Abstract
Study Design
Retrospective cohort analysis of Medicare claims for 2006-2009.
Objective
To examine whether interspinous distraction procedures are used selectively in patients with more advanced age or comorbidity; and whether they are associated with fewer complications, lower costs, and less revision surgery than laminectomy or fusion surgery.
Summary of Background Data
A manufacturer-sponsored randomized trial suggested an advantage of interspinous spacer surgery over non-surgical care, but there are few comparisons with other surgical procedures. Furthermore, there are few population-based data evaluating patterns of use of these devices.
Methods
We used Medicare inpatient claims data to compare age and comorbidity for patients with spinal stenosis having surgery (n=99,084) with (1) an interspinous process spacer alone; (2) laminectomy and a spacer; (3) decompression alone; or (4) lumbar fusion (1-2 level). We also compared these four groups for cost of surgery and rates of revision surgery, major medical complications, wound complications, mortality, and 30-day readmission rates.
Results
Patients who received spacers were older than those receiving decompression or fusion, but had little evidence of greater comorbidity. Patients receiving a spacer alone had fewer major medical complications than those undergoing decompression or fusion surgery (1.2% versus 1.8% and 3.3% respectively), but had higher rates of further inpatient lumbar surgery (16.7% versus 8.5% for decompression and 9.8% for fusion at 2 years). Hospital payments for spacer surgery were greater than for decompression alone, but less than for fusion procedures. These associations persisted in multivariate models adjusting for patient age, sex, comorbidity score, and previous hospitalization.
Conclusions
Compared to decompression or fusion, interspinous distraction procedures pose a trade-off in outcomes: fewer complications for the index operation, but higher rates of revision surgery. This information should help patients make more informed choices, but further research is needed to define optimal indications for these new devices.
Keywords: Spinal stenosis, interspinous process spacer, x-stop, revision surgery, complications
Introduction
The interspinous process spacer is a new technology that offers patients with spinal stenosis a less invasive alternative to laminectomy, but comparative data on safety performance of these alternatives in actual practice are lacking. The X-Stop© was approved by the FDA in late 2005 as an alternative to non-surgical care, and similar devices have followed. These devices share the mechanical goal of distracting the spinous processes to provide symptom relief among patients who experience relief in spine flexion. For the typically older population with spinal stenosis, age and comorbidity increase the risks of surgical complications, so a less invasive option for symptom relief is attractive.
Early evaluation of these spacers included a randomized trial1,2 comparing devices to non-surgical therapy, suggesting at least modest advantages in symptoms and function. Some subsequent studies without a comparison group suggested somewhat less favorable results.3 However, randomized trials comparing the spacers to conventional decompression or fusion procedures have not been available. Furthermore, there have been few population-based data for evaluating patterns of use or safety outcomes of these new devices.
We therefore undertook an analysis of Medicare claims data to address these study questions:
Are spacers being used selectively in patients with more advanced age or comorbidity?
Are spacers associated with fewer medical and wound complications than conventional laminectomy or fusion procedures?
Is the likelihood of revision surgery different with use of spacers than with conventional operations for spinal stenosis?
Methods
Data Source
Data were obtained from the Medicare Provider Analysis and Review, or MedPAR database for the years 2005-2009. This uses procedure codes from the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), and includes all Medicare hospital claims. We included only Medicare patients who were eligible through the Old Age and Survivors Insurance program, and not those eligible because of Social Security Disability Income or end-stage renal disease. We also excluded patients enrolled in a health maintenance organization (HMO) because such patients may not have complete claims data for individual episodes of care. This is a common exclusion in studies of Medicare data4,5.
Patient selection
We identified patients aged 66 years and older with any diagnosis of spinal stenosis. This provided at least 1 year of prior Medicare eligibility for most patients, to allow us to identify and exclude those with recent prior spine surgery. We then identified patients with a surgical procedure indicating placement of an interspinous process spacer or any combination of discectomy, laminectomy and fusion. We defined an index operation as the first operation for an individual patient that occurred in 2006-2009. We required that either the diagnosis or the procedure codes specified a lumbar location in the spine. We excluded patents if any diagnosis at the index hospitalization indicated cancer, drug abuse, HIV infection, spinal cord injuries, vehicular accident, spinal infection, inflammatory spondyloarthropathies, vertebral fractures or dislocations, or cervical or thoracic spine procedures. We excluded patients with fusion of 3 or more disc levels, dynamic stabilizing devices, or less than 30 days of available follow-up to identify postoperative complications (i.e., patients with surgery near the very end of the observation period). However, patients who died within the 30-day interval were counted.
We further excluded patients with evidence of previous lumbar surgery. We identified patients with lumbar surgery prior to the index operation in two ways. First, we identified diagnosis or procedure codes implying previous surgery. Examples were postlaminectomy syndrome, aftercare involving an internal fixation device, refusion, reopening of a laminectomy site, or removal of a device from bone. Second, we searched hospitalizations in the previous year to identify previous lumbar spine procedures. For our primary analysis, we also excluded patients with a diagnosis of spondylolisthesis or scoliosis. In a sensitivity analysis, we examined the results of including patients with a secondary diagnosis of spondylolisthesis in addition to lumbar stenosis (additional n=32,020 without other exclusions).
Categorizing Surgical Procedures
We defined broad categories of spine surgery, which we labeled spacer, decompression or fusion. A fourth group was added when we found that there were substantial numbers of patients (n= 1644) who received both a spacer and a decompression. Decompression included any procedure with discectomy or laminectomy codes without fusion. A fusion was any procedure involving fusion procedure codes, with or without a decompression. We excluded a much smaller group (n= 183 without other exclusions) who received both a spacer and a fusion procedure.
Measures of Comorbidity
We used a modification of the comorbidity index of Quan and colleagues,6 which updated our version of the Charlson index.7 We modified their index by removing codes that could represent postoperative complications if listed at the index hospitalization. Examples included codes for congestive heart failure, acute myocardial infarction, acute stroke, and renal dialysis. However, the full Quan list of conditions was used to search for diagnoses during any hospitalization in the year prior to the index hospitalization. Thus, the modified score included comorbid conditions listed in previous hospitalizations and selected conditions identified in the index hospitalization. In addition, we tabulated the number of hospitalizations for one year prior to the index hospitalization (other than those for spine surgery), as a crude additional measure of illness burden.
Reoperations after the index procedure
With the same procedure codes used for selecting patients, we identified lumbar spine operations that occurred after discharge from the index operation. For analysis purposes, we counted only the first re-occurrence of lumbar surgery. We use the term “repeat surgery” or “reoperation” to indicate any of these subsequent operations, even though the nature of the surgery may have been different from the index operation, and we cannot know whether it involved the same spinal levels. We tabulated the diagnosis and procedure codes recorded at these reoperations. Removals of spacer devices that occurred as ambulatory procedures would be missed in our analysis, which identified only inpatient reoperations.
Complications
As in an earlier study,8 we considered 3 categories of complications: major medical complications, wound complications, and mortality. These are generic surgical complications, not specific for lumbar spine surgery. As major medical complications, we included procedure codes for cardiopulmonary resuscitation or repeat post-operative endotracheal intubation and mechanical ventilation. We also included diagnosis codes for cardiorespiratory arrest, acute myocardial infarction, respiratory failure, pulmonary embolism, bacterial pneumonia, aspiration pneumonia, pneumonia with unknown organism, and stroke, excluding late effects. These complications have a major impact on health and are generally more consistently coded than minor complications.8,9 Complications were counted if they were recorded during the index hospitalization, or any rehospitalization within 30 days of discharge. Complications that did not result in hospitalization were not counted.
Wound complications included ICD-9 codes for hemorrhage, hematoma or seroma complicating a procedure; disruption of operation wound; non-healing surgical wound; postoperative infection; and other infection. We also counted events with a procedure code for “excisional debridement of wound, infection or burn,” and those classified in the Diagnosis-Related Group for wound debridement and skin graft.
Using a file that identified date of death, we calculated mortality within 30 days of hospital discharge, including in-hospital death. We also calculated a rate of “life-threatening complications”, which included both mortality within 30 days and the major medical complications described above (not mutually exclusive).
Healthcare utilization
MedPAR includes hospital length-of-stay. We also examined rehospitalizations within 30 days because short-term rehospitalizations are a target for quality improvement.5 Early readmissions may suggest complications, poor discharge planning, inadequate outpatient follow-up, or other problems. Finally, we recorded the amount of Medicare payment (not charges) for the hospital bill, exclusive of professional fees, which are not included in Part A claims.
Analysis
The proportions of patients with complications or rehospitalizations among subgroups were compared using chi-square analyses for bivariate analyses, and logistic regression for multivariate analyses. In regressions, these events were modeled as a function of age, gender, comorbidity, previous hospitalization and type of index surgical procedure.
Length of stay and hospital payments were compared using t-tests or analysis of variance, then modeled in linear regressions. We used untransformed payments, because mean estimates are generally similar to those of approaches that better account for skewed data.10-13 In large datasets, means often prove to be sufficient.11 Significance tests were 2-sided, with an alpha of 0.05.
To study reoperations, we used time-to-an-event statistical methods (“survival” analysis), with the event being a second spinal operation. We focused on comparing the cumulative probability of reoperation for the 4 broad types of surgery performed at the index operation. The time to reoperation was calculated as the number of days between hospital discharge for the index operation and the date of admission for the first subsequent lumbar spine operation.
We used proportional hazards models to examine the cumulative probability of reoperation, adjusted for age, gender, comorbidity score, and previous hospitalization. We assessed the proportional hazards model with hazard plots and tests of Schoenfeld residuals. We found that the proportionality assumption was weakly violated in comparing fusion surgery with the spacer alone. This occurred because of crossovers in the survival curves, especially in the first 1.5 years of follow-up. We therefore included a time-varying coefficient to examine the probability of reoperation in each year of follow-up. The final model fit was confirmed using the Cox-Snell residuals plot for goodness-of-fit; significance testing was performed with a two-sided alpha of 0.05. Statistical analysis was performed with StataMP software, version 11 (Statacorp, College Station, Texas).
Results
Patient and Procedure Characteristics
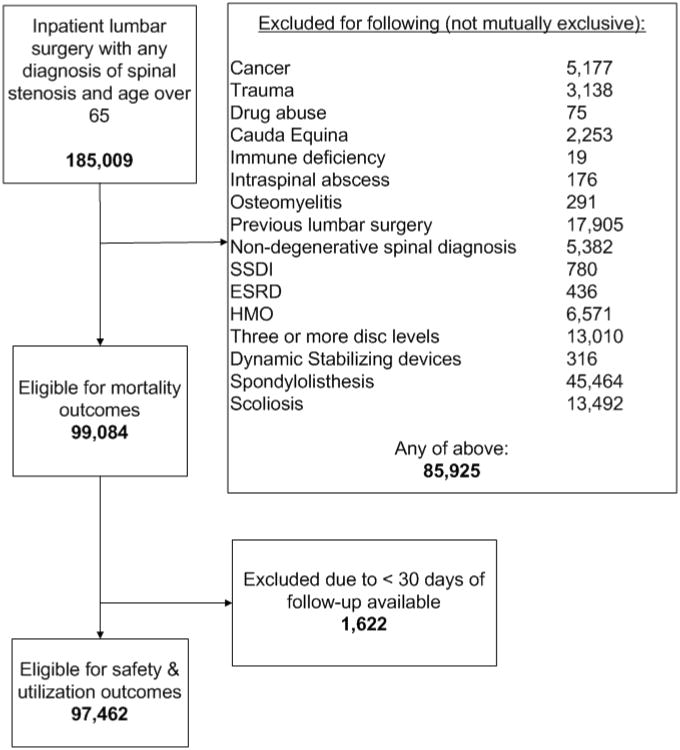
There were 185,009 inpatient operations that included a diagnosis of lumbar stenosis during 2006-2009. There were 85,925 (46%) with one or more exclusion factors, leaving 99,084 patients included in the analysis (Figure 1). There were 1,607 patients with spondylolisthesis who received a spacer, and 488 with a diagnosis of scoliosis who received a spacer, but these were not included in our primary analysis. Because there were 1,622 patients with fewer than 30 days of follow-up (due to index surgery at the end of 2009), we used 97,462 to calculate complications and rehospitalizations.
Figure 1.
Flow diagram for exclusions and analysis.
Among the 99,084 eligible patients, average age was 74.6, and 48.7% were women. There were 93.8% Whites, 3.5% Blacks, and 2.7% other races or ethnicities. Among the index operations, there were 3,965 (4.0%) with a spacer insertion alone, 1,644 (1.7%) with spacer insertion and a decompression, 76,520 (77.2%) with a decompression alone, and 16,955 (17.1%) that included fusion procedures (Table 1).
Table 1. Characteristics of patients undergoing 4 procedure types. Tabled figures are numbers of patients (column %).
| Interspinous process spacer alone | Interspinous process spacer with decompression | Decompression alone | Fusion | All patients | P-value for differences between groups | ||
|---|---|---|---|---|---|---|---|
| n | 3,965 | 1,644 | 76,520 | 16,955 | 99,084 | ||
| Mean age, yr (SD) | 76.1 (6.1) | 75.6 (6.1) | 74.8 (5.9) | 73.6 (5.4) | 74.6 (5.8) | <0.001 | |
| Sex, %* | Male | 1,925 (48.6) | 810 (49.3) | 40,473 (51.4) | 7,580 (44.7) | 50,788 (51.3) | <0.001 |
| Female | 2,037 (51.4) | 833 (50.7) | 36,023 (47.1) | 9,370 (55.3) | 48,263 (48.7) | ||
| Race | White | 3,733 (94.2) | 1,529 (93.0) | 71,914 (94.0) | 15,715 (92.7) | 92,891 (93.8) | <0.001 |
| Black | 119 (3.0) | 60 (3.7) | 2,548 (3.3) | 782 (4.6) | 3,509 (3.5) | ||
| Other | 110 (2.8) | 54 (3.3) | 2,034 (2.7) | 453 (2.7) | 2,651 (2.7) | ||
| Quan comorbidity score | 0 | 2,096 (52.9) | 810 (49.3) | 41,262 (54.0) | 8,764 (51.7) | 52,932 (53.4) | <0.001 |
| 1 | 1,207 (30.4) | 559 (34.0) | 23,348 (30.5) | 5,452 (32.2) | 30,566 (30.9) | ||
| 2 | 662 (16.7) | 275 (16.7) | 11,910 (15.6) | 2,739 (16.2) | 15,586 (15.7) | ||
| Hospitalized in previous year | No | 3,245 (81.8) | 1,331 (81.0) | 62,945 (82.3) | 13,840 (81.6) | 81,361 (82.1) | 0.170 |
| Yes | 720 (18.2) | 313 (19.0) | 13,575 (17.7) | 3,115 (18.4) | 17,723 (17.9) | ||
| Diabetes | No | 3,035 (76.5) | 1,196 (72.8) | 58,359 (76.3) | 12,714 (75.0) | 75,304 (76.0) | <0.001 |
| Yes | 930 (23.5) | 439 (27.2) | 18,161 (23.7) | 4,241 (25.0) | 23,780 (24.0) | ||
| Chronic lung disease | No | 3,317 (83.7) | 1,364 (83.0) | 64,331 (84.1) | 14,043 (82.9) | 83,055 (83.8) | <0.001 |
| Yes | 648 (16.3) | 280 (17.0) | 12,189 (15.9) | 2,912 (17.1) | 16,029 (16.2) | ||
2,784 patients (2.8%) had missing values for gender
Patients who received a spacer alone were significantly older than patients who received either a decompression or fusion. However, the proportion hospitalized in the previous year was similar among groups (Table 1). Although there were significant differences among the surgery groups with regard to comorbidity score and the proportions with diabetes or chronic lung disease, these were not consistently more severe in the spacer groups compared to the decompression or fusion groups.
Complications and Healthcare Utilization
Complications were generally lower in the spacer groups (with or without decompression) than in the decompression or fusion groups (Table 2). Decompression alone was intermediate between the spacer groups and fusion on each measure of complications. For example, life-threatening complications were observed in 1.2% of spacer alone patients, 1.8% of decompression patients, and 3.3% of fusion patients (p<0.001; chisquare). Rehospitalization within 30 days followed the same pattern, occurring in 4.5% of spacer patients, 6.6% of decompression patients, and 9.4% of fusion patients (p<0.001; chi-square). The group with spacer plus decompression was generally intermediate between the groups with spacer alone and decompression alone with regard to complication measures (Table 2).
Table 2.
Complications and utilization according to type of index operation. Tabled figures are numbers (%) of patients with a particular complication, or mean values.
| Interspinous process spacer | Interspinous spacer plus decompression | Decompression alone | Fusion | P-value | |
|---|---|---|---|---|---|
| N for measures that include mortality | 3965 | 1644 | 76520 | 16955 | |
| N for safety & utilization measures | 3912 | 1617 | 75310 | 16623 | |
| Wound complications, 30 days | 30 (0.8) | 21 (1.3) | 1343 (1.8) | 548 (3.3) | <0.001 |
| Cardiopulmonary or stroke complications, 30 days | 39 (1.0) | 21 (1.3) | 1192 (1.6) | 473 (2.9) | <0.001 |
| Death within 30 days | 7 (0.18) | 7 (0.43) | 240 (0.31) | 102 (0.60) | <0.001 |
| Life-threatening complications (either of previous 2 rows) | 45 (1.2) | 25 (1.6) | 1351 (1.8) | 553 (3.3) | <0.001 |
| All cause rehospitalization within 30 days | 175 (4.5) | 92 (5.7) | 4985 (6.6) | 1568 (9.4) | <0.001 |
| OR,‡ wound complications, 30days (95% CI)* | 0.42 (0.29, 0.60) | 0.70 (0.46, 1.09) | 1.00 (ref) | 1.85 (1.67, 2.05) | |
| OR for rehospitalization | 0.62 (0.53, 0.73) | 0.81 (0.65, 1.00) | 1.00 (ref) | 1.53 (1.44, 1.63) | |
| OR,‡ Life-threatening complications (95%CI)* | 0.59 (0.43, 0.79) | 0.79 (0.53, 1.18) | 1.00 (ref) | 2.03 (1.83, 2.24) | |
| Mean hospital stay, d (sd) | 1.5 (1.5) | 1.8 (2.0) | 2.7 (2.4) | 4.1 (3.3) | <0.001 |
| Mean Payment, $ (sd) | 8,312 (3035) | 8,997 (4434) | 5925 (3149) | 20,101 (9117) | |
| Adjusted length of stay† (95% CI) | 1.44 (1.36,1.51) | 1.76 (1.63, 1.88) | 2.67 (2.65 – 2.69) | 4.12 (4.09 – 4.16) | <0.001 |
| Adjusted Payment,$† (95% CI) | 8,227 (8073, 8373) | 8,909 (8679, 9139) | 5,923 (5886, 5955) | 20,140 (20,068, 20212) | <0.001 |
Logistic regression model adjusting for age, sex, comorbidity score, and number of hospitalizations in previous year
Linear regression models adjusting for age, sex, comorbidity score, and number of hospitalizations in previous year; payments do not include professional fees.
OR= odds ratio
The mean hospital length of stay for patients receiving a spacer alone was shorter than for patients receiving a decompression, even after adjusting for other factors (1.44 days vs. 2.67 days). All of the associations of complications, 30-day rehospitalization, and length of stay persisted after multivariate adjustment for age, sex, comorbidity score, and number of previous hospitalizations (Table 2).
Adjusted mean hospital payments for patients receiving spacers ($8,227 for spacer alone) were higher than for patients receiving decompression alone ($5,923); patients receiving fusion procedures had the highest payments ($20,140) (Table 2). Patients who received a spacer alone were more likely to be discharged to home with self-care (83.8%) than were patients who received decompression alone (71.1%) or a fusion procedure (49.6%) (p<0.001). Patients who received a spacer plus decompression were intermediate in their likelihood of discharge to selfcare (78.8%). The vast majority of other discharges were to skilled nursing facilities, home health services, or inpatient rehabilitation programs.
Reoperations
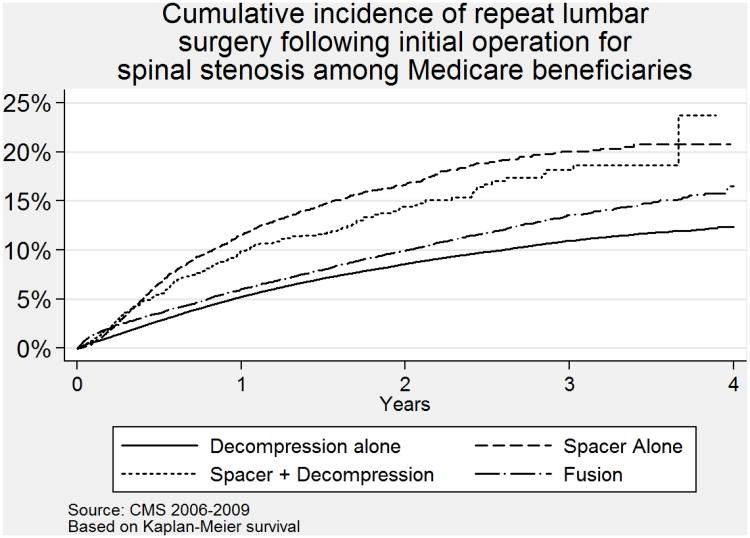
Although patients receiving spacers had the lowest rate of complications, they had the highest rate of revision surgery (Table 3). At 2 years, for example, the cumulative probability of reoperation was 16.7% among those receiving a spacer; 8.5% among those having a decompression, and 9.8% among those having a fusion procedure (p<0.001; chi-square). In a logistic regression model, after adjusting for age, sex, comorbidity score, and previous hospitalizations, these associations persisted (Table 3).
Table 3. Reoperations according to type of index operation.
| Interspinous process spacer only | Spacer plus decompression | Decompression alone | Fusion | ||
|---|---|---|---|---|---|
| Number of reoperations (%) | At 1 year | 383/3,318 (11.5%) | 124/1,230 (10.1%) | 3,100/59,892 (5.2%) | 747/12,528 (6.0%) |
| At 2 years | 397/2,372 (16.7%)‡ | 91/619 (14.7%)‡ | 3,575/41,969 (8.5%)‡ | 823/8,392 (9.8%) | |
| Odds ratio (95% CI) for reoperation* | At 1 year | 2.45 (2.2, 2.7) | 2.09 (1.7, 2.5) | 1.00 (ref) | 1.14 (1.05, 1.23) |
| At 2 years | 2.25 (2.0, 2.5) | 1.93 (1.5, 2.4) | 1.00 (ref) | 1.13 (1.04, 1.22) | |
| Hazard ratio† (95% CI) for reoperation, using all available follow-up | t < 1 year | 2.36 (2.1,2.6) | 2.00 (1.7, 2.4) | 1.00 (ref) | 1.14 (1.06, 1.22) |
| 1<t<2 | 1.77 (1.5,2.1) | 1.40 (1.0, 1.9) | 1.0 (ref) | 1.14 (1.02, 1.27) | |
| t>2 | 1.70 (1.3,2.2) | 1.84 (1.1, 3.0) | 1.00 (ref) | 1.54 (1.35, 1.76) | |
Logistic regression adjusting for age, sex, comorbidity score, and previous hospitalization
Proportional Hazards model, adjusting for age, sex, comorbidity score, and previous hospitalization
Note that 2 year reoperation rates are based on smaller patient numbers than 1 year rates, because of available time of follow-up.
The proportional hazards model with time-varying coefficients confirmed these associations, with hazard ratios generally similar to the odds ratios from logistic regression. Taking decompression as the reference group, the hazard ratio for the spacer alone group having a reoperation within the first year was 2.4 (95% CI 2.1, 2.6), and the hazard ratio for spine fusion was 1.14 (95% CI 1.06, 1.22). The likelihood of reoperation within the first year was similarly high for patients receiving a spacer plus decompression (OR 2.0; 95% CI 1.7, 2.4) (Table 3). The “survival” curves (cumulative probability of reoperation) are presented visually in Figure 2.
Figure 2.
Cumulative incidence of reoperation following index operation for lumbar spinal stenosis among Medicare beneficiaries, 2006-2008.
Among the 397 patients in the spacer only group who had a second operation, 35% underwent a fusion procedure; 52% had a decompression procedure; and 13% had other operations, mostly coded as device revisions, replacements, reinsertions or removals.
In a sensitivity analysis, we examined the results of including patients with a diagnosis of spondylolisthesis in addition to lumbar stenosis (total n=131,104 without other exclusions). The 2-year reoperation rate for spacer alone (16.7%) or for spacer plus decompression (14.4%) remained well above that for decompression alone (8.6%). Including patients with spondylolisthesis lowered the reoperation rate for fusion procedures to 8.5%.
Discussion
Interspinous process spacer procedures were used with patients who were slightly older on average than patients who underwent decompression alone or fusion procedures. However, judging from comorbidity scores and recent hospitalizations, they may not have had a greater burden of medical illness. A substantial fraction of spacer procedures (29%) involved decompression surgery as well. The major findings of this analysis are that, as expected, surgery with a spacer alone had a lower rate of major medical complications and wound complications than either decompression or fusion for patients with spinal stenosis. However, it was also associated with a substantially greater likelihood of requiring revision surgery. Our estimate of revisions may be low, because we could not identify spacer removals that might have been performed as outpatient procedures. Also, some patients may refuse further surgery even when the device is unsuccessful. Payments for spacer procedures exceeded those of decompression alone, but were substantially below those of fusion procedures.
Thus, patients and surgeons face a trade-off in the choice of surgical procedures for spinal stenosis. Complication rates with spacers appear lower than for conventional, more invasive surgery, but reoperation rates are higher. We note that the reoperation rate for spacers in our study (16.8% at 2 years) was also substantially higher than reoperation rates observed for spinal stenosis in the Spine Patient Outcomes Research Trial (SPORT) (7.8% overall at 2 years), for patients mostly having decompression surgery (89%). Thus, the spacer may be a useful option for patients at particularly high risk from surgery. However, for patients of average risk, and with substantial expected longevity, the higher reoperation rate with spacers may argue in favor of conventional decompression surgery.
Because interspinous process spacers are a relatively new technology, the indications and even the design of these devices are likely to evolve. However, our results emphasize the importance of careful evaluation at each step in this evolution. Because they are new, these devices also raise questions about the “learning curve” in acquiring surgical expertise. Complication rates are likely to fall with greater experience, but are low even in these early stages of use. Whether the need for revision surgery will fall with greater experience remains to be seen.
Like other studies using Medicare claims,8,14 this study had some important advantages. It included all eligible Medicare patients having surgery for spinal stenosis in the study years, not selected patients, surgeons, or centers. Data on repeat hospitalizations and surgery were virtually complete, because all patients had insurance coverage, and revisions performed in hospitals other than the original hospital could be identified.
There are some important limitations to this study, as well. We were not able to identify and study patients who had spacers inserted as an outpatient procedure, though we believe most operations in these early years of use were performed on an inpatient basis. Reimbursement incentives and unpublished data from a single state (California) support this belief. We were not able to identify device-specific complications related to interspinous process spacers, such as device dislocations or spinous process fractures.15,16 Some of these events are likely to have resulted in repeat surgery, which we captured. However, because we examined only inpatient claims, we could have missed spacer removal procedures that were performed on an ambulatory surgery basis. Other investigators have suggested that some of the device complications may be related to specific anatomic variants, which we could not identify16.
An important limitation of our observational study is potential confounding by indication, a problem avoided in randomized trials. Patients selected for spacer insertion may systematically differ from those selected for other types of procedures. However, surgeons vary substantially in deciding whether to perform surgery and in their choice of procedures, even for similar patients.17,18 Thus, the differences we observed among groups may result from surgeons' procedure preferences as well as patient characteristics.
Another limitation is that diagnoses and procedures may be miscoded, though the data are used for billing and are subject to audit. Comparisons with medical records suggest that surgical procedures and spine operations in particular are generally coded accurately,19-21 although the ICD-9 system lacks detail.22 Diagnosis coding may be more variable. We were surprised that there was little evidence of greater comorbidity among patients who received spacers, and this may in part be due to limitations in coding practice. Also, if secondary diagnoses are not consistently coded, our exclusion of conditions such as scoliosis and spondylolisthesis may be incomplete.
Our ability to identify surgery prior to the index operation was imperfect, and previous surgery may be unapparent from diagnosis or procedure codes at the index operation.23 This was our rationale for examining a previous year of claims to identify prior surgery, rather than relying solely on coding at the index operation. Even so, we likely missed some more distant previous operations. Finally, we are unable to assess pain relief or functional recovery from these claims data, though these outcomes may partly correlate with revision surgery rates.
Our findings highlight trade-offs in outcomes and important financial stakes in the use of interspinous process spacers. Both device costs and reoperation costs may be important. Thus, the results also highlight the need for more thorough evaluation of this new technology. Comparisons both to more invasive and less invasive treatment options are appropriate. Furthermore, the use of interspinous process spacers in conjunction with other procedures (decompression or fusion) requires closer investigation. Such studies should examine symptom relief and functional recovery as well as complication and revision surgery rates. Finally, costeffectiveness analyses, considering index procedure costs, complication costs, and reoperation costs, may help to identify when spacer operations offer the greatest value. Only with such additional study will the optimal indications for this new technology become clear.
Key Points.
Randomized trials have compared use of interspinous spacers for lumbar stenosis with non-operative care, but not with other surgical procedures. Using Medicare data, we compared complications and repeat surgery rates for patients with lumbar stenosis who received a spacer alone; a spacer plus decompression; decompression alone; or a procedure involving fusion.
Patients receiving a spacer alone had fewer major medical complications than those undergoing decompression or fusion surgery (1.2% versus 1.8% and 3.3% respectively), but had higher rates of revision surgery at 1 or 2 years (16.7% versus 8.5% for decompression and 9.8% for fusion at 2 years).
Hospital payments for surgery involving spacers were greater than for decompression alone, but less than for fusion procedures.
Use of interspinous process spacers poses a trade-off in outcomes: fewer complications for the index operation, but higher rates of subsequent lumbar surgery.
Acknowledgments
Supported by Grants # 1R01AR054912-01A2, NIH/NIAMS, 1 UL1 RR024140-01, NIH/NCRR, NIH/NIA RC1AG036268, and by Grant # R01HS018405 from the Agency for Health Care Policy Research.
References
- 1.Zucherman JF, Hsu KY, Hartjen CA, et al. A prospective randomized multi-center study for the treatment of lumbar spinal stenosis with the X STOP interspinous implant: 1-year results. Eur Spine J. 2004;13:22–31. doi: 10.1007/s00586-003-0581-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zucherman JF, Hsu KY, Hartjen CA, et al. A multicenter, prospective, randomized trial evaluating the X STOP interspinous process decompression system for the treatment of neurogenic intermittent claudication: Two-year follow-up results. Spine. 2005;30:1351–1358. doi: 10.1097/01.brs.0000166618.42749.d1. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui M, Smith FW, Wardlaw D. One year results of X Stop interspinous implant for the treatment of lumbar spinal stenosis. Spine. 2007;32:1345–8. doi: 10.1097/BRS.0b013e31805b7694. [DOI] [PubMed] [Google Scholar]
- 4.Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon EE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464–474. doi: 10.1056/NEJMoa0707348. [DOI] [PubMed] [Google Scholar]
- 5.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 6.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303:1259–1265. doi: 10.1001/jama.2010.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawthers AG, McCarthy E, Davis RB, Teterson LE, Palmer RH, Iezzoni LI. Identification of In-hospital complications from claims data: is it valid? Medical Care. 2000;38:785–795. doi: 10.1097/00005650-200008000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ. 2004;23:525–542. doi: 10.1016/j.jhealeco.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 12.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic evaluation of clinical trials. Oxford University Press; New York: 2007. [Google Scholar]
- 13.Duan N. Smearing estimate: a nonparametric retransformation method. J Am Stat Assoc. 1983;78:605–610. [Google Scholar]
- 14.Deyo RA, Martin BI, Kreuter W, Jarvik JG, Angier H, Mirza SK. Revision surgery following operations for lumbar stenosis. J Bone Joint Surg Am. 2011;93:1979–1986. doi: 10.2106/JBJS.J.01292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DH, Tantorski M, Shaw J, et al. Occult spinous process fractures associated with interspinous process spaces. Spine. 2011;36:E1080–5. doi: 10.1097/BRS.0b013e318204066a. [DOI] [PubMed] [Google Scholar]
- 16.Barbagallo GMV, Olindo G, Corbino L, Albanese V. Analysis of complications in patients treated with the X-Stop interspinous process decompression system: proposal for a novel anatomic scoring system for patient selection and review of the literature. Neurosurgery. 2009;65:111–120. doi: 10.1227/01.NEU.0000346254.07116.31. [DOI] [PubMed] [Google Scholar]
- 17.Irwin ZN, Hilibrand A, Gustavel M, et al. Variation in surgical decision making for degenerative spinal disorders. Part I: lumbar spine. Spine. 2005;30:2208–2213. doi: 10.1097/01.brs.0000181057.60012.08. [DOI] [PubMed] [Google Scholar]
- 18.Katz JN, Lipson SJ, Lew RA, et al. Lumbar laminectomy alone or with instrumented or noninstrumented arthrodesis in degenerative lumbar spinal stenosis. Patient selection, costs, and surgical outcomes. Spine. 1997;22:1123–1131. doi: 10.1097/00007632-199705150-00012. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Taylor VM, Diehr P, Conrad D, Cherkin DC, Ciol M, Kreuter W. Analysis of automated administrative and survey databases to study patterns and outcomes of care. Spine. 1994;185:2083S–2091S. doi: 10.1097/00007632-199409151-00011. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez LF, Thisted R. Using a national health care data base to determine surgical complications in community hospitals: lumbar discectomy as an example. Neurosurgery. 1989;25:218–225. doi: 10.1097/00006123-198908000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Fisher ES, Whaley FS, Krushat M, Malenka DJ, Fleming C, Baron JA, Hsia DC. The accuracy of Medicare's hospital claims data: progress has been made, but problems remain. Am J Public Health. 1992;82:243–248. doi: 10.2105/ajph.82.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faciszewski T, Jensen R, Berg RL. Procedural coding of spinal surgeries (CPT-4 versus ICD-9-C) and decisions regarding standards: a multicenter study. Spine. 2003;28:502–7. doi: 10.1097/01.BRS.0000048671.17249.4F. [DOI] [PubMed] [Google Scholar]
- 23.Faciszewski T, Broste SK, Fardon D. Quality of data regarding diagnoses of spinal disorders in administrative databases. A multicenter study. J Bone Joint Surg Am. 1997;79(10):1481–8. doi: 10.2106/00004623-199710000-00004. [DOI] [PubMed] [Google Scholar]