Abstract
Acid-sensing ion channels (ASICs) are densely expressed in the brain with ASIC1a and ASIC2 channels being the predominant subtypes. These channels are enriched at synaptic sites and are central for the regulation of normal synaptic transmission. Moreover, increasing evidence links ASICs to the pathogenesis of various neurological and neuropsychiatric disorders. In this study, we explore the putative role of ASIC1a and ASIC2 in the regulation of behavioral sensitivity to the psychostimulant cocaine by utilizing ASIC1a or ASIC2 knockout mice. Acute cocaine injection induced a typical dose-dependent increase in locomotor activities in wild-type (WT) mice. However, in ASIC1a and ASIC2 mutant mice, different motor responses to cocaine were observed. In ASIC1a−/− mice, cocaine induced a significantly less motor response at all doses (5, 10, 20, and 30 mg/kg), while in ASIC2−/− mice, cocaine (5–20 mg/kg) stimulated locomotor activity to an extent comparable to WT mice. Only at 30 mg/kg, the cocaine-stimulated motor activity was reduced in ASIC2−/− mice. In a chronic cocaine administration model (20 mg/kg, once daily for 5 days), a challenge injection of cocaine (10 mg/kg, after 2-week withdrawal) caused an evident behavioral sensitization in the cocaine-pretreated WT mice. This behavioral sensitization to challenge cocaine was also displayed in ASIC1a−/− and ASIC2−/− mice. However, ASIC2−/− mice showed less sensitization to challenge cocaine when compared to WT and ASIC1a−/− mice. Our results demonstrate the important role of ASIC1a and ASIC2 channels in the modulation of behavioral sensitivity to cocaine. The two synapse-enriched ASIC subtypes are believed to play distinguishable roles in the regulation of behavioral responses to acute and chronic cocaine administration.
Keywords: acid-sensing ion channels, cocaine, stimulant, addiction, locomotor activity, behavioral sensitization
Drug addiction remains remarkably difficult to treat. This is partially due to the lack of a precise understanding of mechanisms of drug addiction. However, sufficient evidence suggests that many receptors and ion channels in the brain reward circuits are believed to play important roles (Wolf et al., 2004; Kauer & Malenka, 2007; Kalivas, 2009; Mao et al., 2009; Sesack & Grace, 2010; Kalivas & Volkow, 2011). The search for responsible receptors and ion channels that potentially contribute to cocaine addiction persists as an active area of investigation.
Releasing neurotransmitters from presynaptic terminals into the synaptic cleft is an important step in synaptic transmission. The synaptic vesicle in presynaptic terminals that contains transmitters also contains acidic elements (pH 5.7) (Miesenböck et al., 1998), which may lower the pH within the synaptic cleft upon discharge of their contents, an effect seen particularly during rapid, repeated discharge of multiple vesicles. Intense neural activity also produces slower and more sustained acidosis (Sluka et al., 2009). For example, the profound neural activity in seizures lowers pH from 7.35 to 6.8 (Ziemann et al., 2008). Metabolism may also lead to neural acidosis. Mitochondria absorb Ca2+ and extrude H+. The latter can diffuse into the extracellular space (Demaurex et al., 2009), resulting in a decreases in pH and subsequent activation of postsynaptic receptors and ion channels.
Rapid reduction in extracellular pH can activate acid-sensing ion channels (ASICs), a distinct family of membrane ion channels highly expressed in both peripheral sensory as well as central neurons (Krishtal & Pidoplichko, 1981; Waldmann et al., 1997; Alvarez de la Rosa et al., 2003). Four genes (ASIC1–4) encoding at least seven ASIC subunits have been cloned (Waldmann et 1998; Wemmie et al., 2006; Grunder & Chen, 2010). Functional ASICs structurally appear as trimeric complexes of these subunits (Jasti et al., 2007) and most of these subunits can form both homomeric and heteromeric channels (Lingueglia et al., 1997; Askwith et al., 2004; Hesselager et al., 2004; Sherwood et al., 2011). Homomeric ASIC1a and heteromeric ASIC1a/2 channels are the dominant subtypes of ASICs in the brain (Wemmie et al., 2002; Askwith et al., 2004; Chu et al., 2004; Zha et al., 2006, 2009; Jiang et al., 2009; Sherwood et al., 2011). They play critical roles in physiological and pathological conditions (Xiong et al., 2008; Chu et al., 2011; Chu & Xiong, 2012). For example, deletion of the ASIC1a gene impaired synaptic plasticity, learning/memory, and fear behavior in the brain (Wemmie et al., 2002, 2003; Ziemann et al., 2009). Activation of Ca2+-permeable ASIC1a channels resulted in acidosis-mediated ischemic brain injury (Xiong et al., 2004; Gao et al., 2005). In addition, ASIC1a channels also play critical roles in neurodegenerative diseases such as multiple sclerosis (Friese et al., 2007), Parkinson’s disease (Arias et al., 2008), Huntington’s disease (Wong et al., 2008), seizures (Ziemann et al., 2008), and depression (Coryell et al., 2009). While numerous studies delineate some of ASIC1a functions, ASIC2 functions are less clear. Available data show that ASIC2 channels may be involved in brain ischemia and epilepsy (Johnson et al., 2001; Biagina et al., 2001).
The forebrain regions, key structures in reward circuits involved in biological actions of addictive drugs (Sesack & Grace, 2010), densely express ASIC1a and ASIC2 channels (Wemmie et al., 2002; Alvarez de la Rosa et al., 2003). Given the abundance of ASIC1a and ASIC2 channels at synaptic sites (Wemmie et al., 2003; Zha et al., 2006, 2009; Jing et al., 2012) and demonstrated sensitivity of the channels to psychostimulants in our recent studies (Zhang et al., 2009; Suman et al., 2010), these channels are thought to regulate behavioral sensitivity to cocaine. Thus, this study was designed to explore the functional role of ASIC1a and ASIC2 channels in the regulation of locomotor responses to acute and chronic cocaine exposure.
EXPERIMENTAL PROCEDURES
Animals
ASIC1a−/− and ASIC2−/− mice with a congenic C57BL/6J background were obtained from Drs. Wemmie and Welsh’s laboratory at the University of Iowa as gifts and subsequently bred in our facilities. The C57BL/6J wild-type (WT) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) and also bred in our facilities. Animals were maintained on a 12/12 h light/dark cycle with the lights turned on at 7:00 am. The housing environment was maintained at a temperature of 23 °C and humidity of 50% ± 10% with tap water and regular chow available ad libitum. Mice were group-housed (four to five mice per cage) until experiments were conducted. Two- to three-month old male mice weighting 25 to 35 g were used at the onset of experiments. All efforts were made to minimize animal suffering and reduce the number of animals used. All animal use procedures were in strict accordance with the US National Institutes of Health for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Behavioral measurements
Behavioral activity was assessed with an infrared photo-cell-based, automated VersaMax animal activity monitoring system (AccuScan Instruments, Columbus, OH, USA) in a sound-attenuated and light-controlled room as described previously (Mao et al., 2009; Parelkar et al., 2009; Suman et al., 2010). Briefly, mice in acrylic test chambers measuring 30 cm (H) x 20 cm (L) x 20 cm (W) were habituated to the environment for 3 h before testing to exclude the exploratory behavior. Three sensor pairs positioned in x, y (horizontal), and z (vertical, above the animal’s normal height) axes were assigned to each chamber to provide measurements for horizontal and vertical activities. Locomotor activities were recorded at 5-min intervals before and after drug injection. The presence of animals in chambers generated beam interruptions, and corresponding data from the sensors were transferred to a computer with operating VersaMax software.
Exploratory behavior in response to novel environment
Age-matched WT, ASIC1a−/− and ASIC2−/− male mice were brought into the test room and allowed to accommodate to this room for 5 min before being tested. After this period, each mouse was placed individually in its individual test chamber as mentioned above. Their locomotor activity and jumping behavior in response to this novel environment were measured for a period of 180 min. Jumping behavior was counted when the four limbs of the mouse left the chamber ground and went up into the air. A trained observer that was blinded to both genotypes and injection person scored jumping counts.
Acute cocaine administration
After the initial 180-min adjustment period, mice were intraperitoneally (i.p.) injected with either saline (0.9% NaCl, 10 ml/kg) or cocaine (5, 10, 20 and 30 mg/kg). Locomotor activity in response to this single dose was recorded for a period of 90 to 120 min.
Behavioral sensitization
The behavioral sensitization regimen used in this experiment has been described previously (Zhang et al., 2009). Briefly, WT, ASIC1a−/−, and ASIC2−/− received a single i.p. injection of cocaine at a dose of 20 mg/kg (once daily for 5 consecutive days). Mice treated with daily saline injection (10 ml/kg, i.p.) for 5 days served as controls. After a 2-week withdrawal period, mice were brought to the testing room and accommodated to the room as described above. Each mouse then received a challenge injection of cocaine at a dose of 10 mg/kg. Locomotor responses to this challenge dose were measured for 90 min.
Drugs
Cocaine hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO, USA) and dissolved in saline (0.9% NaCl). All drugs were freshly prepared on the day of injection.
Data analysis
The results are presented as means ± S.E.M and were evaluated using unpaired t-test or analysis of variance (ANOVA) followed by a Bonferroni (Dun) comparison of groups using either the least squares-adjusted means or the two-tailed unpaired Student’s t-test. The Fisher’s exact test was used to compare two groups of two categorical variables. Probability levels of < 0.05 were considered statistically significant.
RESULTS
Locomotor and jumping activities under novel conditions
We first examined baseline locomotor activity under novel conditions. WT, ASIC1a−/−, and ASIC2−/− male mice were placed in a testing chamber (open field box). Locomotor activity was recorded for 3 h. Figure 1A shows the time course of distance traveled by these mice. ASIC1a−/− mice displayed increased locomotor activity when compared to WT and ASIC2−/− mice in the first 5 min measurement (p < 0.01). No significant differences in total distance traveled during the 3-h measurement were seen in ASIC1a−/− relative to WT mice (Fig. 1B; p > 0.05). However, ASIC2−/− mice displayed a significantly less motor activity as compared to ASIC1a−/− mice in the 3-h session (Fig. 1B; p < 0.01). ASIC2−/− mice also showed a trend of reduced motor activity as compared to the WT mice.
Fig.1.
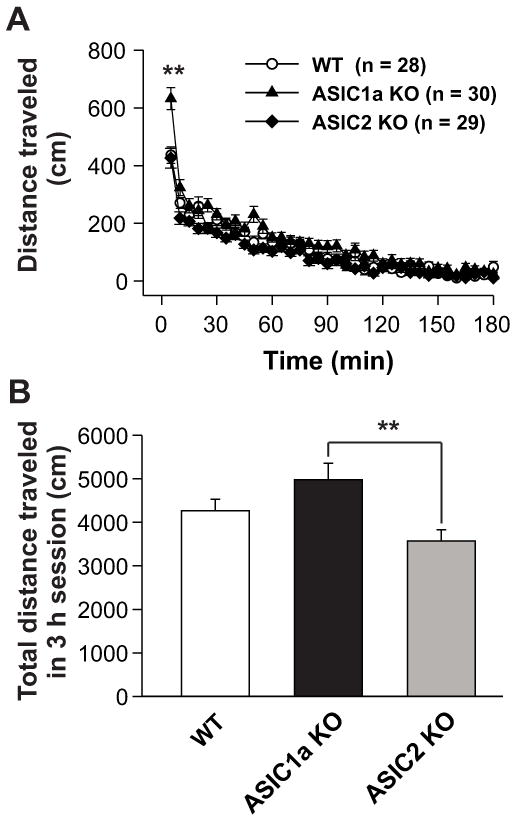
Baseline locomotor activity in WT, ASIC1a−/−, and ASIC2−/− mice. Mice were habituated to the test room for 5 min and then placed in an individual test chamber. Baseline locomotor activity was monitored for 180 min prior to any injection. (A) Time course of distances traveled per 5 min in WT, ASIC1a−/−, and ASIC2−/− mice. ASIC1a−/− mice (n = 28) displayed increased locomotor activity when compared to WT (n = 30) or ASIC2−/− (n = 29) mice in the first 5-min interval. **p < 0.01 vs. WT or ASIC2−/− mice. Open circles: WT mice; Filled triangles: ASIC1a−/− mice; Filled diamonds: ASIC2−/− mice. (B) Total distances traveled during the 180-min session for WT, ASIC1a−/−, and ASIC2−/− mice. Both ASIC1a−/− and WT mice did not differ with respect to locomotor activity during the 180-min session (p > 0.05). ASIC2−/− mice showed reduced locomotor activity when compared to ASIC1a−/− mice (**p < 0.01). Data are means ± S.E.M. WT: wild-type mice; ASIC1a KO: ASIC1a knockout mice; ASIC2 KO: ASIC2 knockout mice. Min: minutes.
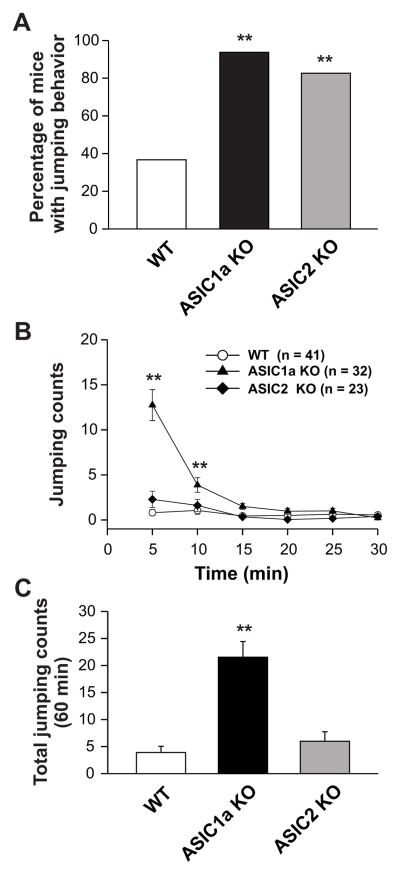
During this exploratory session, we also found that the majority of ASIC1a−/− (30 out of 32) and ASIC2−/− (19 out of 23) mice displayed jumping behavior (Fig. 2A). Conversely, far fewer WT mice displayed this jumping behavior when compared to ASIC1a−/− or ASIC2−/− mice, with only 15 out of 41 WT mice exhibiting jumping behavior in a novel environment (Fig. 2A). The percentage of ASIC1a−/− or ASIC2−/− mice with jumping behavior exceeded that of WT mice (p < 0.01). Figure 2B shows the time course of jumping behavior for the WT, ASIC1a−/−, and ASIC2−/− mice. Jumping activity chiefly appeared in the first 25 min period of the 180-min exploratory period for all genotypes (Fig. 2B). ASIC1a−/− mice displayed the highest frequency of jumping activity when compared to the WT or ASIC2−/− mice in the first 10 min of the exploratory period (p < 0.01). The average numbers of jumps per mouse were 3.9 ± 1.2, 21.5 ± 2.9 and 6.0 ± 1.7 for WT, ASIC1a−/−, and ASIC2−/− mice, respectively (Fig. 2C). The jumping frequency of ASIC1a−/− mice exceeded that of the WT or ASIC2−/− mice (Fig. 2C; p < 0.01).
Fig. 2.
Jumping behavior exploration in WT, ASIC1a−/−, and ASIC2−/− mice. (A) Percentage of mice exhibiting jumping behavior. All genotypes displayed jumping behavior. A higher percentage of ASIC1a−/− (93.75%) and ASIC2−/− (82.61%) mice displayed jumping behavior when compared to WT (36.59%) mice. ** p < 0.01 vs. WT. (B) Time course of jumping counts per 5 min in all genotypes. ASIC1a−/− mice displayed higher frequency than WT or ASIC2−/− mice in the first 10 min. **p < 0.01 vs. WT or ASIC2−/− mice. Open circles: WT mice; Filled triangles: ASIC1a−/− mice; Filled diamonds: ASIC2−/− mice. (C) Total average number of jumps in the 60-min session. ASIC1a−/− mice jumped more frequently than WT or ASIC2−/− mice. ** p < 0.01 vs. WT or ASIC2−/− mice. Data are means ± S.E.M. WT: wild-type mice; ASIC1a KO: ASIC1a knockout mice; ASIC2 KO: ASIC2 knockout mice. Min: minutes.
Acute locomotor responses to cocaine
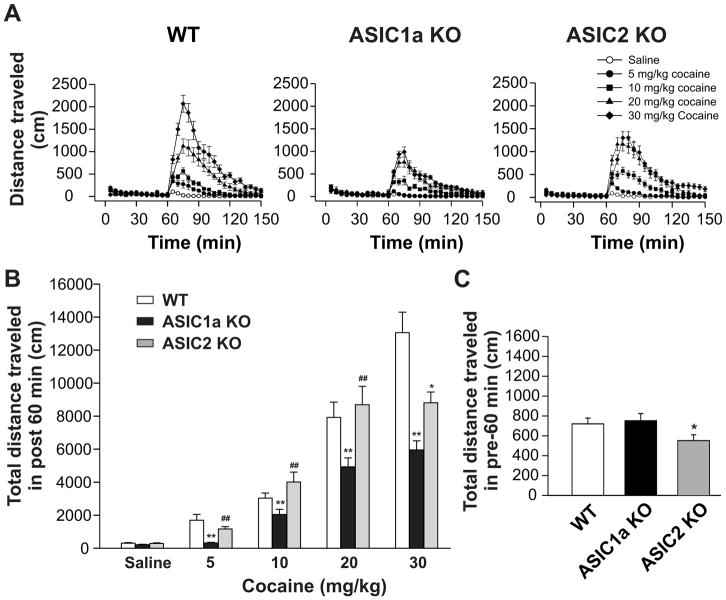
All genotypes underwent a 3-h accommodation in testing chambers prior to saline or cocaine injection. An acute administration of cocaine dose-dependently increased the distance traveled in all WT, ASIC1a−/−, and ASIC2−/− mice (Fig. 3A and 3B). Notably, the overall locomotor response to cocaine at four doses surveyed was significantly reduced in ASIC1a−/− mice relative to WT mice (Fig. 3B). In fact, no significant change was observed after cocaine was injected at 5 mg/kg in ASIC1a−/− mice, while a significant increase in motor responses to 5 mg/kg cocaine was seen in WT mice. In contrast to ASIC1a−/−, the motor response to cocaine at the first three doses (5, 10, and 20 mg/kg) remained unchanged in ASIC2−/− mice compared to WT mice (Fig. 3A and 3B). Only at the highest dose (30 mg/kg), was a significantly less motor response induced in ASIC2−/− mice. All genotypes did not display jumping behavior during the post-injection observation period. These data demonstrate a marked inhibition of overall motor responses to acute cocaine in ASIC1a−/− mice, while in ASIC2−/− mice the inhibition only occurs when applying cocaine stimulation at a high dose (30 mg/kg).
Fig. 3.
Effects of acute cocaine injection on locomotor activity in WT, ASIC1a−/−, and ASIC2−/− mice. Mice were placed in the locomotor test chamber for 180 min for accommodation purpose, with the final 60 min used as the control period. Either cocaine or saline were injected (i.p.) and mice were returned to their test chambers for an additional 90 min. (A) Time courses of locomotor activity of WT (left panel), ASIC1a−/− (middle panel), and ASIC2−/− (right panel) mice. Saline (open circles), 5 mg/kg (filled circles), 10 mg/kg (filled squares), 20 mg/kg (filled triangles) and 30 mg/kg (filled diamonds) doses of cocaine were given at the 60-min mark denoted on the graph. Initial 60 min (0 to 60 min) used as control period. Data shown are the average total distances traveled in 5-min intervals. n = 12 to 24 for each genotype. (B) Total distances traveled in 60 min immediately following injection of either saline or 5, 10, 20, or 30 mg/kg doses of cocaine in the three tested genotypes. Cocaine significantly increased locomotor activity in all genotypes of mice. n = 12 to 24 per each genotype. Data are means ± S.E.M. ANOVA was performed. Comparing WT and ASIC1a−/− mice, cocaine in all tested doses induced less locomotor activity in ASIC1a−/− mice compared to WT mice (** p < 0.01). Comparing WT and ASIC2−/− mice, cocaine at doses of 5, 10, and 20 mg/kg did not produce significant different locomotor activity in ASIC2−/− mice when compared with WT mice (p > 0.05). Cocaine at a dose of 30 mg/kg induced less locomotor activity in ASIC2−/− than in the WT mice (* p < 0.05). Comparing ASIC2a−/− and ASIC1a−/− mice, cocaine in all tested doses induced higher locomotor activity in ASIC2−/− mice compared to ASIC1a−/− mice (## p < 0.01). (C) Total distances traveled in 60 min for WT (n = 67), ASIC1a−/− (n = 71), and ASIC2−/− (n = 66) mice during their habituation to the chambers immediately prior to cocaine or saline injection. No significant differences existed between WT and ASIC1a−/− mice (p > 0.05). ASIC2−/− mice displayed decreased locomotor activity when compared to WT or ASIC1a−/− mice (*p < 0.05). Data are means ± S.E.M. WT: wild-type mice; ASIC1a KO: ASIC1a knockout mice; ASIC2 KO: ASIC2 knockout mice. Min: minutes.
We also analyzed and compared the motor activity measured during a 60-min period prior to drug injection. As shown in Fig. 3C, the total distance traveled by ASIC1a−/− mice during this period of time did not differ from that by WT mice. However, ASIC2−/− mice displayed a significantly less motor activity compared to either the WT or ASIC1a−/− mice. This seems to indicate a reduced level of basal motor activity in ASIC2−/− mice.
Behavioral sensitization
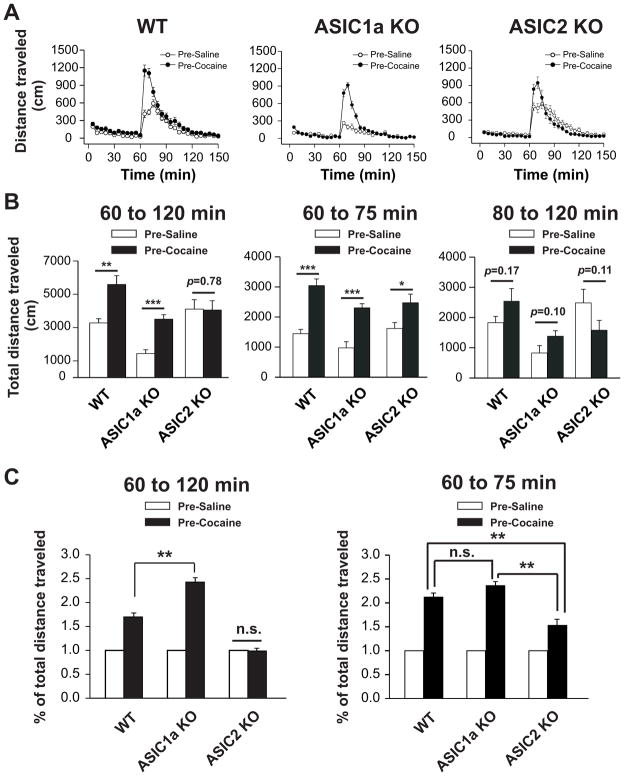
We next wanted to evaluate the role of ASIC1a and ASIC2 in the regulation of behavioral responses to chronic cocaine administration. To this end, we used a typical behavioral sensitization model. We subjected animals to repeated daily injections of cocaine (20 mg/kg, i.p.; once daily for 5 days). We then stopped drug treatments for 2 weeks. After 2-week withdrawal, each animal received a challenge dose of cocaine (10 mg/kg, i.p.). Locomotor responses to this cocaine challenge were measured. As shown in Fig. 4A, cocaine challenge caused a greater motor response in cocaine-pretreated WT mice compared to saline-pretreated WT mice, indicating reliable expression of behavioral sensitization to cocaine. In ASIC1a−/− mice, cocaine challenge induced a smaller increase in motor activity in both saline- and cocaine-pretreated mice when compared to the corresponding values in WT mice. However, noticeably, the motor response to cocaine challenge remain significant greater in cocaine-pretreated ASIC1a−/− mice than in saline-pretreated ASIC1a−/− mice (left panel in Fig. 4B). Due to different responses in locomotor activity to challenge cocaine in saline-pretreated mice of three genotypes, we also used relative response in all genotypes to compare the behavioral sensitization to challenge cocaine. When the locomotor response of post 60 min in cocaine-pretreated mice was normalized by the motor response of post 60 min in saline-pretreated animals, motor stimulation was clearly greater in cocaine-pretreated mice than saline-pretreated mice, indicating the existence of behavioral sensitization (left panel in Fig. 4C). Moreover, the percent increase in cocaine-pretreated over saline-pretreated mice was significantly higher in ASIC1a−/− mice than WT mice (Fig. 4C; p < 0.01). In ASIC2−/− mice, the peak of the motor response to cocaine challenge was higher in cocaine-pretreated mice than in saline-pretreated mice (Fig. 4A, B). However, the motor response recovered in a faster rate in cocaine-pretreated mice. As a result, when total measurements in 60 min after drug injection were analyzed together, no change in motor responses was observed in cocaine-pretreated mice relative to saline-pretreated mice (left panel in Fig. 4B; p > 0.05). Similarly, there was no alteration in percent change in motor responses to cocaine challenge between cocaine- and saline-pretreated animals (left panel in Fig. 4C). We also compared the locomotor activity in mice after challenge injection of cocaine in two time period (see Fig. 4B; from 60 to 75 min and from 80 to 120 min), all three genotypes showed behavioral sensitization to cocaine in the first 15 min (from 60 to 75 min) after challenge injection of cocaine (middle panel in Fig. 4B). There were no significant changes in total distance traveled to cocaine in the rest of time period (from 80 to 120 min) in all genotypes (right panel in Fig. 4B). Furthermore, the percent increase of the first 15 min in cocaine-pretreated over saline-pretreated mice were higher in all genotypes (right panel in Fig. 4C). However, ASIC2−/− mice showed less sensitization to cocaine challenge when compared to WT and ASIC1−/− mice in the first 15 min after cocaine injection (right panel in Fig. 4C). There was no alteration in percent change in motor responses to cocaine challenge in the first 15 min between WT and ASIC1a−/− mice (right panel in Fig. 4C). Collectively, these data demonstrate that differential behavioral sensitization to cocaine challenge in ASIC1a and ASIC2 knockout mice.
Fig. 4.
Locomotor activity after cocaine-induced behavioral sensitization in WT, ASIC1a−/−, and ASIC2−/− mice. Daily cocaine (20 mg/kg, i.p.) or saline control injections began on day 1 until day 5 and sensitization in the form of distances traveled was measured on day 20 following a single challenge cocaine injection (10 mg/kg, i.p.). Locomotor activity was measured for the 60 min (control period) immediately prior to injection and the 90 min immediately following the 10 mg/kg challenge cocaine injection (i.p.). (A) Time courses of distances traveled of WT (left panel), ASIC1a−/− (middle panel), and ASIC2−/− (right panel) mice pretreated with saline (open circles) or cocaine (filled triangles) in the 150-min session. The first 60 min denote pre-injection activity. n = 21 to 28 per group. Data shown are the average total distances traveled given in 5-min intervals. (B) Total distances traveled in the total 60-min (left panel), first 15 min (middle panel, from 60 to 75 min) and following 45 min (right panel, from 80 to 120 min) after challenge cocaine injection for WT, ASIC1a−/−, and ASIC2−/− mice. n = 21 to 28 per group. Data shown are the average total distances traveled in the 60-min session after challenge cocaine injection. * p < 0.05; ** p < 0.01; *** p < 0.001; n.s. means not significant. (C) Relative amplitude of total distances traveled in the total 60-min (left panel) and first 15 min (right panel, from 60 to 75 min) session after challenge cocaine injection in WT, ASIC1a−/−, and ASIC2−/− mice. Mice pretreated with saline were used as control subjects. The relative amplitude represents the ratio of the average total distance traveled in 60 or 15 min by mice pretreated with cocaine divided by the average total distance traveled in 60 or 15 min by mice pretreated with saline. ** p < 0.01.; n.s. means not significant. n = 21 to 28 per group. Pre-Saline: mice pretreated with saline; Pre-Cocaine: mice pretreated with cocaine; WT: wild-type mice; ASIC1a KO: ASIC1a knockout mice; ASIC2 KO: ASIC2 knockout mice. Min: minutes.
DISCUSSION
ASIC1a and ASIC2 channels comprise the majority of ASICs localized to the brain (Waldmann et al., 1997; Wemmie et al., 2002; Askwith et al., 2004; Xiong et al., 2004; Chu et al., 2004; Zha et al., 2009; Sherwood et al., 2011). Several lines of evidence suggest the involvement of ASIC1a channels in learning/memory, fear conditioning, and depression-related behavior (Wemmie et al., 2002, 2003; Coryell et al., 2009). These channels appear subject to the modulation by ASIC2 channels (Askwith et al., 2004; Zha et al., 2009; Sherwood et al., 2011). In this study, we examined the differences in locomotor activity in WT, ASIC1a−/−, and ASIC2−/− mice in response to acute and chronic administration of the psychostimulant cocaine. The main findings of the current study include the following: (1) C57BL/6J-congenic ASIC1a−/− mice demonstrated reduced locomotor activity in response to acute cocaine administration when compared to WT mice, and (2) ASIC2−/− mice displayed decreased cocaine sensitization to chronic cocaine administration as compared to WT controls.
We first observed the baseline locomotor and jumping behavior of mice in a novel environment prior to any injection. ASIC1a−/− mice displayed increased locomotor activity in the first 5 min and exhibited highest frequency of jumps when compared to WT or ASIC2−/− mice. The mechanisms underlying the differences in baseline locomotor activity as well as jumping activity in ASIC1a and ASIC2 mutant mice are unclear. Further studies are needed to delineate their cellular and molecular mechanisms.
Our next finding is that ASIC1a−/− and ASIC2−/− mice demonstrated reduced and normal behavioral activity respectively in response to acute cocaine (5 – 20 mg/kg) stimulation when compared to WT. These results indicate that deletion of ASIC1a or ASIC2 gene differentially alters locomotor activity to acute cocaine injection.
The last finding is that, while ASIC1a−/− mice seemed to retain their sensitized locomotor responses to cocaine challenge, ASIC2−/− mice displayed significant less sensitization to cocaine challenge. This suggests that ASIC1a and ASIC2 play differential roles in cocaine sensitization. The functional importance of ASIC1a in the brain has been widely explored (Chu and Xiong, 2012); however, little research has focused on the function of ASIC2. Our present study demonstrates that ASIC2 channels in the brain are critical to cocaine sensitization.
Cocaine’s profound pharmacologic properties make it a strong psychostimulant and an addictive drug of abuse. Considerable evidence demonstrates that blockade of the dopamine transporter by cocaine and the subsequent elevation of extracellular dopamine primarily mediates the stimulating and rewarding effects of cocaine (Wise and Bozarth, 1987; Di Chiara & Imperato, 1988; Kuhar et al., 1991). The general consensus dictates that drugs of abuse can produce long-lasting adaptations in critical neural circuits and that these actions contribute to development and maintenance of drug addiction (Wolf et al., 2004; Kalivas & Xu, 2006; Kauer & Malenka, 2007; Kalivas, 2009; Sesack & Grace, 2010; Kalivas & Volkow, 2011; Wolf & Tseng, 2012). Given the far-reaching actions of cocaine, different receptors and ion channels at synaptic membrane may certainly contribute to the complex nature of drug addiction.
Earlier studies have shown that cocaine-induced locomotor activity was reduced in dopamine D2 receptor mutant mice (Baik et al., 1995; Kelly et al., 1998; Chausmer et al., 2002). More recently, mice lacking dopamine D2 autoreceptor displayed increased sensitivity to cocaine (Bello et al., 2011). Similar to mice lacking D2 autoreceptor, mice lacking dopamine D4 receptors were supersensitive to cocaine (Rubinstein et al., 1997). In this study, we revealed differential roles of ASIC1a and ASIC2 channels in regulating behavioral sensitivity to acute and chronic cocaine administration. Whether deleting ASIC1a and ASIC2 affects the dopamine receptor function is unclear. This could be an interesting question to be investigated in the future studies.
The molecular mechanisms for behavioral sensitization involve plastic changes in excitatory synapses on medium spiny neurons in the striatum. For example, several types of synaptic plasticity, such as long-term potentiation or depression, in the nucleus accumbens are subject to modifications in response to chronic stimulant exposure (Brebner et al., 2005; Kalivas & Hu, 2006; Kauer & Malenka, 2007; Grueter et al., 2012). Such modifications of excitatory synaptic transmission likely play important roles in drug-induced behavioral plasticity (Wolf et al., 2004; Grueter et al., 2012). Given the apparent abundance of ASIC1a and ASIC2 channels at synaptic sites, these channels are believed to participate in the drug-regulated synaptic plasticity. It is likely that ASICs act in concert with glutamate receptors to control synaptic and behavioral adaptations to cocaine. Our study represents an initial effort towards defining the functional role of ASIC1a and ASIC2 channels in processing stimulant effects. Future studies may help elucidate the precise roles of ASIC1a and ASIC2 channels in this event.
Highlights.
ASIC1a−/− mice showed highest frequency of jumps compared to WT and ASIC2−/− mice.
ASIC1a−/− mice displayed reduced locomotor responses to acute cocaine injection.
ASIC2−/− mice showed locomotor activity comparable to WT mice to acute cocaine.
ASIC2−/− mice showed less sensitization to challenge cocaine.
Acknowledgments
This project was supported by NIH grants DA031259 and DA010355, the University of Missouri Research Board, Saint Luke’s Hospital Foundation-Kansas City, and the University of Missouri-Kansas City School of Medicine Start-up funding to (X.P.C). We thank Drs. John Wemmie and Michael Welsh from the University of Iowa for providing ASIC1a and ASIC2 KO mice. We also thank Vibhav Reddy and Cameran Vakassi for their efforts in data collection during the behavioral studies.
Abbreviations
- ASIC1a
acid-sensing ion channel 1a
- ASIC2
acid-sensing ion channel 2
- ASICs
acid-sensing ion channels
- KO
knockout
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez de la Rosa D, Krueger SR, Kola A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546:77– 87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias RL, Sung ML, Vasylyev D, Zhang MY, Albinson K, Kubek K, Kagan N, Beyer C, Lin Q, Dwyer JM, Zaleska MM, Bowlby MR, Dunlop J, Monaghan M. Amiloride is neuroprotective in an MPTP model of Parkinson’s disease. Neurobiol Dis. 2008;31:334– 341. doi: 10.1016/j.nbd.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. Acid-sensing ion channel 2 (ASIC2) modulates ASIC1 H+-activated currents in hippocampal neurons. J Biol Chem. 2004;279:18296– 18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Nat Neurosci. 2011;14:1033–1040. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagini G, Babinski K, Avoli M, Marcinkiewicz M, Séguéla P. Regional and subunit-specific downregulation of acid-sensing ion channels in the pilocarpine model of epilepsy. Neurobiol Dis. 2001;8:45–58. doi: 10.1006/nbdi.2000.0331. [DOI] [PubMed] [Google Scholar]
- Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science. 2005;310:1340–1343. doi: 10.1126/science.1116894. [DOI] [PubMed] [Google Scholar]
- Chausmer AL, Elmer GI, Rubinstein M, Low MJ, Grandy DK, Katz JL. Cocaine-induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology. 2002;163:54–61. doi: 10.1007/s00213-002-1142-y. [DOI] [PubMed] [Google Scholar]
- Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Papasian CJ, Wang JQ, Xiong ZG. Modulation of acid-sensing ion channels: molecular mechanisms and therapeutic potential. Int J Physiol Pathophysiol Pharmacol. 2011;3:288–309. [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Xiong ZG. Physiological and pathological functions of acid-sensing ion channels in the central nervous system. Curr Drug Targets. 2012;13:263–271. doi: 10.2174/138945012799201685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coryell MW, Wunsch AM, Haenfler JM, Allen JE, Schnizler M, Ziemann AE, Cook MN, Dunning JP, Price MP, Rainier JD, Liu Z, Light AR, Langbehn DR, Wemmie JA. Acid-sensing ion channel-1a in the amygdala, a novel therapeutic target in depression-related behavior. J Neurosci. 2009;29:5381–5388. doi: 10.1523/JNEUROSCI.0360-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Poburko D, Frieden M. Regulation of plasma membrane calcium fluxes by mitochondria. Biochim Biophys Acta. 2009;1787:1383–1394. doi: 10.1016/j.bbabio.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13:1483– 1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48:635– 646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründer S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2:73–94. [PMC free article] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006– 11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A° resolution and low pH. Nature. 2007;449:316– 323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009;162:55–66. doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci. 2012;32:4080–4091. doi: 10.1523/JNEUROSCI.5021-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab. 2001;21:734–740. doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Hu XT. Exciting inhibition in psychostimulant addiction. Trends Neurosci. 2006;29:610– 616. doi: 10.1016/j.tins.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Rubinstein M, Phillips TJ, Lessov CN, Burkhart-Kasch S, Zhang G, Bunzow JR, Fang Y, Gerhardt GA, Grandy DK, Low MJ. Locomotor activity in D2 dopamine receptor-deficient mice is determined by gene dosage, genetic background, and developmental adaptations. J Neurosci. 1998;18:3470–3479. doi: 10.1523/JNEUROSCI.18-09-03470.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. Receptor for protons in the membrane of sensory neurons. Brain Res. 1981;214:150–154. doi: 10.1016/0006-8993(81)90446-7. [DOI] [PubMed] [Google Scholar]
- Kuhar MJ, Ritz MC, Boja JW. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci. 1991;14:299–302. doi: 10.1016/0166-2236(91)90141-g. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Mao LM, Wang W, Chu XP, Zhang GC, Liu XY, Yang YJ, Haines M, Papasian CJ, Fibuch EE, Buch S, Chen JG, Wang JQ. Stability of surface NMDA receptors controls synaptic and behavioral adaptations to amphetamine. Nat Neurosci. 2009;12:602–610. doi: 10.1038/nn.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Parelkar NK, Jiang Q, Chu XP, Guo ML, Mao LM, Wang JQ. Amphetamine alters Ras-guanine nucleotide-releasing factor expression in the rat striatum in vivo. Eur J Pharmacol. 2009;619:50–56. doi: 10.1016/j.ejphar.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein M, Phillips TJ, Bunzow JR, Falzone TL, Dziewczapolski G, Zhang G, Fang Y, Larson JL, McDougall JA, Chester JA, Saez C, Pugsley TA, Gershanik O, Low MJ, Grandy DK. Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell. 1997;90:991–1001. doi: 10.1016/s0092-8674(00)80365-7. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood TW, Lee KG, Gormley MG, Askwith CC. Heteromeric acid-sensing ion channels (ASICs) composed of ASIC2b and ASIC1a display novel channel properties and contribute to acidosis-induced neuronal death. J Neurosci. 2011;31:9723–9734. doi: 10.1523/JNEUROSCI.1665-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Winter OC, Wemmie JA. Acid-sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Devel. 2009;12:693–704. [PMC free article] [PubMed] [Google Scholar]
- Suman A, Mehta B, Guo ML, Chu XP, Fibuch EE, Mao LM, Wang JQ. Alterations in subcellular expression of acid-sensing ion channels in the rat forebrain following chronic amphetamine administration. Neurosci Res. 2010;68:1–8. doi: 10.1016/j.neures.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386:173– 177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418– 424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23:5496– 5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Jr, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34:463– 477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ. Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29:578– 586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong HK, Bauer PO, Kurosawa M, Goswami A, Washizu C, Machida Y, Tosaki A, Yamada M, Knöpfel T, Nakamura T, Nukina N. Blocking acid-sensing ion channel 1 alleviates Huntington’s disease pathology via an ubiquitin-proteasome system-dependent mechanism. Hum Mol Genet. 2008;17:3223– 3235. doi: 10.1093/hmg/ddn218. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Pignataro G, Li M, Chang SY, Simon RP. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr Opin Pharmacol. 2008;8:25– 32. doi: 10.1016/j.coph.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wemmie JA, Price M, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neurosci. 2009;29:8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103:16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GC, Mao LM, Wang JQ, Chu XP. Upregulation of acid-sensing ion channel 1 protein expression by chronic administration of cocaine in the mouse striatum in vivo. Neurosci Lett. 2009;459:119–122. doi: 10.1016/j.neulet.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Allen JE, Dahdaleh NS, Drebot II, Coryell MW, Wunsch AM, Lynch CM, Faraci FM, Howard MA, Welsh MJ, Wemmie JA. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012– 1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816– 822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]