often utilized to mediate homotypic interactions, we speculated that Gle1 might self-associate. To test this, a series of in vitro biochemical experiments were conducted with recombinant purified proteins. First, for in vitro soluble binding assays, a glutathione-S-transferase (GST)-tagged N-terminal region of hGle1 (residues 1–362; GST-hGle1(1–362)) was expressed and purified from bacteria. GST-hGle1(1–362) or GST alone was incubated with [35S]methionine-labeled hGle1(1–362) generated with an in vitro rabbit reticulocyte lysate system and glutathione-Sepharose beads. Bound [35S]-hGle1(1–362) was eluted and analyzed by SDS-PAGE and autoradiography. An increased level of [35S]-hGle1(1–362) was bound with GST-hGle1(1–362) as compared to GST alone (Figure S1A). This suggested that the coiled-coil domain is sufficient to mediate self-association.
As an independent assessment of Gle1 self-association, sedimentation velocity analytical ultracentrifugation (SVAU) was employed. For this, recombinant maltose-binding-protein (MBP)-hGle1(1–362) was purified from bacteria (Figure S1B). MBP-hGle1(1–362) protomer itself has a predicted molecular mass of 0.084 MDa. Strikingly, MBP-hGle1(1–362) sedimented in a series of distinct peaks corresponding to at least eight species with high relative molecular masses ranging from 0.096 MDa to 2.0 MDa (Figure S2A). As controls, we examined purified MBP alone and a purified recombinant yGle1 polypeptide for the C-terminal domain (residues 241–538; yGle1(241–538)) by SVAU. For both, a single peak of low molecular mass was observed at 0.044 and 0.040 MDa, respectively (Figure S2C–D). Overall, the N-terminal coiled-coil domain of hGle1 was both necessary and sufficient to form large in vitro complexes potentially representing higher order oligomers. Next, the oligomeric state of recombinant MBP-h-gle1(1–365)-FinMajor was analyzed by SVAU. Similar to wild-type, MBP-h-gle1(1–365)-FinMajor sedimented in a series of distinct peaks ranging from 0.097 MDa to 1.9 MDa (Figure S2B). Thus, the FinMajor protein also self-associated and formed oligomeric complexes.
hGle1 oligomers form disk structures that are disorganized with FinMajor
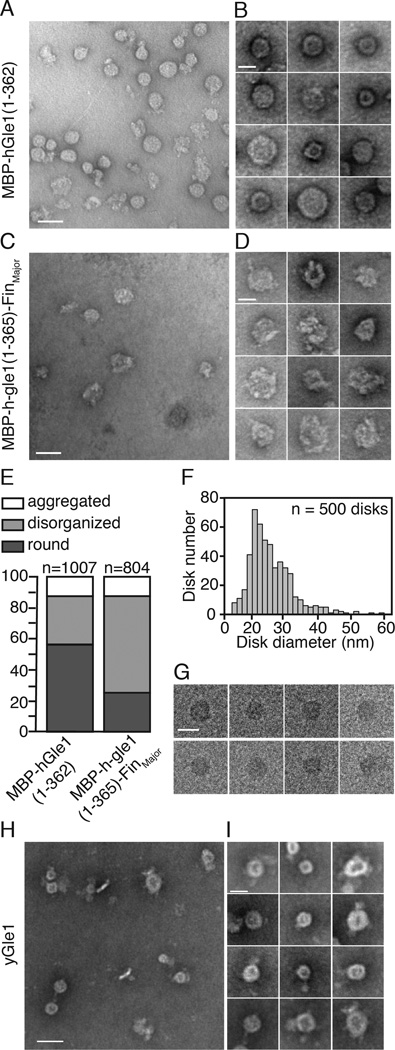
To gain insight into the hGle1 oligomer structure, the high molecular mass MBP-hGle1(1–362) complexes were analyzed using negative stain electron microscopy (EM). Purified, recombinant MBP-hGle1(1–362) samples at ∼0.5 mg/ml were further fractionated by size exclusion chromatography. Negative stain EM revealed that MBP-hGle1(1–362) formed ring/disk structures, as shown in a representative field (Figure 2A) and the montage of individual ring/disks (Figure 2B). Measurements of the diameter for 500 particles ranged from ∼15 nm to 60 nm, with an average size of 25.8 nm (standard deviation of 6.64 nm) (Figure 2F). To further investigate the structures, cryo-electron microscopy (cryo-EM) was conducted, allowing preservation of native protein structure and eliminating negative staining artifacts. The cryo-EM images showed that MBP-hGle1(1–362) oligomers adopted a disk-like shape in solution (Figure 2G).
Figure 2. Gle1 oligomers form disk structures.
(A–B) hGle1 forms large circular structures. (A) Representative EM image for purified MBP-hGle1(1–362). Bar, 50 nm. (B) Gallery of individual MBP-hGle1(1–362) particles. Bar, 25 nm. (C–E) FinMajor particles are malformed and disorganized. (C) Representative EM image of MBP-h-gle1(1–365)-FinMajor. Bar, 50 nm. (D) Gallery of individual MBP-h-gle1(1–365)-FinMajor particles. Bar, 25 nm. (E) Quantification of particle morphology for MBP-hGle1(1–362) and MBP-h-gle1(1–365)-FinMajor samples, categorized as aggregates, disorganized, or round. (F) hGle1 particles vary in diameter. Histogram of the measured diameter of MBP-hGle1(1–362) particles. (G) hGle1 oligomeric particles form disk-like structures. CryoEM images of MBP-hGle1(1–362) disk-shaped structures in vitrified ice. Bar, 25 nm. (H–I) Oligomeric disk structures are conserved through evolution. (H) Representative EM image of recombinant yGle1. Bar, 50 nm. (I) Gallery of individual yGle1 particles. Bar, 25 nm.
We next examined recombinant MBP-h-gle1(1–365)-FinMajor protein by negative stain EM. MBP-h-gle1(1–365)-FinMajor also formed disk-shaped particles; however, these appeared structurally disorganized compared to wild-type disks (Figure 2C–D). Fields of particles from independent purifications were compared and individual particles binned into three distinct structural categories: (1) aggregates, (2) disordered disks, and (3) round disks (Figure 2E). A significantly greater proportion of the MBP-h-gle1(1–365)-FinMajor particle samples were disordered disks. We concluded that the PFQ-insertion perturbs the in vitro oligomeric complex.
Gle1 oligomerization is structurally conserved
We hypothesized that recombinant yGle1 would also form disk-like oligomeric structures. To investigate this, recombinant untagged full-length yGle1 was purified and further fractionated by size exclusion chromatography. By negative stain EM, similar to MBP-hGle1(1–362), yGle1 formed disk-like structures (Figure 2H–I). Importantly, as isolation of untagged full-length hGle1 was technically not possible, analysis of full-length, untagged yGle1 provided strong evidence that disk structure formation was intrinsic to Gle1 and not an artifact of either the MBP tag or an isolated N-terminal domain. Moreover, the structural characteristics of the oligomer were conserved between yGle1 and hGle1.
hGle1 self-associates in living cells
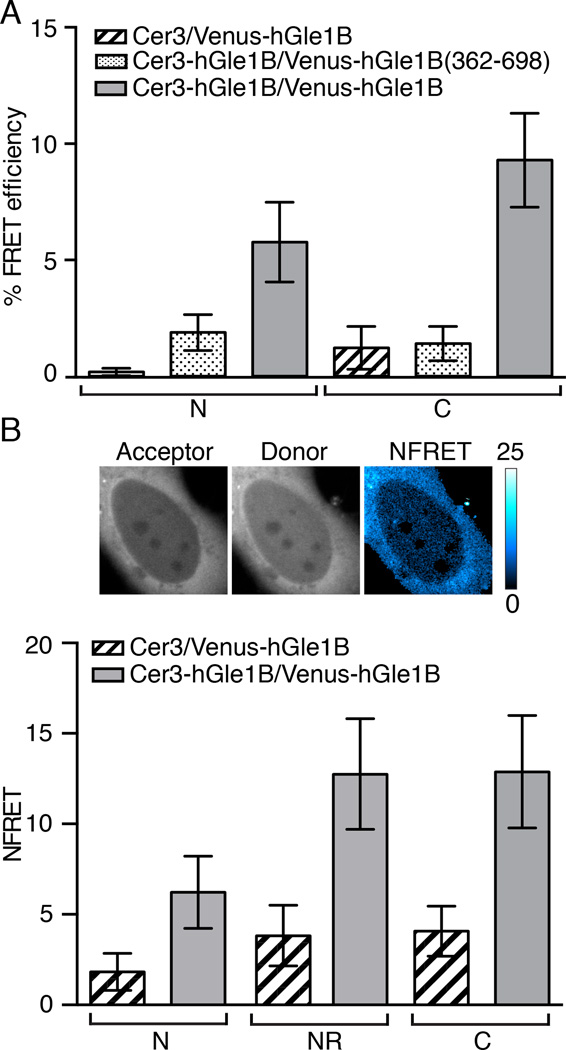
To test whether hGle1 self-associates in human cells, Forster resonance energy transfer (FRET) microscopy was used. Plasmids expressing hGle1B tagged with mVenus (Venus) or mCerulean3 (Cer3) were co-transfected into HeLa cells. Twelve hours post-transfection, FRET measurements were made in living cells using photo-acceptor bleaching FRET microscopy (Figure 3A). Strikingly, a FRET interaction of Cer3-hGle1B and Venus-hGle1B was detected in the cytoplasm (9.19% FRET efficiency) and in the nucleoplasm (5.85% FRET efficiency). In comparison, the percent FRET efficiency between Cer3 alone and Venus-hGle1B was low (<1.25%) in both the cytoplasm and nucleoplasm. As an additional control, a Venus fusion protein for only the hGle1B C-terminal region (residues 362 to 698) was tested. Only low FRET efficiency (<2.0%) for both the nucleoplasm and cytoplasm was detected with co-expression of Cer3-hGle1B and Venus-hGle1B(362–698) (Figure 3A). Thus, the coiled-coil domain was required to mediate hGle1B self-association in living cells.
Figure 3. hGle1B self-associates in living cells.
(A) hGle1B self-associates in living cells. Analysis of hGle1B interactions by acceptor photobleaching FRET microscopy in HeLa cells expressing the indicated fluorescent protein FRET pairs. FRET efficiencies of indicated regions were measured. Nucleoplasm and cytoplasm are designated by “N” and “C”, respectively. Error bars represent mean ± 95% confidence interval (CI) with n ≥ 20 cells from two independent experiments. (B) hGle1B self-associates at the nuclear rim. Analysis of sensitized emission FRET at the nuclear rim in HeLa cells expressing the indicated proteins. Shown are representative images of Venus-hGle1B (acceptor), Cer-hGle1B (donor) and the normalized FRET (NFRET) intensity map signal, top. Bar, 10µm. Bar graph depicts the NFRET signal for indicated regions, bottom. Nucleoplasm, cytoplasm, and nuclear rim are abbreviated as “N”,”C”, and “NR”, respectively. Error bars for each condition represent mean ± 95% CI with n ≥ 15 cells from at least two independent experiments.
During the acceptor bleaching event (∼50 seconds), subtle movement of the nuclear rim prevented accurate FRET measurements using the photo-acceptor bleaching method. Thus, sensitized emission FRET measurements were made. In cells expressing Cer3-hGle1B and Venus-hGle1B, we observed a normalized FRET (NFRET) signal in the cytoplasm (12.9), nucleoplasm (6.22), and at the nuclear rim (12.8) (Figure 3B). In cells expressing Cer3 and Venus-hGle1B cells, low NFRET (<4.08) signal was detected in all respective locations (Figure 3B). Together, these results indicated that hGle1 has the capacity to self-associate, at a minimum, as a dimer pair in living cells.
FinMajor perturbs essential hGle1 function in mRNA export
To investigate whether the FinMajor phenotype is due to perturbed mRNA export, translation initiation, and/or translation termination, several independent tests were conducted. Our previous studies found that hGle1 localization at the NPC is dependent on interactions with both hNup155 and hCG1 (Kendirgi et al., 2005; Rayala et al., 2004), and that hGle1 interacts with the translation initiation factor eIF3f (Bolger et al., 2008). Using the yeast two-hybrid assay, GBD-hGle1B and GBD-FinMajor DNA-binding domain (BD) bait proteins were analyzed with respective activation domain (AD) GAD-hCG1, GAD-hNup155, or GAD-eIF3f prey proteins, with β-galactosidase expression as the interaction readout. In all cases, the GBD-FinMajor was similar to wild-type GBD-hGle1B (Figure S3A). This suggested that the FinMajor protein was properly folded. Furthermore, the hCG1, Nup155 and eIF3f interactions were not perturbed by the PFQ insertion (Figure S3A).
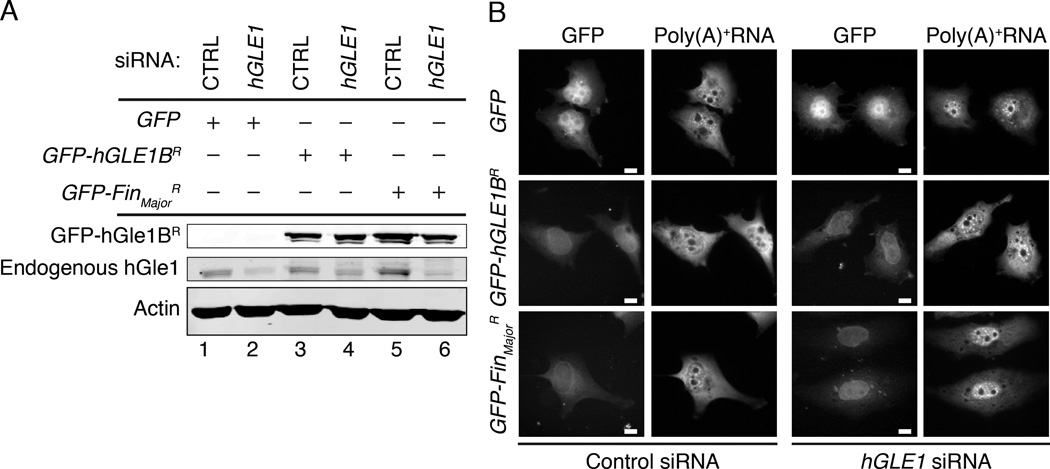
Because the LCCS1 disease is a homozygous recessive condition, we established a siRNA knockdown and add-back, human cell culture model system. As confirmed by immunoblotting, endogenous hGle1 levels were reduced by transfection of a small interfering RNA (siRNA) targeting hGLE1 (Figure 4A, lane 2). As a control, a scrambled siRNA was tested in parallel (CTRL). To assay mRNA export, the cellular distribution of bulk poly(A)+RNA in hGLE1 and CTRL siRNA cells was monitored by in situ hybridization with an oligo (dT) probe. Only cells treated with the hGLE1 siRNA showed robust nuclear accumulation of poly(A)+RNA (Figure 4B).
Figure 4. FinMajor has a defect in nuclear poly(A)+ RNA export.
(A) hGLE1 siRNA treatment depletes endogenous hGle1 protein levels. Immunoblot analysis of hGle1 and actin protein levels in scrambled control (CTRL) or hGLE1 siRNA-treated HeLa cells transfected with the indicated GFP-tagged proteins. (B) Nuclear poly(A)+ RNA accumulation in hGLE1 siRNA treated cells expressing the indicated GFP-tagged proteins, detected by in situ oligo-dT hybridization and direct fluorescence microscopy. See Figure S3B for quantification of the nucleocytoplasmic distribution of poly(A)+ RNA.
Rescue of the mRNA export defect was analyzed by expressing either GFP alone, a GFP-tagged siRNA-resistant (R) hGLE1B gene (GFP-hGLE1BR), or a GFP-tagged siRNA resistant FinMajor gene (GFP-FinMajorR). For each trial, the nuclear/cytoplasmic (N/C) ratio of the poly(A)+RNA distribution was determined by measuring fluorescence intensity. For CTRL siRNA with expression of GFP, GFP-hGLE1BR, or GFP-FinMajorR, the mean N/C ratio was ∼1.2, with no significant difference between the three conditions. In contrast, the mean N/C ratio for expression of only GFP with the hGLE1 siRNA was ∼1.6, reflecting nuclear accumulation. Importantly, expression of GFP-hGLE1BR with the hGLE1 siRNA rescued the mRNA export defect (mean N/C ratio = 1.2) confirming that the hGLE1 siRNA phenotype was not due to off-target effects (Figure 4B, Figure S3B). However, most strikingly, expression of the GFP-FinMajorR did not rescue the hGLE1 siRNA poly(A)+RNA nuclear accumulation defect (mean N/C ratio = 1.5). Immunoblotting confirmed expression of the GFP-hGle1BR (Figure 4A, lane 4) and GFP-FinMajorR (Figure 4A, lane 6) proteins. Moreover, similar to GFP-hGle1BR, the GFP-FinMajorR localized to the nuclear rim in both the CTRL and hGLE1 siRNA cells (Figure 6B). Thus, we concluded that FinMajor is defective for function in mRNA export.
Figure 6. Nucleocytoplasmic shuttling dynamics are altered for the LCCS1 FinMajor disease protein.
(A–B) FinMajor has altered nuclear shuttling activity. (A) FRAP analysis of HeLa cells expressing GFP-hGLE1B and GFP-FinMajor. (B) FRAP analysis of hGLE1 siRNA-treated HeLa cells expressing siRNA-resistant GFP-hGle1BR or GFP-FinMajorR. Bar, 10µm. (C–D) Recovery curves of the experimentally determined bleached region, fit with a one-phase association model. Error bars represent mean ± 95% CI with n ≥ 12 cells from 3 independent experiments.
FinMajor mimic insertions in yGle1 specifically alter mRNA export function
Robust assays for Gle1 roles in translation initiation and translation termination have to date been established only in the yeast S. cerevisiae model. In addition, due to protein solubility issues, reconstitution of Gle1-IP6 activation of Dbp5 has only been possible with the S. cerevisiae proteins. Thus, to further analyze the potential perturbations of FinMajor function in mRNA export, we conducted a series of experiments with yGle1. First, to directly test whether the yGle1 N-terminal region impacts Dbp5 activation, purified recombinant MPB-yGle1 proteins were assayed for in vitro Dbp5 ATPase stimulation. As reported, the C-terminal region of yGle1 (residues 241–538; A240Gle1) with IP6 is sufficient for stimulating Dbp5’s ATPase activity (Figure S4A) (Weirich et al., 2006). In side-by-side assays, the relative stimulation activity level for full-length yGle1 and y-Δ240Gle1 were similar (Figure S4A). Thus, in vitro activation Dbp5 did not require a functional yGle1 N-terminal coiled-coil domain.
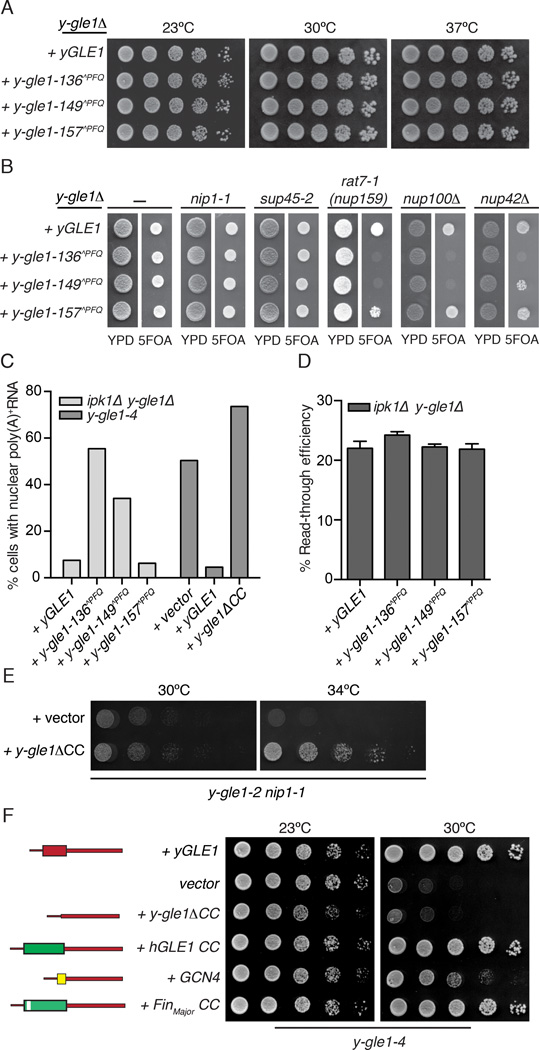
Based on the sequence and structural homologies and the fact that the hGle1 coiled-coil domain can partially complement the role of the yGle1 domain (Watkins et al., 1998), we designed FinMajor mimic insertions in yGle1 for tests of in vivo function. In silico analysis with the Paracoil2 coiled-coil prediction program showed that insertion of a three amino acid PFQ motif after amino acid 136 or 149 in yGle1 would potentially disrupt coiled-coil formation in a manner that would effectively mimic the structural effects of the PFQ found after residue 144 in the FinMajor protein (Figure 1C, Table S1–2) (Nousiainen et al., 2008). Importantly, the modeling analysis also predicted regions of the yGle1 coiled-coil domain that should be impacted less by a PFQ insertion. For example, adding PFQ after amino acid 157 in yGle1 was not predicted to change the potential for the region to form a coiled-coil (Figure 1C, Table S2). Based on this, y-gle1-136^PFQ, y-gle1-149^PFQ, and y-gle1-157^PFQ mutants were generated. The mutant strains showed no growth defects compared to wild-type yGLE1, indicating no global folding defects for the proteins (Figure 5A). Moreover, purified recombinant MBP-y-gle1-136^PFQ and MBP-y-gle1-149^PFQ proteins activated Dbp5 to the same relative level as wild-type MBP-yGle1 (Figure S4A). In addition, live cell direct fluorescence microscopy of yeast cells showed that the GFP-tagged y-gle1^PFQ proteins localized predominantly at the nuclear rim in a similar manner to wild-type GFP-yGle1 (Figure S5G) and GFP-FinMajor in HeLa cells (Figure 6B).
Figure 5. The FinMajor mimic y-gle1^PFQ alleles have specific defects in mRNA export.
(A) y-gle1^PFQ mutants exhibit no growth defect. Growth of the indicated strains in 5-fold serial dilution on YPD was monitored at the temperatures shown.(B) y-gle1^PFQmutants display genetic interactions with mRNA export mutants. Strains bearing the indicated mutation in combination with y-gle1Δ harboring a y-gle1^PFQ-LEU plasmid and a yGLE1/URA3 plasmid were monitored for growth at 23°C. Failure to grow on synthetic complete media containing 5-FOA indicates synthetic lethality. (C) The yGle1 PFQ insertions that mimic FinMajor perturb mRNA export, and y-gle1ΔCC expression does not rescue export defects. Nuclear accumulation of poly(A)+ RNA was detected by in situ oligo-dT hybridization following a shift to 37°C for 2 hours (ipk1Δy-gle1Δ) or 1 hour (y-gle1-4). Calculations were based on >100 cells/condition. See Figure S4D for representative FISH images. (D) y-gle1^PFQ mutants exhibit no defect in translation termination. Ratios of luciferase and β-galactosidase activities were determined and read-through efficiency expressed as the percentage from the reporter with a stop codon inserted in-frame into the linker region between the tandem β-galactosidase and luciferase coding sequences (denoted the TMV reporter) compared to the reporter lacking a stop codon (the TQ control) (Stahl et al., 1995). Standard error of the mean was calculated from 3 independent experiments.(E) y-gle1ΔCC rescues the temperature sensitivity of y-gle1-2 nip1-1. Growth of serially diluted strains on -LEU media was monitored at the indicated temperatures.(F) Oligomerization of Gle1 is required for function in vivo. Mutant y-gle1-4 strains harboring plasmids expressing yGLE1, vector only, y-gle1ΔCC, y-gle1ΔCC+hGLE1-CC, y-gle1ΔCC+GCN4, or y-gle1ΔCC+FinMajor-CC were monitored for growth in 5-fold serial dilution on -LEU media at the temperatures shown. (Left) Schematic representation of the coiled-coil chimeric proteins with yGle1 (red), GCN4 (yellow), hGLE1 (Green), and FinMajor (Green with white bar indicating PFQ insertion).
Next, synthetic fitness defects were tested for double mutants generated from the pairwise combination of the respective y-gle1-^PFQ mutants with mRNA export and/or translation mutants. This included several mRNA export specific mutants (nup100Δ, nup42A, rat7-1(nup159)), a translation initiation mutant (nip1-1), a translation termination mutant (sup45-2), and two mutants with defects in both mRNA export and translation termination (rat8-2(dbp5) and ipk1Δ) (Bolger et al., 2008; Murphy and Wente, 1996; Stutz et al., 1997). The y-gle1-157^PFQ mutant did not have synthetic fitness defects in any of the tested double mutants (Figure 5B, S4B, S4C), correlating with the prediction that it would not perturb the coiled-coil region. Strikingly, the y-gle1-136^PFQ and y-gle1-149^PFQ mutants had no effect when combined with either nip1-1 or sup45-2. However, the y-gle1-1 36^PFQ and y-gle1-149^PFQ mutants were synthetically lethal when combined with the nup100Δ or the rat7-1(nup159) mutant (Figure 5B). The y-gle1-136^PFQ was also synthetically lethal with nup42Δ (Figure 5B). This revealed a separation of function with the y-gle1-136^PFQ and y-gle1-149^PFQ mutants having defects in mRNA export and not in translation.
In combination with the ipk1Δ or the rat8-2(dbp5) mutant, only the y-gle1-136^PFQ allele showed synthetic growth defects compared to the single mutants (Figure S4B–C). Given that ipk1Δ double mutants were viable at the permissive growth temperature and Ipk1 (for IP6 production) is required for both mRNA export and translation termination (Bolger et al., 2008), the y-gle1^PFQ ipk1Δ mutants were excellent candidates for assaying functional effects. After shifting to the nonpermissive growth temperature of 37°C, a significant percentage of the y-gle1-136^PFQ ipk1Δ and the y-gle1-149^PFQ ipk1Δ cells showed nuclear poly(A)+RNA accumulation; whereas, the y-gle1-157^PFQ ipk1Δ cells did not exhibit a defect (Figure 5C and S4C). Next, a plasmid-based reporter assay was used to assess translation termination. By monitoring for production of tandem β-galactosidase/luciferase proteins that are separated by either a stop codon, a stem-loop, or no stop codon in their intervening linker, the level of stop codon read-through was determined (Stahl et al., 1995). As reported (Bolger et al., 2008; Alcazar-Roman et al., 2010), the ipk1Δ single mutant had ∼25% read-through. In comparison, no enhanced defects in termination efficiency were detected for any of the ipk1Δ y-gle1^PFQ double mutants (Figure 5D). As an independent test for translation initiation, we investigated whether expression of a y-gle1ΔCC mutant (with an internal in-frame deletion of the sequence encoding the coiled-coil domain) suppressed the growth defects linked to translation initiation in the y-gle1-2 nip1-1 mutant (Figure 5E). This was indeed observed. In contrast, y-gle1ΔCC expression did not rescue the mRNA export defect in y-gle1-4 cells (Figure 5C). Thus, y-gle1^PFQ mutants that mimic FinMajor had specific defects in mRNA export that correlated with the FinMajor results.
yGle1 oligomerization is required in vivo
We previously showed that expression of a chimeric yeast-human Gle1 protein rescues temperature sensitive growth properties of a y-gle1-4 S. cerevisiae mutant (Watkins et al., 1998). Specifically, when the sequence encoding the essential coiled-coil domain of yGle1 is deleted (y-gle1ΔCC), it can be replaced by an in-frame fragment encoding the coiled-coil domain of hGle1 (+hGLE1 CC) (Figure 5F). We further tested a FinMajor coiled-coil (+FinMajor CC) chimera and it rescued growth of the y-gle1-4 strain at 30°C to a similar level as the hGle1-CC chimera. However, neither the hGle1-CC nor the FinMajor-CC chimera complemented a lethal y-gle1Δ mutant. This indicated that the y-gle1ΔCC+hGLE1-CC was not fully functional and in the context of the chimera, the hGle1-CC domain was potentially perturbed in a manner similar to FinMajor. Building on this, we investigated if swapping in a heterologous oligomerization domain would complement functionality in vivo. Expression (Strikingly expression) of a chimeric protein with the coiled-coil region of yGle1 swapped for that of the well-characterized transcription factor Gcn4 (O'Shea et al., 1991) (+GCN4) partially rescued the temperature sensitive y-gle1-4 phenotype (Figure 5F), revealing that oligomerization is important for yGle1 function in vivo.
Nucleocytoplasmic shuttling of FinMajor is inhibited
Proper hGle1 nucleocytoplasmic shuttling is critical for mRNA export and requires a unique 39-amino acid span in the C-terminal region (Figure 1B) (Kendirgi et al., 2003). Given that FinMajor was defective in mRNA export and oligomer structure but still showed steady state localization at the nuclear rim (Figure 6B), we examined GFP-FinMajor dynamics in living cells using fluorescence recovery after photobleaching (FRAP). Due to the homozygous recessive FinMajor disease phenotype, experiments were conducted in HeLa cells with and without hGLE1 siRNA treatment (as in Figure 3). Nuclei of cells transiently expressing a respective GFP-tagged protein were photobleached and the nuclear GFP fluorescence was monitored over time (Figure 6 and S5). The FRAP data sets were fit with a one-phase exponential association model. Importantly, the relative t1/2 for wild-type GFP-hGle1B nuclear signal after FRAP in untreated HeLa cells (Figure 6A and C) was 12.3 min and correlated with our previous measurements by fluorescence loss in photobleaching (Kendirgi et al., 2003). When GFP-FinMajor, was assayed by FRAP in untreated cells, the t1/2 was not significantly different from wild-type GFP-hGle1B (Figure 6A and C). This was expected due to the homozygous recessive nature of the LCCS1 disease pathology (Nousiainen et al., 2008). FRAP analysis of wild-type GFP-hGle1BR in hGLE1 siRNA treated cells showed similar shuttling dynamics (t1/2 10.3 min) as compared to the presence of endogenous hGle1 (Figure 6B and 6D). However, strikingly, the shuttling dynamics of GFP-FinMajorR were significantly slower (t1/2 20.6 min) in hGLE1 siRNA treated cells (Figure 6B and 6D). In sum, as in the LCCS1 disease state, when expressed as the only hGle1 in the siRNA treated cells, FinMajor had inhibited nucleocytoplasmic shuttling. Thus, the defect in mRNA export was potentially due to the altered FinMajor oligomer structural state impacting nucleocytoplasmic shuttling.
hGle1 requires the coiled-coil domain for NPC localization
The hGle1 coiled-coil domain alone is not sufficient for nuclear rim localization (Kendirgi et al., 2005). To further analyze for roles of the hGle1 coiled-coil domain in subcellular localization, a GFP-tagged h-gle1B lacking the coiled-coil domain (GFP-h-gle1BΔCC) was monitored. HeLa cells were co-transfected with plasmids expressing Pom121-mCherry and either GFP-hGle1B or a GFP-h-gle1BΔCC. As reported, direct fluorescence microscopy in living cells revealed that GFP-hGle1B localized robustly to the nuclear rim overlapping with mCherry-Pom121 (Figure S5E) (Kendirgi et al., 2003). In contrast, the nuclear rim signal intensity for GFP-h-gle1BΔCC was markedly reduced (Figure S5E). Immunoblotting showed similar expression levels for both GFP-tagged proteins (Figure S5E). FRAP analysis revealed that GFP-h-gle1BΔCC shuttled faster than wild-type in both untreated (t1/2 8.0 min versus 12.3 min; Figure S5C and 6C) and hGLE1 siRNA treated (t1/2 5.0 min versus 10.3 min; Figure S5D and Figure 6D) cells. Overall, the hGle1 oligomeric state modulated both NPC localization and nucleocytoplasmic dynamics.
DISCUSSION
In this report, we document a novel requirement for Gle1 self-association during mRNA export and uncover molecular defects underlying a lethal human disease LCCS1. Our results show that wild-type Gle1 protomers form discrete multimers and higher-order disk structures in vitro, with evidence for dimer formation happening in living cells. This self-association occurs through the essential Gle1 coiled-coil domain wherein the LCCS1 FinMajor disease alteration resides. Importantly, disk structures formed with the FinMajor coiled-coil domain are more disordered and malformed. Moreover, in HeLa cells, the FinMajor protein is defective in mRNA export and has slowed nucleocytoplasmic shuttling. We propose that LCCS1 disease pathology is due to perturbations in Gle1 oligomerization and shuttling that disrupt efficient nuclear export of mRNA at NPCs.
Coiled-coil domains are often utilized to mediate the formation of biological homo and hetero-oligomeric complexes and directly impact protein function (Burkhard et al., 2001). We speculate that the FinMajor is a distinct perturbation of the hGle1 oligomeric structure compared to the h-gle1-ΔCC that does not oligomerize. This is based on the different effects on nucleocytoplasmic shuttling and steady state NPC localization. FinMajor shuttles slower and is detected at the nuclear rim, contrasted with h-gle1-ΔCC which shuttles faster and is not rim localized. Thus, oligomerization might regulate Gle1 residence time at the NPC, and hGle1 interactions with hNup155 and hCG1 could potentially facilitate Gle1 self-association. It is striking that the FinMajor analogous alleles in S. cerevisiae (y-gle1^PFQ) specifically disrupt mRNA export function but not yGle1 roles in translation initiation or termination, and that expressing y-gle1ΔCC rescues translation initiation. Thus, proper Gle1 self-association might only be strictly required at the NPC.
Our in vitro studies reveal that Gle1 forms large oligomeric disk structures with an average diameter of 25.8 nm (Figure 5D, 6 and 7A). This was surprising, and such structures have not been previously reported with other isolated NPC associated factors. It is tempting to speculate that these disk structures might be present in the NPC, which measures ∼105 nm in total diameter (Maimon et al., 2012). However, there are no reports of such an NPC-associated disk-like particle in the published structural studies of NPCs in intact cells, or of isolated NPCs or nuclear envelopes (Frenkiel-Krispin et al., 2010; Kiseleva et al., 2004; Maimon et al., 2012; Yang et al., 1998). In intact cells, the electron density of such a disk might not be detected via tomography approaches. It is also possible that the Gle1 oligomer dissociates (partially or fully) during NPC or nuclear envelope isolation. Further studies are needed to investigate these possibilities, and to determine the stoichiometry within the Gle1 oligomer in vitro and in vivo. Additionally, the presence of Gle1 binding partners in vivo will likely play important structural and/or regulatory roles affecting how Gle1 self-associates. Overall, using FRET microscopy, we observed hGle1 self-association in living cells (Figure 4A–B) and can conclude that, at a minimum, a dimer interaction exists at the NPC.
Gle1/IP6 function at the NPC cytoplasmic face triggers Dbp5-mediated mRNP remodeling and facilitates directional mRNA export through the NPC (Alcazar-Roman et al., 2006; Tran et al., 2007; Weirich et al., 2006). As part of the Dbp5 ATPase cycle, release of ADP from Dbp5 is mediated by Nup159 binding at the NPC cytoplasmic face (Noble et al., 2011). Interestingly, Nup159 protomers dimerize by interaction with Dyn2 (Stelter et al., 2007). Thus, both Dbp5 modulators at the NPC (Gle1 for ATP loading and ATPase activation, and Nup159 for ADP release) are at least dimers at the NPC.
As the C-terminal yGle1 domain is sufficient in vitro for stimulating Dbp5 ATPase activity, there are at least two working models by which Gle1 self-association might function in mRNA export. First, oligomerization might promote Gle1 enrichment at the NPC and generate a self-organized platform of multiple C-terminal Gle1 domains. This, in turn, could allow stimulation of the same Dbp5 molecule multiple times, or multiple Dbp5 molecules simultaneously, to promote efficient mRNP remodeling and directional mRNA export. Preliminary support for this model can be drawn from the y-gle1ACC+GCN4 chimera complementation results, wherein the Gcn4 coiled-coil domain facilitates assembly of parallel aligned dimers (O'Shea et al., 1991).
Alternatively, self-association might allow multiple distinct interactions with individual protomers in a Gle1 oligomer. Based on structural analysis of the yGle1 C-terminal domain (Montpetit et al., 2011), it is possible that the binding interfaces for Dbp5 and Nup42 are mutually exclusive. Thus, Gle1 self-association would allow a dimer (or higher order oligomers) to coincidentally bind the NPC and activate Dbp5 for mRNA export. Our ongoing studies will be aimed at investigating the Nup and Dbp5 binding interfaces on the oligomeric complex.
This work implicates Gle1 dysregulation of Dbp5’s mRNP remodeling activity during mRNA export as the key molecular pathological event in LCCS1. As the RNA-binding protein composition of an mRNP dictates both its regulation and function during gene expression (Muller-McNicoll and Neugebauer, 2013), altered mRNP remodeling during mRNA export likely has global cellular impacts. In addition to LCCS1, human genetic linkage analysis has identified additional GLE1 causal mutations that result in the Lethal Arthrogryposis with Anterior horn cell disease (Nousiainen et al., 2008). Further, recent deep sequencing studies report gle1 mutant alleles in other human diseases (Al-Qattan et al., 2012; Tzschach et al., 2012). For these diseases, it is unclear whether or how hGle1 has cell type specific effects. Indeed, our studies of GLE1 depletion in zebrafish show potential impacts on multiple proliferative organ precursors (Jao et al., 2012). With Gle1 uniquely positioned to modulate mRNP composition through regulation of multiple DBPs (Alcazar-Roman et al., 2006; Bolger and Wente, 2011; Weirich et al., 2006), we speculate that disruption of specific Gle1 mechanistic steps in export and/or translation results in different pathological outcomes.
Defective oligomerization of the SMN protein has been causally linked to some cases of Spinal Muscular Atrophy (SMA) (Lorson et al., 1998; Pellizzoni et al., 1999). In this case, defects in SMN oligomerization cause ineffective assembly of the small nuclear (sn)RNPs and disruption of pre-mRNA splicing (Shpargel and Matera, 2005; Wan et al., 2005). Our analysis of Gle1 self-association extends this paradigm for perturbations of RNP effector oligomerization, and highlights altered mRNP remodeling as a new molecular disease mechanism. Taken together, this work provides new evidence for the cellular mechanism underlying the lethal human LCCS1 disease, and impacts the broader understanding of the involvement of defective NPCs, altered mRNA transport and misregulated gene expression in human disease.
EXPERIMENTAL PROCEDURES
Full details of the experimental procedures can be found in the Extended Experimental Procedures.
HeLa Cell Culture and Immunoblotting
HeLa cells were cultured in complete medium (DMEM, Gibco) supplemented with 10% FBS (Atlanta Biologicals) at 37 °C in 5% CO2. Transient transfections of GFP expression vectors were performed using Fugene6 (Promega) according to manufacturer’s instructions. For siRNA experiments, cells were first transfected with a scrambled siRNA or siRNA targeting hGLE1 using HiPerFect (Qiagen). For live cell imaging experiments, cells were plated in 35mm No. 1.5 glass bottom dishes (Mattek). Before imaging, culture medium was replaced with phenol red-free DMEM (Gibco) supplemented with 10% FBS and 25mM HEPES. For paraformaldehyde fixation, cells were plated on No. 1.5 round coverslips in a 24 well plate (Fisher). For immunoblots, HeLa cells were processed as described (Bolger et al., 2008). Protein bands were visualized with a Li-COR Odyssey scanner (Lincoln, NE).
Yeast Strains, Plasmids, and Two Hybrid
Supplemental Tables S3 and S4 list the plasmids and S. cerevisiae strains used in this study. Yeast strains were grown at indicated temperatures in either YPD (2% peptone, 2% dextrose, 1% yeast extract) or selective minimal media lacking appropriate amino acids and supplemented with 2% dextrose, and 5-fluoroorotic acid (5-FOA, US Biological) as needed at 1.0 mg/ml. Yeast two hybrid analysis was performed as described (Clontech, Protocol #PT3024-1), with activities reported normalized to GBD-hGle1B β-galactosidase levels.
In situ Hybridization
S. cerevisiae cells were processed as described (Wente and Blobel, 1993). To localize poly(A)+RNA in HeLa cells, cells were fixed seventy-two hours post siRNA treatment and processed as described (Watkins et al, 1998). Both human and yeast cells were incubated with Cy3-conjugated oligo d(T) in hybridization for two hours. Images were acquired using a microscope (BX50; Olympus), Olympus 100×/1.3 UPlanF1 objective (Yeast samples) or Olympus 40×/1.3 UPlanF1 objective (HeLa samples), and digital charge coupled device camera (Orca-R2; Hamamatsu). Images were processed with ImageJ (NIH) or Adobe Photoshop CS6.
Translation Termination
Translation termination experiments using the tandem β-galactosidase-luciferase reporters were performed as described (Alcazar-Roman et al., 2010; Bolger et al., 2008).
Biochemical analysis of recombinant proteins
MBP-TEV-yGle1(241–528), MBP-TEV-yGle1, MBP-TEV-y-gle1-136^PFQ, MBP-TEV-y-gle1-149^PFQ, and GST-Dbp5 were purified as described (Tran et al., 2007). The GST-hGle1(1–362), MBP-hGle1(1–362), and MBP-h-gle1(1–365)-FinMajor were expressed in E. coli BL21-RIL (DE3) cells (Stratagene). Bacteria were lysed by sonication in buffer (200mM NaCl, 20mM Tris, pH 7.5), and the soluble fraction was used for affinity chromatography with either amylose resin (New England Biolabs) or glutathione-coupled sepharose (GE Healthcare) according to manufacturer recommendations. Size exclusion chromatography with a S200 column (GE Healthcare) was used to further isolate complexes. PK/LDH-coupled ATPase assays (Noble et al., 2011), soluble binding assays (Kendirgi et al., 2005), and SVAU analysis (Roberts-Galbraith et al., 2010) were performed as described. Velocity scans were analyzed using Sedfit (version 14.0) (Schuck and Rossmanith, 2000). Size distributions were determined for a confidence level of p = 0.95, a resolution of n = 200, and sedimentation coefficients between 0 and 80 S.
Live Cell Microscopy
All images were processed with ImageJ (NIH) or Adobe Photoshop CS6. Wild-type S. cerevisiae (W303) with plasmids harboring either y-GLE1-GFP (pBRR118b) or y-gle1-136^PFQ-GFP (pSW3779) were imaged as described (Noble et al., 2011) using a microscope (BX50; Olympus), Olympus 100×/1.3 UPlanF1 oil immersion objective, and digital charge coupled device camera (Orca-R2; Hamamatsu). For HeLa cells, photo-acceptor bleaching and sensitized emission FRET microscopy experiments were conducted on a confocal microscope (LSM710, Zeiss) using a Zeiss 40×/1.1 C-Apochromat water objective. Photo-acceptor bleaching was performed on HeLa cells co-transfected with: (1) pSW3775 (Cer-hGLE1B) and pSW3774 (Venus-hGLE1B), (2) mCerulean3-C1 and pSW3774, or (3) pSW3932 (Venus-hGle1B(362–698)) and pSW3775. Cell volume was bleached by exciting at 514 nm throughout the targeted region, and FRET efficiency was calculated. For sensitized emission FRET measurements, HeLa cells were co-transfected with either pSW3775 and pSW3774 or mCerulean3 and pSW3774. A normalized FRET (NFRET) signal for indicated regions was determined using standard methods. FRAP was performed on untreated HeLa cells co-transfected with Pom121-mCherry and either GFP-hGLE1B (pSW1831), GFP-FinMajor (pSW3903), or GFP-h-gle1BΔCC (pSW3894), or on hGLE1 siRNA treated HeLa cells co-transfected with Pom121-mCherry and either siRNA-resistant GFP-hGLE1BR (pSW3908), GFP-FinMajorR (pSW3945), or GFP-h-gle1BΔCC (pSW3894). All FRAP microscopy experiments were conducted on a confocal microscope (LSM710, Zeiss) using a Zeiss 40×/1.1 C-Apochromat water objective configured for time-lapse acquisition. Bleaching was achieved by exciting at 488 nm throughout the targeted region.
Electron Microscopy
Uranyl formate stained samples were prepared as described (Ohi et al., 2004). Samples were imaged on a FEI Morgagni electron microscope operated at an acceleration voltage of 100 kV. Images were recorded at a magnification of 22–36,000x and collected using a 1K × 1K CCD camera (ATM). To prepare samples in vitrified ice, a holey carbon grid (Quantifoil Micro Tools GmbH, Germany) was glow-discharged and used to adsorb gel-filtration purified human Gle1 particles. Grids were blotted and frozen in liquid ethane using a Vitrobot (FEI, Hillsboro, OR). Vitrified specimens were imaged under low-dose conditions at a nominal magnification of 100,000x at defocus values ranging from −3 to −5 µm using a Gatan cryo-transfer holder in a FEI Tecnai 200 kV electron microscope equipped with a field emission electron source (FEI, Hillsboro, OR) and 4K x 4K Gatan Ultrascan CCD.
Supplementary Material
HIGHLIGHTS.
Gle1 self-associates via its conserved, essential coiled-coil domain
Oligomerization of Gle1 is required for mRNA export and not translation
Gle1 oligomers form disk structures that are perturbed in the LCCS1 disease variant
mRNA export dysregulation at nuclear pore complexes is linked to LCCS1 pathology
ACKNOWLEDGEMENTS
The authors thank T. R. Dawson for advice and critical reading of the manuscript, the Wente laboratory, M. Rout, and C. Cole for discussions, C. Cole for yeast strains, and Y. Takizawa for technical assistance with cryoEM. This work was supported in part by the NCI Cancer Center Support Grant #P30 CA068485 utilizing the Cell Imaging Shared Resource, and grants NIH R37GM051219 (to S.R.W), NIH F31NS070431 (to A.W.F), NIH 1DP2OD004483 (to M.D.O.), and training positions on T32CA119925 (to A.W.F.) and T32GM008320 (to S.E.C).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL DATA
Supplemental Information includes Extended Experimental Procedures, four tables, and five figures.
REFERENCES
- Al-Qattan MM, Shamseldin HE, Alkuraya FS. Familial dorsalization of the skin of the proximal palm and the instep of the sole of the foot. Gene. 2012;500:216–219. doi: 10.1016/j.gene.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Alcazar-Roman AR, Bolger TA, Wente SR. Control of mRNA export and translation termination by inositol hexakisphosphate requires specific interaction with Gle1. J. Biol. Chem. 2010;285:16683–16692. doi: 10.1074/jbc.M109.082370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcazar-Roman AR, Tran EJ, Guo S, Wente SR. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat. Cell Biol. 2006;8:711–716. doi: 10.1038/ncb1427. [DOI] [PubMed] [Google Scholar]
- Bolger TA, Folkmann AW, Tran EJ, Wente SR. The mRNA export factor Gle1 and inositol hexakisphosphate regulate distinct stages of translation. Cell. 2008;134:624–633. doi: 10.1016/j.cell.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger TA, Wente SR. Gle1 is a multifunctional DEAD-box protein regulator that modulates Ded1 in translation initiation. J. Biol. Chem. 2011;286:39750–39759. doi: 10.1074/jbc.M111.299321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard P, Stetefeld J, Strelkov SV. Coiled-coils: a highly versatile protein folding motif. Trends Cell Biol. 2001;11:82–88. doi: 10.1016/s0962-8924(00)01898-5. [DOI] [PubMed] [Google Scholar]
- Cooper TA, Wan L, Dreyfuss G. RNA and disease. Cell. 2009;136:777–793. doi: 10.1016/j.cell.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkiel-Krispin D, Maco B, Aebi U, Medalia O. Structural analysis of a metazoan nuclear pore complex reveals a fused concentric ring architecture. J. Mol. Biol. 2010;395:578–586. doi: 10.1016/j.jmb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Hall JG. Genetic aspects of arthrogryposis. Clin Orthop Relat Res. 1985:44–53. [PubMed] [Google Scholar]
- Herva R, Leisti J, Kirkinen P, Seppanen U. A lethal autosomal recessive syndrome of multiple congenital contractures. Am. J. Med. Genet. 1985;20:431–439. doi: 10.1002/ajmg.1320200303. [DOI] [PubMed] [Google Scholar]
- Hurt JA, Silver PA. mRNA nuclear export and human disease. Dis. Model Mech. 2008;1:103–108. doi: 10.1242/dmm.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao LE, Appel B, Wente SR. A zebrafish model of lethal congenital contracture syndrome 1 reveals Gle1 function in spinal neural precursor survival and motor axon arborization. Development. 2012;139:1316–1326. doi: 10.1242/dev.074344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendirgi F, Barry DM, Griffis ER, Powers MA, Wente SR. An essential role for hGle1 nucleocytoplasmic shuttling in mRNA export. J. Cell Biol. 2003;160:1029–1040. doi: 10.1083/jcb.200211081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendirgi F, Rexer DJ, Alcazar-Roman AR, Onishko HM, Wente SR. Interaction between the shuttling mRNA export factor Gle1 and the nucleoporin hCG1: a conserved mechanism in the export of Hsp70 mRNA. Mol. Biol. Cell. 2005;16:4304–4315. doi: 10.1091/mbc.E04-11-0998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiseleva E, Allen TD, Rutherford S, Bucci M, Wente SR, Goldberg MW. Yeast nuclear pore complexes have a cytoplasmic ring and internal filaments. J. Struct. Biol. 2004;145:272–288. doi: 10.1016/j.jsb.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Lorson CL, Strasswimmer J, Yao JM, Baleja JD, Hahnen E, Wirth B, Le T, Burghes AH, Androphy EJ. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nat. Genet. 1998;19:63–66. doi: 10.1038/ng0598-63. [DOI] [PubMed] [Google Scholar]
- Maimon T, Elad N, Dahan I, Medalia O. The human nuclear pore complex as revealed by cryo-electron tomography. Structure. 2012;20:998–1006. doi: 10.1016/j.str.2012.03.025. [DOI] [PubMed] [Google Scholar]
- Montpetit B, Thomsen ND, Helmke KJ, Seeliger MA, Berger JM, Weis K. A conserved mechanism of DEAD-box ATPase activation by nucleoporins and InsP6 in mRNA export. Nature. 2011;472:238–242. doi: 10.1038/nature09862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-McNicoll M, Neugebauer KM. How cells get the message: dynamic assembly and function of mRNA-protein complexes. Nat. Rev. Genet. 2013;14:275–287. doi: 10.1038/nrg3434. [DOI] [PubMed] [Google Scholar]
- Murphy R, Wente SR. An RNA-export mediator with an essential nuclear export signal. Nature. 1996;383:357–360. doi: 10.1038/383357a0. [DOI] [PubMed] [Google Scholar]
- Noble KN, Tran EJ, Alcazar-Roman AR, Hodge CA, Cole CN, Wente SR. The Dbp5 cycle at the nuclear pore complex during mRNA export II: nucleotide cycling and mRNP remodeling by Dbp5 are controlled by Nup159 and Gle1. Genes Dev. 2011;25:1065–1077. doi: 10.1101/gad.2040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nousiainen HO, Kestila M, Pakkasjarvi N, Honkala H, Kuure S, Tallila J, Vuopala K, Ignatius J, Herva R, Peltonen L. Mutations in mRNA export mediator GLE1 result in a fetal motoneuron disease. Nat. Genet. 2008;40:155–157. doi: 10.1038/ng.2007.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea EK, Klemm JD, Kim PS, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled-coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- Ohi M, Li Y, Cheng Y, Walz T. Negative Staining and Image Classification - Powerful Tools in Modern Electron Microscopy. Biol. Proced. Online. 2004;6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni L, Charroux B, Dreyfuss G. SMN mutants of spinal muscular atrophy patients are defective in binding to snRNP proteins. Proc. Natl. Acad. Sci. USA. 1999;96:11167–11172. doi: 10.1073/pnas.96.20.11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayala HJ, Kendirgi F, Barry DM, Majerus PW, Wente SR. The mRNA export factor human Gle1 interacts with the nuclear pore complex protein Nup155. Mol. Cell. Proteomics. 2004;3:145–155. doi: 10.1074/mcp.M300106-MCP200. [DOI] [PubMed] [Google Scholar]
- Renoux AJ, Todd PK. Neurodegeneration the RNA way. Prog. Neurobiol. 2012;97:173–189. doi: 10.1016/j.pneurobio.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Galbraith RH, Ohi MD, Ballif BA, Chen JS, McLeod I, McDonald WH, Gygi SP, Yates JR, 3rd, Gould KL. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol. Cell. 2010;39:86–99. doi: 10.1016/j.molcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck P, Rossmanith P. Determination of the sedimentation coefficient distribution by least-squares boundary modeling. Biopolymers. 2000;54:328–341. doi: 10.1002/1097-0282(20001015)54:5<328::AID-BIP40>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Shpargel KB, Matera AG. Gemin proteins are required for efficient assembly of Sm-class ribonucleoproteins. Proc. Natl. Acad. Sci. USA. 2005;102:17372–17377. doi: 10.1073/pnas.0508947102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl G, Bidou L, Rousset JP, Cassan M. Versatile vectors to study recoding: conservation of rules between yeast and mammalian cells. Nucleic Acids Res. 1995;23:1557–1560. doi: 10.1093/nar/23.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelter P, Kunze R, Flemming D, Hopfner D, Diepholz M, Philippsen P, Bottcher B, Hurt E. Molecular basis for the functional interaction of dynein light chain with the nuclear-pore complex. Nat. Cell Biol. 2007;9:788–796. doi: 10.1038/ncb1604. [DOI] [PubMed] [Google Scholar]
- Strahm Y, Fahrenkrog B, Zenklusen D, Rychner E, Kantor J, Rosbach M, Stutz F. The RNA export factor Gle1p is located on the cytoplasmic fibrils of the NPC and physically interacts with the FG-nucleoporin Rip1p, the DEAD-box protein Rat8p/Dbp5p and a new protein Ymr 255p. EMBO J. 1999;18:5761–5777. doi: 10.1093/emboj/18.20.5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutz F, Kantor J, Zhang D, McCarthy T, Neville M, Rosbash M. The yeast nucleoporin rip1p contributes to multiple export pathways with no essential role for its FG-repeat region. Genes Dev. 1997;11:2857–2868. doi: 10.1101/gad.11.21.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran EJ, Zhou Y, Corbett AH, Wente SR. The DEAD-box protein Dbp5 controls mRNA export by triggering specific RNA:protein remodeling events. Mol. Cell. 2007;28:850–859. doi: 10.1016/j.molcel.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Tzschach A, Grasshoff U, Schaferhoff K, Bonin M, Dufke A, Wolff M, Haas-Lude K, Bevot A, Riess O. Interstitial 9q34.11-q34.13 deletion in a patient with severe intellectual disability, hydrocephalus, and cleft lip/palate. American Journal of Medical Genetics Part A. 2012;158A:1709–1712. doi: 10.1002/ajmg.a.35398. [DOI] [PubMed] [Google Scholar]
- Wan L, Battle DJ, Yong J, Gubitz AK, Kolb SJ, Wang J, Dreyfuss G. The survival of motor neurons protein determines the capacity for snRNP assembly: biochemical deficiency in spinal muscular atrophy. Mol. Cell Biol. 2005;25:5543–5551. doi: 10.1128/MCB.25.13.5543-5551.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JL, Murphy R, Emtage JL, Wente SR. The human homologue of Saccharomyces cerevisiae Gle1p is required for poly(A)+ RNA export. Proc. Natl. Acad. Sci. USA. 1998;95:6779–6784. doi: 10.1073/pnas.95.12.6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirich CS, Erzberger JP, Flick JS, Berger JM, Thorner J, Weis K. Activation of the DExD/H-box protein Dbp5 by the nuclear-pore protein Gle1 and its coactivator InsP6 is required for mRNA export. Nat. Cell Biol. 2006;8:668–676. doi: 10.1038/ncb1424. [DOI] [PubMed] [Google Scholar]
- Wente SR, Blobel G. A temperature-sensitive NUP116 null mutant forms a nuclear envelope seal over the yeast nuclear pore complex thereby blocking nucleocytoplasmic traffic. J. Cell Biol. 1993;123:275–284. doi: 10.1083/jcb.123.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Rout MP, Akey CW. Three-dimensional architecture of the isolated yeast nuclear pore complex: functional and evolutionary implications. Mol. Cell. 1998;1:223–234. doi: 10.1016/s1097-2765(00)80023-4. [DOI] [PubMed] [Google Scholar]
- York JD, Odom AR, Murphy R, Ives EB, Wente SR. A phospholipase C-dependent inositol polyphosphate kinase pathway required for efficient messenger RNA export. Science. 1999;285:96–100. doi: 10.1126/science.285.5424.96. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.