Abstract
Tripeptidyl-peptidase 1 (TPP1) null or residual activity occurs in neuronal ceroid lipofuscinosis (NCL) with underlying TPP1/CLN2 mutations. A survey of 25 South American CLN2 affected individuals enabled the differentiation of two phenotypes: classical late-infantile and variant juvenile, each in approximately 50% of patients, with residual TPP1 activity occurring in approximately 32%. Each individual was assigned to one of three subgroups: (I) n=11, null TPP1 activity in leukocytes; (II) n=8, residual TPP1 activity of 0.60–15.85 nmol/h/mg (nr 110–476); (III) n=6, activity not measured in leukocytes. Curvilinear bodies (CB) appeared in almost all studied CLN2 subjects; the only exceptions occurred in cases of subgroup II: two individuals had combined CBs/fingerprints (FPs), and one case had pure FPs. There were 15 mutations (4 first published in this paper, 3 previously observed in South America by our group, and 8 previously observed by others). In subgroup I, mutations were either missense or nonsense; in subgroups II and III, mutations prevailed at the non-conserved intronic site, c.887-10A>G (intron 7), and to a lesser extent at c.89+5G>C (intron 2), in heterozygous combinations. Grouping phenotypically and genetically known individuals on the basis of TPP1 activity supported the concept that residual enzyme activity underlies a protracted disease course. The prevalence of intronic mutations at nonconserved sites in subgroup II individuals indicates that some alternative splicing might allow some residual TPP1 activity.
Keywords: Neuronal ceroid lipofuscinoses, Tripeptidyl-peptidase 1, TPP1/CLN2 gene, phenotype, South American population, mutational spectrum
1. Introduction
The neuronal ceroid lipofuscinoses (NCLs) are the most common group of inherited progressive neurodegenerative diseases of childhood (Goebel, 1995), and are classified as lysosomal disorders (LDs) (Cox and Cachon-Gonzalez, 2012). The childhood forms are characterized clinically by progressive motor and mental deterioration, vision loss, behavioral changes, and severe seizures, resulting in a persistent vegetative state and ending in premature death (Rider and Rider, 1999). Dementia is the main clinical symptom in the adult forms (Siintola et al., 2006a; Noskova et al., 2011; Velinov et al., 2012), and is often confused with other neurodegenerative diseases of adulthood. The storage of autofluorescent ceroid-lipofuscin-like bodies in the brain and in peripheral tissues is characteristic of these diseases (Goebel, 1995; Kida et al., 2001), which can exhibit various fine structural phenotypes, such as granular osmiophilic deposits (GRODs), curvilinear bodies (CBs), fingerprint profiles (FPs), rectilinear bodies (RBs), or mixed. NCLs are inherited in an autosomal recessive manner, except for one autosomal dominant adult form (Noskova et al., 2011; Velinov et al., 2012). According to the newly proposed classification scheme recently published by Williams and Mole (2012), nine NCL-causative genes have been identified and can be functionally grouped as “soluble lysosomal enzyme deficiencies” PPT1/CLN1 (Vesa et al., 1995), TPP1/CLN2 (Sleat et al., 1997; Liu et al., 1998), and CTSD/CLN10 (Siintola et al., 2006b; Steinfeld et al., 2006), or “nonenzyme deficiencies” CLN3 (The International Batten Disease Consortium, 1995), DNAJC5/CLN4 (Noskova et al., 2011; Velinov et al., 2012), CLN5 (Savukoski et al., 1998), CLN6 (Gao et al., 2002; Wheeler et al., 2002; Arsov et al., 2011), MFSD8/CLN7 (Siintola et al., 2007), and CLN8 (Ranta et al., 1999). Remaining genes whose classification is still uncertain are CLCN6 (Poet et al., 2006), SGSH (Sleat et al., 2009), CLN9 (Schulz et al., 2004), GRN/CLN11 (Smith et al., 2012), ATP13A2/CLN12 (Bras et al., 2012), CTSF/CLN13 (Tang et al., 2006), and KCTD7/CLN14 (van Bogaert et al., 2007; Krabichler et al., 2012; Staropoli et al., 2012).
Mutations in the TPP1/CLN2 gene located on chromosome 11p15.4 (Gene ID: 1200) result in a deficiency of the lysosomal enzyme Tripeptidyl-peptidase 1 (TPP1; EC 3.4.14.9) (CLN2; OMIM ID: 204500) (Zhong, 2005). Pathogenic TPP1/CLN2 mutations were assumed to typically result in the classical late infantile phenotype (LI-NCL), with onset of symptoms between 2 and 4 years of age, including seizures, progressive encephalopathy, visual failure, and motor abnormalities (Jalanko and Braulke, 2009). Pathologic material has been characterized in the literature by lysosomal deposits with mainly CB morphology (Wisniewski et al., 1999). Variant forms with a later onset or protracted disease course have been reported worldwide in a small number of patients (Hartikainen et al., 1999; Wisniewski et al., 1999; Zhong et al., 2000; Steinfeld et al., 2002; Bessa et al., 2008; Elleder et al., 2008). In most cases, the phenotype of these individuals resembled juvenile forms (J-NCL) and the patients were usually compound heterozygotes (Mole et al., 2005). In addition to the typical CB morphology observed in the “classical” LI-NCL phenotype, electron microscopy revealed FPs (Kohan et al., 2009) and occasional GRODs (Williams et al., 1999). A variant infantile phenotype described in a few CLN2 patients, with onset before the first year of age, was also reported (Simonati et al., 2000; Ju et al., 2002).
CLN2 is the prevalent genotype in South America, and is recognized in individuals with the classical LI and milder vJ presentations (Kohan et al., 2009; Kohan, 2011). Null or residual TPP1 activity occurs in NCLs with underlying TPP1/CLN2 mutations. The aim of this study was to determine whether the two subtypes, null or residual TPP1 activity, correlate with the mutational spectrum, clinical phenotypes, and morphotypes, to support consistent differences among them.
2. Material and methods
2.1. Study participants
We profiled the phenotypes and genotypes of 25 CLN2 individuals (n=9 retrospective (Kohan et al., 2009) and n=16 prospective) belonging to Argentinean and Chilean families. Controls for enzyme and molecular studies were obtained from volunteer donors from a similar ethnic population. Each individual was assigned to one of three subgroups: (I) null TPP1 activity in leukocytes; (II) residual TPP1 activity; and (III) activity not measured in leukocytes. Subgroup III comprised three Chilean patients for whom dried blood spots (DBSs) were the only available sample, and three additional retrospective individuals related to other NCL patients, who died before the implementation of the NCL Program in Argentina. All procedures were performed in accordance with the Universal Declaration on Bioethics and Human Rights, UNESCO-2005. The investigation was approved by the CIEIS - Polo Hospitalario of the Province of Córdoba, Argentina (registration no. 132). All study participants provided written informed consent for participation in the research project and authorization to release medical information.
2.2. Determining TPP1 enzyme activity
TPP1 activity was evaluated in samples of leukocytes (the gold standard) (Sohar et al., 1999; Sohar et al., 2000), saliva (Kohan et al., 2005), and/or DBSs (Lukacs et al., 2003), according to previously published methods. Palmitoyl protein thioesterase 1 (PPT1; EC 3.1.2.22) activity was measured as a control of the sample’s integrity (van Diggelen et al., 1999). Briefly, the substrate for TPP1, 0.25 mM Ala-Ala-Phe-7-amido-4-methylcoumarin (Sigma-Aldrich, Buenos Aires, Argentina) diluted fresh from a 25 mM stock solution in dimethyl sulfoxide and kept at -20°C) was prepared in 0.15 M sodium chloride, 1 g/L Triton X-100, 0.1 M sodium acetate, at pH 4.0 at 20°C (Sohar et al., 1999; Sohar et al., 2000). The leukocyte pellets were prepared as published (Kohan, 2011). The saliva (+/−0.3 ml) was collected with a disposable Pasteur pipette from the mouth under the tongue after at least 3 h of fasting, without any mouth cleansing. It was cleared by centrifugation at 15,000 × g for 45 min. The samples were stored at -20°C until assayed (Kohan et al., 2005). Protein measurements were performed according to the method ofLowry et al. (1951). The assay fractions contained 3 µg/10µL protein in a final volume of 0.5 mL. DBSs (3 mm) were punched from filter paper (S&S903) into Eppendorf tubes (Lukacs et al., 2003). Each spot was eluted with 20 µL substrate buffer and 40 µL NaCl (9 g/L). The tubes were shaken for 45 min at room temperature. Incubation times were 1 h for leukocyte pellets, 24 h for saliva, and 45 h for DBSs at 37°C. The reaction was stopped by the addition of 0.1 M monochloroacetic acid/0.13 M NaOH/0.1 M acetic acid, pH 4.3. Measurements were performed using an LS 50 B fluorometer (Perkin Elmer, Waltham, MA, USA) at an excitation wavelength of 360 nm and an emission wavelength of 460 nm. Product formation was converted from fluorescent units to nanomoles using 7-amino-4- methylcoumarin calibrators.
2.3. Transmission electron microscopy
Transmission electron microscopy (TEM) was performed on skin biopsies. These were fixed in 1.5% Karnowsky/1% osmium tetroxide in 0.1M sodium cacodylate buffer (pH 7.2), and embedded in Durcupan (Sigma-Aldrich, Buenos Aires, Argentina). Thin sections were cut with a diamond knife on a JEOL JUM-7 ultramicrotome (Nikon, Tokyo, Japan), stained with uranyl acetate and lead citrate, and examined with a Zeiss LEO 906-E electron microscope equipped with a Megaview III digital camera (Oberkochen, Germany).
2.4. PCR amplification
Blood samples were collected in tubes containing ethylenediamine tetraacetic acid (EDTA), and genomic DNA was extracted using the Wizard® DNA Purification Kit (Promega, Fitchburg, WI, USA), according to the manufacturer’s instructions. Each of the 13 exons and the adjacent intronic sequences in the TPP1/CLN2 gene (GenBank ID: AF017456.1, with nucleotide 1 counted as the first nucleotide of the translation initiation codon) was amplified using previously published intron-based primers (Liu et al., 1998). PCR amplification was performed using AmpliTaq Gold (Roche, NJ, USA) and an amplification protocol comprising an initial hold of 5 min at 95°C; 30 cycles of 30 sec at 95°C, 30 sec at 62°C, and 1 min at 72°C; and an extension step of 5 min at 72°C. PCR products were purified using the QIAquick Multiwell PCR Purification System (Qiagen, Valencia, CA, USA), and the purified products were sequenced bi-directionally on an ABI 3730XL capillary gel electrophoresis system (Applied Biosystems, Inc., Foster City, CA, USA) using the same primer sets mentioned above (Liu et al., 1998). Positive mutations and polymorphisms were identified by comparing bi-directional sequence data against normal control sequence, and were confirmed by independent re-amplification and bi-directional sequencing from the patients’ original DNA. Polymorphisms were re-named according to the SNP database (http://www.ncbi.nlm.nih.gov/snp?term=tpp1%20human).
2.5. Experimental validation of DNA changes
A series of complementary methods were used to differentiate pathogenic mutations from common polymorphisms in the population (den Dunnen and Antonarakis, 2000). New missense changes were presumed pathogenic through analysis of co-segregation of the change in the parents. Direct sequencing was used to verify their absence in 200 chromosomes from healthy individuals of the same ethnic group.
2.6. Bioinformatic analysis
A variety of web tools were used for bioinformatic analysis of the new missense mutations. We used the ClustalW2 program (http://www.ebi.ac.uk/Tools/clustalw2/index.html) to set the alignment of TPP1 sequences from different species in the protein sequence region of the new missense changes with UniproKW (http://www.uniprot.org). Polymorphism Phenotyping-2 (PolyPhen-2, http://genetics.bwh.harvard.edu/pph2) (Adzhubei et al., 2010) predicted the possible impact of an amino acid substitution on the structure and function of a human protein through direct physical and comparative considerations. The program Sorting Intolerant from Tolerant (SIFT, http://sift.jcvi.org) (Ng and Henikoff, 2003; Kumar et al., 2009) sorted missense substitutions and classified them as tolerated or deleterious. The program PopMusic 2.1 (http://babylone.ulb.ac.be/popmusic) (Dehouck et al., 2011) predicted changes in the thermodynamic stability of proteins caused by mutations, using a statistical potential linear combination whose coefficients depend on the solvent accessibility of the mutated residue. Finally, the Protein Data Bank (PDB, http://www.rcsb.org/pdb/home/home.do) and PyMOL version 1.1 (http://www.pymol.org) were used to analyze the three-dimensional structure of TPP1 protein (PDB ID: 3EDY) and the location of the new mutations.
3. Results
3.1. Patient classification
The phenotypes and genotypes of the 25 CLN2 affected individuals were profiled through clinical data collection, enzymology, electron microscopy, and genotyping. The individuals were separated into three subgroups: I, null TPP1 activity; II, residual TPP1 activity of any magnitude in leukocytes (exceptionally, one result in saliva); and III, enzyme activity obtained in DBSs or not measured (Table 1). Because the DBS technique often gives false negative results owing to its low sensitivity, DBS results were not considered for the classification of individuals in subgroups I or II. The residual TPP1 activity in leukocytes ranged from 0.60 to 15.85 nmol/h/mg protein, for a control range of 110–476 nmol/h/mg protein. Among subgroup II individuals, the percentage of residual TPP1 activity in leukocytes ranged from 0.35% to 9.30% of the mean for the controls, occurring in approximately 32% of the studied CLN2 subjects (n=8 of 25).
Table 1.
Subgroups I, II, and III: phenotypes and genotypes of CLN2 individuals from Argentina and Chile.
| ID Number- COUNTRY |
ONSET AGE |
CURRENT AGE / † AGE AT DEATH |
TPP1 ACTIVITYa |
MORPHO TYPE |
ALLELE 1 | ALLELE 2 | POLYMORPHISMS (SNPs) |
|
|---|---|---|---|---|---|---|---|---|
| SUBGROUP I: NO RESIDUAL TPP1 ACTIVITY | ||||||||
| 016-0303-AR | 3 y | † 9 y | 0.00 | CB | E6 p.Arg208*e | E7 p.Asp276Vale | - | - |
| 070-0701-AR | 3 y | 15 y | 0.00b | CB | E7 p.Asp276Vale | E7 p.Asp276Vale | - | - |
| 073-0312-AR | 3 y | 12 y | 0.00 | CB | E7 p.Asp276Vale | E7 p.Asp276Vale | - | - |
| 082-2219-AR | 4 y | 11 y | 0.00 | CB | E4 p.Leu104*e | E11 p.Ala453Vale |
I6 c.687+32T>C, (rs1800710)e |
- |
| 117-1312-AR | 3 y | 10 y | 0.00 | CB | E4 p.Leu104* | E4 p.Leu104* |
I6 c.687+32T>C, (rs1800710)e |
I6 c.687+32T>C, (rs1800710)e |
| 127-0414-AR | 3.5 y | 8 y | 0.00 | CB | E6 p.Arg208* | E7 p.Asp276Val | - | - |
| 134-1107-AR | 4 y | 11 y | 0.00 | CB | E7 p.Asp276Val | E7 p.Asp276Val | - | - |
| 147-1807-AR | 5 y | 7 y | 0.00 | nd | E8 p.Arg350Trp | E13 p.Gly535Arg | - | - |
| 153-0601-AR | ? | ? | 0.00 | CB | E6 p.Arg208* | E6 p.Arg208* | - | - |
| 154-2010-AR | 3.5 y | 10 y | 0.00 | CB | I5 c.509-1G>C | E7 p.Asp276Val |
I6 c.687+32T>C, (rs1800710) |
- |
| 162-0411-AR | 4 y | 7 y | 0.00 | CB | E8 p.Arg339Gln | E8 p.Arg339Gln |
I5 c.508+26A>T, (rs1800738) |
I5 c.508+26A>T, (rs1800738) |
| SUBGROUP II: RESIDUAL TPP1 ACTIVITY | ||||||||
| 005-1507-AR | 9 y | 36 y | 4.80 | nd | E3 p.Gln66*e | I7 c.887-10A>Ge | - | - |
| 005-1503-AR | 9 y | † 27 y | 0.60 | CB+FP | E3 p.Gln66*e | I7 c.887-10A>Ge | E11 p.Val426Vale | I2 c.89+4A>Ge |
| 081-1004-CL | 2 y | 9 y | 2.90 | CB | E7 p.Asp276Val | E9 c.1107- 1108delTG |
I5 c.508+26A>T, (rs1800738) |
I8 c.1075+28C>T, (rs7943955) |
| 147-1805-AR | 5 y | 17 y | 9.70 | FP | E8 p.Arg350Trp | E13 p.Gly535Arg |
I5 c.508+26A>T, (rs1800738) I8 c.1075+42C>T, (rs2072651) |
I6 c.687+32T>C, (rs1800710) E12 p.Gly514Gly, (rs1128396) |
| 149-0110-AR | 3 y | 8 y | 8.76 | nd | E11 p.Ala453Asp | I2 c.89+5G>C | E11 p.Ser472Ser |
I5 c.508+26A>T, (rs1800738) |
| 149-0112-AR | 2.5 y | 6 y | 15.85 | nd | E11 p.Ala453Asp | I2 c.89+5G>C | E11 p.Ser472Ser | - |
| 173-1307-AR | 9 y | 19 y | 6.45 | CB+FP+ GROD |
E6 p.Arg208* | I7 c.887-10A>G | - | - |
| 185-1903-AR | 5 y | 13 y | 4.16 | CB | E11 p.Arg447His | E11 p.Ser475Leu | - | - |
| SUBGROUP III: TPP1 ACTIVITY DETERMINED IN DBSc/ NOT DETERMINED | ||||||||
| 005-1501-AR | 9 y | † 27 y | nd | nd | E3 p.Gln66*d | I7 c.887-10A>Gd | - | - |
| 082-1806-AR | 4.5 y | † 13 y | nd | nd | E7 p.Asp276Vald | E11 p.Ala453Vald | - | - |
| 099-1303-CL | 3 y | 16 y | 0.07 | CB | E11 p.Ser475Leu | I7 c.887-10A>G | - | - |
| 123-0713-CL | 4 y | 10 y | (0.11#) | CB | E7 p.Asp276Val | E7 p.Asp276Val | - | - |
| 126-0113-CL | 10y | 16 y | (0.15 #) | CB | E7 p.Asp276Val | I7 c.887-10A>G | - | - |
| 173-1308-AR | 5 y † | 21 y | nd | nd | E6 p.Arg208*d | I7 c.887-10A>Gd | - | - |
Reference sequence: GenBank ID: AF017456.1; AR, Argentina; CL, Chile; y, years; nd, not determined;
TPP1 measured in leukocytes: χ 170.5 nmol/h/mg protein; normal range 110–476;
TPP1 measured in saliva: χ 218 nmol/24 h/mg protein; normal range 92–476;
PP1 measured in DBS: χ 0.26 nmol/spot; normal range 0.1–0.81;
These results correspond to activities in DBSs in the control range (false negatives);
Molecular results deducted from studies performed on other family members or using banked DNA samples;
Results published previously by the authors. Note: DNA pathogenic mutations in intronic splice sites and other DNA changes in non-coding positions are designated in bold type in the table. Siblings or other family members share the first three ciphers of the notation.
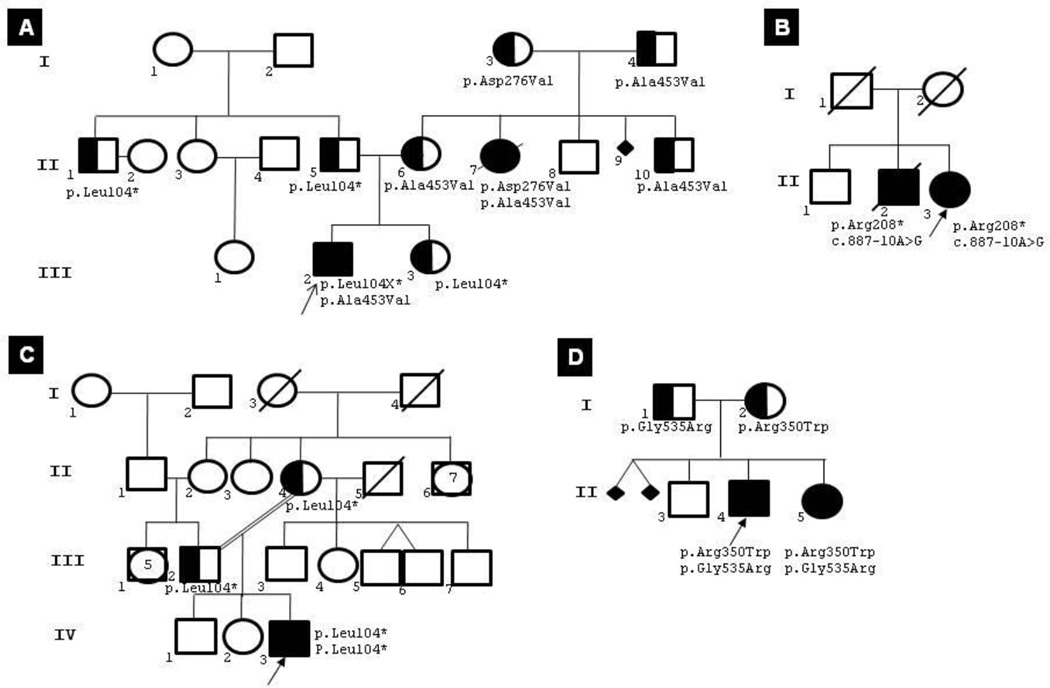
Table 1 shows the relevant phenotypic data and the mutational spectrum of each individual in the three subgroups. The presented data are age at onset, present age or age at death, TPP1 activity, morphotypes with CBs or combined with FPs, designation of mutations, and polymorphisms. The genotypes of all CLN2 patients rendered a total of n=15 mutations; of these, eight were known worldwide, three had been observed previously in South America by our group (Kohan et al., 2008; Kohan et al., 2009), and four were novel (Table 2). The novel mutations comprised three missense mutations (E8 p.Arg350Trp, E11 p. Ala453Asp, and E13 p.Gly535Arg) and one deletion (E9 c.1107-1108delTG). Pedigrees of some interesting families are shown in Fig. 1.
Table 2.
Frequency of mutated TPP1/CLN2 alleles in the three subgroups of TPP1-deficient patients.
| Mutations | No. of alleles | Subgroup I | Subgroup II | Subgroup III | Overall % | ||
|---|---|---|---|---|---|---|---|
| Worldwide mutations |
I7 c.887-10A>G (Bessa et al., 2008) |
7 | n=0 | n=3 | n=4 | 14 | |
| E6 p.Arg208* (Sleat et al., 1999) |
6 | n=4 | n=1 | n=1 | 12 | ||
| E3 p.Gln66* (Sleat et al., 1999) |
3 | n=0 | n=2 | n=1 | 6 | ||
| E8 p.Arg339Gln (Kousi et al., 2012) |
2 | n=2 | n=0 | n=0 | 4 | ||
| E11 p.Ser475Leu (Sleat et al., 1999) |
2 | n=0 | n=1 | n=1 | 4 | ||
| I2 c.89+5G>C (Kousi et al., 2012) |
2 | n=0 | n=2 | n=0 | 4 | ||
| I5 c.509-1G>C (Sleat et al., 1999) |
1 | n=1 | n=0 | n=0 | 2 | ||
| E11 p.Arg447His (Sleat et al., 1999) |
1 | n=0 | n=1 | n=0 | 2 | ||
| South American mutations |
E7 p.Asp276Val (Kohan et al., 2009) |
14 | n=9 | n=1 | n=4 | 28 | |
| E4 p.Leu104* (Kohan et al., 2008; Kohan et al., 2009) |
3 | n=3 | n=0 | n=0 | 6 | ||
| E8 p.Arg350Trp | 2 | n=1 | n=1 | n=0 | 4 | ||
| E11 p.Ala453Val (Kohan et al., 2009) |
2 | n=1 | n=0 | n=1 | 4 | ||
| E11 p.Ala453Asp | 2 | n=0 | n=2 | n=0 | 4 | ||
| E13 p.Gly535Arg | 2 | n=1 | n=1 | n=0 | 4 | ||
| E9 c.1107-1108delTG | 1 | n=0 | n=1 | n=0 | 2 | ||
| 50 | n=22 | n=16 | n=12 | 100% | |||
Reference sequence: GenBank ID: AF017456.1
Fig. 1.
Genealogies. (A) 82-2219 (III-2) and 82-1806 (II-7); (B) 173-1307 (II-3) and 173-1308 (II-2); C) 117-1312 (IV-3); and (D) 147-1805 (II-4) and 147-1807 (II-5). Although II-7 and II-2 were not screened, their genotypes were deduced from those of their relatives.
3.2. Experimental validation of novel missense mutations in the TPP1/CLN2 gene
To rule out the possibility of common South American sequence polymorphisms, direct sequence analysis of 200 samples from healthy individuals in Argentina’s general population was performed for each of the three missense mutations identified for the first time in the prospective subjects of this study. No mutation change was identified in any of the 200 control samples. Co-segregation studies among direct family members and bioinformatics programs were also applied to support their validation as disease-causing mutations.
3.3. Bioinformatic predictions for new missense pathogenic mutations
Three novel missense mutations were analyzed using bioinformatics tools: p.Arg350Trp (exon 8), p.Ala453Asp (exon 11), and p.Gly535Arg (exon 13). The scores obtained for each of the analyzed missense mutations applying Polymorphism Phenotyping-2 indicated the likelihood of harmful effects, as they were all within a value that was likely damaging (data not shown). SIFT results showed that no substitutions were tolerated by the TPP1 protein structure (data not shown). Popmusic 2.1 predicted that E8 p.Arg350Trp, E11 p.Ala453Asp, and E13 p.Gly535Arg would be destabilizing mutations (data not shown). ClustalW2 alignment of the TPP1 sequence with the mutations showed the selected residues to be highly conserved when compared among nine different species (Supplementary Fig. 1). The three-dimensional structure of TPP1 (PBD ID: 3EDY) obtained through the application of PDB Protein Workshop 3.3 and PyMOL 1.1 programs is shown in Supplementary Fig. 2 (the positions of the different South American new missense mutations are depicted by arrowheads). The proximity between the sites of the new missense mutations and the TPP1 active site is notable.
4. Discussion
The occurrence of 30 cases of NCL disorders was first published in Argentina by Taratuto et al. almost 20 years ago (Taratuto et al., 1995). It was later observed that the disease, caused by mutations in the TPP1/CLN2 gene, is prevalent in the region, and genetic findings were published for CLN1 and CLN2 by our group as part of a systematic detection program implemented in Córdoba, Argentina (Kohan et al., 2005; Noher de Halac et al., 2005; Kohan et al., 2009; Kohan, 2011).
In the literature, TPP1-deficient individuals were usually classified as one of three phenotypes according to their age at onset (Wisniewski et al., 2001; Williams et al., 2006): late infantile (LI), variant juvenile (vJ), or variant infantile (vI). The LI-NCL phenotype is the most abundant CLN2 form globally, characterized by early-onset seizures (2–4.5 years onset age), progressive encephalopathy, and visual failure; the vJ-NCL phenotype is characterized by a protracted course and later onset age (2.5–10 years of age), and the rare infantile form features onset at approximately 1 year of age (Simonati et al., 2000; Ju et al., 2002). We propose a new rationale for grouping the CLN2 phenotypes under the indicator trait of a quantifiable feature, such as the amount of TPP1 enzyme activity (Table 1): subgroup I “null TPP1 activity”, subgroup II “residual TPP1 activity”, and subgroup III “TPP1 activity not measured in leukocytes”. Notably, the residual TPP1 activity in leukocytes corresponded to a milder phenotype in five of eight individuals classified in subgroup II; although the remaining three subjects experienced an earlier age of onset than the other individuals in subgroup II, it is still too early to know whether the later course of the disease will exhibit a milder phenotype in these patients. Although significant variation in the ability of different observers to detect the onset of signs is highly likely, it is remarkable that the clinical data of the South American patients show a clear tendency: a more severe phenotype appeared within subgroup I, with ages of onset ranging from 3 to 5 years (mean age of onset 3.60 years; SD=0.65 years) and a lifespan of 15 years or fewer. In subgroup II, the ages of onset ranged from 2 to 9 years (mean age of onset 5.56 years; SD=3.04 years) and the lifespan was up to 36 years. In the six individuals in subgroup III, with TPP1 enzyme assays not performed or performed only using DBSs, molecular studies were performed because the clinical picture and morphotypes were compatible with a TPP1 deficiency. Although DBSs are useful for a first diagnostic approach, the results must be confirmed in leukocytes and/or saliva (Lukacs et al., 2003; Kohan et al., 2005). However, the clinical data from patients of subgroup III reinforce the high frequency of the CLN2 vJ phenotype in our region.
Morphotypes different from the typical CBs were observed in three individuals from subgroup II (Table 1), who exhibited combined or pure FPs. The significance of these findings is still unclear, and more detailed studies are needed to determine correlation traits for this variability.
A total of 15 pathogenic mutations were recognized (seven apparently unique to the South American population, and eight identified throughout the world). The eight pan-ethnic mutations include two nonsense (E3 p.Gln66* and E6 p.Arg208*), three missense (E8 p.Arg339Gln, E11 p.Arg447His, and E11 p.Ser475Leu), and three intronic (I2 c.89+5G>C, I5 c.509-1G>C, and I7 c.887-10A>G) mutations, all of which were described earlier in Europe, while the seven South American mutations include one nonsense (E4 p.Leu104*), one deletion (E9 c.1107-1108delTG), and five missense (E7 p.Asp276Val, E8 p.Arg350Trp, E11 p.Ala453Val/p.Ala453Asp, and E13 p.Gly535Arg) mutations (Tables 1 and 2). Of these, the E8 p.Arg350Trp, E11 p. Ala453Asp, E13 p.Gly535Arg, and E9 c.1107-1108delTG mutations are first being documented in this publication.
The most frequent mutations were E7 c.827A>T/p.Asp276Val, in 14 alleles (28%), followed by I7 c.887-10A>G in seven alleles (14%), E6 c.622C>T/p.Arg208* in six alleles (12%), and E4 c.311T>A/p.Leu104* and E3 c.196C>T/p.Gln66* in three alleles each (6% each). The allele frequency of each globally known or South American mutation with regard to enzyme phenotypes is shown in Table 2. The three new missense mutations were proved to be disease-causing mutations, following the recommendations provided by the Human Genome Organization (HUGO) (http://www.hgvs.org/entry.html): 1) the entire TPP1/CLN2 gene was sequenced, and no other DNA changes were observed (with exception of polymorphisms); 2) every mutation was validated by co-segregation studies (parents and other family members were evaluated for carrier status); 3) they were excluded as common regional polymorphisms by direct sequencing performed in 200 control chromosomes from individuals of the same ethnic background. The pathogenicity of mutations was also deduced by the use of a combination of bioinformatics experiments to predict the theoretical degree of pathogenic potential. The consistency and coherence of the results achieved by the different resources is remarkable (Supplementary Figs. 1 and 2).
The only two intronic mutations located in non-consensus sites (I7 c.887-10A>G and I2 c.89+5G>C, both found in heterozygous combinations) correlate well with the presence of residual TPP1 activity within subgroup II, independent of the age of onset and the second allele (Table 1). Aberrant splicing may be caused by intronic or exonic DNA changes that exclude an entire exon, activate cryptic splice sites within the intron that led to inclusion of intronic sequences, exclude exonic sequences, promote inclusion of pseudo-exons in the mature transcript, generate multiple abnormal transcripts, or result in a combination of several of these events (Wilton and Fletcher, 2011). It is generally accepted that mutations at the conserved splice dinucleotide consensus sites GU (donor) and AG (acceptor) disrupt pre-mRNA splicing, and those creating alternative novel acceptor and donor sites may interfere with splicing efficiency at wild-type sites. The presence of novel splice sites that compete with the wild-type sites would allow the alternative existence of normal and mutated mRNAs, which may in turn be related to some residual enzyme activity that ameliorates the pathological phenotype. In fact, the I7 c.887-10A>G mutation was extensively studied byBessa et al. (2008). They found this intronic mutation in homozygous condition in one Portuguese patient exhibiting a protracted phenotype and residual TPP1 activity. After conducting in vivo and in vitro TPP1 mRNA and protein assays and in silica studies, the authors concluded that the translation of the mutant transcript produced a polypeptide with three extra amino acids after Proline 295 (p.Pro295_Gly296insGluAsnPro). Therefore, the residual enzyme activity might be directly related to the mild phenotypes observed in homozygous (in the case of the Portuguese patient) or heterozygous (in the Latin American patients) conditions because of a defectively folded protein that retains some enzyme activity, or to the possibility of alternative splicing among the wild type and the novel splice sites that generate a small percentage of TPP1 with normal activity.
There is a growing realization that splicing efficiency is a significant contributor to phenotypic variability, and the contribution of splicing to phenotype has become particularly apparent through its impact on modifying the severity of human disease and its contribution to disease susceptibility (Wang and Cooper, 2007). The identification of abnormal splicing as the primary mechanism of disease raises the possibility of therapeutic approaches that specifically target splicing. For example, antisense oligomer-mediated splice manipulation promises potential therapies for a wide range of inherited or acquired disorders (Wilton and Fletcher, 2011), and successful experiments have been described regarding in vitro studies of β-thalassemia (Sierakowska et al., 1996) and cystic fibrosis (Friedman et al., 1999), among other genetic entities.
Eight polymorphisms were found in the population. Of these, five were previously recognized in the SNP database (http://www.ncbi.nlm.nih.gov/snp?term=tpp1%20human) and three were newly identified (Table 1). It is now clear that genetic variants that are linked with a phenotype, whether these are disease-causing mutations or common SNPs, must be evaluated for disruption of the correct splicing pattern. Normal genetic variation in the splicing code creates differences in splicing efficiency that may modify disease severity (Wang and Cooper, 2007).
The E7 p.Asp276Val mutation is the most common of these mutations in South America, as it is present in 14 (28%) of the TPP1/CLN2 alleles (Table 2). To date, it has only been reported in families from Argentina and Chile (https://www.ucl.ac.uk/ncl/cln2mutationtable.htm). It was shared among all patient subgroups, although its homozygous state was only observed in patients without residual TPP1 activity. Only one subgroup II individual exhibited this mutation in heterozygous combination with a novel deletion (081-1004-CL, Table 1). The p.Asp276Val mutation is assumed to severely affect enzyme activity, as the Asp276 amino acid has been proposed as part of the TPP1 active site (Guhaniyogi et al., 2009; Pal et al., 2009). The pathogenic nature of p.Asp276Val is supported by studies revealing that the catalytic activity of TPP1 is reduced by 79% when Asp276 is replaced with alanine (Walus et al., 2005).
A comparative summary of the clinical, enzymatic, morphological, and genetic data from the two CLN2 subgroups in South American patients is shown in Table 3.
Table 3.
Comparative summary of CLN2 South American patients grouped according to their null/residual TPP1 activity.
| CLN2 | Subgroup I | Subgroup II |
|---|---|---|
| Onset age | 3–5 y (average: 3.6 y) | 2–9 y (average: 5.5 y) |
| Evolution time | 2–12 y (average: 6.4 y) | 3.5–27 y (average: 11.3 y) |
| Presenting symptoms | Seizures (85%) | Seizures (75%) |
| Epileptic syndrome | 100% | 83% |
| Altered MRI | 3.5–5 y | 7–11 y |
| Altered EEG | 3 y - | 11–13 y |
| Visual failure | 5 y (77%); no (23%) | 10 y (66.7%); no (33.3%) |
| Ataxia | 3.5 y (85.7%); no (14.3%) | 10 y - (100%) |
| Motor regression | 2.5 y (100%) | 7 y - (100%) |
| Language difficulties | 2.5 y (100%) | 11 y - (88.9%); no (11.1%) |
| Mental regression | 3 y (100%) | 5–11 y (100%) |
| TPP1 residual activity | No (100%) | Yes (100%) |
| Vacuolated lymphocytes | Negative (100%) | Negative (87.5%); positive (12.5%) |
| TEM | Pure CB (100%) | Pure CB (40%); atypical deposits (60%): CB+FP/CB+FP+GROD/FP |
| Mutations | Novel: | Novel: |
| Missense: | Missense: | |
| E8 p.Arg350Trp, E13 p.Gly535Arg | E8 p.Arg350Trp, | |
| Known: | E11 p.Ala453Asp, E13 p.Gly535Arg | |
| Missense: | Deletion: | |
| E7 p.Asp276Vala, E8 p.Arg339Glnb, | E9 c.1107-1108delTG | |
| E11 p.Ala453Vala | Known: | |
| Nonsense: | Missense: | |
| E4 p.Leu104*a, E6 p.Arg208*b | E7 p.Asp276Vala, E11 p.Ser475Leub, | |
| Intronic: | E11 p.Arg447Hisb | |
| I5 c.509-1G>Cb | Nonsense: | |
| E6 p.Arg208*b, E3 p.Gln66*b | ||
| Intronic: | ||
| I7 c.887-10A>Gb; I2 c.89+5G>Cb | ||
| Allele combination | Homozygous (54.5%); heterozygous (45.5%) |
Heterozygous 100% |
MRI, Magnetic resonance images; EEG, electroencephalogram; TEM, transmission electron microscopy; y, years;
mutations previously published by our group in South American patients;
globally recognized mutations.
Table 1 lists the mutations found in this South American cohort, including those published in earlier works by our group (Kohan et al., 2008; Kohan et al., 2009) or other authors (Sleat et al., 1999; Bessa et al., 2008; Kousi et al., 2012). We have added to the mutational spectrum those of 18 additional subjects, identifying four novel mutations. The global CLN2 mutational spectrum is shown in Supplementary Fig. 3. The mutations found in our cohort (15.3% of all globally recognized TPP1/CLN2 mutations) are highlighted. It is worth emphasizing the heterogeneity of mutations, which is in keeping with the multiethnic population of South America: five missense mutations and 1 nonsense mutation, one deletion, and three polymorphisms not previously described in patients from other parts of the world. The mutations were observed in both homozygous (28%, n=7) and heterozygous (72%, n=18) combinations (Table 1).
5. Conclusions
CLN2 is the most frequently observed NCL form in South America. TPP1-deficient individuals exhibit either null enzyme activity (in relation to a generally more severe clinical phenotype) or residual enzyme activity (associated with a mostly protracted phenotype). Clustering the data into three groups, “null TPP1 activity”, “residual TPP1 activity” and “activity not measured in leukocytes”, suggested that slight residual TPP1 activity of any magnitude might have an effect on the attenuation of the phenotype. On the basis of the concept that residual enzyme activity is expected to underlie a protracted disease course, this paper creates subgroups of clinically and genetically defined CLN2 individuals for the first time. Residual TPP1 enzyme activity may allow a therapeutic approach based on modifier molecules (Pierret et al., 2008; Kohan et al., 2011).
The protracted CLN2 phenotype was previously considered globally rare. In South America, the frequency is approximately 50% of affected individuals, with phenotypic characteristics not published before in a relevant number of subjects from other parts of the world.
The heterozygous combination of intronic mutations in non-consensus sites with missense/nonsense mutations correlated with residual TPP1 activity, demonstrating that splice site mutations may allow some enzyme activity either by generating a variable percentage of alternative splicing using the wild type or mutated splice site, or by producing an incorrectly folded enzyme that still retains some activity. Some mild missense mutations may also cause incorrect protein folding that would enable a small percentage of enzyme activity in correlation with a more protracted phenotype.
The molecular studies rendered a total of 15 mutations in South America, including one deletion mutation and three novel missense mutations (Tables 1 and 2). To simplify the future genetic analysis of TPP1-deficient patients in our region, one might consider ruling out E7 p.Asp276Val, I7 c.887-10A>G, E6 p.Arg208X, E3 p.Gln66* and E4 p.Leu104* before starting costly complete TPP1/CLN2 screening. At present, these five most common South American mutations comprise 66% of the detected pathological alleles.
Supplementary Material
Acknowledgments
We are especially grateful to the NCL families of South America. We also acknowledge the personnel of the Center for the Study of Inborn Errors of Metabolism (CEMECO) at the Children’s Hospital, Córdoba, Argentina; Hugo Arroyo, Liliana Czornij, Soledad Monges, and Mariana Loos from the Hospital Garrahan, Buenos Aires, Argentina; Alejandra Barile and Adriana Becerra from the Children’s Hospital, Córdoba, Argentina; Ana Lia Taratuto, Mercedes Villanueva, and Santiago Vazquez from the Foundation for Neurological Disease Control for Children (FLENI), Buenos Aires, Argentina; and all of the pediatricians, ophthalmologists, radiologists, physiotherapists, and other professionals that care for the patients and their families. We also thank the laboratory personnel in the Neurogenetics DNA Diagnostic Laboratory, Massachusetts General Hospital, Boston; Michael Fietz and Viv Muller from Women’s and Children’s Hospital, Adelaide, Australia; Hans H. Goebel, Alfried Kohlschutter, Zoltan Luckacs, and Robert Steinfeld from Mainz and Hamburg Universities, Germany; and Sara E. Mole from the MRC Laboratory for Molecular Cell Biology, University College London, UK. We thank the organizations that provided grant support to the investigation during the last 5 years: Argentinean National Research Council (CONICETRA); Secretary of Science and Technology of the National University Córdoba (SECyT-UNC); Batten Disease Support and Research Association (BDSRA-US), especially its former executive director, Mr. Lance Johnston; NIH-Fogarty R21TW00843301-US; and the von Humboldt Foundation, which provided grant support for INH’s travels to Germany.
Abbreviations
- CB
curvilinear body
- DBS
dried blood spot
- FP
fingerprint profile
- GROD
granular osmiophilic deposit
- J-NCL
juvenile NCL
- LD
lysosomal disorder
- LI-NCL
late-infantile NCL
- NCLs
neuronal ceroid lipofuscinoses
- PDB
protein data bank
- PolyPhen-2
polymorphism phenotyping-2
- PPT1
palmitoyl protein thioesterase 1
- RB
rectilinear body
- SIFT
sorting intolerant from tolerant
- SNP
single nucleotide polymorphism
- TEM
transmission electron microscopy
- TPP1
tripeptidyl-peptidase 1
- vI-NCL
variant infantile NCL
- vJ-NCL
variant juvenile NCL
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
We declare no conflicts of interest.
Website references
http://www.ncbi.nlm.nih.gov/snp?term=tpp1%20human
http://www.ebi.ac.uk/Tools/clustalw2/index.html
http://genetics.bwh.harvard.edu/pph2
http://babylone.ulb.ac.be/popmusic
http://www.rcsb.org/pdb/home/home.do
http://www.hgvs.org/entry.html
https://www.ucl.ac.uk/ncl/cln2mutationtable.htm
Supplementary Fig. 1.
ClustalW2 analysis demonstrates evolutionary conservation. (A) Arg350; (B) Ala453; and (C) Gly535.
Supplementary Fig. 2.
The location of missense mutations in the TPP1 three-dimensional structure, obtained with PyMol 1.1. (A) Arg350 (light blue); (B) Ala453 (yellow); and (C) Gly535 (yellow). Amino acids depicted in pink correspond to the pro-segment (residues 20 to 195), while those in green correspond to the catalytic domain (residues 196–563). The active site according to Pal et al. 2009 (Ser475-Glu272- Asp360) is depicted in red. According to Guhaniyogi et al. 2009, the active site would be formed by Ser475-Glu272-Asp276.
Supplementary Fig.3.
Global TPP1/CLN2 mutations. Red circles highlight those found in South American families. Countries of origin (red): AR, Argentina; BE, Belgium; BR, Brazil; CA, Canada; CH, Switzerland; CL, Chile; CN, China; CZ, Czech Republic; DE, Germany; DK, Denmark; ES, Spain; FI, Finland; FR, France; GB, England; HU, Hungary; IE, Ireland; IL, Israel; IN, India; IQ, Iraq; IT, Italy; LB, Lebanon; MX, Mexico; NED, Netherlands; NO, Norway; OMA, Oman; PL, Poland; PT, Portugal; RO, Romania; RU, Russia; SE, Sweden; SK, Slovakia; TR, Turkey; US, United States; ZA, Africa. Phenotypes (blue): LINCL, late infantile, JNCL, juvenile; v, variant; prot, protracted; unk, unknown. The number of affected families is depicted in green. Global information: http://www.ucl.ac.uk/ncl/cln2.shtm; Country codes: http://inf.ufrgs.br/~cabral/Paises.html.
References
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [PubMed: 20354512] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsov T, Smith KR, Damiano J, Franceschetti S, Canafoglia L, Bromhead CJ, Andermann E, Vears DF, Cossette P, Rajagopalan S, McDougall A, Sofia V, Farrell M, Aguglia U, Zini A, Meletti S, Morbin M, Mullen S, Andermann F, Mole SE, Bahlo M, Berkovic SF. Kufs disease, the major adult form of neuronal ceroid lipofuscinosis, caused by mutations in CLN6. Am. J Hum. Genet. 2011;88:566–573. doi: 10.1016/j.ajhg.2011.04.004. [PubMed: 21549341] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa C, Teixeira CA, Dias A, Alves M, Rocha S, Lacerda L, Loureiro L, Guimaraes A, Ribeiro MG. CLN2/TPP1 deficiency: the novel mutation IVS7-10A>G causes intron retention and is associated with a mild disease phenotype. Mol. Genet. Metab. 2008;93:66–73. doi: 10.1016/j.ymgme.2007.08.124. [PubMed: 17959406] [DOI] [PubMed] [Google Scholar]
- Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ. Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum. Mol. Genet. 2012;21:2646–2650. doi: 10.1093/hmg/dds089. [PubMed: 22388936] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TM, Cachon-Gonzalez MB. The cellular pathology of lysosomal diseases. J Pathol. 2012;226:241–254. doi: 10.1002/path.3021. [PubMed: 21990005] [DOI] [PubMed] [Google Scholar]
- Dehouck Y, Kwasigroch JM, Gilis D, Rooman M. PoPMuSiC 2.1: a web server for the estimation of protein stability changes upon mutation and sequence optimality. BMC. Bioinformatics. 2011;12:151. doi: 10.1186/1471-2105-12-151. [PubMed: 21569468] [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Dunnen JT, Antonarakis SE. Mutation nomenclature extensions and suggestions to describe complex mutations: a discussion. Hum. Mutat. 2000;15:7–12. doi: 10.1002/(SICI)1098-1004(200001)15:1<7::AID-HUMU4>3.0.CO;2-N. [PubMed: 10612815] [DOI] [PubMed] [Google Scholar]
- Elleder M, Dvorakova L, Stolnaja L, Vlaskova H, Hulkova H, Druga R, Poupetova H, Kostalova E, Mikulastik J. Atypical CLN2 with later onset and prolonged course: a neuropathologic study showing different sensitivity of neuronal subpopulations to TPP1 deficiency. Acta Neuropathol. 2008;116:119–124. doi: 10.1007/s00401-008-0349-3. [PubMed: 18283468] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman KJ, Kole J, Cohn JA, Knowles MR, Silverman LM, Kole R. Correction of aberrant splicing of the cystic fibrosis transmembrane conductance regulator (CFTR) gene by antisense oligonucleotides. J Biol. Chem. 1999;274:36193–36199. doi: 10.1074/jbc.274.51.36193. [PubMed: 10593905] [DOI] [PubMed] [Google Scholar]
- Gao H, Boustany RM, Espinola JA, Cotman SL, Srinidhi L, Antonellis KA, Gillis T, Qin X, Liu S, Donahue LR, Bronson RT, Faust JR, Stout D, Haines JL, Lerner TJ, MacDonald ME. Mutations in a novel CLN6-encoded transmembrane protein cause variant neuronal ceroid lipofuscinosis in man and mouse. Am. J. Hum. Genet. 2002;70:324–335. doi: 10.1086/338190. [PubMed: 11791207] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH. The neuronal ceroid-lipofuscinoses. J Child Neurol. 1995;10:424–437. doi: 10.1177/088307389501000602. [PubMed: 8576551] [DOI] [PubMed] [Google Scholar]
- Guhaniyogi J, Sohar I, Das K, Stock AM, Lobel P. Crystal structure and autoactivation pathway of the precursor form of human tripeptidyl-peptidase 1, the enzyme deficient in late infantile ceroid lipofuscinosis. J Biol Chem. 2009;284:3985–3997. doi: 10.1074/jbc.M806943200. [PubMed: 19038967] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartikainen JM, Ju W, Wisniewski KE, Moroziewicz DN, Kaczmarski AL, McLendon L, Zhong D, Suarez CT, Brown WT, Zhong N. Late infantile neuronal ceroid lipofuscinosis is due to splicing mutations in the CLN2 gene. Mol. Genet. Metab. 1999;67:162–168. doi: 10.1006/mgme.1999.2853. [PubMed: 10356316] [DOI] [PubMed] [Google Scholar]
- Jalanko A, Braulke T. Neuronal ceroid lipofuscinoses. Biochim. Biophys. Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [PubMed: 19084560] [DOI] [PubMed] [Google Scholar]
- Ju W, Zhong R, Moore S, Moroziewicz D, Currie JR, Parfrey P, Brown WT, Zhong N. Identification of novel CLN2 mutations shows Canadian specific NCL2 alleles. J. Med. Genet. 2002;39:822–825. doi: 10.1136/jmg.39.11.822. [PubMed: 12414822] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida E, Golabek AA, Wisniewski KE. Cellular pathology and pathogenic aspects of neuronal ceroid lipofuscinoses. Adv. Genet. 2001;45:35–68. doi: 10.1016/s0065-2660(01)45003-6. [PubMed: 11332776] [DOI] [PubMed] [Google Scholar]
- Kohan R, Noher de Halac I, Tapia Anzolini V, Cismondi IA, Oller Ramirez AM, Paschini Capra A, Dodelson de Kremer R. Palmitoyl Protein Thioesterase1 (PPT1) and Tripeptidyl Peptidase-I (TPP-I) are expressed in the human saliva. A reliable and noninvasive source for the diagnosis of infantile (CLN1) and late infantile (CLN2) neuronal ceroid lipofuscinoses. Clin. Biochem. 2005;38:492–494. doi: 10.1016/j.clinbiochem.2004.12.007. [PubMed: 15820783] [DOI] [PubMed] [Google Scholar]
- Kohan R, Muller VJ, Fietz MJ, Cismondi A, Oller Ramirez AM, Noher de Halac I. Novel human pathological mutations. Gene symbol: TPPI. Disease: Neuronal Ceroid Lipofuscinosis, Late infantile. Hum. Genet. 2008;123:537–555. [PubMed: 20960655] [PubMed] [Google Scholar]
- Kohan R, Cismondi IA, Dodelson de Kremer R, Muller VJ, Guelbert N, Tapia Anzolini V, Fietz MJ, Oller Ramirez AM, Noher de Halac I. An integrated strategy for the diagnosis of neuronal ceroid lipofuscinosis types 1 (CLN1) and 2 (CLN2) in eleven Latin American patients. Clin. Genet. 2009;76:372–382. doi: 10.1111/j.1399-0004.2009.01214.x. [PubMed: 19793312] [DOI] [PubMed] [Google Scholar]
- Kohan R. PhD thesis. National University Córdoba; 2011. Caracterización morfológica, bioquímica y molecular de las Lipofuscinosis Ceroideas Neuronales tipos CLN1 y CLN2 en pacientes argentinos con proyección hacia Latinoamérica. [Google Scholar]
- Kohan R, Cismondi IA, Oller-Ramirez AM, Guelbert N, Anzolini Tapia V, Alonso G, Mole SE, Dodelson de Kremer R, Noher de Halac I. Therapeutic Approaches to the Challenge of Neuronal Ceroid Lipofuscinoses. Curr. Pharm. Biotechnol. 2011;12:867–883. doi: 10.2174/138920111795542633. [PubMed: 21235444] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum. Mutat. 2012;33:42–63. doi: 10.1002/humu.21624. [PubMed: 21990111] [DOI] [PubMed] [Google Scholar]
- Krabichler B, Rostasy K, Baumann M, Karall D, Scholl-Burgi S, Schwarzer C, Gautsch K, Spreiz A, Kotzot D, Zschocke J, Fauth C, Haberlandt E. Novel mutation in potassium channel related gene KCTD7 and progressive myoclonic epilepsy. Ann. Hum. Genet. 2012;76:326–331. doi: 10.1111/j.1469-1809.2012.00710.x. [PubMed: 22606975] [DOI] [PubMed] [Google Scholar]
- Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009;4:1073–1081. doi: 10.1038/nprot.2009.86. [PubMed: 19561590] [DOI] [PubMed] [Google Scholar]
- Liu CG, Sleat DE, Donnelly RJ, Lobel P. Structural organization and sequence of CLN2, the defective gene in classical late infantile neuronal ceroid lipofuscinosis. Genomics. 1998;50:206–212. doi: 10.1006/geno.1998.5328. [PubMed: 9653647] [DOI] [PubMed] [Google Scholar]
- Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed: 14907713] [PubMed] [Google Scholar]
- Lukacs Z, Santavuori P, Keil A, Steinfeld R, Kohlschutter A. Rapid and simple assay for the determination of tripeptidyl peptidase and palmitoyl protein thioesterase activities in dried blood spots. Clin. Chem. 2003;49:509–511. doi: 10.1373/49.3.509. [PubMed: 12600970] [DOI] [PubMed] [Google Scholar]
- Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107–126. doi: 10.1007/s10048-005-0218-3. [PubMed: 15965709] [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [PubMed: 12824425] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noher de Halac I, Dodelson de Kremer R, Kohan R, Tapia Anzolini V, Guelbert N, Cismondi IA, Oller Ramirez AM, Paschini Capra A. Clinical, Morphological, Biochemical and Molecular Study of Atypical Forms of Neuronal Ceroid Lipofuscinoses in Argentina. In: Noher de Halac I, Dodelson de Kremer R, editors. Neuronal Ceroid Lipofuscinoses (Batten Disease) in Latin America- an update. Córdoba: Secretaría de Extensión; Universidad Nacional de Córdoba; 2005. pp. 103–116. [Google Scholar]
- Noskova L, Stranecky V, Hartmannova H, Pristoupilova A, Baresova V, Ivanek R, Hulkova H, Jahnova H, van der Zee J, Staropoli JF, Sims KB, Tyynela J, Van BC, Nijssen PC, Mole SE, Elleder M, Kmoch S. Mutations in DNAJC5, encoding cysteine-string protein alpha, cause autosomal-dominant adult-onset neuronal ceroid lipofuscinosis. Am. J Hum. Genet. 2011;89:241–252. doi: 10.1016/j.ajhg.2011.07.003. [PubMed: 21820099] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Kraetzner R, Gruene T, Grapp M, Schreiber K, Gronborg M, Urlaub H, Becker S, Asif AR, Gartner J, Sheldrick GM, Steinfeld R. Structure of tripeptidyl-peptidase I provides insight into the molecular basis of late infantile neuronal ceroid lipofuscinosis. J Biol Chem. 2009;284:3976–3984. doi: 10.1074/jbc.M806947200. [PubMed: 19038966] [DOI] [PubMed] [Google Scholar]
- Pierret C, Morrison JA, Kirk MD. Treatment of lysosomal storage disorders: Focus on the neuronal ceroid-lipofuscinoses. Acta Neurobiol. Exp. (Wars. ) 2008;68:429–442. doi: 10.55782/ane-2008-1709. [PubMed: 18668166] [DOI] [PubMed] [Google Scholar]
- Poet M, Kornak U, Schweizer M, Zdebik AA, Scheel O, Hoelter S, Wurst W, Schmitt A, Fuhrmann JC, Planells-Cases R, Mole SE, Hubner CA, Jentsch TJ. Lysosomal storage disease upon disruption of the neuronal chloride transport protein ClC-6. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13854–13859. doi: 10.1073/pnas.0606137103. [PubMed: 16950870] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranta S, Zhang Y, Ross B, Lonka L, Takkunen E, Messer A, Sharp J, Wheeler R, Kusumi K, Mole S, Liu W, Soares MB, Bonaldo MF, Hirvasniemi A, de la Chapelle A, Gilliam TC, Lehesjoki AE. The neuronal ceroid lipofuscinoses in human EPMR and mnd mutant mice are associated with mutations in CLN8. Nat. Genet. 1999;23:233–236. doi: 10.1038/13868. [PubMed: 10508524] [DOI] [PubMed] [Google Scholar]
- Rider JA, Rider DL. Thirty years of Batten disease research: present status and future goals. Mol. Genet. Metab. 1999;66:231–233. doi: 10.1006/mgme.1999.2827. [PubMed: 10191106] [DOI] [PubMed] [Google Scholar]
- Savukoski M, Klockars T, Holmberg V, Santavuori P, Lander ES, Peltonen L. CLN5, a novel gene encoding a putative transmembrane protein mutated in Finnish variant late infantile neuronal ceroid lipofuscinosis. Nat. Genet. 1998;19:286–288. doi: 10.1038/975. [PubMed: 9662406] [DOI] [PubMed] [Google Scholar]
- Schulz A, Dhar S, Rylova S, Dbaibo G, Alroy J, Hagel C, Artacho I, Kohlschutter A, Lin S, Boustany RM. Impaired cell adhesion and apoptosis in a novel CLN9 Batten disease variant. Ann. Neurol. 2004;56:342–350. doi: 10.1002/ana.20187. [PubMed: 15349861] [DOI] [PubMed] [Google Scholar]
- Sierakowska H, Sambade MJ, Agrawal S, Kole R. Repair of thalassemic human beta-globin mRNA in mammalian cells by antisense oligonucleotides. Proc Natl. Acad. Sci. U. S. A. 1996;93:12840–12844. doi: 10.1073/pnas.93.23.12840. [PubMed: 8917506] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siintola E, Lehesjoki AE, Mole SE. Molecular genetics of the NCLs -- status and perspectives. Biochim. Biophys. Acta. 2006a;1762:857–864. doi: 10.1016/j.bbadis.2006.05.006. [PubMed: 16828266] [DOI] [PubMed] [Google Scholar]
- Siintola E, Partanen S, Stromme P, Haapanen A, Haltia M, Maehlen J, Lehesjoki AE, Tyynela J. Cathepsin D deficiency underlies congenital human neuronal ceroid-lipofuscinosis. Brain. 2006b;129:1438–1445. doi: 10.1093/brain/awl107. [PubMed: 16670177] [DOI] [PubMed] [Google Scholar]
- Siintola E, Topcu M, Aula N, Lohi H, Minassian BA, Paterson AD, Liu XQ, Wilson C, Lahtinen U, Anttonen AK, Lehesjoki AE. The novel neuronal ceroid lipofuscinosis gene MFSD8 encodes a putative lysosomal transporter. Am. J. Hum. Genet. 2007;81:136–146. doi: 10.1086/518902. [PubMed: 17564970] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonati A, Santorum E, Tessa A, Polo A, Simonetti F, Bernardina BD, Santorelli FM, Rizzuto N. A CLN2 gene nonsense mutation is associated with severe caudate atrophy and dystonia in LINCL. Neuropediatrics. 2000;31:199–201. doi: 10.1055/s-2000-7453. [PubMed: 11071145] [DOI] [PubMed] [Google Scholar]
- Sleat DE, Donnelly RJ, Lackland H, Liu CG, Sohar I, Pullarkat RK, Lobel P. Association of mutations in a lysosomal protein with classical late-infantile neuronal ceroid lipofuscinosis. Science. 1997;277:1802–1805. doi: 10.1126/science.277.5333.1802. [PubMed: 9295267] [DOI] [PubMed] [Google Scholar]
- Sleat DE, Gin RM, Sohar I, Wisniewski K, Sklower-Brooks S, Pullarkat RK, Palmer DN, Lerner TJ, Boustany RM, Uldall P, Siakotos AN, Donnelly RJ, Lobel P. Mutational analysis of the defective protease in classic late-infantile neuronal ceroid lipofuscinosis, a neurodegenerative lysosomal storage disorder. Am. J. Hum. Genet. 1999;64:1511–1523. doi: 10.1086/302427. [PubMed: 10330339] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleat DE, Ding L, Wang S, Zhao C, Wang Y, Xin W, Zheng H, Moore DF, Sims KB, Lobel P. Mass spectrometry-based protein profiling to determine the cause of lysosomal storage diseases of unknown etiology. Mol. Cell Proteomics. 2009;8:1708–1718. doi: 10.1074/mcp.M900122-MCP200. [PubMed: 19383612] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Damiano J, Franceschetti S, Carpenter S, Canafoglia L, Morbin M, Rossi G, Pareyson D, Mole SE, Staropoli JF, Sims KB, Lewis J, Lin WL, Dickson DW, Dahl HH, Bahlo M, Berkovic SF. Strikingly different clinicopathological phenotypes determined by progranulin-mutation dosage. Am. J Hum. Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [PubMed: 22608501] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohar I, Sleat DE, Jadot M, Lobel P. Biochemical characterization of a lysosomal protease deficient in classical late infantile neuronal ceroid lipofuscinosis (LINCL) and development of an enzyme-based assay for diagnosis and exclusion of LINCL in human specimens and animal models. J. Neurochem. 1999;73:700–711. doi: 10.1046/j.1471-4159.1999.0730700.x. [PubMed: 10428067] [DOI] [PubMed] [Google Scholar]
- Sohar I, Lin L, Lobel P. Enzyme-based diagnosis of classical late infantile neuronal ceroid lipofuscinosis: comparison of tripeptidyl peptidase I and pepstatin-insensitive protease assays. Clin. Chem. 2000;46:1005–1008. [PubMed: 10894849] [PubMed] [Google Scholar]
- Staropoli JF, Karaa A, Lim ET, Kirby A, Elbalalesy N, Romansky SG, Leydiker KB, Coppel SH, Barone R, Xin W, MacDonald ME, Abdenur JE, Daly MJ, Sims KB, Cotman SL. A Homozygous Mutation in KCTD7 Links Neuronal Ceroid Lipofuscinosis to the Ubiquitin-Proteasome System. Am. J Hum. Genet. 2012;91:202–208. doi: 10.1016/j.ajhg.2012.05.023. [PubMed: 22748208] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinfeld R, Heim P, von Gregory H, Meyer K, Ullrich K, Goebel HH, Kohlschutter A. Late infantile neuronal ceroid lipofuscinosis: quantitative description of the clinical course in patients with CLN2 mutations. Am. J. Med. Genet. 2002;112:347–354. doi: 10.1002/ajmg.10660. [PubMed: 12376936] [DOI] [PubMed] [Google Scholar]
- Steinfeld R, Reinhardt K, Schreiber K, Hillebrand M, Kraetzner R, Bruck W, Saftig P, Gartner J. Cathepsin D deficiency is associated with a human neurodegenerative disorder. Am. J. Hum. Genet. 2006;78:988–998. doi: 10.1086/504159. [PubMed: 16685649] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang CH, Lee JW, Galvez MG, Robillard L, Mole SE, Chapman HA. Murine cathepsin F deficiency causes neuronal lipofuscinosis and late-onset neurological disease. Mol. Cell Biol. 2006;26:2309–2316. doi: 10.1128/MCB.26.6.2309-2316.2006. [PubMed: 16508006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taratuto AL, Saccoliti M, Sevlever G, Ruggieri V, Arroyo H, Herrero M, Massaro M, Fejerman N. Childhood neuronal ceroid-lipofuscinoses in Argentina. Am. J. Med. Genet. 1995;57:144–149. doi: 10.1002/ajmg.1320570207. [PubMed: 7668319] [DOI] [PubMed] [Google Scholar]
- The International Batten Disease Consortium. Isolation of a novel gene underlying Batten disease, CLN3. Cell. 1995;82:949–957. doi: 10.1016/0092-8674(95)90274-0. [PubMed: 7553855] [DOI] [PubMed] [Google Scholar]
- van Bogaert P, Azizieh R, Desir J, Aeby A, De Meirleir L, Laes JF, Christiaens F, Abramowicz MJ. Mutation of a potassium channel-related gene in progressive myoclonic epilepsy. Ann. Neurol. 2007;61:579–586. doi: 10.1002/ana.21121. [PubMed: 17455289] [DOI] [PubMed] [Google Scholar]
- van Diggelen OP, Keulemans JL, Winchester B, Hofman IL, Vanhanen SL, Santavuori P, Voznyi YV. A rapid fluorogenic palmitoyl-protein thioesterase assay: pre- and postnatal diagnosis of INCL. Mol. Genet. Metab. 1999;66:240–244. doi: 10.1006/mgme.1999.2809. [PubMed: 10191108] [DOI] [PubMed] [Google Scholar]
- Velinov M, Dolzhanskaya N, Gonzalez M, Powell E, Konidari I, Hulme W, Staropoli JF, Xin W, Wen GY, Barone R, Coppel SH, Sims K, Brown WT, Zuchner S. Mutations in the Gene DNAJC5 Cause Autosomal Dominant Kufs Disease in a Proportion of Cases: Study of the Parry Family and 8 Other Families. PLoS. One. 2012;7:e29729. doi: 10.1371/journal.pone.0029729. [PubMed: 22235333] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [PubMed: 7637805] [DOI] [PubMed] [Google Scholar]
- Walus M, Kida E, Wisniewski KE, Golabek AA. Ser475, Glu272, Asp276, Asp327, and Asp360 are involved in catalytic activity of human tripeptidyl-peptidase I. FEBS Lett. 2005;579:1383–1388. doi: 10.1016/j.febslet.2005.01.035. [PubMed: 15733845] [DOI] [PubMed] [Google Scholar]
- Wang GS, Cooper TA. Splicing in disease: disruption of the splicing code and the decoding machinery. Nat. Rev Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [PubMed: 17726481] [DOI] [PubMed] [Google Scholar]
- Wheeler RB, Sharp JD, Schultz RA, Joslin JM, Williams RE, Mole SE. The gene mutated in variant late-infantile neuronal ceroid lipofuscinosis (CLN6) and in nclf mutant mice encodes a novel predicted transmembrane protein. Am. J. Hum. Genet. 2002;70:537–542. doi: 10.1086/338708. [PubMed: 11727201] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RE, Gottlob I, Lake BD, Goebel HH, Winchester B, Wheeler R. CLN2 Classic Late Infantile NCL. In: Goebel HH, Mole SE, Lake BD, editors. The Neuronal Ceroid Lipofuscinoses (Batten Disease) Amsterdam: IOS Press; 1999. pp. 37–54. [Google Scholar]
- Williams RE, Aberg L, Autti T, Goebel HH, Kohlschutter A, Lonnqvist T. Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochim. Biophys. Acta. 2006;1762:865–872. doi: 10.1016/j.bbadis.2006.07.001. [PubMed: 16930952] [DOI] [PubMed] [Google Scholar]
- Williams RE, Mole SE. New nomenclature and classification scheme for the neuronal ceroid lipofuscinoses. Neurology. 2012;79:183–191. doi: 10.1212/WNL.0b013e31825f0547. [PubMed: 22778232] [DOI] [PubMed] [Google Scholar]
- Wilton SD, Fletcher S. RNA splicing manipulation: strategies to modify gene expression for a variety of therapeutic outcomes. Curr. Gene Ther. 2011;11:259–275. doi: 10.2174/156652311796150381. [PubMed: 21453280] [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Kaczmarski A, Kida E, Connell F, Kaczmarski W, Michalewski MP, Moroziewicz DN, Zhong N. Reevaluation of neuronal ceroid lipofuscinoses: atypical juvenile onset may be the result of CLN2 mutations. Mol. Genet. Metab. 1999;66:248–252. doi: 10.1006/mgme.1999.2814. [PubMed: 10191110] [DOI] [PubMed] [Google Scholar]
- Wisniewski KE, Kida E, Golabek AA, Kaczmarski W, Connell F, Zhong N. Neuronal ceroid lipofuscinoses: classification and diagnosis. Adv. Genet. 2001;45:1–34. doi: 10.1016/s0065-2660(01)45002-4. [PubMed: 11332767] [DOI] [PubMed] [Google Scholar]
- Zhong N, Moroziewicz DN, Ju W, Jurkiewicz A, Johnston L, Wisniewski KE, Brown WT. Heterogeneity of late-infantile neuronal ceroid lipofuscinosis. Genet. Med. 2000;2:312–318. doi: 10.1097/00125817-200011000-00002. [PubMed: 11339651] [DOI] [PubMed] [Google Scholar]
- Zhong N. Clinical and Molecular Approaches for Genotype and Phenotype Correlations Studies. In: Noher de Halac I, Dodelson de Kremer R, editors. Neuronal Ceroid Lipofuscinoses (Batten Disease) in Latin America- an update. Córdoba: Secretaría de Extensión; Universidad Nacional de Córdoba; 2005. pp. 35–52. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.