Abstract
Both embryonic and adult neurogenesis involves the self-renewal/proliferation, survival, migration and lineage differentiation of neural stem/progenitor cells. Such dynamic process is tightly regulated by intrinsic and extrinsic factors and complex signaling pathways. Misregulated neurogenesis contributes much to a large range of neurodevelopmental defects and neurodegenerative diseases. The signaling of NFκB regulates many genes important in inflammation, immunity, cell survival and neural plasticity. During neurogenesis, NFκB signaling mediates the effect of numerous niche factors such as cytokines, chemokines, growth factors, extracellular matrix molecules, but also crosstalks with other signaling pathways such as Notch, Shh, Wnt/β-catenin. This review summarizes current progress on the NFκB signaling in all aspects of neurogenesis, focusing on the novel role of NFκB signaling in initiating early neural differentiation of neural stem cells and embryonic stem cells.
Keywords: neural stem cells, transcriptional factors, NFκB, neurogenesis, embryonic stem cells, signal transduction
Introduction
Neurogenesis is a process of neural cell formation and includes three terms based on the lineage differentiation into neurons, astrocytes and oligodendrocytes: neuronogenesis, astrocytogenesis and oligodendrocytogenesis. Neurogenesis occurs not only during embryonic and perinatal development but also in adult nervous system including central (Conti and Cattaneo, 2010; Ming and Song, 2011), peripheral (Le Douarin et al., 2008; Pardal et al., 2010) and enteric nervous system (Heanue and Pachnis, 2007; Metzger, 2010). Defects or impairments in embryonic and neonatal neurogenesis contribute significantly to a large range of neuropsychiatric diseases including intellectual disability (mental retardation), autism spectrum disorders, childhood-onset schizophrenia, epilepsy, pediatric bipolar disorder, attention-deficit hyperactivity disorder, as well as genetic or neurodevelopmental disorders such as Fragile X syndrome, Rett syndrome, Down syndrome, Hirschsprung’s disease (Heanue and Pachnis, 2007; Vaillend et al., 2008; Ma et al., 2009; Schäfer et al., 2009; Kishi and Macklis, 2010; Callan and Zarnescu, 2011). Adult neurogenesis is a relatively new concept and abnormal adult neurogenesis plays an important role in the pathogenesis of many cognitive/mood dysfunction in adult such as depression (Hanson et al., 2011), schizophrenia (Inta et al., 2011), epilepsy (Andres-Mach et al., 2011), stroke (Zhang et al., 2011), HIV-associated neurocognitive disorders (Okamoto et al., 2007; Kaul, 2008) and neurodegenerative diseases including Alzheimer’s disease (Mu and Gage, 2011), Parkinson’s disease (van den Berge et al., 2011), multiple sclerosis (Huehnchen et al., 2011; Tepavčević et al., 2011), and others (Winner et al., 2011; Curtis et al., 2012).
Both embryonic and adult neurogenesis includes the self-renewal/proliferation, survival, migration and lineage differentiation of neural stem/progenitor cells (NSCs/NPCs). For better understanding, neurogenesis can be divided into early, middle and late phases though they are indistinguishable in most cases. Early neurogenesis includes self-renewal/proliferation of NSCs/NPCs and cell fate commitment. Middle neurogenesis covers the migration of lineage-restricted cells (neuroblast or glioblast) and terminal differentiation. Late neurogenesis includes the maturation and functional integrity of nascent cells. The duration of each phase depends upon the neurogenic region, developmental stage, and species. The progression from the early to late neurogenesis is tightly regulated by intrinsic and extrinsic factors and complex signaling pathways (Ma et al., 2009; Pathania et al., 2010). At the early stage, NSCs represent a very small population of quiescent, slow dividing cells while NPCs represent a large population of amplifying, rapid dividing cells (Encinas and Sierra, 2012). NSCs are the most primitive and normally quiescent neural precursor cells and are exceptionally sensitive to cosmic radiation (Encinas et al., 2008). Distinguishing NSCs from NPCs both in vitro and in vivo remains a big challenge (Bonaguidi et al., 2011; Siebzehnrubl et al., 2011).
Neurogenesis is regulated on various conceptual levels, from behavior, systemic response, specific nervous system activity, niche factors to intracellular/molecular regulation. Although many extrinsic factors and intrinsic epigenic/transcriptional proteins to regulate both embryonic and adult neurogenesis have been identified (Zhao et al., 2008; Conti and Cattaneo, 2010), the signaling pathways and molecular mechanisms remain poorly understood. In particular, what factors control the initiation of NSC differentiation into NPCs? After initial differentiation, what factors guide lineage-specific terminal differentiation? Several signaling pathways such as Wnt/β-catenin, Notch, sonic hedgehog (Shh) as well as transcriptional factors such as Sox2, Pax6, Mash1, TLX, Hes1/5, NeuroD, Tbr2, etc. in neurogenesis have been identified (Ma et al., 2009; Hodge and Hevner, 2011), but little is known about the signaling of nuclear factor κB (NFκB) during neurogenesis (Schölzke and Schwaninger, 2007; Widera et al., 2008; Kaltschmidt and Kaltschmidt, 2009; Gutierrez and Davies, 2011; Zhang et al., 2011). Recently, inflammatory mediators including cytokines, chemokines, growth factors, adhesion molecules, are receiving increased attentions in the field of embryonic and adult neurogenesis because inflammatory and immune responses are known to play critical roles in various diseases and injuries of the nervous system (Das and Basu, 2008; Taupin, 2008; Widera et al., 2008; Whitney et al., 2009; Teng and Tang, 2010). Most inflammatory mediators act as both sources and targets of NFκB signaling. The role of NFκB signaling in regulating the proliferation/apoptosis of NSCs/NPCs, migration of neuroblast, maturation and plasticity of nascent neurons has been reviewed in several previous publications (Widera et al., 2006b; Schölzke and Schwaninger, 2007; Widera et al., 2008; Gutierrez and Davies, 2011; Zhang et al., 2011). In this review, we will focus on the role of NFκB signaling in regulating selfrenewal and early differentiation of NSCs.
Signaling pathways of NFκB activation
The transcriptional factor NFκB plays a pivotal role in inflammation, immunity, cancer and neural plasticity (Häcker and Karin, 2006; Perkins, 2007). Constitutive and inducible activation of NFκB has been reported in many types of human tumors and chronic diseases (Wong and Tergaonkar, 2009; Boyce et al., 2010; Lin et al., 2010; Mancino and Lawrence, 2010). Like other signaling transduction pathways, NFκB is activated through a series of signaling cascades. The NFκB family contains 5 members including RelA(p65), RelB, c-Rel, p50/p105 (NFκB1) and p52/p100 (NFκB2), which form various combinations of homodimers or heterodimers (Chen and Greene, 2004; Perkins, 2007). All contain REL homology domain (RHD, 300 aa) responsible for DNA binding, dimerization, IκB binding and nuclear translocation. The class I (p65, RelB, c-Rel) contains transactivation domain (TAD) and thus has intrinsic ability to activate transcription. The class II (p50 and p52) lacks TAD and thus their homodimers generally repress transcription of target genes but their heterodimers with class I have transcriptional activity. In non-stimulated cells, the NFκB dimer is retained in the cytoplasm by the inhibitor of NFκB (IκB). Upon stimulation, IκB is degraded via a phosphorylation-dependent proteasome-mediated mechanism and the released NFκB is translocated to the nucleus where it binds to the κB-sites and regulates the transcription of target genes. The degradation of IκB is regulated by the IκB kinase (IKK) that is activated by its upstream IKK kinases (Fig. 1). The IκB family contains 8 members including the classical inhibitors IκBα, IκBβ, IκBγ (p105), IκBδ (p100) and IκBε, as well as the atypical regulators Bcl-3, IκBξ and IκBns. All of them are characterized by the hallmark 5–7 ankyrin-repeats that mediate the interaction with NFκB. The classical IKK complex contains 2 catalytic subunits IKK1/2 or IKKα/β and 1 regulatory subunit IKKγ (Chen and Greene, 2004; Perkins, 2007).
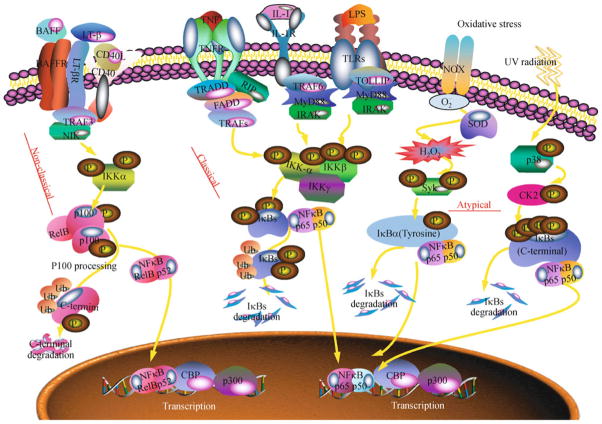
Figure 1.
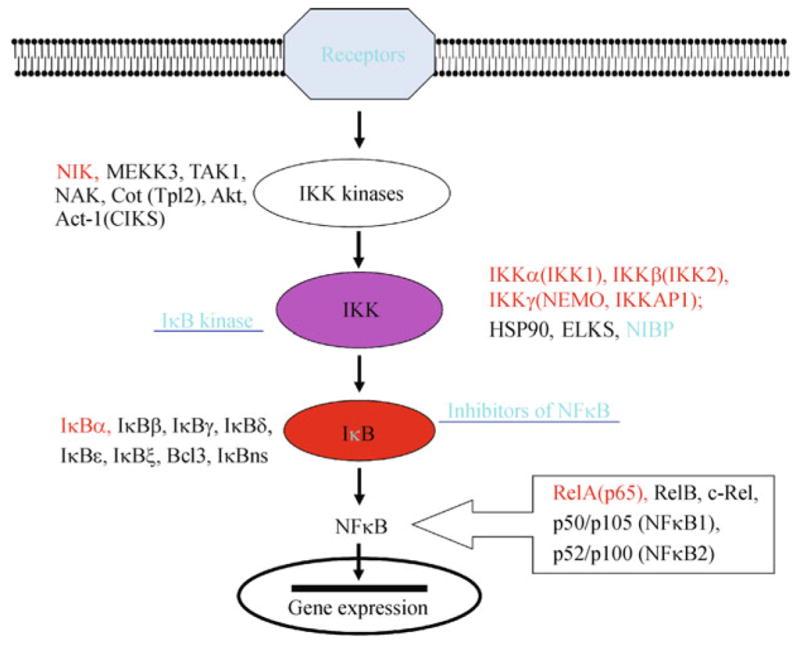
Signaling cascade of NFκB activation and identified members of each family. The red highlighted text denotes the best-studied original member(s) for each family.
Three distinct signaling pathways for NFκB activation have been identified: classical (canonical), non-classical (non-canonical, alternative) and atypical pathways (Viatour et al., 2005), all of them rely on sequentially activated kinases (Fig. 2). The classical pathway is a well-established original pathway and is crucial for the activation of innate immunity and inflammation. It involves the activation of the classical IKK complex (Häcker and Karin, 2006). This pathway generally regulates the activation of NFκB complexes (e.g. p65/p50), in response to a wide range of stimuli such as pro-inflammatory cytokines tumor necrosis factor α (TNFα) and interleukin (IL) 1β, Toll-like receptor agonists (LPS), antigens, or viruses (HTLV1, EBV). The activated IKK complex phosphorylates IκB members (IκBα, IκBβ, IκBε and p105) on a consensus motif DSGFxS (e.g. Ser-32/Ser-36 for IκBα and Ser-19/Ser-23 for IκBβ) and the phosphorylated serines act as binding site for β-TrCP, the substrate recognition subunit of a Skp1-Cullin-F-box (SCF)–type E3 ubiquitin-protein ligase complex, named SCFβ-TrCP. This process, then, leads to the ubiquitination on specific lysine and the ubiquitinated IκBs are directed to 26S proteasome for full degradation, leaving free NFκB complexes to enter into the nucleus. The kinetics of phosphorylation and degradation of IκBβ or IκBε are much slower than that of IκBα and may reflect different substrate specificities of the IKK complex. The non-classical pathway is critical for the secondary lymphoid organ development, maturation of B cells and adaptive humoral immunity. It involves TRAF3-mediated activation of the NFκB-inducing kinase (NIK) and IKKα (Razani et al., 2011; Sun, 2012). Activated IKKα phosphorylates p100 on specific serine residues. After phosphorylation, p100 is ubiquitinated by SCFβ-TrCP E3 ligase and cleaved by 19S proteasome, instead of completely degraded by 26S proteasome, to generate the NFκB subunit p52. This process is generally slower than the activation of the classical pathway and leads to a delayed activation of nuclear p52-containing complexes, such as RelB/p52. The mechanisms of p52 generation are either constitutive (by cotranslational processing) or inducible (by post-translational cleavage). The non-classical pathway is triggered by some particular members of TNF family (Lymphotoxin β, B cell activation factor, CD40 ligand). Early studies demonstrated that almost all inducers of NFκB lead to activation of the classical IKKα/β/γ complex. Additional IKK complex, NIK/IKKα (Woronicz et al., 1997; Senftleben et al., 2001) and IKKβ/IKKγ (Quirling et al., 2004), has been identified. NIK/IKKα mediates the non-classical NFκB pathway while IKKβ/IKKγ subcomplex remains functional for the classical NFκB pathway. Our studies identified a novel NFκB signaling regulator, NIBP (NIK-and IKKβ binding protein) that forms a novel subcomplex comprised of NIK-NIBP-IKKβ without IKKα and IKKγ (Hu et al., 2005). Such subcomplex may represent a novel signaling pathway of NFκB activation and regulate protein transport and subcellular trafficking (Cox et al., 2007; Kümmel et al., 2008; Zong et al., 2011; Westlake et al., 2011; Zong et al., 2012).
Figure 2.
Signaling pathways of NFκB activation. In the classical pathway, the classical stimulators like TNFα bind to receptor, leading to recruitment of the adaptor molecule TRADD and the signaling proteins TRAF2 and RIP1. TRAF2 recruits the IKK complex that phosphorylates IκBα at serine residue 32 and 36, leading to its ubiquitination and degradation by the proteasome, allowing the release of the classical heterodimer of p65/p50, which then translocate to the nucleus to regulate the transcription of target genes. In the non-classical pathway, NIK-induced phosphorylation of IKKα leads to the phosphorylation of p100 and proteasome-mediated processing of p100 into p52, allowing the nuclear translocation of RelB/p52 to regulate target gene expression. In the atypical pathway, the p38-activated casein kinase 2 (CK2) phosphorylates IκBα at a cluster of serine residues within C-terminal domain, while oxidative stress induces spleen tyrosine kinase (Syk)-mediated phosphorylation of IκBα at tyrosine residue (Y-42) within N-terminal domain.
Most stimuli activate NFκB through IKK-mediated IκB phosphorylation on N-terminal serine residues. A third signaling pathway for NFκB activation is classified as atypical because it is independent of IKK (Fig. 2). Although the phosphorylation and proteasome-mediated degradation of IκB are involved, the phosphorylation sites are different. One atypical pathway is triggered by DNA damage such as UVor doxorubicin, leading to IκBα degradation via the proteasome, but the targeted serine residues are located within a C-terminal cluster, which is recognized by the p38 MAP kinase-activated casein kinase 2 (CK2) (Kato et al., 2003; Huillard et al., 2010). Another atypical pathway is stimulated by oxidative stress, which involves the activation of NADPH oxidase (NOX), generation of superoxide and hydrogen peroxide (H2O2) that leads to NFκB activation via phosphorylation of IκBα on the N-terminal Tyr-42 residue (Gloire et al., 2006). The upstream kinases for tyrosine phosphorylation of IκBα remain unclear, and the spleen tyrosine kinase (Syk) may be involved (Gallagher et al., 2007).
NFκB signaling in embryonic neurogenesis (neurodevelopment)
During early embryonic development, a subset of cells in the ectoderm overlying the notochord of the mesoderm proliferate/differentiate and become neural plate in response to the diffusible inhibitory signals (neural inducer) produced from the notochord. Neural plate folds to form the neural groove, which fuses to form the neural tube. Within the neural tube, NSCs (radial glia cells) derive from neuroepithelial cells (NECs) and differentiate sequentially into NPCs and various lineage-restricted precursor cells (RPCs), which include neuroblast and glioblast. These RPCs migrate to the marginal zone and beyond to assume their terminal fate. The generation of different lineage occurs in a temporally distinct yet overlapping pattern. In rodents, neuronogenesis peaks at embryonic day (E) 14, astrocytogenesis at postnatal day (P) 2, and oligodendrocytogenesis at P14 (Wang and Bordey, 2008; Sauvageot and Stiles, 2002).
The self-renewal and cell fate decision of NSCs during embryonic neurogenesis are regulated by various transcription factors and their signaling pathways including the nuclear hormone receptor TLX (tailless), the high-mobility-group transcription factor Sox2, the basic helix-loop-helix transcriptional factor Hes (hairy and enhancer of split), the tumor suppressor phosphatase Pten (phosphatase and tensin homolog deleted on chromosome 10), and the Drosophila membrane-associated protein Numb homologs, Numb and Numblike (Shi et al., 2008). The Hes family plays key but opposing role in regulating neurodevelopment. Hes1 and Hes5 are activated by Notch signaling and repress the expression of proneuronal factors such as Mash1, Neurogenin, Math and NeuroD (Cau et al., 2000; Kageyama et al., 2008). In contrast, Hes6 promotes neuronal differentiation but inhibits astrocyte differentiation (Fior and Henrique, 2005; Vilas-Boas and Henrique, 2010).
NFκB is essential for embryonic development because p65 knockout mice died on E15 and p65/p50 or p65/c-rel double knockout mice died on E13 due to liver degeneration (Beg et al., 1995; Grossmann et al., 1999). Such embryonic lethality precluded further investigation on the role of NFκB in late embryonic brain development. Additional knockout of TNF receptor 1 (TNFR1) in these p65 null mice rescued embryonic lethality (Alcamo et al., 2001), providing an opportunity to investigate the role of NFκB signaling in regulating embryonic neurogenesis (Young et al., 2006). However, the distribution pattern of NSCs/NPCs and cell lineage analysis in neurogenic zones of these mutants have not yet been examined. IKKα/IKKβ double knockout mice died on E12 due to apoptosis of NECs leading to impairments in neurogenesis (Li et al., 2000). In Drosophila (Chen et al., 2000; Ayyar et al., 2007; DeLotto et al., 2007; Reeves and Stathopoulos, 2009) and Xenopus laevis (Lake et al., 2001), the graded activation of NFκB/c-rel protein in dorsal region determine the dorsal-ventral patterning during the very early embryonic development. During mouse embryogenesis, virtually all members of the NFκB pathway are expressed in embryonic, trophoblastic, and uterine cells (Torchinsky and Toder, 2004). NFκB may protect the embryos exposed to embryopathic stresses, possibly through its anti-apoptotic effect (Torchinsky and Toder, 2004).
Although little is known regarding the role of NFκB in the early step of embryonic neurogenesis, several lines of evidence demonstrate that NFκB is implicated in early differentiation of other stem cells. During murine spermatogenesis, NFκB is activated in a stage-specific manner (Lilienbaum et al., 2000). During oocyte maturation and early embryonic development, NFκB is activated (Nishikimi et al., 1999; Paciolla et al., 2011). In Drosophila melanogaster, the mRNA of the p65 homolog, named Dorsal, is maternally expressed and is concentrated in the egg cortex (Chen et al., 2000). In Xenopus, NFκB activation is observed during oocyte maturation (Dominguez et al., 1993) and in late blastulae and gastrulae (Richardson et al., 1994). In mouse embryos, NFκB activation is crucial to engage development beyond the 2-cell stage (Nishikimi et al., 1999). NFκB mediates the neurogenic effect of erythropoietin in neurosphere cultures from E14 mouse ganglionic eminence (Shingo et al., 2001). Neural induction from cultured embryonic stem (ES) cells or induced pluripotent stem (iPS) cells has been established (Osakada and Takahashi, 2011; Denham et al., 2012), but the signaling mechanisms remain largely unknown. Such induction is an excellent in vitro model to recapture the embryonic neurogenesis. The signaling pathways identified during endogenous embryonic morphogenesis and neurogenesis can be applied to the neural induction and patterning, such as bone morphogenetic protein (BMP), fibroblast growth factor (FGF), Wnt, Shh and Notch signaling. We speculate that NFκB signaling play an important role in the neuronal induction from ES or iPS cells. Recently, it has been shown that murine and human ES cells possess a low level of NFκB activity that increases significantly during the differentiation process (Kang et al., 2007; Kim et al., 2008; Torres and Watt, 2008). In human ES cells, the classical NFκB pathway regulates differentiation while the non-classical pathway maintains pluripotency (Yang et al., 2010). The transcription factor Nanog is essential in maintaining pluripotency of ES cells (Mitsui et al., 2003). During ES cell differentiation, endogenous NFκB activity and target-gene expression increased. NFκB inhibition increased expression of pluripotency markers. Nanog binds to NFκB proteins, inhibits NFκB activity and cooperates with Stat3 to maintain pluripotency (Torres and Watt, 2008). ES cell-specific miR-290 maintains the pluripotency and self-renewal of ES cells through repressing NFκB classical signaling (Lüningschrör et al., 2012). Forced expression of p65 causes loss of pluripotency, promotes differentiation of ES cells, and leads to an epithelial to mesenchymal transition. These data define p65 as a novel target gene of miR-290 cluster and provide new insight into the function of ES cell-specific miRNAs (Lüningschrör et al., 2012). Taken altogether, NFκB signaling is activated and required during the early differentiation of various stem cells including ES/iPS, hematopoietic (Xiao et al., 2009; Montano-Almendras et al., 2012), mesenchymal (Hess et al., 2009; Cho et al., 2010) and cancer stem cells (Reikvam et al., 2009; Nogueira et al., 2011).
NFκB signaling in adult neurogenesis
NFκB signaling is well known to regulate cell survival or apoptosis in many cell types, depending upon the generation of its target genes. In adult nervous system, inflammation-related NFκB signaling plays a sword-edge role after injuries or diseases. Neuronal NFκB signaling is neuroprotective via its crucial role in maintaining neuronal survival, synaptogenesis, neural plasticity, learning and memory (Fridmacher et al., 2003; Mattson and Meffert, 2006; Camandola and Mattson, 2007; Boersma et al., 2011). A striking enrichment of phosphorylated IκBα and IKK is observed in the axon initial segment (Schultz et al., 2006; Sanchez-Ponce et al., 2008) and the nodes of Ranvier (Politi et al., 2008), suggesting a novel role of NFκB signaling in regulating axonal polarity and initial axonal formation. In the zones of active neurogenesis in both postnatal and adult mouse brain, various members of the NFκB family are highly expressed (Denis-Donini et al., 2005), indicating for the first time that NFκB is actively involved in the proliferation, migration and differentiation of adult NSCs/NPCs (Rolls et al., 2007; Widera et al., 2008). The presence of NFκB in adult neurogenic zone is further validated using immunofluorescent microscopy (Meneghini et al., 2010; Zhang et al., 2012b). Early studies using κB-dependent lacZ transgenic mice show that NFκB activation in the central nervous system starts on day E12.5 and major changes are not observed until after birth, when the structures start their final maturation processes, but details about neurogenic zones are not reported (Schmidt-Ullrich et al., 1996). Recent studies in the hippocampal subgranular zone of adult NFκB lacZ reporter mice have identified the activation of NFκB signaling in NSCs instead of NPCs (Koo et al., 2010), suggesting a potential role of NFκB signaling in early neurogenesis. Direct evidence for the in vivo effect of NFκB signaling on the proliferation of NSCs/NPCs derives from p65 and p50 double knockout mice (Young et al., 2006) as well as overexpression of super inhibitor IκBα mutant in NSCs/NPCs (Wu et al., 2000; Widera et al., 2006a; Rubio-Araiz et al., 2008; Zhu et al., 2008).
Little is known about the role of NFκB signaling in regulating neural differentiation of NSCs/NPCs. In PC12 cells, both NIK and IKKβ and their binding protein NIBP are required for nerve growth factor-induced neuronal differentiation via NFκB signaling (Foehr et al., 2000; Azoitei et al., 2005; Hu et al., 2005), though several other signaling pathways are also involved (Wooten et al., 2000; Azoitei et al., 2005). In neuroblastoma cells, NFκB promotes neuronal differentiation (Feng and Porter, 1999). Toll-like receptor 2 (TLR2) induces neuronal differentiation via protein kinase C (PKC)-dependent activation of NFκB whereas TLR4 inhibits proliferation and neuronal differentiation of NPCs (Rolls et al., 2007). In p50-deficient mice, the neuronal differentiation of adult hippocampal NSCs is reduced by 50% while the proliferation does not change (Denis-Donini et al., 2008). In our recent study, we demonstrate that NFκB signaling regulates the early differentiation of NSCs (Zhang et al., 2012b). During early differentiation, NFκB signaling becomes active. Addition of TNFα to activate NFκB signaling under proliferation conditions induces neural differentiation of NSCs/NPCs (Lou et al., 2003; Bernardino et al., 2008; Zhang et al., 2012b). TNF-like weak inducer of apoptosis (TWEAK) induces neuronal differentiation of NSCs/NPCs, under proliferation condition, through NFκB-dependent downregulation of Hes1 that prevents neuronal differentiation (Schölzke et al., 2011). Selective inhibition of canonical NFκB signaling by various pharmacologic inhibitors, shRNA and NSC-specific transgene dnIκBα retain the tripotential ability of differentiation and restore or enhance self-renewal capability of NSCs, suggesting that NFκB signaling is essential for early neural differentiation (Zhang et al., 2012b). The critical role of NFκB in the initial differentiation step of NSCs highlights a novel molecular mechanism for neurogenesis. Under physiologic conditions, moderate activation of NFκB signaling promotes NSC differentiation into NPCs and maintains a continuous source for adult neurogenesis. In a mouse inducible IκBα transgenic model, NFκB in NSCs/NPCs is necessary for axogenesis and maturation (Imielski et al., 2012). However, persistent and repeated overactivation of NFκB signaling in NSCs may exhaust the NSC pool and thus lead to reduced neurogenesis as seen in aging patients (Villeda et al., 2011; Artegiani and Calegari, 2012; Encinas and Sierra, 2012) and chronic stress (Koo et al., 2010).
Cytokine-induced regulation of NFκB signaling during neurogenesis
Several cytokines have been shown to regulate neurogenesis, such as TNFα, IL-1β, IL-6, TWEAK, and IFNγ (Das and Basu, 2008; Whitney et al., 2009; Schölzke et al., 2011; Yirmiya and Goshen, 2011), and most of them stimulate NFκB signaling. TNFα is extensively studied in affecting neurogenesis but the reports remain controversial. The enhancing effect of TNFα on neuronal differentiation is first reported in rat mesencephalic NSCs (Lou et al., 2003). TNFα stimulation promotes the reaggregation of 3D neurospheres in vitro (Widera et al., 2006c). TNFR1-derived peptide promotes neuronal differentiation of the hippocampal NSCs (Kajiwara et al., 2005). However, TNFα has no effect on neuronal differentiation and neurite outgrowth of murine adult NSCs/NPCs (Wong et al., 2004; Keohane et al., 2010) and mouse ES-derived NPCs (Ideguchi et al., 2008). Other data suggest that TNFα promote gliogenesis but not neuronogenesis in the NSCs/NPCs (Ricci-Vitiani et al., 2006; Ideguchi et al., 2008; Johansson et al., 2008; Peng et al., 2008). Recent study demonstrates that TNFα-induced neuronal differentiation in NSCs/NPCs is dose-dependent (Bernardino et al., 2008). These opposite results regarding the effects of TNFα on neural differentiation may have resulted from the use of different protocols (culture media, coating matrix, and exposure time), passage numbers, brain regions, species, or doses (Keohane et al., 2010). In addition, these reports have targeted the late stages (from NPCs to RPCs and terminal cells) of neural differentiation. Using stemness assay and pharmacological approach, we demonstrate that TNFα treatment under proliferation conditions induced neural differentiation through NFκB activation at a very early stage of neurogenesis. We also confirmed previous reports (Bernardino et al., 2008; Keohane et al., 2010) showing that TNFα controls the survival and neuronal differentiation of neural cells at some late stages during neural differentiation. The neurogenic effects of inflammatory cytokines highlight the important role of inflammatory and immune responses in early neurogenesis after injury or diseases of the nervous system (Rolls et al., 2007).
IL-1β, another major proinflammatory cytokine, also plays an important role in neurogenesis. Several lines of evidence implicate that IL-1β mediates stress-induced depression via inhibiting adult neurogenesis (Ben Menachem-Zidon et al., 2008; Goshen et al., 2008; Koo and Duman, 2008; Goshen and Yirmiya, 2009). Blockade of IL-1β signaling could be a novel therapeutic approach for the treatment of depression (Koo and Duman, 2009). Further studies demonstrate a critical role of NFκB signaling in mediating IL-1β-induced antineurogenic effect by exhausting NSC pool (Koo et al., 2010). IL-1β treatment decreases the proliferation and self-renewal of proliferating NSCs/NPCs cultured from embryonic rat hippocampus, but inhibits neuronal differentiation and promotes astroglial differentiation (Green et al., 2012), consistent with the effect of TNFα (Ricci-Vitiani et al., 2006; Ideguchi et al., 2008; Johansson et al., 2008; Peng et al., 2008).
Increasing evidence shows that another cytokine, IL-6, inhibits neuronogenesis but promotes gliogenesis both in vitro and in vivo (Okano, 2006; Nakanishi et al., 2007; Ideguchi et al., 2008; Johansson et al., 2008; Mukaino et al., 2008; Islam et al., 2009). However, interferon-γ is shown to inhibit the proliferation but promote neuronal differentiation of NSCs (Ben-Hur et al., 2003;Wong et al., 2004; Ideguchi et al., 2008; Johansson et al., 2008; Lum et al., 2008).
Taken together, transient NFκB activation by these cytokines under normal conditions or early stage of injuries or diseases may be beneficial by promoting NSC differentiation for neural repair or maintaining daily neurogenesis. However, chronic NFκB activation may delete the NSC pool, leading to aberrant and insufficient neurogenesis (Koo et al., 2010; Artegiani and Calegari, 2012; Schwarz et al., 2012).
Non-classical and atypical NFκB signaling pathways during neurogenesis
Most studies in terms of neurogenesis target the classical IKKβ/IκBα/p65 pathway of NFκB activation (Widera et al., 2008; Kaltschmidt and Kaltschmidt, 2009). Little is known about the role of non-classical and atypical pathways in neurogenesis. RelB is expressed in migrating neuroblast, c-Rel is present in a few cells located at the edges of the rostral migratory stream, but only the classical p65/p50 is detectable in NSCs/NPCs (Denis-Donini et al., 2005). The p65 knockout mice dies on E15 (Beg et al., 1995), but RelB (Weih et al., 1997), c-Rel (Köntgen et al., 1995) or p50 (Sha et al., 1995) knockout mice are not embryonically lethal. There are no reports on the neurogenic pattern and lineage differentiation in the neurogenic zones of these knockout mice. In NSCs/NPCs, the pigment epithelium-derived factor (PEDF) induces a non-canonical activation of the NFκB pathway, leading to the dismissal of the transcriptional co-repressor N-CoR from specific Notch-responsive promoters (Andreu-Agulló et al., 2009). However, several studies have shown that non-classical pathway plays important role in the differentiation of ES cells and other tissue stem cells. For example, the canonical NFκB pathway regulates differentiation while the noncanonical pathway maintains the pluripotency of human ES cells (Yang et al., 2010). The p100/RelB signaling positively and intrinsically regulates self-renewal of hematopoietic stem/progenitor cell (Zhao et al., 2012). NIK/RelB signaling is required for osteoclast differentiation (Vaira et al., 2008). In mesenchymal stem cells, the non-classical NFκB pathway promotes but the classical pathway inhibits the late and sustained CD69 expression (Saldanha-Araujo et al., 2011). LIGHT, a stimulator for non-classical NFκB pathway, negatively regulates neurite growth from developing sensory neurons via inhibition of classical NFκB pathway (Gavaldà et al., 2009). The mechanisms underlying the different and frequently opposing effects of the classical and non-classical NFκB pathways in stem cell seflrenewal and differentiation remain to be determined. NIBP may act as a switch between two pathways by binding to IKKβ (classical) and NIK (nonclassical) (Hu et al., 2005).
Although there is no direct evidence, we speculate that the atypical NFκB pathway is involved in neurogenesis, because the key components of these atypical pathways have been shown to regulate neurogenesis. NOX-mediated ROS is well known to activate NFκB in various cell types (Gloire and Piette, 2009; Morgan and Liu, 2011). NSCs/NPCs maintain a higher level of ROS compared to differentiated neurons (Le Belle et al., 2011). Endogenous ROS and nitric oxide are essential for the proliferation and differentiation of NSCs/NPCs (Yoneyama et al., 2010; Le Belle et al., 2011). Controlled generation of ROS at low-to-moderate levels stimulates neural differentiation of NSCs/NPCs (Vieira et al., 2011; Kennedy et al., 2012). Numb-interacting protein 1 (Nip1), a component of the ROS-generating Duox system, directs neuronal differentiation of NSCs through ROS generation (Kennedy et al., 2010). The mechanisms underlying ROS-mediated neurogenesis may involve ROS-stimulated signaling pathways such as PI3K/Akt (Le Belle et al., 2011), p38 MAPK (Kim and Wong, 2009), ERK (Kim et al., 2011a), JNK (Yeo and Kang, 2007) and NFκB signaling (Piao et al., 2005; Li et al., 2009; Qin and Crews, 2012). Syk mediates ciliary neurotrophic factor (CNTF)-induced tyrosine phosphorylation of IκBα and promotes neurite growth of developing neurons (Gallagher et al., 2007; Gutierrez and Davies, 2011). Syk is involved in ephrinA-induced promotion of neuritogenesis (Richards et al., 2006; Angibaud et al., 2011). CK2β functions as a positive regulator for NSC proliferation and multipotency (Ziercher et al., 2011) and controls oligodendrocytogenesis (Huillard et al., 2010).
Crosstalk between NFκB and other signaling pathways
Many signaling pathways have been identified to regulate both embryonic and adult neurogenesis, including Notch, Shh, Wnt/β-catenin, etc. (Mu et al., 2010). The functional interaction of these pathways with NFκB signaling has been reported in neuron, microglia, astrocytes and other cells (Cao et al., 2011; Maniati et al., 2011; Zhang et al., 2012a). Such crosstalk may occur also to NSCs/NPCs (Bonini et al., 2011), but experimental evidence is needed. For example, Notch signaling is well known to maintain NSC selfrenewal and multipotency, and thus it is speculated that NFκB signaling may regulate NSC stemness through Notch signaling (Ang and Tergaonkar, 2007; Fujita et al., 2011). PEDF modulates Notch-dependent stemness of mouse NSCs through activation of non-canonical NFκB pathway (Andreu-Agulló et al., 2009). In mouse mesencephalic neural crest cells, NFκB signaling promotes gliogenesis through the interaction with Notch signaling (Fujita et al., 2011). In Xenopus laevis, NFκB activates Shh signaling for anterior neural patterning (Lake et al., 2001). In mouse, Shh signaling regulates neural induction from ES cells (Cai et al., 2008) or NSCs (Dave et al., 2011). TLR3 regulates NSC proliferation by modulating Shh signaling (Yaddanapudi et al., 2011), perhaps through activation of NFκB (Kasperczyk et al., 2009). Many studies have demonstrated the importance of Wnt/β-catenin signaling in neurogenesis (Kondo et al., 2011; Pei et al., 2012) and its interaction with NFκB signaling in cancer stem cells (Gavert et al., 2011; Pan et al., 2012) and mesenchymal stem cells (Hyun Hwa et al., 2008; Kim et al., 2011b). However, the crosstalk between NFκB and Wnt/β-catenin signaling during neural induction and neurogenesis need further investigation.
Correlation of NFκB dysfunction to neurodevelopmental and neurodegenerative diseases
The link of NFκB signaling defects to various neurodevelopmental disorders remains to be determined, though many immune and inflammatory responses have been implicated in these diseases and NFκB signaling mediates most actions of these immune/inflammatory factors. Among 6 genes associated with nonsyndromic autosomal-recessive mental retardation (Mir et al., 2009; Mochida et al., 2009; Philippe et al., 2009), two, NIBP (Mir et al., 2009; Mochida et al., 2009; Philippe et al., 2009) and CC2D1A (Noor et al., 2008; Zhao et al. 2010), have been shown to regulate NFκB signaling through the classical IKKβ pathway, implying the important role of NFκB signaling in mental retardation. In the cerebellum and front cortex from autism patients and animal model, IKKα expression is significantly increased, but the expression and activity of the classical NFκB signaling components such as IκBα and p65 do not display any significant change (Song et al., 2009), although the role of non-classical and atypical NFκB pathways need further investigation. In schizophrenia patients, NFκB activity is increased in peripheral blood mononuclear cells (Song et al., 2009) and the expression of NFκB-responsive genes is also increased (Hashimoto et al., 2011). Three single nucleotide polymorphism variants of the p65 gene are associated with schizophrenia in a Japanese population (Hashimoto et al., 2011).
NFκB signaling has been widely shown to regulate the pathogenesis of many neurodegenerative diseases such as Alzheimer’s (Granic et al., 2009; Mu and Gage, 2011), Parkinson’s (Ghosh et al., 2007; Sha et al., 2010), Huntington’s (Marcora and Kennedy, 2010) diseases and multiple sclerosis (Huehnchen et al., 2011; Tepavčević et al., 2011), as well as traumatic or ischemic injuries of nervous system (Dong et al., 2011; Zhang et al., 2011). NFκB signaling has been targeted as a therapeutic strategy for neurodegenerative diseases (Camandola and Mattson, 2007; Mattson et al., 2000). On the other hand, adult neurogenesis has been shown to contribute to these neurodegenerative diseases (Mu and Gage, 2011; Winner et al., 2011). NFκB signaling acts as a double-edge sword during neurodegenerative disease, and the outcome depends upon the cell type, environmental factors and disease stages (Massa et al., 2006; Yang et al., 2007; Teng and Tang, 2010). As discussed above, NFκB signaling regulates all aspects of adult neurogenesis, which may contribute to the development and progression of nervous system injury and diseases.
Concluding remark and future direction
NFκB signaling is a key mediator for numerous niche factors that regulate various stages or phases of embryonic and adult neurogenesis. The classical pathway of NFκB activation plays important role in regulating selfrenewal/multipotency and early differentiation of NSCs, as well as the proliferation/apoptosis of NPCs, migration of neuroblast, maturation and plasticity of nascent neurons. NFκB signaling is also important in regulating the early differentiation of other stem cells such as embryonic stem cells, hematopoietic stem cell, and mesenchymal stem cells. During neural induction both in vitro and in vivo, NFκB signaling is required. Further investigation of the upstream regulation and downstream mechanism underlying the essential role of NFκB signaling in initiating early differentiation of both neural induction and neurogenesis will open a potential avenue for the development of therapeutics for the treatment of neurodevelopmental disorders and neurodegenerative diseases. Emerging evidence suggests that non-classical and atypical NFκB pathways are implicated in ES cell differentiation. It will be important to evaluate the different role of three NFκB pathways during neurogenesis. NFκB signaling selectively regulates cell fate decision and lineage differentiation. The major barrier for NSC-based transplantation is the predominant gliogenesis in vivo when NSCs/NPCs encounter the complicate microenvironmental niches. Thus, selective blockade of gliogenesis and promotion of neuronal differentiation will be an important strategy for stem cell transplantation.
Acknowledgments
This work was supported by the National Institutes of Diabetes and Digestive and Kidney Diseases (WH, DK075964) and the National Center for Research Resources (WH, RR032123).
References
- Alcamo E, Mizgerd JP, Horwitz BH, Bronson R, Beg AA, Scott M, Doerschuk CM, Hynes RO, Baltimore D. Targeted mutation of TNF receptor I rescues the RelA-deficient mouse and reveals a critical role for NF-κB in leukocyte recruitment. J Immunol. 2001;167(3):1592–1600. doi: 10.4049/jimmunol.167.3.1592. [DOI] [PubMed] [Google Scholar]
- Andres-Mach M, Fike JR, Łuszczki JJ. Neurogenesis in the epileptic brain: a brief overview from temporal lobe epilepsy. Pharmacol Rep. 2011;63(6):1316–1323. doi: 10.1016/s1734-1140(11)70696-x. [DOI] [PubMed] [Google Scholar]
- Andreu-Agulló C, Morante-Redolat JM, Delgado AC, Fariñas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nat Neurosci. 2009;12(12):1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- Ang HL, Tergaonkar V. Notch and NFκB signaling pathways: Do they collaborate in normal vertebrate brain development and function? Bioessays. 2007;29(10):1039–1047. doi: 10.1002/bies.20647. [DOI] [PubMed] [Google Scholar]
- Angibaud J, Louveau A, Baudouin SJ, Nerrière-Daguin V, Evain S, Bonnamain V, Hulin P, Csaba Z, Dournaud P, Thinard R, Naveilhan P, Noraz N, Pellier-Monnin V, Boudin H. The immune molecule CD3zeta and its downstream effectors ZAP-70/Syk mediate ephrin signaling in neurons to regulate early neuritogenesis. J Neurochem. 2011;119(4):708–722. doi: 10.1111/j.1471-4159.2011.07469.x. [DOI] [PubMed] [Google Scholar]
- Artegiani B, Calegari F. Age-related cognitive decline: Can neural stem cells help us? Aging (Albany NY) 2012;4(3):176–186. doi: 10.18632/aging.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyar S, Pistillo D, Calleja M, Brookfield A, Gittins K, Goldstone C, Simpson P. NF-κB/Rel-mediated regulation of the neural fate in Drosophila. PLoS ONE. 2007;2(11):e1178. doi: 10.1371/journal.pone.0001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoitei N, Wirth T, Baumann B. Activation of the IκB kinase complex is sufficient for neuronal differentiation of PC12 cells. J Neurochem. 2005;93(6):1487–1501. doi: 10.1111/j.1471-4159.2005.03148.x. [DOI] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376(6536):167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Ben-Hur T, Ben-Menachem O, Furer V, Einstein O, Mizrachi-Kol R, Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci. 2003;24(3):623–631. doi: 10.1016/s1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Ben Menahem Y, Reinhartz E, Ben Hur T, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33(9):2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26(9):2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- Boersma MC, Dresselhaus EC, De Biase LM, Mihalas AB, Bergles DE, Meffert MK. A requirement for nuclear factor-κB in developmental and plasticity-associated synaptogenesis. J Neurosci. 2011;31(14):5414–5425. doi: 10.1523/JNEUROSCI.2456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonini SA, Ferrari-Toninelli G, Uberti D, Montinaro M, Buizza L, Lanni C, Grilli M, Memo M. Nuclear factor κB-dependent neurite remodeling is mediated by Notch pathway. J Neurosci. 2011;31 (32):11697–11705. doi: 10.1523/JNEUROSCI.1113-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce BF, Yao Z, Xing L. Functions of nuclear factor κB in bone. Ann N Y Acad Sci. 2010;1192(1):367–375. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C, Thorne J, Grabel L. Hedgehog serves as a mitogen and survival factor during embryonic stem cell neurogenesis. Stem Cells. 2008;26(5):1097–1108. doi: 10.1634/stemcells.2007-0684. [DOI] [PubMed] [Google Scholar]
- Callan MA, Zarnescu DC. Heads-up: new roles for the fragile X mental retardation protein in neural stem and progenitor cells. Genesis. 2011;49(6):424–440. doi: 10.1002/dvg.20745. [DOI] [PubMed] [Google Scholar]
- Camandola S, Mattson MP. NF-κB as a therapeutic target in neurodegenerative diseases. Expert Opin Ther Targets. 2007;11(2):123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Cao Q, Kaur C, Wu CY, Lu J, Ling EA. Nuclear factor-κβ regulates notch signaling in production of proinflammatory cytokines and nitric oxide in murine BV-2 microglial cells. Neuroscience. 2011;192:140–154. doi: 10.1016/j.neuroscience.2011.06.060. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127(11):2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Chen G, Handel K, Roth S. The maternal NF-κB/dorsal gradient of Tribolium castaneum: dynamics of early dorsoventral patterning in a short-germ beetle. Development. 2000;127(23):5145–5156. doi: 10.1242/dev.127.23.5145. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5(5):392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Cho HH, Shin KK, Kim YJ, Song JS, Kim JM, Bae YC, Kim CD, Jung JS. NF-κB activation stimulates osteogenic differentiation of mesenchymal stem cells derived from human adipose tissue by increasing TAZ expression. J Cell Physiol. 2010;223(1):168–177. doi: 10.1002/jcp.22024. [DOI] [PubMed] [Google Scholar]
- Conti L, Cattaneo E. Neural stem cell systems: physiological players or in vitro entities? Nat Rev Neurosci. 2010;11(3):176–187. doi: 10.1038/nrn2761. [DOI] [PubMed] [Google Scholar]
- Cox R, Chen SH, Yoo E, Segev N. Conservation of the TRAPPII-specific subunits of a Ypt/Rab exchanger complex. BMC Evol Biol. 2007;7(1):12. doi: 10.1186/1471-2148-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Low VF, Faull RL. Neurogenesis and progenitor cells in the adult human brain: a comparison between hippocampal and subventricular progenitor proliferation. Dev Neurobiol. 2012 doi: 10.1002/dneu.22028. Online Available April 27, 2012. [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86(6):1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Dave RK, Ellis T, Toumpas MC, Robson JP, Julian E, Adolphe C, Bartlett PF, Cooper HM, Reynolds BA, Wainwright BJ. Sonic hedgehog and notch signaling can cooperate to regulate neurogenic divisions of neocortical progenitors. PLoS ONE. 2011;6(2):e14680. doi: 10.1371/journal.pone.0014680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLotto R, DeLotto Y, Steward R, Lippincott-Schwartz J. Nucleocytoplasmic shuttling mediates the dynamic maintenance of nuclear Dorsal levels during Drosophila embryogenesis. Development. 2007;134(23):4233–4241. doi: 10.1242/dev.010934. [DOI] [PubMed] [Google Scholar]
- Denham M, Parish CL, Leaw B, Wright J, Reid CA, Petrou S, Dottori M, Thompson LH. Neurons derived from human embryonic stem cells extend long-distance axonal projections through growth along host white matter tracts after intra-cerebral transplantation. Front Cell Neurosci. 2012;6:11. doi: 10.3389/fncel.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis-Donini S, Caprini A, Frassoni C, Grilli M. Members of the NF-κB family expressed in zones of active neurogenesis in the postnatal and adult mouse brain. Brain Res Dev Brain Res. 2005;154(1):81–89. doi: 10.1016/j.devbrainres.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Denis-Donini S, Dellarole A, Crociara P, Francese MT, Bortolotto V, Quadrato G, Canonico PL, Orsetti M, Ghi P, Memo M, Bonini SA, Ferrari-Toninelli G, Grilli M. Impaired adult neurogenesis associated with short-term memory defects in NF-κB p50-deficient mice. J Neurosci. 2008;28(15):3911–3919. doi: 10.1523/JNEUROSCI.0148-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I, Sanz L, Arenzana-Seisdedos F, Diaz-Meco MT, Virelizier JL, Moscat J. Inhibition of protein kinase C zeta subspecies blocks the activation of an NF-κB-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993;13(2):1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Liu B, Song L, Lu L, Xu H, Gu Y. Neural stem cells in the ischemic and injured brain: endogenous and transplanted. Cell Tissue Bank. 2011 doi: 10.1007/s10561-011-9283-z. Online Available Dec 21, 2011. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Sierra A. Neural stem cell deforestation as the main force driving the age-related decline in adult hippocampal neurogenesis. Behav Brain Res. 2012;227(2):433–439. doi: 10.1016/j.bbr.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Vazquez ME, Switzer RC, Chamberland DW, Nick H, Levine HG, Scarpa PJ, Enikolopov G, Steindler DA. Quiescent adult neural stem cells are exceptionally sensitive to cosmic radiation. Exp Neurol. 2008;210(1):274–279. doi: 10.1016/j.expneurol.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Porter AG. NF-κB/Rel proteins are required for neuronal differentiation of SH-SY5Y neuroblastoma cells. J Biol Chem. 1999;274 (43):30341–30344. doi: 10.1074/jbc.274.43.30341. [DOI] [PubMed] [Google Scholar]
- Fior R, Henrique D. A novel hes5/hes6 circuitry of negative regulation controls Notch activity during neurogenesis. Dev Biol. 2005;281(2):318–333. doi: 10.1016/j.ydbio.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Foehr ED, Bohuslav J, Chen LF, DeNoronha C, Geleziunas R, Lin X, O’Mahony A, Greene WC. The NF-κB-inducing kinase induces PC12 cell differentiation and prevents apoptosis. J Biol Chem. 2000;275(44):34021–34024. doi: 10.1074/jbc.C000507200. [DOI] [PubMed] [Google Scholar]
- Fridmacher V, Kaltschmidt B, Goudeau B, Ndiaye D, Rossi FM, Pfeiffer J, Kaltschmidt C, Israël A, Mémet S. Forebrain-specific neuronal inhibition of nuclear factor-κB activity leads to loss of neuroprotection. J Neurosci. 2003;23(28):9403–9408. doi: 10.1523/JNEUROSCI.23-28-09403.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita K, Yasui S, Shinohara T, Ito K. Interaction between NF-κB signaling and Notch signaling in gliogenesis of mouse mesencephalic neural crest cells. Mech Dev. 2011;128(7–10):496–509. doi: 10.1016/j.mod.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Gutierrez H, Gavalda N, O’Keeffe G, Hay R, Davies AM. Nuclear factor-κB activation via tyrosine phosphorylation of inhibitor κB-alpha is crucial for ciliary neurotrophic factor-promoted neurite growth from developing neurons. J Neurosci. 2007;27(36):9664–9669. doi: 10.1523/JNEUROSCI.0608-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavaldà N, Gutierrez H, Davies AM. Developmental regulation of sensory neurite growth by the tumor necrosis factor superfamily member LIGHT. J Neurosci. 2009;29(6):1599–1607. doi: 10.1523/JNEUROSCI.3566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavert N, Vivanti A, Hazin J, Brabletz T, Ben-Ze’ev A. L1-mediated colon cancer cell metastasis does not require changes in EMT and cancer stem cell markers. Mol Cancer Res. 2011;9(1):14–24. doi: 10.1158/1541-7786.MCR-10-0406. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA. 2007;104(47):18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: fifteen years later. Biochem Pharmacol. 2006;72(11):1493–1505. doi: 10.1016/j.bcp.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Gloire G, Piette J. Redox regulation of nuclear post-translational modifications during NF-κB activation. Antioxid Redox Signal. 2009;11 (9):2209–2222. doi: 10.1089/ars.2009.2463. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol Psychiatry. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Goshen I, Yirmiya R. Interleukin-1 (IL-1): a central regulator of stress responses. Front Neuroendocrinol. 2009;30(1):30–45. doi: 10.1016/j.yfrne.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Granic I, Dolga AM, Nijholt IM, van Dijk G, Eisel UL. Inflammation and NF-κB in Alzheimer’s disease and diabetes. J Alzheimers Dis. 2009;16(4):809–821. doi: 10.3233/JAD-2009-0976. [DOI] [PubMed] [Google Scholar]
- Green HF, Treacy E, Keohane AK, Sullivan AM, O’Keeffe GW, Nolan YM. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol Cell Neurosci. 2012;49(3):311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- Grossmann M, Metcalf D, Merryfull J, Beg A, Baltimore D, Gerondakis S. The combined absence of the transcription factors Rel and RelA leads to multiple hemopoietic cell defects. Proc Natl Acad Sci USA. 1999;96(21):11848–11853. doi: 10.1073/pnas.96.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez H, Davies AM. Regulation of neural process growth, elaboration and structural plasticity by NF-κB. Trends Neurosci. 2011;34 (6):316–325. doi: 10.1016/j.tins.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006(357):re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Hanson ND, Owens MJ, Nemeroff CB. Depression, antidepressants, and neurogenesis: a critical reappraisal. Neuropsychopharmacology. 2011;36(13):2589–2602. doi: 10.1038/npp.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Ohi K, Yasuda Y, Fukumoto M, Yamamori H, Takahashi H, Iwase M, Okochi T, Kazui H, Saitoh O, Tatsumi M, Iwata N, Ozaki N, Kamijima K, Kunugi H, Takeda M. Variants of the RELA gene are associated with schizophrenia and their startle responses. Neuropsychopharmacology. 2011;36(9):1921–1931. doi: 10.1038/npp.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-κB signaling pathway. Bone. 2009;45(2):367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- Hodge RD, Hevner RF. Expression and actions of transcription factors in adult hippocampal neurogenesis. Dev Neurobiol. 2011;71(8):680–689. doi: 10.1002/dneu.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WH, Pendergast JS, Mo XM, Brambilla R, Bracchi-Ricard V, Li F, Walters WM, Blits B, He L, Schaal SM, Bethea JR. NIBP, a novel NIK and IKK(beta)-binding protein that enhances NF-(κ)B activation. J Biol Chem. 2005;280(32):29233–29241. doi: 10.1074/jbc.M501670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huehnchen P, Prozorovski T, Klaissle P, Lesemann A, Ingwersen J, Wolf SA, Kupsch A, Aktas O, Steiner B. Modulation of adult hippocampal neurogenesis during myelin-directed autoimmune neuroinflammation. Glia. 2011;59(1):132–142. doi: 10.1002/glia.21082. [DOI] [PubMed] [Google Scholar]
- Huillard E, Ziercher L, Blond O, Wong M, Deloulme JC, Souchelnytskyi S, Baudier J, Cochet C, Buchou T. Disruption of CK2beta in embryonic neural stem cells compromises proliferation and oligodendrogenesis in the mouse telencephalon. Mol Cell Biol. 2010;30(11):2737–2749. doi: 10.1128/MCB.01566-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun Hwa C, Hye Joon J, Ji Sun S, Yong Chan B, Jin Sup J. Crossregulation of beta-catenin/Tcf pathway by NF-κB is mediated by lzts2 in human adipose tissue-derived mesenchymal stem cells. Biochim Biophys Acta. 2008;(3):419–428. doi: 10.1016/j.bbamcr.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ideguchi M, Shinoyama M, Gomi M, Hayashi H, Hashimoto N, Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J Neurosci Res. 2008;86(9):1936–1943. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- Imielski Y, Schwamborn JC, Lüningschrör P, Heimann P, Holzberg M, Werner H, Leske O, Püschel AW, Memet S, Heumann R, Israel A, Kaltschmidt C, Kaltschmidt B. Regrowing the adult brain: NF-κB controls functional circuit formation and tissue homeostasis in the dentate gyrus. PLoS ONE. 2012;7(2):e30838. doi: 10.1371/journal.pone.0030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Meyer-Lindenberg A, Gass P. Alterations in postnatal neurogenesis and dopamine dysregulation in schizophrenia: a hypothesis. Schizophr Bull. 2011;37(4):674–680. doi: 10.1093/schbul/sbq134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam O, Gong X, Rose-John S, Heese K. Interleukin-6 and neural stem cells: more than gliogenesis. Mol Biol Cell. 2009;20(1):188–199. doi: 10.1091/mbc.E08-05-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Price J, Modo M. Effect of inflammatory cytokines on major histocompatibility complex expression and differentiation of human neural stem/progenitor cells. Stem Cells. 2008;26(9):2444–2454. doi: 10.1634/stemcells.2008-0116. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Kobayashi T. Roles of Hes genes in neural development. Dev Growth Differ. 2008;50(Suppl 1):S97–S103. doi: 10.1111/j.1440-169X.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Ogata S, Tanihara M. Promotion of neurite outgrowth from fetal hippocampal cells by TNF-alpha receptor 1-derived peptide. Cell Transplant. 2005;14(9):665–672. [PubMed] [Google Scholar]
- Kaltschmidt B, Kaltschmidt C. NF-κB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1(3):a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HB, Kim YE, Kwon HJ, Sok DE, Lee Y. Enhancement of NF-κB expression and activity upon differentiation of human embryonic stem cell line SNUhES3. Stem Cells Dev. 2007;16(4):615–624. doi: 10.1089/scd.2007.0014. [DOI] [PubMed] [Google Scholar]
- Kasperczyk H, Baumann B, Debatin KM, Fulda S. Characterization of sonic hedgehog as a novel NF-κB target gene that promotes NF-κB-mediated apoptosis resistance and tumor growth in vivo. FASEB J. 2009;23(1):21–33. doi: 10.1096/fj.08-111096. [DOI] [PubMed] [Google Scholar]
- Kato T, Jr, Delhase M, Hoffmann A, Karin M. CK2 Is a C-Terminal IκB Kinase Responsible for NF-κB Activation during the UV Response. Mol Cell. 2003;12(4):829–839. doi: 10.1016/s1097-2765(03)00358-7. [DOI] [PubMed] [Google Scholar]
- Kaul M. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13(13):2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KA, Ostrakhovitch EA, Sandiford SD, Dayarathna T, Xie X, Waese EY, Chang WY, Feng Q, Skerjanc IS, Stanford WL, Li SS. Mammalian numb-interacting protein 1/dual oxidase maturation factor 1 directs neuronal fate in stem cells. J Biol Chem. 2010;285(23):17974–17985. doi: 10.1074/jbc.M109.084616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KA, Sandiford SD, Skerjanc IS, Li SS. Reactive oxygen species and the neuronal fate. Cell Mol Life Sci. 2012;69(2):215–221. doi: 10.1007/s00018-011-0807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keohane A, Ryan S, Maloney E, Sullivan AM, Nolan YM. Tumour necrosis factor-alpha impairs neuronal differentiation but not proliferation of hippocampal neural precursor cells: Role of Hes1. Mol Cell Neurosci. 2010;43(1):127–135. doi: 10.1016/j.mcn.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Kim J, Wong PK. Loss of ATM impairs proliferation of neural stem cells through oxidative stress-mediated p38 MAPK signaling. Stem Cells. 2009;27(8):1987–1998. doi: 10.1002/stem.125. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park SH, Park SG, Choi JS, Xia Y, Sung JH. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011a;20(10):1753–1761. doi: 10.1089/scd.2010.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Song JS, Cho HH, Shin KK, Bae YC, Lee BJ, Jung JS. Effect of the modulation of leucine zipper tumor suppressor 2 expression on proliferation of various cancer cells functions as a tumor suppressor. Mol Cell Biochem. 2011b;346(1–2):125–136. doi: 10.1007/s11010-010-0599-y. [DOI] [PubMed] [Google Scholar]
- Kim YE, Kang HB, Park JA, Nam KH, Kwon HJ, Lee Y. Upregulation of NF-κB upon differentiation of mouse embryonic stem cells. BMB Rep. 2008;41(10):705–709. doi: 10.5483/bmbrep.2008.41.10.705. [DOI] [PubMed] [Google Scholar]
- Kishi N, Macklis JD. MeCP2 functions largely cell-autonomously, but also non-cell-autonomously, in neuronal maturation and dendritic arborization of cortical pyramidal neurons. Exp Neurol. 2010;222(1):51–58. doi: 10.1016/j.expneurol.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Matsuoka AJ, Shimomura A, Koehler KR, Chan RJ, Miller JM, Srour EF, Hashino E. Wnt signaling promotes neuronal differentiation from mesenchymal stem cells through activation of Tlx3. Stem Cells. 2011;29(5):836–846. doi: 10.1002/stem.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köntgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9(16):1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr Opin Investig Drugs. 2009;10(7):664–671. [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci USA. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel D, Oeckinghaus A, Wang C, Krappmann D, Heinemann U. Distinct isocomplexes of the TRAPP trafficking factor coexist inside human cells. FEBS Lett. 2008;582(27):3729–3733. doi: 10.1016/j.febslet.2008.09.056. [DOI] [PubMed] [Google Scholar]
- Lake BB, Ford R, Kao KR. Xrel3 is required for head development in Xenopus laevis. Development. 2001;128(2):263–273. doi: 10.1242/dev.128.2.263. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8 (1):59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Douarin NM, Calloni GW, Dupin E. The stem cells of the neural crest. Cell Cycle. 2008;7(8):1013–1019. doi: 10.4161/cc.7.8.5641. [DOI] [PubMed] [Google Scholar]
- Li Q, Estepa G, Memet S, Israel A, Verma IM. Complete lack of NF-κB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 2000;14(14):1729–1733. [PMC free article] [PubMed] [Google Scholar]
- Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-κB by TNF-alpha. Antioxid Redox Signal. 2009;11(6):1249–1263. doi: 10.1089/ars.2008.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienbaum A, Sage J, Mémet S, Rassoulzadegan M, Cuzin F, Israël A. NF-κB is developmentally regulated during spermatogenesis in mice. Dev Dyn. 2000;219(3):333–340. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1064>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Lin Y, Bai L, Chen W, Xu S. The NF-κB activation pathways, emerging molecular targets for cancer prevention and therapy. Expert Opin Ther Targets. 2010;14(1):45–55. doi: 10.1517/14728220903431069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou SJ, Gu P, Xu H, Xu XH, Wang MW, He C, Lu CL. Effect of tumor necrosis factor-alpha on differentiation of mesencephalic neural stem cells and proliferation of oligodendrocytes in the rat. Sheng Li Xue Bao. 2003;55(2):183–186. [PubMed] [Google Scholar]
- Lum M, Croze E, Wagner C, McLenachan S, Mitrovic B, Turnley AM. Inhibition of neurosphere proliferation by IFNgamma but not IFNbeta is coupled to neuronal differentiation. J Neuroimmunol. 2008;206 (1–2):32–38. doi: 10.1016/j.jneuroim.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Lüningschrör P, Stöcker B, Kaltschmidt B, Kaltschmidt C. miR-290 cluster modulates pluripotency by repressing canonical NF-κB signaling. Stem Cells. 2012;30(4):655–664. doi: 10.1002/stem.1033. [DOI] [PubMed] [Google Scholar]
- Ma DK, Bonaguidi MA, Ming GL, Song H. Adult neural stem cells in the mammalian central nervous system. Cell Res. 2009;19(6):672–682. doi: 10.1038/cr.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancino A, Lawrence T. Nuclear factor-κB and tumor-associated macrophages. Clin Cancer Res. 2010;16(3):784–789. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniati E, Bossard M, Cook N, Candido JB, Emami-Shahri N, Nedospasov SA, Balkwill FR, Tuveson DA, Hagemann T. Crosstalk between the canonical NF-κB and Notch signaling pathways inhibits Pparγ expression and promotes pancreatic cancer progression in mice. J Clin Invest. 2011;121(12):4685–4699. doi: 10.1172/JCI45797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcora E, Kennedy MB. The Huntington’s disease mutation impairs Huntingtin’s role in the transport of NF-κB from the synapse to the nucleus. Hum Mol Genet. 2010;19(22):4373–4384. doi: 10.1093/hmg/ddq358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa PT, Aleyasin H, Park DS, Mao X, Barger SW. NFκB in neurons? The uncertainty principle in neurobiology. J Neurochem. 2006;97 (3):607–618. doi: 10.1111/j.1471-4159.2006.03810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Culmsee C, Yu Z, Camandola S. Roles of nuclear factor κB in neuronal survival and plasticity. J Neurochem. 2000;74(2):443–456. doi: 10.1046/j.1471-4159.2000.740443.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Meffert MK. Roles for NF-κB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- Meneghini V, Francese MT, Carraro L, Grilli M. A novel role for the receptor for advanced glycation end-products in neural progenitor cells derived from adult SubVentricular Zone. Mol Cell Neurosci. 2010;45 (2):139–150. doi: 10.1016/j.mcn.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Metzger M. Neurogenesis in the enteric nervous system. Arch Ital Biol. 2010;148(2):73–83. [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir A, Kaufman L, Noor A, Motazacker MM, Jamil T, Azam M, Kahrizi K, Rafiq MA, Weksberg R, Nasr T, Naeem F, Tzschach A, Kuss AW, Ishak GE, Doherty D, Ropers HH, Barkovich AJ, Najmabadi H, Ayub M, Vincent JB. Identification of mutations in TRAPPC9, which encodes the NIK-and IKK-beta-binding protein, in nonsyndromic autosomal-recessive mental retardation. Am J Hum Genet. 2009;85(6):909–915. doi: 10.1016/j.ajhg.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 2003;113(5):631–642. doi: 10.1016/s0092-8674(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mochida GH, Mahajnah M, Hill AD, Basel-Vanagaite L, Gleason D, Hill RS, Bodell A, Crosier M, Straussberg R, Walsh CA. A truncating mutation of TRAPPC9 is associated with autosomalrecessive intellectual disability and postnatal microcephaly. Am J Hum Genet. 2009;85(6):897–902. doi: 10.1016/j.ajhg.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano-Almendras CP, Essaghir A, Schoemans H, Varis I, Noel LA, Velghe AI, Latinne D, Knoops L, Demoulin JB. ETV6-PDGFRB and FIP1L1-PDGFRA stimulate human hematopoietic progenitor proliferation and differentiation into eosinophils: role of NF-κB. Haematologica, Online Available Jan. 2012;22:2012. doi: 10.3324/haematol.2011.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Gage FH. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol Neurodegener. 2011;6(1):85. doi: 10.1186/1750-1326-6-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y, Lee SW, Gage FH. Signaling in adult neurogenesis. Curr Opin Neurobiol. 2010;20(4):416–423. doi: 10.1016/j.conb.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaino M, Nakamura M, Okada S, Toyama Y, Liu M, Okano H. Role of IL-6 in regulation of inflammation and stem cell differentiation in CNS trauma. Nihon Rinsho Meneki Gakkai Kaishi. 2008;31(2):93–98. doi: 10.2177/jsci.31.93. (Role of IL-6 in regulation of inflammation and stem cell differentiation in CNS trauma) [DOI] [PubMed] [Google Scholar]
- Nakanishi M, Niidome T, Matsuda S, Akaike A, Kihara T, Sugimoto H. Microglia-derived interleukin-6 and leukaemia inhibitory factor promote astrocytic differentiation of neural stem/progenitor cells. Eur J Neurosci. 2007;25(3):649–658. doi: 10.1111/j.1460-9568.2007.05309.x. [DOI] [PubMed] [Google Scholar]
- Nishikimi A, Mukai J, Yamada M. Nuclear translocation of nuclear factor κB in early 1-cell mouse embryos. Biol Reprod. 1999;60(6):1536–1541. doi: 10.1095/biolreprod60.6.1536. [DOI] [PubMed] [Google Scholar]
- Nogueira L, Ruiz-Ontañon P, Vazquez-Barquero A, Lafarga M, Berciano MT, Aldaz B, Grande L, Casafont I, Segura V, Robles EF, Suarez D, Garcia LF, Martinez-Climent JA, Fernandez-Luna JL. Blockade of the NFκB pathway drives differentiating glioblastoma-initiating cells into senescence both in vitro and in vivo. Oncogene. 2011;30(32):3537–3548. doi: 10.1038/onc.2011.74. [DOI] [PubMed] [Google Scholar]
- Noor A, Windpassinger C, Patel M, Stachowiak B, Mikhailov A, Azam M, Irfan M, Siddiqui ZK, Naeem F, Paterson AD, Lutfullah M, Vincent JB, Ayub M. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet. 2008;82(4):1011–1018. doi: 10.1016/j.ajhg.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1(2):230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Okano H. Adult neural stem cells and central nervous system repair. Ernst Schering Res Found Workshop. 2006;60:215–228. doi: 10.1007/3-540-31437-7_14. [DOI] [PubMed] [Google Scholar]
- Osakada F, Takahashi M. Neural induction and patterning in Mammalian pluripotent stem cells. CNS Neurol Disord Drug Targets. 2011;10(4):419–432. doi: 10.2174/187152711795563958. [DOI] [PubMed] [Google Scholar]
- Paciolla M, Boni R, Fusco F, Pescatore A, Poeta L, Ursini MV, Lioi MB, Miano MG. Nuclear factor-κ-B-inhibitor alpha (NFKBIA) is a developmental marker of NF-κB/p65 activation during in vitro oocyte maturation and early embryogenesis. Hum Reprod. 2011;26(5):1191–1201. doi: 10.1093/humrep/der040. [DOI] [PubMed] [Google Scholar]
- Pan JX, Ding K, Wang CY. Niclosamide, an old antihelminthic agent, demonstrates antitumor activity by blocking multiple signaling pathways of cancer stem cells. Chin J Cancer. 2012 doi: 10.5732/cjc.011.10290. Online Available Jan 9, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Ortega-Sáenz P, Durán R, Platero-Luengo A, López-Barneo J. The carotid body, a neurogenic niche in the adult peripheral nervous system. Arch Ital Biol. 2010;148(2):95–105. [PubMed] [Google Scholar]
- Pathania M, Yan LD, Bordey A. A symphony of signals conducts early and late stages of adult neurogenesis. Neuropharmacology. 2010;58 (6):865–876. doi: 10.1016/j.neuropharm.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Y, Brun SN, Markant SL, Lento W, Gibson P, Taketo MM, Giovannini M, Gilbertson RJ, Wechsler-Reya RJ. WNT signaling increases proliferation and impairs differentiation of stem cells in the developing cerebellum. Development. 2012;139(10):1724–1733. doi: 10.1242/dev.050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Whitney N, Wu Y, Tian C, Dou H, Zhou Y, Zheng J. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia. 2008;56(8):903–916. doi: 10.1002/glia.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins ND. Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol. 2007;8(1):49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- Philippe O, Rio M, Carioux A, Plaza JM, Guigue P, Molinari F, Boddaert N, Bole-Feysot C, Nitschke P, Smahi A, Munnich A, Colleaux L. Combination of linkage mapping and microarray-expression analysis identifies NF-κB signaling defect as a cause of autosomal-recessive mental retardation. Am J Hum Genet. 2009;85(6):903–908. doi: 10.1016/j.ajhg.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao YJ, Seo YH, Hong F, Kim JH, Kim YJ, Kang MH, Kim BS, Jo SA, Jo I, Jue DM, Kang I, Ha J, Kim SS. Nox 2 stimulates muscle differentiation via NF-κB/iNOS pathway. Free Radic Biol Med. 2005;38(8):989–1001. doi: 10.1016/j.freeradbiomed.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Politi C, Del Turco D, Sie JM, Golinski PA, Tegeder I, Deller T, Schultz C. Accumulation of phosphorylated IκB alpha and activated IKK in nodes of Ranvier. Neuropathol Appl Neurobiol. 2008;34(3):357–365. doi: 10.1111/j.1365-2990.2007.00901.x. [DOI] [PubMed] [Google Scholar]
- Qin L, Crews FT. NADPH oxidase and reactive oxygen species contribute to alcohol-induced microglial activation and neurodegeneration. J Neuroinflammation. 2012;9(1):5. doi: 10.1186/1742-2094-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirling M, Page S, Jilg N, Plenagl K, Peus D, Grubmüller C, Weingärtner M, Fischer C, Neumeier D, Brand K. Detection of IKKbeta-IKKgamma subcomplexes in monocytic cells and characterization of associated signaling. J Biol Chem. 2004;279(36):37452–37460. doi: 10.1074/jbc.M312119200. [DOI] [PubMed] [Google Scholar]
- Razani B, Reichardt AD, Cheng G. Non-canonical NF-κB signaling activation and regulation: principles and perspectives. Immunol Rev. 2011;244(1):44–54. doi: 10.1111/j.1600-065X.2011.01059.x. [DOI] [PubMed] [Google Scholar]
- Reeves GT, Stathopoulos A. Graded dorsal and differential gene regulation in the Drosophila embryo. Cold Spring Harb Perspect Biol. 2009;1(4):a000836. doi: 10.1101/cshperspect.a000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reikvam H, Olsnes AM, Gjertsen BT, Ersvar E, Bruserud O. Nuclear factor-κB signaling: a contributor in leukemogenesis and a target for pharmacological intervention in human acute myelogenous leukemia. Crit Rev Oncog. 2009;15(1–2):1–41. doi: 10.1615/critrevoncog.v15.i1-2.10. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani L, Casalbore P, Petrucci G, Lauretti L, Montano N, Larocca LM, Falchetti ML, Lombardi DG, Gerevini VD, Cenciarelli C, D’Alessandris QG, Fernandez E, De Maria R, Maira G, Peschle C, Parati E, Pallini R. Influence of local environment on the differentiation of neural stem cells engrafted onto the injured spinal cord. Neurol Res. 2006;28(5):488–492. doi: 10.1179/016164106X115134. [DOI] [PubMed] [Google Scholar]
- Richards GR, Smith AJ, Cuddon P, Ma QP, Leveridge M, Kerby J, Roderick HL, Bootman MD, Simpson PB. The JAK3 inhibitor WHI-P154 prevents PDGF-evoked process outgrowth in human neural precursor cells. J Neurochem. 2006;97(1):201–210. doi: 10.1111/j.1471-4159.2006.03723.x. [DOI] [PubMed] [Google Scholar]
- Richardson JC, Garcia Estrabot AM, Woodland HR. XrelA, a Xenopus maternal and zygotic homologue of the p65 subunit of NF-κ B. Characterisation of transcriptional properties in the developing embryo and identification of a negative interference mutant. Mech Dev. 1994;45(2):173–189. doi: 10.1016/0925-4773(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Ziv Y, Ronen A, Levy R, Schwartz M. Toll-like receptors modulate adult hippocampal neurogenesis. Nat Cell Biol. 2007;9(9):1081–1088. doi: 10.1038/ncb1629. [DOI] [PubMed] [Google Scholar]
- Rubio-Araiz A, Arévalo-Martín A, Gómez-Torres O, Navarro-Galve B, García-Ovejero D, Suetterlin P, Sánchez-Heras E, Molina-Holgado E, Molina-Holgado F. The endocannabinoid system modulates a transient TNF pathway that induces neural stem cell proliferation. Mol Cell Neurosci. 2008;38(3):374–380. doi: 10.1016/j.mcn.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Saldanha-Araujo F, Haddad R, Malmegrim de Farias KC, Alves Souza AD, Palma PV, Araujo AG, Orellana MD, Voltarelli JC, Covas DT, Zago MA, Panepucci RA. Mesenchymal stem cells promote the sustained expression of CD69 on activated T-lymphocytes: roles of canonical and non-canonical NF-κB signaling. J Cell Mol Med, Online Available July. 2011;21:2011. doi: 10.1111/j.1582-4934.2011.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Ponce D, Tapia M, Muñoz A, Garrido JJ. New role of IKK alpha/beta phosphorylated I κB alpha in axon outgrowth and axon initial segment development. Mol Cell Neurosci. 2008;37(4):832–844. doi: 10.1016/j.mcn.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Sauvageot CM, Stiles CD. Molecular mechanisms controlling cortical gliogenesis. Curr Opin Neurobiol. 2002;12(3):244–249. doi: 10.1016/s0959-4388(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Schäfer KH, Micci MA, Pasricha PJ. Neural stem cell transplantation in the enteric nervous system: roadmaps and roadblocks. Neurogastroenterol Motil. 2009;21(2):103–112. doi: 10.1111/j.1365-2982.2008.01257.x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Mémet S, Lilienbaum A, Feuillard J, Raphaël M, Israel A. NF-κB activity in transgenic mice: developmental regulation and tissue specificity. Development. 1996;122(7):2117–2128. doi: 10.1242/dev.122.7.2117. [DOI] [PubMed] [Google Scholar]
- Schölzke MN, Röttinger A, Murikinati S, Gehrig N, Leib C, Schwaninger M. TWEAK regulates proliferation and differentiation of adult neural progenitor cells. Mol Cell Neurosci. 2011;46(1):325–332. doi: 10.1016/j.mcn.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Schölzke MN, Schwaninger M. Transcriptional regulation of neurogenesis: potential mechanisms in cerebral ischemia. J Mol Med (Berl) 2007;85(6):577–588. doi: 10.1007/s00109-007-0196-z. [DOI] [PubMed] [Google Scholar]
- Schultz C, König HG, Del Turco D, Politi C, Eckert GP, Ghebremedhin E, Prehn JH, Kögel D, Deller T. Coincident enrichment of phosphorylated IκBalpha, activated IKK, and phosphorylated p65 in the axon initial segment of neurons. Mol Cell Neurosci. 2006;33(1):68–80. doi: 10.1016/j.mcn.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Schwarz TJ, Ebert B, Lie DC. Stem cell maintenance in the adult mammalian hippocampus: A matter of signal integration? Dev Neurobiol, Online Available April. 2012;5:2012. doi: 10.1002/dneu.22026. [DOI] [PubMed] [Google Scholar]
- Senftleben U, Cao Y, Xiao G, Greten FR, Krähn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-κ B signaling pathway. Science. 2001;293(5534):1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- Sha D, Chin LS, Li L. Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NF-κB signaling. Hum Mol Genet. 2010;19(2):352–363. doi: 10.1093/hmg/ddp501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80(2):321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- Shi Y, Sun G, Zhao C, Stewart R. Neural stem cell self-renewal. Crit Rev Oncol Hematol. 2008;65(1):43–53. doi: 10.1016/j.critrevonc.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21(24):9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebzehnrubl FA, Vedam-Mai V, Azari H, Reynolds BA, Deleyrolle LP. Isolation and characterization of adult neural stem cells. Methods Mol Biol. 2011;750:61–77. doi: 10.1007/978-1-61779-145-1_4. [DOI] [PubMed] [Google Scholar]
- Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-κB and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65(6):481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Sun SC. The noncanonical NF-κB pathway. Immunol Rev. 2012;246 (1):125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int J Med Sci. 2008;5(3):127–132. doi: 10.7150/ijms.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng FY, Tang BL. NF-κB signaling in neurite growth and neuronal survival. Rev Neurosci. 2010;21(4):299–314. doi: 10.1515/revneuro.2010.21.4.299. [DOI] [PubMed] [Google Scholar]
- Tepavčević V, Lazarini F, Alfaro-Cervello C, Kerninon C, Yoshikawa K, Garcia-Verdugo JM, Lledo PM, Nait-Oumesmar B, Baron-Van Evercooren A. Inflammation-induced subventricular zone dysfunction leads to olfactory deficits in a targeted mouse model of multiple sclerosis. J Clin Invest. 2011;121(12):4722–4734. doi: 10.1172/JCI59145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchinsky A, Toder V. To die or not to die: the function of the transcription factor NF-κB in embryos exposed to stress. Am J Reprod Immunol. 2004;51(2):138–143. doi: 10.1046/j.8755-8920.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- Torres J, Watt FM. Nanog maintains pluripotency of mouse embryonic stem cells by inhibiting NFκB and cooperating with Stat3. Nat Cell Biol. 2008;10(2):194–201. doi: 10.1038/ncb1680. [DOI] [PubMed] [Google Scholar]
- Vaillend C, Poirier R, Laroche S. Genes, plasticity and mental retardation. Behav Brain Res. 2008;192(1):88–105. doi: 10.1016/j.bbr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Vaira S, Johnson T, Hirbe AC, Alhawagri M, Anwisye I, Sammut B, O’Neal J, Zou W, Weilbaecher KN, Faccio R, Novack DV. RelB is the NF-κB subunit downstream of NIK responsible for osteoclast differentiation. Proc Natl Acad Sci USA. 2008;105(10):3897–3902. doi: 10.1073/pnas.0708576105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, van de Berg WD, Hol EM. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134(Pt 11):3249–3263. doi: 10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- Viatour P, Merville MP, Bours V, Chariot A. Phosphorylation of NF-κB and IκB proteins: implications in cancer and inflammation. Trends Biochem Sci. 2005;30(1):43–52. doi: 10.1016/j.tibs.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Vieira HL, Alves PM, Vercelli A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog Neurobiol. 2011;93(3):444–455. doi: 10.1016/j.pneurobio.2011.01.007. [DOI] [PubMed] [Google Scholar]
- Vilas-Boas F, Henrique D. HES6-1 and HES6-2 function through different mechanisms during neuronal differentiation. PLoS ONE. 2010;5 (12):e15459. doi: 10.1371/journal.pone.0015459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DD, Bordey A. The astrocyte odyssey. Prog Neurobiol. 2008;86 (4):342–367. doi: 10.1016/j.pneurobio.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weih F, Durham SK, Barton DS, Sha WC, Baltimore D, Bravo R. p50-NF-κB complexes partially compensate for the absence of RelB: severely increased pathology in p50−/−relB−/− double-knockout mice. J Exp Med. 1997;185(7):1359–1370. doi: 10.1084/jem.185.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, Jackson PK. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA. 2011;108(7):2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney NP, Eidem TM, Peng H, Huang Y, Zheng JC. Inflammation mediates varying effects in neurogenesis: relevance to the pathogenesis of brain injury and neurodegenerative disorders. J Neurochem. 2009;108(6):1343–1359. doi: 10.1111/j.1471-4159.2009.05886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Kaus A, Kaltschmidt C, Kaltschmidt B. Neural stem cells, inflammation and NF-κB: basic principle of maintenance and repair or origin of brain tumours? J Cell Mol Med. 2008;12(2):459–470. doi: 10.1111/j.1582-4934.2007.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Elvers M, Kaltschmidt C, Kaltschmidt B. Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-κB signaling. BMC Neurosci. 2006a;7(1):64. doi: 10.1186/1471-2202-7-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Kaltschmidt B, Kaltschmidt C. Potential role of NF-κB in adult neural stem cells: the underrated steersman? Int J Dev Neurosci. 2006b;24(2–3):91–102. doi: 10.1016/j.ijdevneu.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Widera D, Mikenberg I, Kaus A, Kaltschmidt C, Kaltschmidt B. Nuclear factor-κB controls the reaggregation of 3D neurosphere cultures in vitro. Eur Cell Mater. 2006c;11:76–84. doi: 10.22203/ecm.v011a08. discussion 85. [DOI] [PubMed] [Google Scholar]
- Winner B, Kohl Z, Gage FH. Neurodegenerative disease and adult neurogenesis. Eur J Neurosci. 2011;33(6):1139–1151. doi: 10.1111/j.1460-9568.2011.07613.x. [DOI] [PubMed] [Google Scholar]
- Wong ET, Tergaonkar V. Roles of NF-κB in health and disease: mechanisms and therapeutic potential. Clin Sci (Lond) 2009;116(6):451–465. doi: 10.1042/CS20080502. [DOI] [PubMed] [Google Scholar]
- Wong G, Goldshmit Y, Turnley AM. Interferon-gamma but not TNF alpha promotes neuronal differentiation and neurite outgrowth of murine adult neural stem cells. Exp Neurol. 2004;187(1):171–177. doi: 10.1016/j.expneurol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Wooten MW, Seibenhener ML, Neidigh KB, Vandenplas ML. Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells. Mol Cell Biol. 2000;20(13):4494–4504. doi: 10.1128/mcb.20.13.4494-4504.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IκB kinase-beta: NF-κB activation and complex formation with IκB kinase-alpha and NIK. Science. 1997;278(5339):866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- Wu JP, Kuo JS, Liu YL, Tzeng SF. Tumor necrosis factor-alpha modulates the proliferation of neural progenitors in the subventricular/ventricular zone of adult rat brain. Neurosci Lett. 2000;292(3):203–206. doi: 10.1016/s0304-3940(00)01472-5. [DOI] [PubMed] [Google Scholar]
- Xiao M, Inal CE, Parekh VI, Li XH, Whitnall MH. Role of NF-κB in hematopoietic niche function of osteoblasts after radiation injury. Exp Hematol. 2009;37(1):52–64. doi: 10.1016/j.exphem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Yaddanapudi K, De Miranda J, Hornig M, Lipkin WI. Toll-like receptor 3 regulates neural stem cell proliferation by modulating the Sonic Hedgehog pathway. PLoS ONE. 2011;6(10):e26766. doi: 10.1371/journal.pone.0026766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Atkinson SP, Vilella F, Lloret M, Armstrong L, Mann DA, Lako M. Opposing putative roles for canonical and noncanonical NFκB signaling on the survival, proliferation, and differentiation potential of human embryonic stem cells. Stem Cells. 2010;28(11):1970–1980. doi: 10.1002/stem.528. [DOI] [PubMed] [Google Scholar]
- Yang L, Tao LY, Chen XP. Roles of NF-κB in central nervous system damage and repair. Neurosci Bull. 2007;23(5):307–313. doi: 10.1007/s12264-007-0046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo JE, Kang SK. Selenium effectively inhibits ROS-mediated apoptotic neural precursor cell death in vitro and in vivo in traumatic brain injury. Biochim Biophys Acta. 2007;(11–12):1199–1210. doi: 10.1016/j.bbadis.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25(2):181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K. Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int. 2010;56(6–7):740–746. doi: 10.1016/j.neuint.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Young KM, Bartlett PF, Coulson EJ. Neural progenitor number is regulated by nuclear factor-κB p65 and p50 subunit-dependent proliferation rather than cell survival. J Neurosci Res. 2006;83(1):39–49. doi: 10.1002/jnr.20702. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu H, Zhu X, Wang Y, Guo J. Role of transcription factors in neurogenesis after cerebral ischemia. Rev Neurosci. 2011;22(4):457–465. doi: 10.1515/RNS.2011.034. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Wang C, Liu Z, Liu X, Han C, Cao X, Li N. Notch signal suppresses Toll-like receptor-triggered inflammatory responses in macrophages by inhibiting extracellular signal-regulated kinase 1/2-mediated nuclear factor κB activation. J Biol Chem. 2012a;287 (9):6208–6217. doi: 10.1074/jbc.M111.310375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu J, Yao S, Li F, Xin L, Lai M, Bracchi-Ricard V, Xu H, Yen W, Meng W, Liu S, Yang L, Karmally S, Liu J, Zhu H, Gordon J, Khalili K, Srinivasan S, Bethea JR, Mo X, Hu W. Nuclear factor κB signaling initiates early differentiation of neural stem cells. Stem Cells. 2012b;30(3):510–524. doi: 10.1002/stem.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zhao C, Xiu Y, Ashton J, Xing L, Morita Y, Jordan CT, Boyce BF. Noncanonical NF-κB signaling regulates hematopoietic stem cell self-renewal and microenvironment interactions. Stem Cells. 2012;30 (4):709–718. doi: 10.1002/stem.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Li XD, Chen Z. CC2D1A, a DM14 and C2 domain protein, activates NF-κB through the canonical pathway. J Biol Chem. 2010;285(32):24372–24380. doi: 10.1074/jbc.M109.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Liu Z, Gui L, Yao W, Qian W, Zhang C. Mutated IκBalpha represses proliferation of immortalized neural progenitor cells and prevents their apoptosis after oxygen-glucose deprivation. Brain Res. 2008;1244:24–31. doi: 10.1016/j.brainres.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Ziercher L, Filhol O, Laudet B, Prudent R, Cochet C, Buchou T. Structure-function analysis of the beta regulatory subunit of protein kinase CK2 by targeting embryonic stem cell. Mol Cell Biochem. 2011;356(1–2):75–81. doi: 10.1007/s11010-011-0955-6. [DOI] [PubMed] [Google Scholar]
- Zong M, Satoh A, Yu MK, Siu KY, Ng WY, Chan HC, Tanner JA, Yu S. TRAPPC9 mediates the interaction between p150 and COPII vesicles at the target membrane. PLoS ONE. 2012;7(1):e29995. doi: 10.1371/journal.pone.0029995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong M, Wu XG, Chan CW, Choi MY, Chan HC, Tanner JA, Yu S. The adaptor function of TRAPPC2 in mammalian TRAPPs explains TRAPPC2-associated SEDT and TRAPPC9-associated congenital intellectual disability. PLoS ONE. 2011;6(8):e23350. doi: 10.1371/journal.pone.0023350. [DOI] [PMC free article] [PubMed] [Google Scholar]