Abstract
Objectives
Irritable bowel syndrome (IBS) patients show pain hypersensitivity and hypercontractility in response to colonic or rectal distention. Aims were to determine whether predominant bowel habits and IBS symptom severity are related to pain sensitivity, colon motility, or smooth muscle tone.
Methods
129 patients classified as IBS with diarrhea (IBS-D, n=44), IBS with constipation (IBS-C, n=29), mixed IBS (IBS-M, n=45) and unspecified IBS (IBS-U, n=11) based on stool consistency, and 30 healthy controls (HC) were studied. A manometric catheter containing a 600-ml capacity plastic bag was positioned in the descending colon. Pain threshold was assessed using a barostat. Motility was assessed for 10 min with the bag minimally inflated (individual operating pressure or IOP), 10 min at 20 mmHg above the IOP, and for 15-min recovery following bag inflation. Motility was also recorded for 30 min following an 810-kcal meal.
Results
Compared to HC, IBS patients had lower pain thresholds (medians: 30 vs. 40 mmHg, p<0.01), but IBS subtypes were not different. IBS symptom severity was correlated with pain thresholds (rho=-0.36, p<0.001). During distention, the motility index (MI) was significantly higher in IBS compared to HC (909±73 vs. 563±78, p<0.01). Average barostat bag volume at baseline was higher (muscle tone lower) in HC compared to IBS-D and IBS-M but not compared to IBS-C. The baseline MI and bag volume differed between IBS-D and IBS-C and correlated with symptoms of abdominal distention and dissatisfaction with bowel movements. Pain thresholds and MI during distention were uncorrelated.
Conclusions
Pain sensitivity and colon motility are independent factors contributing to IBS symptoms. Treatment may need to address both and to be specific to predominant bowel habit.
Keywords: irritable bowel syndrome, visceral hypersensitivity, colonic motility, symptom severity, subtypes of bowel habit
Introduction
There is no consensus on the pathophysiology of IBS. In several studies, IBS patients had lower perception thresholds and increased sensations of discomfort to intraluminal distention in the sigmoid colon1-3 and the rectum4-6 (visceral hypersensitivity) or more exaggerated GI motility responses to experimental stress7, 8, phasic intraluminal distention9, 10 or eating11, 12 (hyper-reactive motility) compared to healthy controls (HC). However, there is only limited data on the extent to which visceral hypersensitivity and hyper-reactive motility correlate with each other and influence clinical symptoms of abdominal pain and altered bowel habits13.
Some reports suggest that patients with diarrhea-dominant IBS (IBS-D) have increased visceral sensitivity compared with those with constipation-dominant IBS (IBS-C)14, 15, but others failed to find differences between subtypes16, 17. Previous reports may be inconsistent due to a small number of subjects or other methodological differences. The guidelines for sub-classifying IBS into IBS-C and IBS-D were recently revised18. The former Rome II criteria19 used multiple signs and symptoms to make this sub-classification, which were complex and possibly unreliable, but the Rome III criteria simplified this classification by using only stool consistency18. Most patients with IBS have a stool frequency within the normal range regardless of bowel pattern20, but stool consistency (from watery to hard) reflects intestinal transit time21. In this study, we used the Rome III criteria to identify subtypes of IBS.
Aims of the present study were to (1) compare IBS patients to healthy controls (HC) with respect to pain sensitivity and tonic and phasic colon motility, (2) determine whether IBS subtypes defined by usual stool consistency differ on these variables, (3) determine whether pain sensitivity is related to (i.e., correlated with) phasic and tonic motility, and (4) determine whether IBS symptom severity is related to pain sensitivity, or tonic or phasic colon motility.
Methods
Subjects
This was a prospective study. Subjects were recruited by advertisements or physician referrals and screened by telephone. The study population consisted of 136 patients who fulfilled Rome II criteria for IBS, received a physician diagnosis of IBS, and had current symptoms (abdominal pain or discomfort at least one-fourth of the time in the last 3 months). These subjects had no history of gastrointestinal surgery (other than appendectomy or cholecystectomy), inflammatory bowel disease, celiac disease, lactose malabsorption, heart disease or diabetes mellitus, and they were not pregnant at the time of study.
Patients with IBS were classified by usual stool consistency into subtypes according to Rome III guidelines18: IBS-D was defined as loose (mushy) or watery stool ≥25% and hard or lumpy stool <25% of bowel movements, and IBS-C was defined as hard or lumpy stool ≥25% and loose or watery stool <25% of bowel movements. IBS with mixed bowel habits (IBS-M) was defined as loose or watery stools ≥25% of the time plus hard or lumpy stools ≥25% of the time, and IBS with normal bowel habits (IBS-U, for unclassifiable) was defined by neither loose/watery nor hard/lumpy stools 25% or more of the time. The Rome III descriptions of stool consistencies were adapted from the Bristol Stool Scale, but the pictures of different stool forms were not provided to subjects.
The control population was recruited by advertisement and consisted of 33 subjects without any significant or recurring gastrointestinal symptoms. Exclusion criteria were average stool frequency of less than 3/week or more than 3/day, abdominal pain or use of a laxative or anti-diarrheal agent on more than two occasions over the prior year, history of alcohol or substance abuse, a psychiatric diagnosis, or any of the medical conditions listed above for the IBS patients. The study was approved by the Institutional Review Board of the University of North Carolina (UNC), and all subjects provided written informed consent.
Study design
Subjects were admitted to the General Clinical Research Center at the University of North Carolina for a 24-30 hour period. They were asked to fast for at least 4 hours prior to reporting for admission. On the day of admission, a medical history, physical examination, and breath test for lactose intolerance and small intestinal bacterial overgrowth were completed. The Irritable Bowel Syndrome Severity Scale22 (IBS-SS), the Brief Symptom Inventory-1823 (BSI-18), and other questionnaires were completed on a computer terminal during the breath test. A low fiber meal was consumed at approximately 5:00 pm. Day 1 ended with a bowel cleanout consisting of 1.5 oz of Fleet's phosphosoda consumed at 6:00 pm and repeated at 9:00 pm.
Questionnaires
The IBS-SS22 is a validated scale for measuring the overall severity of IBS symptoms. It consists of 5 equally weighted questions. Subjects are asked to indicate for the past 10 days the average intensity of abdominal pain, the number of days with any abdominal pain, the average severity of abdominal distention, dissatisfaction with bowel habits, and the degree to which bowel symptoms interfered with their usual activities. Response to all except the pain frequency question are on a 1-100 numeric scale (“none” to “worst ever”), and the number of days of pain in the past 10 days is multiplied by 10 to arrive at a 0-100 score for this item. The 5 questions are added to arrive at a total score of 0-500.
The BSI-1823 is a validated questionnaire for measuring the degree of psychological distress over the past week. Subjects are asked how much they were bothered by each of 18 symptoms, and they respond on a 5-point ordinal scale from “not at all bothered” to “extremely.” There are 3 subscales – anxiety, depression, and somatization – as well as a global severity index (GSI). Sum scores for each subscale and the GSI are converted to standardized scores where the mean for the healthy population is a score of 50 and each standard deviation from the mean is equal to 10 scale points; thus, a score of 60 is one standard deviation above the mean. These standardized scores adjust for sex differences in the reporting of psychological symptoms, so BSI-18 scores for males and females can be pooled together.
Equipment
The barostat is a computer-controlled pump (Distender II model, G&J Electronics, Willodale, Ontario, Canada) used for testing sensory thresholds and smooth muscle tone in the lumen of the bowel. It inflates a plastic bag to a predefined pressure and holds this pressure constant for a fixed period of time by adding or subtracting air. Volumes and pressures are recorded 16 times per second and are displayed graphically in real time. The rate of inflation and deflation was 38 ml/sec. Controlling the pump by means of a computer program made it possible to present complex sequences of distentions.
The motility catheter (Model C7-CB-0026, Mui Scientific, Mississauga, Ontario, Canada) is 5 mm in outside diameter. It consists of a bundle of smaller polyethylene tubes bonded together and includes a central lumen which accommodates a guide wire, 2 lumens which open inside the bag (one to inflate/deflate the bag and a second to monitor pressure inside the bag), plus 4 small catheters used to measure pressures 2.5 and 5 cm from the proximal and distal edges of the bag. A disposable, 10 cm long, 600 ml capacity polyethylene bag (Model CT-BP600R, Mui Scientific) was attached to the surface of the motility catheter and tied with surgical thread.
The pneumohydraulic pump (8 channel hydraulic capillary infusion system, Arndorfer Inc, Greendale, Wisconsin) uses a tank of compressed air to force de-gassed sterile water from a reservoir through 4 capillary (very small diameter) catheters which are connected to 4 pressure transducers. These pressure transducers are also connected to the 4 small catheters in the motility catheter which open above and below the barostat bag. Water is perfused through the pressure transducers and the perfusion catheters at a rate of 0.37 ml/min. Because there is a continuous column of water connecting the pressure transducer to the openings on the outside of the motility catheter, pressure changes occurring at the openings are transmitted up the column of water to the pressure transducers. The outputs of these pressure transducers were continuously recorded (see below).
The physiological recorder used to record phasic and tonic motility changes above and below the balloon was a Synectics Polygram (Medtronic Inc., Minneapolis, MN). This instrument continuously recorded pressure changes above and below the bag and stored them in a digital file. A research nurse marked these recordings to indicate which experimental condition was in effect.
Colonic sensory and motility testing
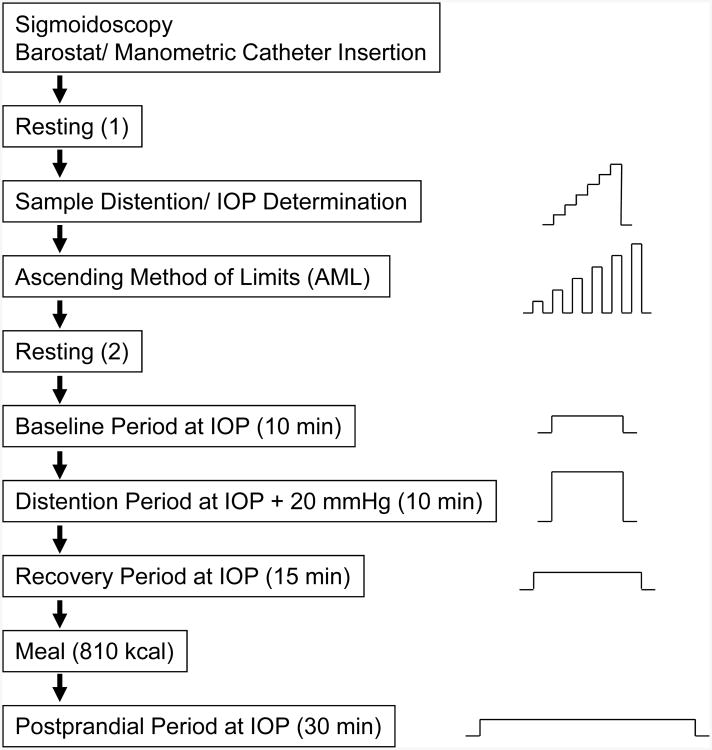
All physiological and sensory testing was performed on Day 2 according to the protocol in Figure 1. On the morning of Day 2 at approximately 8:00 AM, the barostat catheter was placed in the descending colon for sensory and motility testing. First, a guide wire was inserted to the level of the splenic flexure using a flexible sigmoidoscope. The sigmoidoscope was then withdrawn and the motility catheter was guided over this wire. The guide wire was then withdrawn and barostat placement was confirmed by fluoroscopy. Following catheter placement, the subject rested for 90 min before testing began. No sedation was used during sigmoidoscopy. Subjects were not permitted to have food until the test meal (see Figure 1).
Fig.1. Study protocol.
Graphs on right show sequence of pressure changes during each phase of testing. IOP (individual operating pressure) is the pressure required to overcome the weight of overlying tissue and minimally inflate the barostat bag.
Sample distention and determination of individual operating pressure
Subjects were instructed to give separate ratings of the intensity of pain and urgency to defecate experienced at the end of each distention, using a six point scale (0= no sensation; 1 = weak; 2= mild; 3 = moderate; 4 = strong; 5 = intense). The scale was visible to subjects during the procedure. Sample distentions were then performed during which the barostat bag was inflated in a stepwise fashion by increasing bag pressure by 4 mmHg every 15 seconds until the subject reported moderate pain (rating of 3). The sample distentions served three purposes: (1) to insure that the barostat bag was unfolded; (2) to teach the subject how to use the rating scale to describe the intensity of colonic sensations; and (3) to decrease anticipatory anxiety. The barostat bag was then slowly inflated with 30 ml of air and the pressure was allowed to equilibrate for 3 minutes. The average bag pressure during the last 15 seconds defined the individual operating pressure (IOP)24, which is the minimum pressure required to overcome mechanical forces and inflate the bag with 30 ml of air. All sensory and motility testing was done with the subject lying in a left-lateral position to minimize pressures due to the weight of overlying body tissues compressing the bowel.
Ascending method of limits (AML)
Pain thresholds in the colon were assessed using the ascending method of limits24. Phasic distentions were 30 seconds in duration and were separated by 30 second rest intervals. Distentions starting at the IOP and progressively increased in 2 mmHg steps until either the subject requested the research nurse to stop the protocol or 48 mmHg was reached. The pain threshold was defined as the amount of pressure above IOP at which the subject first reported moderate pain (absolute distending pressure minus the IOP). If the subject reached 48 mmHg without reporting moderate pain, then the pain threshold was defined as 50 mmHg minus the IOP. After measuring pain thresholds, there was a 15 min rest period. Individual pain thresholds are shown in Figure 2.
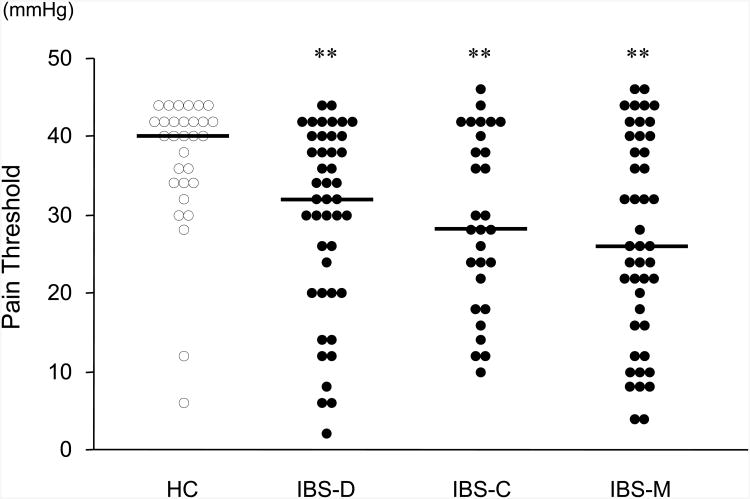
Fig.2. Pain threshold to intraluminal distention in the descending colon.
Pain threshold in the descending colon was assessed using an electronic barostat by the ascending method of limit (AML). Each horizontal bar indicates the median value. The pain threshold was defined as the amount of pressure above IOP at which the subject first reported moderate pain (absolute distending pressure minus the IOP). If the subject reached 48 mmHg without reporting moderate pain, then the pain threshold was defined as 50 mmHg minus the IOP. HC, healthy controls (n=30); IBS-D, IBS with diarrhea (n=44); IBS-C, IBS with constipation (n=29); IBS-M, mixed IBS (n=45). *p<0.05, **p<0.01, vs. controls.
Colonic phasic motility
Phasic contractions were measured from the perfusion ports above and below the bag under the following conditions: (a) during the fasting baseline for 10 min at the IOP, (b) during distention for 10 min at a pressure of IOP+20 mmHg, (c) during a recovery period after intraluminal distention for 15 min at the IOP, and (d) following the meal for 30 min at the IOP. These tracings were visually screened to exclude artifact, defined as wave amplitudes less than 5 mmHg or with durations less than 6 sec. The beginning and ending inflection points for each individual contraction were identified visually and the area under the curve was calculated using computer software (Polygram, Lower GI Edition, Version 5.06; Synectics Medical, now Medtronic, Inc., Minneapolis, MN). These areas were added together, then divided by recording time in seconds (excluding the time occupied by movement artifact), and multiplied by 100. The motility index (MI) was the average of phasic contractions at 4 perfusion ports.
Colonic tonic motility
Average barostat bag volume in each period was recorded as a measure of smooth muscle tone24. Muscle tone was measured during the fasting baseline, recovery period after intraluminal distention, and following the meal. The average volume required to maintain the barostat bag at a constant pressure was recorded. The average barostat bag volumes in successive 5 min blocks constitutes an index of smooth muscle tone.
Postprandial motility
The test meal was standardized and contained 810 kcals and 38 grams of fat. Subjects were asked to consume the meal within 10 minutes. Immediately after completing the meal, the patient returned to the prone position and bag pressure was maintained at the IOP for 30 min (the post-prandial period). Phasic and tonic motility were recorded throughout this period.
Data analysis
Phasic and tonic motility were assessed at baseline and in response to stimulation with intraluminal distention and meal ingestion. Because there were differences between groups at baseline, the response to stimulation had to be assessed after adjusting for baseline differences. To accomplish this, we expressed stimulated values as a percent of baseline values. Absolute values are also presented in Table 2 to allow the reader to compare these two methods of data presentation. Statistical comparisons were performed on both the percent-of-baseline values and the absolute values.
Table 2. Sensory thresholds and colonic motility responses in subtypes of IBS.
| Controls (n=30) | IBS total (n=129) | IBS-D (n=44) | IBS-C (n=29) | IBS-M (n=45) | IBS-U¶ (n=11) | |
|---|---|---|---|---|---|---|
| Pain Threshold (mmHg) | 40 [6-44] | 30 [2-46]** | 32 [2-44]** | 28 [10-46]** | 26 [4-46]** | 36 [6-42] |
| Motility Index | ||||||
| Baseline | 280±33 | 311±19 | 370±37 | 271±35§ | 290±32 | 263±40 |
| Distention | 563±78 b | 909±73**,b | 840±127*,b | 1001±208b | 914±99**,b | 927±162 |
| Recovery | 302±30 | 430±31b | 531±61*,b | 422±65a | 372±45a | 288±40 |
| Post-meal | 429±49 b | 481±28 b | 542±60 a | 422±47b | 477±45§, b | 410±61 |
| IOP (mmHg) | 9.1±0.6 | 9.3±0.3 | 9.8±0.6 | 9.4±0.6 | 8.8±0.5 | 8.4±0.8 |
| Tone (ml) | ||||||
| Baseline | 54.8±7.9 | 40.1±2.8* | 32.5±3.1* | 48.7±6.9§ | 38.3±4.5* | 55.3±14.5 |
| Recovery | 54.2±7.9 | 38.6±2.8* | 34.5±4.0* | 42.8±5.8 | 36.6±4.7* | 52.2±14.6 |
| Post-meal | 21.1±2.5 b | 18.8±1.2 b | 18.6±1.6 b | 20.5±3.3 b | 17.6±2.0 b | 19.6±5.0 |
Note.
No statistical comparisons were made to the IBS-U group because of a small sample size. Sensory thresholds are shown as medians with range and motility data are shown as means ± SEM.
p<0.05,
p<0.01, compared with controls;
p<0.05, compared with IBS-D;
p<0.05,
p<0.01, compared with baseline, Mann-Whitney U test.
Comparisons between groups were made using the Kruskal-Wallis test, followed by Mann-Whitney U test. Correlations between variables were limited to the IBS patients, and employed the Spearman non-parametric correlation coefficient to account for non-normal distributions of some variables. Multiple linear regression was used to determine which variables were most strongly associated with IBS symptom severity. In this analysis, dummy regression terms to code for usual stool consistency (hard or lumpy stool ≥25% and loose or watery stool ≥25%) were used (1=yes, 0=no). For all analyses, a p-value of 0.05 without adjustment for multiple comparisons, defined statistical significance.
Although this is the largest study to be reported comparing subgroups of IBS patients on physiological parameters, the subgroups were unequal in size (n=45 for IBS-D, n=29 for IBS-C. n=45 for IBS-M, and n=11 for IBS-U), and consequently the statistical power of between-group comparisons and correlations varied and could lead to spurious conclusions. Three steps were taken to avoid this: (1) No statistical comparisons were made to the IBS-U group and no correlations were reported for this subgroup because it was too small. (2) For other between-group comparisons, we identified the minimum Z-score for a Mann-Whitney U test involving the smallest group (IBS-C) that was significant at p<0.05 and interpreted other comparisons as statistically significant only if they exceeded this critical value. (3) Similarly for Spearman nonparametric correlations, we identified the smallest correlation of a group of size n=29 that was significant at p<0.05 and interpreted other correlations as statistically significant only if they exceeded this critical value.
Results
Eligible subjects
129 IBS patients (20 males; mean age 35.8 years) and 30 healthy control subjects (8 males; 37.2 years) underwent both colonic sensory and motility tests. Seven additional IBS patients were enrolled but did not undergo colonic sensory and motility testing: three refused flexible sigmoidscopy, two began but could not tolerate completion of unsedated sigmoidscopy, one had an extremely elevated blood pressure, and one had colonic inflammation detected on sigmoidscopy. Three additional control subjects were similarly screened but excluded: one did not tolerate the flexible sigmoidscopy and two had exclusionary medical conditions that were detected during the study (lactose intolerance in one and prior colonic surgery in the other).
Two of 129 eligible patients did not complete the postprandial assessment due to unpleasant symptoms experienced during the test meal. One patient did not complete only the postprandial tonic motility assessment due to equipment failure. Two patients and one control subject did not report symptom severity on the IBS-SS. No serious adverse events were observed.
Table 1 shows demographic characteristics of the sample. There were no differences between HC and IBS patients in gender, age, or race/ethnicity.
Table 1. Demographics, IBS symptom severity, and psychological distress scales.
| Controls | IBS total | IBS-D | IBS-C | IBS-M | IBS-U¶ | |
|---|---|---|---|---|---|---|
| Number | 30 | 129 | 44 | 29 | 45 | 11 |
| Female n. %) | 22 (73) | 109 (84) | 34 (77) | 26 (90) | 38 (84) | 11 (100) |
| Age (yr) | 37.2±2.2 | 35.8±1.1 | 36.6±2.0 | 34.9±2.0 | 35.1±1.9 | 38.4±3.5 |
| Race | ||||||
| White | 13 | 90 | 31 | 16 | 37 | 6 |
| Non-white | 14 | 36 | 12 | 12 | 7 | 5 |
| Unknown | 3 | 3 | 1 | 1 | 1 | 0 |
| IBS-SS | ||||||
| Overall | 27.9±9.8 | 273.2±8.1** | 255.8±15.1** | 281.1±16.5** | 282.2±13.6** | 287.9±13.2 |
| Pain Severity | 4.7±2.4 | 47.0±2.2** | 43.3±3.8** | 45.5±4.5** | 48.2±3.9** | 64.4±5.6 |
| Pain Frequency | 4.5±2.3 | 47.2±2.1** | 43.4±3.6** | 46.6±4.5** | 48.9±3.7** | 58.9±5.1 |
| Distention | 3.3±1.5 | 44.8±2.6** | 38.5±4.7** | 51.4±5.3** | 48.4±4.1** | 35.7±8.8 |
| Bowel Dissatisfaction | 18.0±5.8 | 77.6±2.2** | 72.9±4.3** | 80.7±4.0** | 80.7±3.2** | 75.0±9.1 |
| QOL | 2.7±1.9 | 56.4±2.6** | 57.2±4.6** | 56.9±5.3** | 55.9±4.1** | 53.9±11.0 |
| BSI-18 | ||||||
| Global Scale | 42.3±1.5 | 52.4±0.8** | 53.4±1.5** | 51.6±1.6** | 52.5±1.2** | 49.8±3.6 |
| Somatization | 44.8±1.2 | 54.1±0.7** | 55.5±1.1** | 52.8±1.6** | 53.9±1.0** | 52.9±3.4 |
| Depression | 46.0±1.5 | 51.0±1.0* | 52.6±1.9** | 52.0±1.8* | 49.4±1.6 | 49.0±5.1 |
| Anxiety | 43.3±1.2 | 51.1±0.8** | 51.7±1.4** | 50.6±1.7** | 51.8±1.4** | 45.8±2.6 |
Note.
No statistical comparisons were made to the IBS-U group because of a small sample size. Data were shown as mean ± SEM.
p<0.05,
p<0.01, compared with controls, Mann-Whitney U test. IBS-SS: IBS severity scale, BSI-18: Brief Symptom Index 18.
IBS Symptom severity and psychological distress
Scores on the IBS-SS and the BSI-18 are shown in Table 1. IBS patients scored significantly higher than HC on the IBS-SS total score and all subscales of the IBS-SS. Figure A1 in the appendix shows IBS-SS scores for all subjects separated into groups. This figure demonstrates that the IBS-SS scores were normally distributed within the IBS subgroups and that there was no tendency for the subtypes of IBS to differ from each other. On the BSI-18, IBS patients also scored significantly higher than HC on all subscales and on the global symptom index. However, there were no significant differences among the IBS subtypes.
Pain sensitivity
IBS patients had significantly lower thresholds for pain on the barostat test compared to HC (Table 2). Figure 2 shows the distribution of pain thresholds for each group. All IBS subtypes had significantly lower pain thresholds than HC, and for 57% of IBS patients, pain thresholds were below the 95 percent confidence interval for controls (34 mmHg). There were no significant differences in pain thresholds between the subtypes of IBS.
Phasic Colon motility
Baseline
When tested under baseline conditions (fasting, no intraluminal distention), the MI (phasic contractions) was no different in the total IBS patient group vs. HC (Table 2). However, the IBS-D group showed significantly more phasic contractions than IBS-C.
Response to intraluminal distention
As shown in Table 2, both HC and IBS groups showed a significant increase in MI during intraluminal distention. The magnitude of this increase was greater in the IBS patients compared to HC. The magnitude of the increase in MI from baseline to distention was significantly greater in the IBS-C and IBS-M groups compared to HC (p<0.05), but the subtypes of IBS did not differ from each other in magnitude of increase in phasic motility from baseline. Figure A2 in the appendix shows responses to distention as a percent of baseline values. Statistical analysis of these baseline-adjusted values showed the same significant comparisons as did analysis of the absolute values.
During the recovery period following intraluminal distention, the MI decreased and was not significantly different from baseline for either HC or IBS patients (all subtypes combined). When the IBS subtypes were compared to each other during recovery from distention, there were no significant differences between the subtypes.
Response to eating
As shown in Table 2, both HC and IBS showed significant increases in MI following the meal, but the magnitude of this increase was similar in IBS vs. HC. There was no significant difference between IBS subtypes in the magnitude of the meal-stimulated increase in phasic contractions. Figure A2 in the appendix shows responses to the meal as a percent of baseline values. Statistical analysis of these baseline-adjusted values also failed to show differences between HC vs. IBS or between IBS subgroups.
Smooth muscle tone measured by barostat bag volume
Baseline: When tested in the fasting state and without intraluminal distention, barostat bag volumes were smaller (i.e., smooth muscle tone was greater) in IBS compared to HC (Table 2). Comparison of IBS subtypes showed that the IBS-D subgroup had significantly lower bag volumes than the IBS-C group. Since IBS-M patients, like IBS-D patients, have loose or watery stools at least 25% of the time, we performed a post-hoc comparison of the IBS-D and IBS-M groups combined vs. the IBS-C and IBS-U groups combined and found that this was significant; as a group, patients who reported ≥25% of bowel movements as loose or watery had lower barostat bag volumes (35.4±2.7 mL) than IBS patients whose bowel movements were rarely loose or watery (50.5±6.3 mL, p<0.05).
Recovery period following distention
Muscle tone could not be measured during distention because barostat bag volumes during distention reflect compliance rather than tone. Barostat bag volumes during recovery from distention were approximately the same as during baseline and again showed lower bag volumes in the total IBS sample and in the IBS-D and IBS-M subgroups compared to the HC group (Table 2).
Response to eating
As shown in Table 2, both HC and IBS exhibited a statistically significant and profound decrease in bag volumes (i.e., an increase in smooth muscle tone) following the meal. The magnitude of this change in bag volume was not significantly different between IBS and HC. When the IBS subtypes were compared to each other, there was no difference in muscle tone between the subtypes (Table 2). Figure A3 in the appendix shows meal-related changes in bag volume as a percent of baseline values. Statistical analysis of these baseline-adjusted values also failed to show differences between IBS and HC or differences between IBS subtypes.
Relationship between motility and sensory thresholds
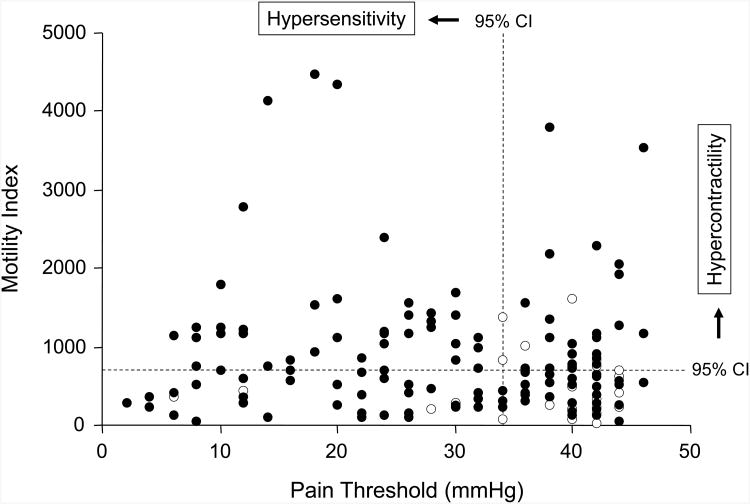
Figure 3 shows the relationship between the MI and the threshold for pain perception. Dotted vertical and horizontal lines show where the 95 percent confidence interval for HC lies on each of these dimensions. The overall correlation between motility during distention and pain sensory threshold in IBS patients was rho=-0.06 (p>0.1, see Figure 3), suggesting that these are independent dimensions.
Fig.3. Relationship between colonic motility during distention period and pain threshold.
No significant relationship was observed between colonic motility during distention period and visceral sensitivity in IBS patients (n=129; closed circles) or healthy subjects (n=30; open circles). Dashed lines show the 95% confidence intervals (CI): 49% of IBS patients showed contractile activity above the CI for controls in response to intraluminal distention, and 57% of patients had pain thresholds below the CI for controls. Of these patients, 31% showed both visceral hyper-contractivity and pain hypersensitivity.
Relationship of IBS symptom severity to visceral perception, colonic motility, and psychological symptoms
When all subtypes of IBS were pooled together, the pain threshold was significantly correlated with overall symptom severity (rho=-0.36, p<0.001), intensity of abdominal pain (rho=-0.34, p<0.001), frequency of abdominal pain (rho=-0.32, p<0.001), and severity of abdominal distention (rho=-0.31, p<0.001). Neither phasic motility nor smooth muscle tone was significantly correlated with overall symptom severity or with individual symptoms on the IBS-SS. However, because there were differences in motility between IBS-D and IBS-C (as shown in Table 2), it was possible that associations between motility and clinical symptoms were obscured by pooling all IBS subtypes together. Therefore, the correlations between motility parameters and key clinical symptoms (frequency of clinical pain, intensity of abdominal distention, and dissatisfaction with bowel habits) were computed for each IBS subtype separately. These data are summarized below.
Abdominal pain
Clinical pain frequency was significantly correlated with pain threshold in IBS-D (rho=-0.33, p<0.05) and this correlation was even stronger in IBS-M (rho=-0.50, p<0.001). However, there was no correlation between pain threshold and clinical pain frequency in IBS-C (rho=-0.13, NS).
Abdominal distention
The motility index was negatively correlated with severity of abdominal distention in IBS-C (rho=-0.39, p<0.05), but the motility index was unrelated to distention severity in IBS-D and IBS-M. The negative correlation indicates that as the amount of phasic contractions increased in IBS-C, the severity of distention decreased.
Dissatisfaction with bowel habits
The symptom of dissatisfaction with bowel habits correlated negatively (rho=-0.40, p<0.01) with postprandial smooth muscle tone in IBS-D (i.e., smaller bag volumes due to greater smooth muscle tone were associated with greater dissatisfaction with bowel habits). Among IBS-M and IBS-C, there was no association with muscle tone.
Psychological symptoms
When all IBS patients were analyzed together as one group, somatization (the tendency to notice and report somatic sensations possibly indicative of disease) was significantly correlated with overall IBS symptom severity (rho=0.30, p<0.01), and with the IBS-SS subscales for pain frequency (rho=0.23, p<0.05) and abdominal distention (rho=0.21, p<0.05). Anxiety and depression were not significantly related to bowel symptoms. When the subtypes of IBS were analyzed separately, the association between somatization and bowel symptom severity was found to be significant only for the IBS-M subtype: (rho=0.36 for pain frequency, rho=0.30 for abdominal distention, and rho=-0.30 for dissatisfaction with bowel movements; all significant at p<0.05). Depression was significantly correlated with dissatisfaction with bowel movements, but only in the IBS-C group.
Multiple linear regression (Table 3) was used to determine whether pain threshold, phasic motility, tonic motility, BSI psychological scales for somatization, depression, and anxiety and dominant stool consistency make independent contributions to IBS symptom severity after adjusting for the intercorrelations among these independent measures. Separate regression models were run for overall IBS symptom severity and the component clinical symptoms of pain intensity, pain frequency, abdominal distention severity, and dissatisfaction with bowel movements. The regression analyses showed that a significant amount of variance in IBS symptom severity was explained by these models for all dependent variables except dissatisfaction with bowel movements; the amount of variance explained (R2) ranged from 0.22 to 0.26 for the 4 significant models. Pain threshold was a significant independent predictor (p<0.001) in all of these models except dissatisfaction with bowel movements, and motility index during recovery was a significant predictor for pain intensity (β=0.27, p<0.05) and abdominal distention (β =-0.29, p<0.05). Somatization was a significant independent predictor for overall symptom severity (β =0.23, p<0.05) and abdominal distention (β =0.24, p<0.05). For abdominal distention, having hard or lumpy stools at least 25% of the time was also a significant predictor (β =0.20, p<0.05). Smooth muscle tone was not a significant predictor in any of the models.
Table 3. Multiple linear regression analyses for IBS symptoms severity in patients with IBS (n=129).
| IBS-SS Overall | Pain Intensity | Pain Frequency | Distention Severity | Bowel Dissatisfaction | |
|---|---|---|---|---|---|
| R2 | .26** | .23** | .22* | .26** | .07 |
| Covariates (β) | |||||
| Pain threshold | -.36*** | -.35*** | -.33*** | -.35*** | .06 |
| Baseline MI | -.01 | -.08 | -.12 | .18 | .08 |
| Distention MI | .00 | .02 | .05 | -.10 | -.03 |
| Recovery MI | .01 | .27* | .15 | -.29* | .01 |
| Post-meal MI | -.08 | -.18 | -.11 | .05 | -.02 |
| Baseline Tone | .10 | .11 | .17 | .01 | .10 |
| Recovery Tone | -.09 | -.02 | -.15 | -.12 | -.07 |
| Post-meal Tone | .06 | .01 | .08 | -.04 | -.14 |
| BSI Somatization | .23* | .12 | .13 | .24* | -.07 |
| BSI Depression | .11 | -.04 | .03 | .03 | -.06 |
| BSI Anxiety | -.08 | -.03 | -.06 | -.01 | .00 |
| Hard/lumpy stools ≥25% | .08 | -.02 | .01 | .20* | -.13 |
| Loose/watery stools ≥25% | -.04 | -.07 | -.04 | -.07 | -.02 |
NOTE.
p<0.05,
p<0.01,
p<0.001.
IBS-SS, IBS severity scale; MI, motility index; BSI, brief symptom index. The model included the following variables: Pain threshold, phasic and tonic motility, T-scores of BSI psychological scales for somatization, depression, and anxiety, hard or lumpy stools >25% (yes=1, no=0) and loose or watery stools >25% (yes=1, no=0).
Discussion
This large, carefully conducted study yielded three important and novel findings: First, we confirmed that visceral pain hypersensitivity is associated with (and likely contributes to) greater severity of IBS clinical symptoms, especially the frequency and the typical intensity of abdominal pain. This association was not explained by psychological influences on symptom reporting. Second, when IBS patients were divided into subtypes based on the frequency of loose or watery stools and/or the frequency of hard or lumpy stools, we found significant differences between the subtypes in both phasic and tonic motility of the descending colon. (Differences in phasic motility have been previously described but differences in muscle tone have not.) Third, the relationship between clinical symptoms and motility differed depending on predominant bowel habits. Thus, both pain thresholds and motility have an impact on the severity of specific IBS symptoms: pain thresholds show their strongest associations with clinical pain while motility is more strongly associated with abdominal distention and dissatisfaction with bowel movements.
Hypersensitivity for visceral pain
Our data are consistent with a large body of research which shows that IBS patients exhibit hypersensitivity to intra-luminal distention: 57% of our IBS patients had pain thresholds that were below the normal range, i.e., below the confidence interval for pain thresholds in healthy controls (Figure 2). A strength of this study is that sensory thresholds were studied in the descending colon rather than the rectum; most previous studies of visceral perception have tested pain sensitivity in the rectum even though it is assumed that the symptoms of IBS originate predominantly from the colon or small intestine.
Pain thresholds were significantly correlated with the overall severity of IBS symptoms. Individual symptoms that correlated with pain threshold were clinical pain intensity, pain frequency, and the severity of distention. Somatization (the psychological tendency to notice and report symptoms) was also correlated with clinical pain, abdominal distention, and overall symptom severity. However, the association between pain threshold and clinical symptoms remained significant after adjusting for the correlation of clinical symptoms with somatization, anxiety, and depression (Table 3).
We observed no significant differences in pain thresholds between IBS subtypes defined by Rome III criteria. Our findings are in agreement with previous barostat studies in the rectum which showed no significant differences between IBS-D and IBS-C for pain thresholds15, 25, although previous studies showed that rectal urge thresholds were lower in IBS-D patients compared to IBS-C patients15, 25. Earlier studies using volume distentions instead of pressure or tension-scaled distentions, reported lower pain thresholds in IBS-D compared to IBS-C, which is consistent with our observation that IBS-D patients have increased muscle tone compared to IBS-C (Table 2). These data extend earlier studies by showing that in the colon as well as the rectum, pressure-scaled pain thresholds are similar in IBS-D vs. IBS-C.
Phasic motility
Previous reports have been inconsistent as to whether IBS patients have more phasic contractions than healthy controls under baseline conditions (i.e., fasting and without stimulation by distention, stress, or exogenous hormones): there have been reports that IBS patients show more baseline motility26, 27, less motility28, and about the same amount9, 10 compared to HC. We found no difference when comparing HC to all IBS patients combined. However, the IBS-D group showed significantly more baseline contractile activity than IBS-C. We believe our results are generalizable because our study was relatively large (n=129 IBS patients and 30 HC) and we visually identified each phasic contraction and calculated its area under the curve rather than relying on the less precise method of allowing a computer program to estimate the MI by integrating the area of all activity above an arbitrary baseline.
Sustained intraluminal distention simulates a frequently occurring physiological stimulus to the colon, namely distention of the colon by fecal material or gas. This stimulus evokes an increase in phasic motility in both healthy controls and IBS patients, but the increase is significantly greater in IBS patients. This exaggerated response to intraluminal distention has been termed “hyper-reactivity” 29, and our data suggest that it is characteristic of all IBS patients rather than being limited to one subtype. We first reported that IBS is characterized by this exaggerated response to intraluminal distention in 198010 and other laboratories have replicated this observation9. The response to distention is reversible – MI returns to baseline when the distending stimulus is removed (Table 2) – and in other studies we have shown that it is reproducible on a second occasion of testing10. These characteristics make the response to intraluminal distention an attractive probe of motility for investigations of IBS. However, there is overlap between IBS and HC, rendering MI of limited value as a diagnostic marker for IBS.
Eating also stimulates an increase in the MI in both HC and IBS, as others have also shown11. We did not find that the magnitude of the meal stimulation was significantly greater in IBS patients as a group compared to healthy controls. There were no differences in postprandial stimulation of MI between IBS subgroups.
To summarize our findings with respect to phasic contractions of the colon: (1) Phasic contractions increase in reaction both to intraluminal distention and eating. (2) Compared to healthy controls, IBS patients show an exaggerated response to intraluminal distention but a similar response to eating. (3) IBS-D patients have more baseline phasic motility than IBS-C, but the differences are modest and there is overlap.
Barostat bag volumes
Average barostat bag volumes measured at the IOP provide an indirect measure of smooth muscle tone. As previously reported33, IBS patients have lower bag volumes than HC, indicating that IBS is associated with increased smooth muscle tone. Our data confirm this observation and extend it by showing that elevated smooth muscle tone is limited to patients with loose or watery stools at least 25% of the time. Patients with IBS-D had significantly lower bag volumes than patients with IBS-C (Table 2).
As previously reported27, 34, barostat bag volumes decrease substantially following a meal (Table 2), indicating an increase in smooth muscle tone in the descending colon. However, the magnitude of the increase in smooth muscle tone is similar in IBS vs. HC, and it does not distinguish patients with IBS-D from those with IBS-C. This increase in smooth muscle tone, along with the increase in phasic motility following a meal, are believed to be part of the physiological mechanism resulting in a tendency for flatus and defecation to occur shortly after a meal.
We anticipated that phasic motility and smooth muscle tone would be related to IBS symptoms, especially to dissatisfaction with bowel habits. No associations between motility and symptoms were seen when all IBS patients were considered together. However, when IBS subtypes defined by Rome III criteria were analyzed separately, we found significant but contrasting associations: (a) Bowel dissatisfaction was significantly correlated with smooth muscle tone in the IBS-D group. (b) Abdominal distention was negatively correlated with phasic motility in IBS-C (decreased muscle contraction associated with increased distention severity) but not IBS-D. In general, the associations between motility parameters and clinical symptoms were weaker than the associations between pain threshold and clinical symptoms, and some of these univariate associations could not be confirmed by regression analysis. Other variables such as somatization are also significant predictors of clinical symptoms in IBS.
Relationship between pain sensitivity and motility
IBS patients demonstrate both hypersensitivity and hyper-reactive motility in comparison to healthy controls. These appear to be independent pathophysiological mechanisms because (a) there is no correlation between them and (b) they show different relationships to the symptoms of IBS. Pain hypersensitivity is associated with more severe clinical pain and distention but is unrelated to dissatisfaction with bowel movements, and pain thresholds do not differ between IBS-D and IBS-C. Phasic motility and smooth muscle tone, on the other hand, are greater in IBS-D than they are in IBS-C and may play a role in regulating usual or predominant bowel habits. We did not, however, find that hyper-reactive motility could reliably differentiate between patients with IBS-D and those with IBS-C. The apparent independence of pain hypersensitivity and motility suggests that different etiologies – genetic, inflammatory, psychosocial, or other factors – are likely to be found for motility hyper-reactivity and pain sensitivity, and different treatments or management strategies may be required.
Study limitations
It is possible that the invasive nature of the test protocol, which required unsedated sigmoidoscopy to place the motility catheter and led subjects to anticipate pain and/or anxiety, may have biased recruitment. Consistent with this possibility, the average age of the IBS sample was 35.8 years, which is younger than average for the IBS patients we have studied in other settings35. Secondly, the prevalence of pain hypersensitivity may have been underestimated since 5 IBS patients and one HC were unable or unwilling to undergo unsedated flexible sigmoidoscopy after volunteering. Both of these study limitations could have led to an under-representation of IBS patients with the greatest pain sensitivity, but this would not affect the conclusions that pain sensitivity and motility reactivity are independent pathophysiological mechanisms for IBS and that pain sensitivity is the more important determinant of clinical symptoms. A third limitation is that the motility parameters tested were restricted to phasic and tonic motility in the descending colon and did not include small bowel motility36 or high amplitude propagating contractions30, 31Thus, we may have overlooked motility patterns that show a stronger association with IBS symptom severity or altered bowel habits.
Significance
This study shows that both pain thresholds and motility have an impact on the severity of specific IBS symptoms: pain thresholds show their strongest associations with clinical pain while motility is more strongly associated with abdominal distention and dissatisfaction with bowel movements. Our data further suggest that pain sensitivity and motility are independent physiological mechanisms for the symptoms of IBS. The implication of these findings for clinical practice is that treatments may have to be selected based on the patient's predominant bowel habit in order to maximize clinical benefit. Furthermore, since most drugs currently approved for the treatment of IBS have a greater impact on motility than on pain, clinicians may want to supplement current drug treatments for IBS with a pain management strategy. The implication for pharmaceutical companies is that it may be advantageous to target pain sensitivity in future drug development programs.
Study Highlights.
What is Current Knowledge
Patients with irritable bowel syndrome (IBS) show visceral hypersensitivity or hyper-reactive motility in the sigmoid colon and the rectum.
What is New Here
Visceral pain sensitivity in the descending colon is associated with greater severity of IBS clinical symptoms, especially abdominal pain.
Phasic and tonic colon motility are related to predominant bowel habits and to the clinical symptoms of abdominal distention and dissatisfaction with bowel movements.
Visceral hypersensitivity and motility are independent physiological mechanisms for the symptoms of IBS.
Acknowledgments
This study was supported by RO1 DK31369, R24 DK67674, and MO1 RR00046.
Appendix.
Fig.A1 IBS symptom severity scale (IBS-SS) in subtypes of IBS and healthy controls
IBS-SS total score for each subject was shown. Each horizontal bar indicates the median value. HC, healthy controls (n=30); IBS-D, IBS with diarrhea (n=44); IBS-C, IBS with constipation (n=29); IBS-M, mixed IBS (n=45). **p<0.01, vs. controls.
Fig. A2 Changes in motility index during each stimulus period in subtypes of IBS and healthy controls
Distention, the MI during recovery and postprandial periods, is expressed as a percent of the baseline MI for the subgroup to adjust for any baseline differences. HC, healthy controls (light blue: n=30); IBS-D, IBS with diarrhea (n=44); IBS-C, IBS with constipation (n=29); IBS-M, mixed IBS (n=45). Data were expressed as mean + SEM. *p<0.05 vs. controls in same phase of testing. During distention and postprandial periods, all groups are significantly (p<0.05) greater than baseline.
Fig. A3 Changes in Colonic Muscle Tone during each stimulus period in subtypes of IBS and healthy controls
The colonic muscle tone was measured as mean bag volume. For the recovery and postprandial periods, each bar shows percent of baseline value for the subgroup. HC, healthy controls; IBS-D, IBS with diarrhea; IBS-C, IBS with constipation; IBS-M, mixed IBS. Data were expressed as mean +/- SEM. Groups are not significantly different from each other within conditions, but postprandial values are significantly (p<0.05) lower than baseline for all groups.
Footnotes
Conflicts of Interest and Author Roles: William E. Whitehead, Ph.D., is the guarantor of this paper, and he participated in study design, data collection, data analysis, and manuscript preparation. Motoyori Kanazawa, M.D., Ph.D., participated in study design, data analysis, and preparation of the manuscript. Olafur S. Palsson, Psy.D., participated in study design and data analysis. Syed IM Thiwan, M.D., participated in data collection. Marsha J. Turner, M.A., participated in data collection. Miranda AL van Tilburg, Ph.D., participated in data collection and data analysis. Lisa M. Gangarosa, M.D., participated in data collection. Denesh K. Chitkara, M.D., participated in data collection. Shin Fukudo, M.D., Ph.D., participated in data analysis and manuscript preparation. Douglas A. Drossman, M.D., participated in data collection. All financial support is declared in the acknowledgment, and none of the authors have competing interests.
Reference List
- 1.Ritchie J. Pain from distension of the pelvic colon by inflating a balloon in the irritable colon syndrome. GUT. 1973;14:125–132. doi: 10.1136/gut.14.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitehead WE, Holtkotter B, Enck P, Hoelzl R, Holmes KD, Anthony J, Shabsin HS, Schuster MM. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterol. 1990;98:1187–1192. doi: 10.1016/0016-5085(90)90332-u. [DOI] [PubMed] [Google Scholar]
- 3.Dorn SD, Palsson OS, Thiwan SIM, Kanazawa M, Clark WC, van Tilburg MAL, Drossman DA, Scarlett Y, Levy RL, Ringel Y, Crowell MD, Olden KW, Whitehead WE. Colonic pain sensitivity in Irritable Bowel Syndrome is the result of an increased tendency to report pain rather than increased neurosensory sensitivity. GUT. 2007;56:1202–1209. doi: 10.1136/gut.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mertz H, Naliboff B, Munakata J, Niazi N, Mayer EA. Altered rectal perception is a biological marker of patients with irritable bowel syndrome. Gastroenterol. 1995;109:40–52. doi: 10.1016/0016-5085(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 5.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganiere M, Verrier P, Poitras P. Rectal distention testing in patients with irritable bowel syndrome: sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterol. 2002;122:1771–1777. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 6.Distrutti E, Salvioli B, Azpiroz F, Malagelada JR. Rectal function and bowel habit in irritable bowel syndrome. Am J Gastroenterol. 2004;99:131–137. doi: 10.1046/j.1572-0241.2003.04012.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukudo S, Suzuki J. Colonic motility, autonomic function, and gastrointestinal hormones under psychological stress on irritable bowel syndrome. Tohoku J Exp Med. 1987;151:373–385. doi: 10.1620/tjem.151.373. [DOI] [PubMed] [Google Scholar]
- 8.Welgan P, Meshkinpour H, Hoehler F. The effect of stress on colon motor and electrical activity in irritable bowel syndrome. Psychosom Med. 1985;47:139–149. doi: 10.1097/00006842-198503000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Fukudo S, Kanazawa M, Kano M, Sagami Y, Endo Y, Utsumi A, Nomura T, Hongo M. Exaggerated motility of the descending colon with repetitive distention of the sigmoid colon in patients with irritable bowel syndrome. J Gastroenterol. 2002;37(Suppl 14):145–150. doi: 10.1007/BF03326434. [DOI] [PubMed] [Google Scholar]
- 10.Whitehead WE, Engel BT, Schuster MM. Irritable Bowel Syndrome - Physiological and Psychological Differences Between Diarrhea-Predominant and Constipation-Predominant Patients. Digestive Diseases and Sciences. 1980;25:404–413. doi: 10.1007/BF01395503. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan MA, Cohen S, Snape WJ., Jr Colonic myoelectrical activity in irritable-bowel syndrome. Effect of eating and anticholinergics. N Engl J Med. 1978;298:878–883. doi: 10.1056/NEJM197804202981604. [DOI] [PubMed] [Google Scholar]
- 12.Di Stefano M, Miceli E, Missanelli A, Mazzocchi S, Corazza GR. Meal induced rectosigmoid tone modification: a low caloric meal accurately separates functional and organic gastrointestinal disease patients. GUT. 2006;55:1409–1414. doi: 10.1136/gut.2005.076323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drossman DA, Whitehead WE, Toner BB, Diamant N, Hu YJ, Bangdiwala SI, Jia H. What determines severity among patients with painful functional bowel disorders? Am J Gastroenterol. 2000;95:974–980. doi: 10.1111/j.1572-0241.2000.01936.x. [DOI] [PubMed] [Google Scholar]
- 14.Prior A, Maxton DG, Whorwell PJ. Anorectal Manometry in Irritable Bowel Syndrome -Differences Between Diarrhea and Constipation Predominant Subjects. GUT. 1990;31:458–462. doi: 10.1136/gut.31.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steens J, Van Der Schaar PJ, Penning C, Brussee J, Masclee AA. Compliance, tone and sensitivity of the rectum in different subtypes of irritable bowel syndrome. Neurogastroenterol Motil. 2002;14:241–247. doi: 10.1046/j.1365-2982.2002.00332.x. [DOI] [PubMed] [Google Scholar]
- 16.Simren M, Abrahamsson H, Bjornsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. GUT. 2001;48:20–27. doi: 10.1136/gut.48.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caldarella MP, Milano A, Laterza F, Sacco F, Balatsinou C, Lapenna D, Pierdomenico SD, Cuccurullo F, Neri M. Visceral sensitivity and symptoms in patients with constipation- or diarrhea-predominant irritable bowel syndrome (IBS): effect of a low-fat intraduodenal infusion. Am J Gastroenterol. 2005;100:383–389. doi: 10.1111/j.1572-0241.2005.40100.x. [DOI] [PubMed] [Google Scholar]
- 18.Longstreth GF, Thompson WG, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. In: Drossman DA, Corazziari E, Delvaux M, Spiller RC, Talley NJ, Thompson WG, Whitehead WE, editors. Rome III: The Functional Gastrointestinal Disorders. 3rd. McLean, Virginia: Degnon Associates; 2006. pp. 487–555. [Google Scholar]
- 19.Thompson WG, Longstreth G, Drossman DA, Heaton K, Irvine EJ, Muller-Lissner S. Functional bowel disorders and functional abdominal pain. In: Drossman DA, Corazziari E, Talley NJ, Thompson WG, Whitehead WE, editors. Rome II: The Functional Gastrointestinal Disorders. 2nd. McLean, VA: Degnon Associates; 2000. pp. 351–432. [Google Scholar]
- 20.Mearin F, Balboa A, Badia X, Baro E, Caldwell E, Cucala M, az-Rubio M, Fueyo A, Ponce J, Roset M, Talley NJ. Irritable bowel syndrome subtypes according to bowel habit: revisiting the alternating subtype. Eur J Gastroenterol Hepatol. 2003;15:165–172. doi: 10.1097/00042737-200302000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scandinavian Journal of Gastroenterology. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 22.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Alimentary Pharmacology & Therapeutics. 1997;11:395–402. doi: 10.1046/j.1365-2036.1997.142318000.x. [DOI] [PubMed] [Google Scholar]
- 23.Derogatis LR. BSI 18 Brief Symptom Inventory 18: Administration, scoring, and procedures manual. NCS Pearson Inc; 2000. [Google Scholar]
- 24.Whitehead WE, Delvaux M, Azpiroz F, Barlow J, Bradley L, Camilleri M, Crowell MD, Enck P, Fioramonti J, Track J, Mayer EA, Morteau O, Phillips SF, Thompson DG, Wingate DL. Standardization of barostat procedures for testing smooth muscle tone and sensory thresholds in the gastrointestinal tract. Digestive Diseases and Sciences. 1997;42:223–241. doi: 10.1023/a:1018885028501. [DOI] [PubMed] [Google Scholar]
- 25.Zar S, Benson MJ, Kumar D. Rectal afferent hypersensitivity and compliance in irritable bowel syndrome: differences between diarrhoea-predominant and constipation-predominant subgroups. Eur J Gastroenterol Hepatol. 2006;18:151–158. doi: 10.1097/00042737-200602000-00007. [DOI] [PubMed] [Google Scholar]
- 26.Rogers J, Henry MM, Misiewicz JJ. Increased segmental activity and intraluminal pressures in the sigmoid colon of patients with the irritable bowel syndrome. GUT. 1989;30:634–641. doi: 10.1136/gut.30.5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassallo MJ, Camilleri M, Phillips SF, Steadman CJ, Talley NJ, Hanson RB, Haddad AC. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725–731. doi: 10.1016/s0025-6196(12)60796-4. [DOI] [PubMed] [Google Scholar]
- 28.Misiewicz JJ, Connell AM, Pontes FA. Comparison of the effect of meals and prostigmine on the proximal and distal colon in patients with and without diarrhoea. GUT. 1966;7:468–473. doi: 10.1136/gut.7.5.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterol. 2002;123:2108–2131. doi: 10.1053/gast.2002.37095. [DOI] [PubMed] [Google Scholar]
- 30.Bassotti G, de RG, Castellani D, Sediari L, Morelli A. Normal aspects of colorectal motility and abnormalities in slow transit constipation. World J Gastroenterol. 2005;11:2691–2696. doi: 10.3748/wjg.v11.i18.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassotti G, de RG, Chistolini F, Sietchiping-Nzepa F, Morelli O, Morelli A. Twenty-four-hour manometric study of colonic propulsive activity in patients with diarrhea due to inflammatory (ulcerative colitis) and non-inflammatory (irritable bowel syndrome) conditions. Int J Colorectal Dis. 2004;19:493–497. doi: 10.1007/s00384-004-0604-6. [DOI] [PubMed] [Google Scholar]
- 32.Bassotti G, Chistolini F, Marinozzi G, Morelli A. Abnormal colonic propagated activity in patients with slow transit constipation and constipation-predominant irritable bowel syndrome. Digestion. 2003;68:178–183. doi: 10.1159/000075554. [DOI] [PubMed] [Google Scholar]
- 33.Whitehead WE, Crowell MD, Davidoff AL, Palsson OS, Schuster MM. Pain from rectal distension in women with irritable bowel syndrome: relationship to sexual abuse. Dig Dis Sci. 1997;42:796–804. doi: 10.1023/a:1018820315549. [DOI] [PubMed] [Google Scholar]
- 34.Simren M, Abrahamsson H, Bjornsson ES. Lipid-induced colonic hypersensitivity in the irritable bowel syndrome: the role of bowel habit, sex, and psychologic factors. Clin Gastroenterol Hepatol. 2007;5:201–208. doi: 10.1016/j.cgh.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Whitehead WE, Levy RL, Von Korff MV, Feld AD, Palsson OS, Turner MJ, Drossman DA. Usual medical care for irritable bowel syndrome. Alimentary Pharmacology & Therapeutics. 2004;20:1305–1315. doi: 10.1111/j.1365-2036.2004.02256.x. [DOI] [PubMed] [Google Scholar]
- 36.Simren M, Castedal M, Svedlund J, Abrahamsson H, Bjornsson E. Abnormal propagation pattern of duodenal pressure waves in the irritable bowel syndrome (IBS) [correction of (IBD)] Dig Dis Sci. 2000;45:2151–2161. doi: 10.1023/a:1010770302403. [DOI] [PubMed] [Google Scholar]