Abstract
Secretion by neutrophils contributes to acute inflammation following injury or infection. Vimentin has been shown to be important for secretion by neutrophils but little is known about its dynamics during secretion, which is regulated by cyclin-dependent kinase 5 (Cdk5). In this study, we sought to examine the vimentin dynamics and its potential regulation by Cdk5 during neutrophil secretion. We show that vimentin is a Cdk5 substrate that is specifically phosphorylated at Ser56. In response to neutrophil stimulation with GTP, vimentin Ser56 was phosphorylated and colocalized with Cdk5 in the cytoplasmic compartment. Vimentin pSer56 and Cdk5 colocalization was consistent with coimmunoprecipitation from stimulated cells. Vimentin Ser56 phosphorylation occurred immediately after stimulation, and a remarkable increase in phosphorylation was noted later in the secretory process. Decreased GTP-induced vimentin Ser56 phosphorylation and secretion resulted from inhibition of Cdk5 activity by roscovitine or olomoucine or by depletion of Cdk5 by siRNA, suggesting that GTP-induced Cdk5-mediated vimentinSer56 phosphorylation may be related to GTP-inducedCdk5-mediated secretion by neutrophils. Indeed, inhibition of vimentin Ser56 phosphorylation led to a corresponding inhibition of GTP-induced secretion, indicating a link between these two events. While fMLP also induced vimentin Ser56 phosphorylation, such phosphorylation was unaffected by roscovitine, which nonetheless, inhibited secretion, suggesting that Cdk5 regulates fMLP-induced secretion via a mechanism independent of Cdk5-mediated vimentin Ser56 phosphorylation. These findings demonstrate the distinct involvement of Cdk5 in GTP- and fMLP-induced secretion by neutrophils, and support the notion that specific targeting of Cdk5 may serve to inhibit the neutrophil secretory process.
The neutrophil is the first cell type recruited to a site of infection or inflammation. One of the mechanisms used by the neutrophil to destroy infectious agents or to mediate the inflammatory process in a variety of clinical scenarios is to secrete its granule contents. Regulation of secretion from the neutrophil is key to the balance between host defense and tissue injury (Nathan, 2006). Previously, we have demonstrated the existence of a calcium-independent GTP-regulated mechanism of secretion in neutrophils (Rosales and Ernst, 2000). Other nucleotide-mediated mechanisms of activation in neutrophils such as adenosine-5′-triphosphate (ATP)- and uridine-5′-triphosphate (UTP)-induced stimulation have been shown to be regulated by protein kinases, including the p38 mitogen-activated protein kinase (p38MAPK) and the extracellular signal-regulated kinase (ERK1/2) (Meshki et al., 2004). Our studies showed that GTP-dependent secretion from neutrophils is mediated by cyclin-dependent kinase 5 (Cdk5) (Rosales et al., 2004a). However, the mechanisms underlying this process remain to be investigated.
Cdk5, a small proline-directed serine/threonine kinase, has close structural homology with cell cycle Cdks (Dhavan and Tsai, 2001) but is unique among the Cdks in that its functions have been associated with post-mitotic events. Cdk5 requires its regulatory partner, p35 (or its potent truncated form, p25) (Lew and Wang, 1995; Lee et al., 1997) or its isoform, p39 (Tang et al., 1995) for activity. Although Cdk5 activity has mostly been associated with brain, more recent studies indicate that Cdk5 plays an important role in other tissues as well such as muscle (Sahlgren et al., 2003), ocular lens (Gao et al., 2002) and testis (Rosales et al., 2004b). It appears that Cdk5 regulates cell differentiation and certain specialized cell functions (Rosales and Lee, 2006), including wound healing (Gao et al., 2004), gene transcription (Fu et al., 2004), senescence (Alexander et al., 2004), and secretion (Rosales et al., 2004a).
Secretion involves cytoskeletal rearrangements that allow mobilization of secretory organelles towards the plasma membrane. Several studies have shown that actin and microtubules regulate transport and potentially, docking and fusion of these secretory organelles. Similarly, the vimentin intermediate filaments have been shown to regulate secretion (Quintanar, 2000), and are required for lysosomal vesicle transport and positioning (Styers et al., 2004). While intermediate filaments are believed to be relatively more stable compared to microfilaments and microtubules, they do possess dynamic properties (Chou et al., 2007). For example, chemotactic peptides have been shown to alter the organization of the vimentin intermediate filaments (Pryzwansky and Merricks, 1998), and protein kinases and phosphatases have been shown to regulate the intracellular organization of vimentin (Inada et al., 1999; Cheng et al., 2000; Lee et al., 2005). Indeed, vimentin is phosphorylated by protein kinase A, protein kinase C, and CaMKII at specific serine sites (Izawa and Inagaki, 2006) and this has been associated with disassembly of the vimentin intermediate filaments (Eriksson et al., 1992). In addition, the cell cycle p34cdc2 (Cdk1) was shown to phosphorylate vimentin during mitosis (Chou et al., 1990), and this has been associated with vimentin disassembly as well. In this study, we investigated the vimentin dynamics and its potential regulation by the unique Cdc2 homologue, Cdk5, during secretion by post-mitotic neutrophils which have undetectable levels of Cdc2 (Wei et al., 1996).
Our studies indicate that vimentin Ser56 phosphorylation depends on Cdk5 in GTP- but not fMLP-stimulated neutrophils but that Cdk5 is involved in both GTP- and fMLP-induced secretory processes, albeit Cdk5-mediated fMLP-induced secretion appears to be independent of vimentin Ser56 phosphorylation. Together, our findings indicate the discrete involvement of Cdk5 in GTP- and fMLP-induced secretion by neutrophils, but suggest that Cdk5 could be targeted for inhibition of neutrophil secretion.
Materials and Methods
Neutrophil isolation, permeabilization, stimulation, and reconstitution
Human neutrophils obtained from normal and healthy volunteers that gave informed consent were permeabilized as we described previously (Rosales and Ernst, 1997). Briefly, permeabilization using streptolysin O (SO; Murex Diagnostics, Inc., Norcross, GA) was performed in two steps: (i) SO binding at 4°C for 10 min followed by washing to remove unbound SO, and (ii) pore formation by resuspending cells in prewarmed (37°C) permeabilization buffer (PB; 50 mM HEPES, pH 7.0, with 100 mM KCl, 20 mM NaCI, 1 mM EGTA, and 0.1% dextrose) and incubation at 37°C for 10 min. Stimulation with 300 μM GTP (Boehringer Mannheim, Indianapolis, IN) in the presence or absence of 10 μM roscovitine (Calbiochem-Novabiochem, San Diego, CA) or 40 μM olomoucine (Calbiochem-Novabiochem) was performed simultaneously with the second step of permeabilization. As indicated in some experiments, incubation at 37°C was continued for an additional 5 min after the pore formation step for a total stimulation time of 15 min. To examine the relevance of vimentin phosphorylation in GTP-dependent secretion, vimentin antibody (1 μg/2 × 106 cells; MyBiosource, San Diego, CA) was introduced to permeabilized cells for 30 min at RT prior to stimulation. As GTP-induced secretion requires cytosolic factor(s), which leaks out during the permeabilization/antibody introduction step, GTP-dependent secretion was reconstituted using crude cytosol from HL60 cells as we described previously (Rosales and Ernst, 2000). Stimulation with fMLP (1 mM) was performed in non-permeabilized cells suspended in HBSS in the presence of 1 mM calcium. Cells treated with roscovitine or olomoucine during stimulation with GTP or fMLP were also pre-incubated with 10 μM roscovitine or 40 μM olomoucine for 20 min at 37°C prior to permeabilization.
Expression, purification, and reconstitution of GST-Cdk5 and GST-p35
GST-Cdk5 and GST-p35 were purified and reconstituted as described (Lee et al., 1996). Briefly, pGEX-2T recombinant plasmid containing either Cdk5 or p25, a truncated form of p35, was transformed into E. coli strain DH5α. Expression of the proteins was induced with 0.4 mM isopropyl-β-D-thiogalactopyranoside (Invitrogen, Burlington, ON) at RT overnight. The supernatant of lysed cells was incubated for 1 h at 4°C with 4 ml of glutathione-agarose (Sigma, St. Louis, MO), and after loading the slurry onto a 10-ml column and extensive washing, the expressed proteins were eluted by 5 mM reduced glutathione in 50 mM Tris–HCl, pH 8.0, containing 1 mM DTT. Reconstitution of Cdk5 and p35 (1:1 molar ratio) was performed by overnight incubation at 4°C in the presence of BSA (0.25 mg/ml).
Phosphorylation assay and autoradiography
Phosphorylation of GST-vimentin (1–84; Santa Cruz Biotech., Santa Cruz, CA) by Cdk5 was assessed by incubating the peptide (2 μg) with reconstituted bacterially expressed Cdk5/p25 (20 ng Cdk5, 40 ng p25) in the presence of [γ-32P] ATP (specific activity of at least 1,000 dpm/pmol in each experiment) and 100 μM cold ATP in kinase assay buffer (Lee et al., 1996) at 30°C for 40 min. The reaction was stopped by adding SDS–PAGE sample buffer and heating for 5 min at 95°C. Samples were then resolved by SDS–PAGE, transferred to a nitrocellulose membrane, and phosphorylation of vimentin was detected by autoradiography. The exposure time for autoradiography was 16 h.
Cdk5 siRNA transfection
Cdk5 siRNA was generated using an ON-TARGETplus™ modification (Dharmacon, Lafayette, CO) to enhance siRNA specificity and reduce off-target effects. Four Cdk5 siRNAs were tested in this study. Data presented are from the use of the most effective siRNA (most effectively depleted Cdk5 as assessed by Western blotting, data not shown) sequence: GGGCUGGGAUUCUGUCAUAUU. The siRNA sequence, UAAGGCUAUGAAGAGAUAC, was used as a negative control to further exclude non-specific effects on gene expression. The Cdk5 siRNA used in this study was conjugated (DNA core facility, University of Calgary) with 6-carboxy-2′,4,4′,5′,7,7′-hexachlorofluorescein (HEX, Invitrogen, Carlsbad, CA) to assess transfection efficiency by fluorescence microscopy. Neutrophils were transfected with siRNA using siLentFect (Bio-Rad, Hercules, CA), following the manufacture’s protocol and, after 16 h, were stimulated with GTP.
Immunoprecipitation
Neutrophil lysates in phosphate buffer (PBS: 10 mM Tris–HCl, pH 7.2, 150 mM NaCl) containing 1% NP-40 and 50 mM sodium fluoride, were pre-cleared with protein G plus agarose beads prior to incubation with fresh beads pre-adsorbed with the indicated antibody. The mixture was incubated for 2 h on a rocker at 4°C. The immunocomplexes were washed with the PBS lysis buffer and analyzed by Western blotting for the indicated protein of interest. The use of phosphate buffer and sodium fluoride was aimed at preventing dephosphorylation.
Western blotting and protein determination
For Western blot analysis, proteins were transferred onto a nitrocellulose membrane (Millipore, Bedford, MA), probed with a primary antibody (anti-Cdk5 DC-17 or anti-vimentin V9 from Santa Cruz Biotech.; anti-vimentin pSer56 from StressGen Biotech., Victoria, BC, Canada) then incubated with a horseradish peroxidase conjugated secondary antibody (Pierce, Rockford, IL). Immunoreactive protein bands were visualized using the ECL detection system (Amersham Life Sciences, Arlington Heights, IL) with exposure times of 30–60 sec. For membranes blotted with radioactive samples, radioactivity was allowed to decay prior to immunoblotting with an antibody. Protein concentration was determined using the Bradford microassay.
Deconvolution/immunofluorescence microscopy
Cells suspended in Hanks balanced salt solution (HBSS) containing 0.15% bovine serum albumin (BSA) were allowed to adhere to coverslips at 37°C in the presence of 5% CO2. After 45 min, cells were gently washed with warm (37°C) PB and incubated with warm PB ± 300 μM GTP ± microcystin. Cells that were treated with GTP in the presence of microcystin (50 nM) were also pre-treated with microcystin for 20 min prior to stimulation with GTP. Following treatment, cells were fixed in 3.7% paraformaldehyde for 10 min at room temperature. After washing, cells were incubated with vimentin (V9), vimentin pSer56, and/or Cdk5 (DC-17) antibody, washed, and incubated with Cy3- (Jackson ImmunoResearch Labs., West Grove, PA) or Alexa 488-(Invitrogen) conjugated secondary antibody. Coverslips were mounted on glass slides using PermaFluor mounting medium (Fisher Scientific, Ottawa, ON, Canada). Immunofluorescence staining was visualized by standard widefield microscopy (using Olympus IX70 for Figs. 1 and 2D, and Olympus BX-60 for Fig. 3A), and images were acquired using a cooled charge-coupled device camera. Deconvolution images were obtained using a Leica DM RXA2 microscope with in house software for semiautomatic collection of multichannel image series. The EXFOS Xcite-120 (Photonic Solutions, Inc., Mississauga, ON, Canada) light source and iXon+EMCCD camera (Andor Technology, Belfast, Northern Ireland) were used.
Fig. 1.

Neutrophils exhibit vimentin reorganization following stimulation with GTP in the presence and absence of microcystin. Vimentin in unstimulated (first panel), microcystin-treated (second panel), GTP-stimulated (third panel), and GTP plusmicrocystin-stimulated (fourth panel) neutrophils was visualized by immunofluorescence microscopy. Arrows in the third panel are directed at focal increases in intensity of vimentin staining in both the cytoplasmic region and the periphery of cells stimulated with GTP. Arrows in the fourth panel are directed at vimentin networks which appear to pack together in filamentous pattern in the cytoplasmic region of GTP plus microcystin-treated cells. Images were taken 10 min after treatment, and are representative data from three separate sets of experiments.
Fig. 2.
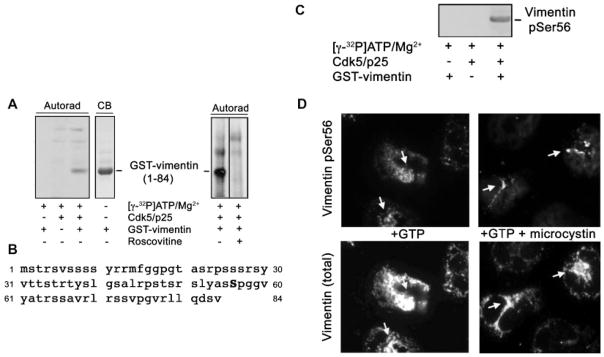
Vimentin is specifically phosphorylated at Ser56 by Cdk5. A, Left panel: Autoradiogram (Autorad) following SDS–PAGE and blotting of samples subjected to radioactive kinase assay in the presence or absence of GST-vimentin peptide (1–84), reconstituted Cdk5/p25 and [γ-32P]ATP-Mg2+. GST-vimentin did not show any non-specific binding with [γ-32P] ATP-Mg2+ (first lane). Cdk5/p25 incubated with [γ-32P] ATP-Mg2+ shows two non-specific bands at and higher than 49 kDa (second lane). GST-vimentin alone run on the same gel was visualized by Coomassie blue (CB) staining (lane 4). The right panel shows another autoradiogram following a phosphorylation assay where GST-vimentin peptide (1–84) was incubated with reconstituted recombinant Cdk5/p25 and [γ-32P]ATP-Mg2+ in the presence (second lane) or absence (first lane) of roscovitine. The exposure time for autoradiography was 16 h. B: The sequence representing vimentin peptide (1–84) contains one proline-directed serine site, Ser56, the most likely phosphorylation target of Cdk5. C: After radioactive decay, the membrane in (A), left panel, was subjected to immunoblotting using a vimentin pSer56-specific antibody. The immunoreactive band was visualized following an exposure time (to the ECL reagent) of 30 sec. D: Immunofluorescence staining for vimentin pSer56 (top panel) and total vimentin (bottom panel) of neutrophils stimulated with GTP in the presence (right panel) and absence (left panel) of microcystin.
Fig. 3.
Vimentin pSer56 and Cdk5 are partially colocalized and functionally associated with each other in neutrophils stimulated with GTP. A: Detection of Cdk5 (left panel) and vimentin pSer56 (middle panel) by standard widefield immunofluorescence microscopy in neutrophils stimulated with GTP at different time points. Merged images are shown at the right panel. B: Deconvolution microscopy (using the same antibodies and samples used in [A]) shows that vimentin pSer56 partially colocalizes with Cdk5 in neutrophils stimulated with GTP for 10 min. C: The Cdk5 activator protein, p35, was immunoprecipitated (IP) from lysates of non-stimulated neutrophils (second lane) and neutrophils stimulated with GTP (third lane). The immunoprecipitates were then resolved by SDS–PAGE, Western blotted, and probed with p35, Cdk5, and vimentin pSer56 antibodies. The first lane was loaded with a non-specific rabbit IgG immunoprecipitate (IgG IP) from cells stimulated with GTP. The right panel shows the densitometry results of the blots on the left panel. D: Cdk5 was also immunoprecipitated from lysates of unstimulated neutrophils (second lane) and neutrophils stimulated with GTP (third lane). The resulting immunoprecipitates were subjected to SDS–PAGE and immunoblotted for Cdk5 and vimentin pSer56. The sample in the first lane was a non-specific rabbit IgG immunoprecipitate from cells stimulated with GTP. Densitometric analysis of the blots is shown on the right panel.
Lactoferrin and β-hexosaminidase secretion assays
Cell-free supernatants of stimulated neutrophils were examined for secreted granule contents. The method of Gaudreault et al. (2005) was followed to assess secretion of β-hexosaminidase from neutrophil primary granules. The extent of lactoferrin secretion from secondary granules was performed as we described previously (Rosales and Ernst, 1997).
Zymography
Secretion of matrix-metalloproteinase-9 (MMP-9 or gelatinase B) was assessed by zymography. Samples in non-reducing loading buffer (126 mM Tris–HCl, 20% glycerol, 4% SDS, 0.05% bromophenol blue, pH 6.8) were loaded into an SDS–PAGE gel containing gelatin (1 mg/ml). Following electrophoresis, the gel was rinsed for 15 min in wash buffer (50 mM Tris, pH 7.5) containing 2.5% Triton X-100 and 5 mM CaCl2, then incubated overnight at room temperature in wash buffer alone. After extensive washing with water, the gel was incubated for an additional 24 h at 37°C in digestion buffer (wash buffer without Triton X-100). Clear bands revealed after staining with Coomassie brilliant blue R-250 indicate the presence of gelatinase activity.
Densitometry
Densitometric analysis was performed using the NIH ImageJ software.
Statistics
Statistical analysis was performed using the Student’s t-test.
Results
Neutrophils exhibit vimentin rearrangement following stimulation with GTP
We previously showed that a calcium-independent GTP-mediated mechanism of secretion exists in neutrophils (Rosales and Ernst, 2000) but further investigation is required to characterize this secretory system. While many studies have shown that microfilaments play an important role in secretion by neutrophils, there is very limited study on the involvement of the vimentin intermediate filament in this process. Here, we initially sought to examine a potential change in neutrophil vimentin organization following stimulation with GTP. We found that resting, non-stimulated cells have a somewhat diffuse and filamentous vimentin staining pattern (Fig. 1, left panel). On the other hand, cells exposed to GTP for 10 min showed more focal increases in intensity of staining in both the cytoplasmic region and the cell periphery (arrows, Fig. 1, third panel). Since vimentin is known to be a widespread major phosphoprotein, we looked at the effect of pre-treating neutrophils with microcystin which inhibits serine/threonine phosphatases PP2A and PP1. In unstimulated, microcystin-treated cells (Fig. 1, second panel), vimentin remained to have both diffuse and filamentous pattern but there seemed to have some aggregation of cytoplasmic vimentin as well. Conversely, neutrophils treated with GTP and microcystin (Fig. 1, last panel) showed further rearrangement of the vimentin network, appearing to form filamentous bundles (arrows) in the cytoplasmic region. Thus, vimentin phosphorylation/ dephosphorylation and reorganization appear to be strategic events in neutrophils stimulated with GTP.
Vimentin is a Cdk5 substrate that is specifically phosphorylated at Ser56
Reversible phosphorylation of vimentin has been demonstrated to play an important role in their dynamic intracellular organization (Chou et al., 2007). To determine whether the phosphorylation and rearrangement of vimentin intermediate filaments in GTP-stimulated neutrophils are potentially mediated by the serine/threonine kinase, Cdk5, we initially performed a Cdk5 phosphorylation assay using GST-vimentin (1–84) as substrate. Autoradiography showed a distinct phosphorylated band when GST-vimentin (1–84) was incubated with Cdk5/p35 in the presence of [γ-32P] ATP-Mg2+ (Fig. 2A, lane 3). This band migrated to a distance identical to that of GST-vimentin run on the same gel but visualized by Coomassie blue staining (Fig. 2A, lane 4), indicating that vimentin acts as a substrate of Cdk5. Indeed, the potent inhibitor of Cdk5, roscovitine, completely blocked GST-vimentin peptide (1–84) phosphorylation (second lane on the right panel), indicating specific phosphorylation by Cdk5. While vimentin possess a number of potential serine/ threonine phosphorylation sites, including those present in the vimentin (1–84) peptide, it contains only one proline-directed serine residue at position 56 (Fig. 2B), a likely phosphorylation target of Cdk5. Using a pSer56-specific vimentin antibody, we detected an immunoreactive band in the same spot where the specific phosphorylated band in Figure 2A (lane 3) was detected (Fig. 2C), indicating that Cdk5 specifically phosphorylates Ser56 in vimentin. To determine whether the rearrangement of vimentin induced by GTP ± microcystin is related to phosphorylation of vimentin Ser56, we examined the staining pattern of both pSer56 and total vimentin in treated neutrophils. In Figure 2D, cells stimulated with GTP ± microcystin show vimentin pSer56 staining (top panel) that overlaps regions with total vimentin staining (bottom panel), including in regions with focal increases in total vimentin (e.g., arrows in bottom panel) and in regions with filamentous vimentin bundles (arrows in GTP plus microcystin-treated cells), suggesting that vimentin pSer56 is panel of the rearranged vimentin network.
Vimentin pSer56 and Cdk5 are functionally associated with each other in neutrophils stimulated with GTP
Following neutrophil stimulation with GTP (Fig. 3A), some vimentin phosphorylation at Ser56 was detectable after 1 and 3 min. At the same time points, no obvious change in Cdk5 staining was observed, and merged images did not show apparent localization of Cdk5 and phosphoSer56 vimentin in the same cellular compartment, suggesting that another kinase may be responsible for the immediate phosphorylation of vimentin Ser56 following neutrophil stimulation with GTP. By 5 min and until at least 15 min after stimulation, there was increasing staining for vimentin pSer56 which, as shown in the merged panel, appeared to increasingly localize in the same cellular compartment as Cdk5 in neutrophils stimulated with GTP. Deconvolution microscopy (Fig. 3B; using the same antibodies and set of samples used in Fig. 3A) removed the image blur seen in Figure 3A and allowed clear visualization of the partial colocalization of Cdk5 and vimentin pSer56 in neutrophils stimulated with GTP for 10 min. This suggests that at a later time following stimulation with GTP, Cdk5 contributes to vimentin Ser56 phosphorylation. This is consistent with our current finding that Cdk5 serves as a vimentin kinase that specifically phosphorylates vimentin Ser56. The staining pattern of the Cdk5 activator, p35, was similar to that of Cdk5 but of lesser intensity (data not shown).
To further determine whether Cdk5/p35 associates with vimentin pSer56 in cells stimulated with GTP, we immunoprecipitated p35 from non-stimulated and GTP-stimulated neutrophils, and by Western blotting, checked whether the immunoprecipitates were associated with Cdk5 and vimentin pSer56. As shown in Figure 3C, the presence of GTP resulted in increased interaction between Cdk5/p35 and vimentin pSer56 (third lane). No relevant signal was detected in the non-specific IgG immunoprecipitate (IgG IP) from cells stimulated with GTP (first lane). These findings support the notion that Cdk5/p35 and vimentin pSer56 coimmunoprecipitates are functionally associated with each other. Consistent with this observation, we found that immunoprecipitation using a Cdk5 antibody coimmunoprecipitated vimentin pSer56 with increased coimmunoprecipitation when cells were stimulated with GTP (Fig. 3D).
Vimentin Ser56 phosphorylation accompanies secretion by neutrophils stimulated with GTP: both decrease upon inhibition of Cdk5 activity
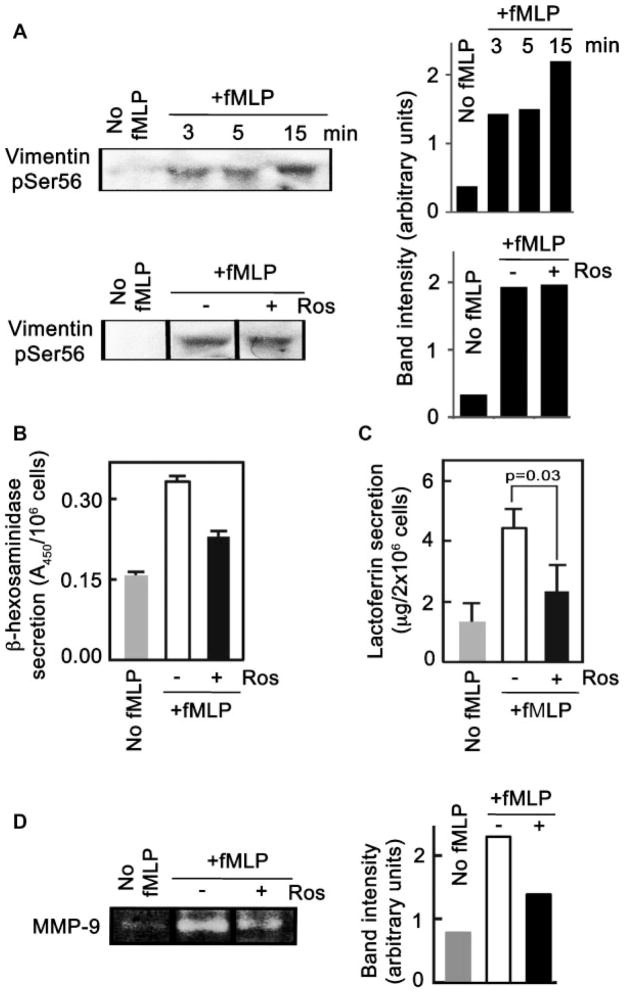
Since vimentin Ser56 is a phosphorylation target of Cdk5, and these proteins colocalize and associate with each other in neutrophils stimulated with GTP, we examined whether vimentin dynamics during GTP-induced secretion by neutrophils could be regulated by Cdk5. For this study, we again took advantage of the availability of the potent Cdk5 inhibitor, roscovitine. Figure 4A shows that at 3 min following stimulation with GTP, neutrophils exhibited phosphorylation of vimentin Ser56. Increased level of phosphorylation was observed at 5 and 10 min and by 15 min, the level of phosphorylation has increased dramatically. Upon addition of roscovitine, inhibition of GTP-induced vimentin phosphorylation was observed beginning at 3 min (right panel), and the additional increase in GTP-induced phosphorylation at 5, 10, and 15 min (left panel) was inhibited by roscovitine (right panel). While a relatively small amount of phosphorylation at 3–15 min seemed to result from activity of another kinase (i.e., phosphorylation that cannot be completely inhibited by roscovitine as shown in the right panel), the majority of the phosphorylation at 5, 10, and 15 min (left panel) was inhibited by roscovitine (right panel), indicating that vimentin phosphorylation, particularly at 5–15 min, was predominantly due to Cdk5. Interestingly, the time-dependent increase in intensity of vimentin Ser56 phosphorylation coincided with increasing extent of secretion of β-hexosaminidase (Fig. 4B), lactoferrin (Fig. 4C) and MMP-9 (gelatinase B; Fig. 4D) from azurophil (primary), specific (secondary) and gelatinase (tertiary) granules, respectively, starting at 5 min following stimulation (Fig. 4B–D, open bars), the time at which secretion begins to be observed. Similar to its effect on vimentin Ser56 phosphorylation, roscovitine resulted in considerable but incomplete inhibition of β-hexosaminidase, lactoferrin, and MMP-9 secretion (Fig. 4B–D). The different extent and pattern of secretory response to GTP and inhibitory effect of roscovitine on the three granule populations is consistent with previous findings from our group (Rosales and Ernst, 1997) and others (Nusse et al., 1998), indicating that the secretory mechanisms in the different granule populations are distinct. Thus, roscovitine appears to have the greatest inhibitory effect on β-hexosaminidase secretion after 5 min of treatment while roscovitine inhibition of lactoferrin secretion was noticeable at 10 min following treatment. As with roscovitine, olomoucine, another Cdk5 inhibitor, showed an inhibitory effect on GTP-induced vimentin Ser56 phosphorylation (Fig. 5A) and secretion from neutrophils (Fig. 5B–D). The incomplete inhibitory effect of roscovitine and olomoucine on both vimentin Ser56 phosphorylation and secretion suggests that cellular proteins other than Cdk5 are also involved in these processes following neutrophil stimulation with GTP. Indeed, colocalization of vimentin pSer56 and Cdk5 was also incomplete in neutrophils stimulated with GTP (Fig. 3B). Nonetheless, it is possible that GTP-induced Cdk5-mediated vimentin Ser56 phosphorylation is linked to GTP-induced Cdk5-mediated secretion by neutrophils.
Fig. 4.
GTP-induced time-dependent vimentin Ser56 phosphorylation and secretion from neutrophils are inhibited by roscovitine. A: Neutrophils stimulated with GTP for 3, 5, 10, and 15 min in the presence (right side) and absence (left side) of roscovitine (Ros) were pelleted, lysed, and subjected to Western blotting for pSer56 (upper panel) and total (lower panel) vimentin. The non-stimulated cells (No GTP) were incubated for 15 min at 37°C. The pattern of multiple vimentin bands is consistent with several previous studies, indicating that this is caused by proteolytic cleavage (e.g., Mor-Vaknin et al., 2003). Data represent results from three separate sets of experiments. Supernatants from cells stimulated with GTP ± roscovitine treatment were examined for secreted (B) β-hexosaminidase, (C) lactoferrin, and (D) MMP-9 from primary, secondary, and tertiary granules, respectively. Values in (B) and (C) are means ± SD from three experiments. The left panel in (D) shows a representativezymogramofMMP-9 secretion. The right panel in (D) shows the mean values ± SD of the densitometry of three MMP-9 zymograms from three experiments. The area of the pro-MMP-9 band, which represents the global MMP-9 activity (Ernens et al., 2006) was used to evaluate the enzyme’s activity (expressed in arbitrary units). The differences in GTP-induced lactoferrin, β-hexosaminidase, and MMP-9 secretion in the presence and absence of roscovitine were found to be significant (P < 0.05 by Student’s t-test), particularly at 10 and 15 min.
Fig. 5.

Olomoucine (Olo) inhibits GTP-induced vimentin Ser56 phosphorylation and secretion from neutrophils. A: Streptolysin O permeabilized neutrophils stimulated with GTP for 10 min in the presence or absence of olomoucine were pelleted, lysed, and subjected to Western blotting for pSer56 (upper panel) and total (lower panel) vimentin. Supernatants from these cells were examined for secreted (B) β-hexosaminidase, (C) lactoferrin, and (D) MMP-9 from primary, secondary, and tertiary granules, respectively. Densitometry values (band intensity) of the blots (A) and zymogram (D) were obtained using the NIH Image J software.
Vimentin Ser56 phosphorylation and secretion from neutrophils stimulated with GTP are inhibited following Cdk5 siRNA transfection
Although short-lived cells, neutrophils have previously been successfully transfected with siRNA to examine adenosine inhibition of MMP-9 secretion by neutrophils (Ernens et al., 2006), and to characterize p38MAPK activation pathways in fMLP-induced superoxide production and chemotaxis (Azuma et al., 2007). Certainly, we have successfully transfected Cdk5 siRNA into neutrophils (Fig. 6A), depleting Cdk5 to a certain extent within 16 h of transfection (Fig. 6B, top panel, 2nd lane). Following stimulation with GTP, Cdk5 siRNA transfected cells showed reduced phosphorylation of vimentin Ser56 (Fig. 6B, 2nd from the top panel, 2nd lane) compared to cells transfected with control siRNA (Fig. 6B, 2nd from the top panel, 1st lane). Consistent with roscovitine treatment (Fig. 4B–D, solid bars), Cdk5 siRNA treatment resulted in decreased secretion of β-hexosaminidase, lactoferrin, and MMP-9 from cells stimulated with GTP (Fig. 6C–E, respectively, solid bars). The seemingly greater effect on secretion by Cdk5 siRNA compared to roscovitine may be attributed to a possible increase in sensitivity of the siRNA-treated cells following 16 h in culture.
Fig. 6.
GTP-induced vimentin Ser56 phosphorylation and secretion from neutrophils are reduced in Cdk5-siRNA transfected cells. A: Neutrophils transfected for 16 h with Cdk5 siRNA (top panel) and control (Cx) siRNA (bottom panel) were analyzed by phase contrast (left panel) and fluorescence (middle panel) microscopy. The right panel shows merged phase and fluorescence images. B: Following stimulation with GTP for 10 min, neutrophils transfected with control (first lane) and Cdk5 (second lane) siRNA were pelleted, lysed, and subjected to immunoblottingforCdk5, vimentinpSer56, total vimentin, and actin. Densitometric analyses of the blots (on the left panel) are shown on the right panel. D at are present results from are presentative experiment (n =3). The Cdk5 siRNA used in this study was the most effective in depleting Cdk5 compared to three others as assessed by Western blotting (data not shown). However, incomplete depletion of Cdk5 was most likely due to the short transfection period of 16 h to allow examination of cells that are still competent to secrete their granule contents. After 16 h, an average of 75% of neutrophils transfected with either control or Cdk5 siRNA remained viable in RPMI 1640 medium containing non-essential amino acids, L-glutamine and sodium pyruvate. The total number of viable cells, as measured by membrane integrity (i.e., trypan blue exclusion), after transfection was also equivalent in both treatments and thus, the overall cell loss was equivalent in the control and Cdk5 siRNA transfected cells. Supernatants from siRNA-transfected cells stimulated with GTP were examined for secreted (C) β-hexosaminidase, (D) lactoferrin, and (E) MMP-9. Values in (C) and (D) are means ±SD from a representative experiment (n =3). Values in (E) are densitometry results from the accompanying zymogram shown on the top panel.
Roscovitine inhibits fMLP-induced secretion but not fMLP-induced vimentin Ser56 phosphorylation
Since fMLP, which mimics the activity of bacterially derived peptides with formylated N-terminal methionionine groups, induced vimentin Ser56 phosphorylation over time (Fig. 7A, top panel), we examined whether this phosphorylation event is also mediated by Cdk5. As shown in Figure 7A (bottom panel), treatment of cells with roscovitine did not affect the phosphorylation of vimentin Ser56, indicating that Cdk5 is not involved in the phosphorylation process and that another vimentin Ser56 kinase is activated during neutrophil stimulation with fMLP. However, roscovitine caused a partial but significant inhibition of fMLP-induced secretion of β-hexosaminidase (Fig. 7B), lactoferrin (Fig. 7C), and MMP-9 (Fig. 7D), indicating that Cdk5 is, nonetheless, involved in the fMLP-induced secretory process but likely through a mechanism that is not related to vimentin Ser56 phosphorylation.
Fig. 7.
Roscovitine caused a decrease in fMLP-induced secretion but not fMLP-induced vimentin Ser56 phosphorylation. A: Vimentin Ser56 phosphorylation was examined in neutrophils stimulated with fMLP at different time points (top panel), and in neutrophils stimulated with fMLP for 15 min with or without roscovitine (Ros) treatment (bottom panel). The right panel shows the densitometric analysis of the blots on the left panel. Supernatants from cells stimulated for 15 min with fMLP ± roscovitine treatment were examined for secreted (B) β-hexosaminidase, (C) lactoferrin, and (D) MMP-9. Values in (B) and (C) are means ± SD of are presentative experiment (n =3). Values in (D) are densitometry results from the representative zymogram on the top panel.
Vimentin Ser56 phosphorylation is important for GTP-induced secretion by neutrophils
To further examine whether phosphorylation of vimentin Ser56 is related to GTP-induced secretion, we took advantage of the SO permeabilized cell system to introduce vimentin antibody into neutrophils and determine whether this would inhibit vimentin Ser56 phosphorylation and GTP-induced secretion. We (unpublished observation) and others (Ferry et al., 2001) have observed that antibodies can effectively traverse SO-permeabilized cells, and the strategy of blocking phosphorylation of intermediate filaments using antibodies has been used previously (Tao and Ip, 1991). Indeed, introduction of vimentin antibody into permeabilized neutrophils resulted in loss of vimentin Ser56 phosphorylation (Fig. 8A) and inhibition of GTP-induced secretion from neutrophil granules (Fig. 8B, C), suggesting a link between vimentin Ser56 phosphorylation and GTP-induced secretion.
Fig. 8.

Inhibition of vimentin Ser56 phosphorylation causes inhibition of GTP-induced secretion from neutrophils. After introduction of vimentin antibody into permeabilized neutrophils, GTP-dependent secretion was reconstituted using crude cytosol from HL60 cells as described in the Materials and Methods Section. A: The vimentin antibody introduced into permeabilized cells inhibited GTP-induced vimentin Ser56 phosphorylation. Duration of stimulation with GTP was 15 min. After centrifugation of stimulated neutrophils, supernatants were examined for release of (B) lactoferrin and (C) MMP-9. HL60 cytosol contains endogenous β-hexosaminidase and therefore, analysis of this protein was omitted. Values in (B) are means ±SD of triplicate experiments from a representative set of experiments (n =3). Values in (C) are densitometry results from the representative gel at the bottom panel.
Discussion
Although vimentin−/− mice were initially found to develop and reproduce without any apparent defects (Colucci-Guyon et al., 1994), subsequent studies using these mice and cells derived from these mice revealed that vimentin, in fact, plays crucial roles in various cellular functions such as in cellular mechanical stability, migration and contractile capacity (Eckes et al., 1998), and in lymphocyte adhesion and transmigration (Nieminen et al., 2006). Therefore, it is possible that compensatory mechanisms exist in vimentin−/− mice to produce a normal phenotype, but that under normal conditions in wt mice, vimentin is a significant player in many cellular processes. In neutrophils, vimentin has been found to be important for secretion (Pryzwansky and Merricks, 1998), and indeed, our current studies demonstrate the occurrence of vimentin Ser56 phosphorylation during neutrophil secretion induced by fMLP or GTP. Interestingly, we also show that although Cdk5 mediates vimentin Ser56 phosphorylation induced by GTP and not by fMLP, it regulates both GTP- and fMLP-induced secretion.
Certainly, a role for Cdk5 in various secretory systems has been documented (Rosales and Lee, 2006). Initial implication resulted from the finding that Cdk5 phosphorylation of Munc-18 causes dissociation of the latter from syntaxin 1, which then associates with other SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) proteins to promote secretion (Shuang et al., 1998). In neutrophils, we previously found that Cdk5 activity is required to elicit the maximum GTP-induced secretory response (Rosales et al., 2004a). Our premise is that Cdk5 is involved in vimentin dynamics during GTP-induced secretion by neutrophils.
Several studies support the notion that depolymerization and disassembly of cytoskeletal components allow vesicles and granules to move towards the cell periphery to dock and fuse with the plasma membrane and to secrete their contents to the extracellular space. During secretion by neutrophils, little is known about the mechanisms regulating the structural dynamics of vimentin intermediate filaments although there is increasing evidence that intracellular organization of vimentin networks is under the control of protein kinases and phosphatases (Chou et al., 2007). Indeed, we found different staining patterns of vimentin in resting non-stimulated cells, and in cells exposed to GTP with and without the serine/threonine phosphatase inhibitor, microcystin, further demonstrating the impact of the phosphorylation state in the cellular arrangement of vimentin. In support of this premise, in vitro reconstituted vimentin filaments undergo complete disassembly when subjected to phosphorylation by protein kinase A or protein kinase C (Inagaki et al., 1987). In addition, phosphorylation of vimentin by p34cdc2 contributes to the reorganization of vimentin during M phase of the cell cycle (Chou et al., 1991). Using a phospho-specific antibody, we found that Ser56, the only Ser residue immediately before Pro in vimentin, is phosphorylated by Cdk5. This finding is consistent with proline-directed phosphorylation of its substrates by the Cdk family of proteins. Apparently, the pyrrolidine ring of proline is required for Cdks (Cdk5 included) to recognize their substrates (Ando et al., 1997), including vimentin.
In neutrophils stimulated with GTP± microcystin, the relatively modest staining for vimentin pSer56 compared to total rearranged vimentin concurs with previous reports that disassembly or disorganization of vimentin is associated with its phosphorylation at specific serine sites by different protein kinases (Eriksson et al., 1992; Izawa and Inagaki, 2006). For example, ser56 is specifically phosphorylated by Cdc2 in mitotic cells (Izawa and Inagaki, 2006). However, post-mitotic neutrophils do not express relevant levels of Cdc2 (Wei et al., 1996), and thus, Cdc2 could not account for vimentin Ser56 phosphorylation in these cells. On the other hand, we found colocalization of the Cdc2 homologue, Cdk5, with GTP-induced vimentin pSer56, particularly at a time when secretion from neutrophils is evident, pointing to a novel functional role of Cdk5 in these cells. Indeed, physical association of Cdk5 with vimentin pSer56 in neutrophils stimulated with GTP supports the notion that Cdk5 contributes to vimentin Ser56 phosphorylation and subsequent secretion from neutrophils. The apparent concentration of highly phosphorylated vimentin Ser56 at the inner cytoplasmic region is consistent with its disassembly and collapse, allegedly to facilitate the transport of granules towards the plasma membrane to allow fusion and subsequent secretion. Thus, it appears that Cdk5 mediates the phosphorylation of vimentin Ser56 and the secretion from neutrophil granules following stimulation with GTP.
However, the fact that there is incomplete colocalization of vimentin pSer56 and Cdk5 after GTP stimulation, more so at the initial state of Ser56 phosphorylation, further suggests that another kinase is involved in the phosphorylation event. As indicated above, protein kinase A, protein kinase C and CaMKII have been shown to phosphorylate vimentin at multiple serine sites but not at Ser56 (Inagaki et al., 1996). On the other hand, while Cdc2 specifically phosphorylates vimentin Ser56 in mitotic cells (Inagaki et al., 1996), it is not detectable in post-mitotic neutrophils (Wei et al., 1996) and, therefore, is an irrelevant vimentin kinase in these cells. Conversely, p21-activated kinase (PAK) has been shown to also specifically phosphorylate vimentin Ser56 in smooth muscle cells stimulated with 5-hydroxytryptamine (Tang et al., 2005). Potentially, early phosphorylation of vimentin Ser56 in neutrophils stimulated with GTP is a result of PAK activity but this remains to be determined. In contrast, it is possible that sustained Cdk5-independent phosphorylation of vimentin Ser56 in fMLP plus roscovitine-treated neutrophils is related to the previous finding that cGMP causes vimentin phosphorylation in neutrophils stimulated with fMLP (Wyatt et al., 1991). Nonetheless, although Cdk5 does not account for vimentin Ser56 phosphorylation in neutrophils stimulated with fMLP, it appears to have a novel role in fMLP-induced secretion as roscovitine causes inhibition of the process. Further studies are required to explain this occurrence but it seems that Cdk5 regulates fMLP-induced secretion via a mechanism independent of vimentin Ser56 phosphorylation. Indeed, in other systems, Cdk5 has been shown to regulate the secretory process by phosphorylating other components of the secretory apparatus such as Munc-18 (Fletcher et al., 1999) and synapsin 1 (Matsubara et al., 1996; Fletcher et al., 1999).
Unlike neutrophils stimulated with fMLP, neutrophils stimulated with GTP showed reduced vimentin Ser56 phosphorylation and secretion following roscovitine, olomoucine, or Cdk5 siRNA treatment. The fact that the extent of inhibition of vimentin Ser56 phosphorylation did not correlate proportionally with the degree of inhibition of secretion supports specific vimentin Ser56 targeting by Cdk5 and the existence of other regulators of GTP-induced secretion. In other words, considerable inhibition of vimentin Ser56 phosphorylation by roscovitine indicates that such phosphorylation predominantly depends on Cdk5, but a less dramatic effect of roscovitine on secretion indicates that Cdk5 is only one of multiple regulators of GTP-induced secretion. These findings led us to believe that vimentin Ser56 phosphorylation is highly dependent on Cdk5 in GTP-stimulated cells but not in fMLP-stimulated cells, and that the involvement of vimentin in GTP-induced secretion by neutrophils may occur through its phosphorylation at Ser56. Indeed, this premise is supported by the fact that antibody-mediated inhibition of vimentin Ser56 phosphorylation results in inhibition of GTP-induced secretion. It thus appears that vimentin Ser56 phosphorylation is linked to GTP-induced neutrophil granule secretion. Together, our findings further support the notion that GTP- and fMLP-induced secretion are distinctly regulated by Cdk5.
Indeed, the Cdk5 inhibitor, roscovitine has been shown to enhance the resolution of neutrophil-dependent inflammation (Rossi et al., 2006). However, while the suggestion was that this is achieved through induction of apoptosis (Rossi et al., 2006), and thus, inhibition of calcium influx (Ayub et al., 2004) and associated signaling pathways (Whyte et al., 1993), the GTP-induced secretion that we present here is independent of calcium (Rosales and Ernst, 2000). Moreover, in the current study, exposure of neutrophils to 10 μM roscovitine for 25–35 min is insufficient to cause apoptosis. In fact, it has been shown that it takes 4–8 h for 20 μM roscovitine to promote apoptosis (Leitch et al., 2011). Thus, while longer exposure to roscovitine may resolve inflammation by inducing apoptosis, its more immediate effects likely include inhibition of its specific target, Cdk5, and consequently, inhibition of Cdk5-mediated secretion. Certainly, the apparent involvement of Cdk5 in both the GTP- and fMLP-induced secretory processes supports the view that it could serve as a valuable target in controlling neutrophil secretion and thus, the neutrophil inflammatory pathway.
Acknowledgments
Contract grant sponsor: Natural Sciences and Engineering Research Council of Canada.
Contract grant sponsor: University Research Grants Committee.
Contract grant sponsor: Canadian Institutes of Health Research.
We thank Jamie McElgunn and Smaranda Abaco for technical assistance. This study was supported by the Natural Sciences and Engineering Research Council of Canada, and the University Research Grants Committee (JLR), and the Canadian Institutes of Health Research (KYL).
Literature Cited
- Alexander K, Yang HS, Hinds PW. Cellular senescence requires CDK5 repression of Rac1 activity. Mol Cell Biol. 2004;24:2808–2819. doi: 10.1128/MCB.24.7.2808-2819.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando S, Ikuhara T, Kamata T, Sasaki Y, Hisanaga S, Kishimoto T, Ito H, Inagaki M. Role of the pyrrolidine ring of proline in determining the substrate specificity of cdc2 kinase or cdk5. J Biochem. 1997;122:409–414. doi: 10.1093/oxfordjournals.jbchem.a021768. [DOI] [PubMed] [Google Scholar]
- Ayub K, Laffafian I, Dewitt S, Hallett MB. Ca influx shutdown in neutrophils induced by Fas (CD95) cross-linking. Immunology. 2004;112:454–460. doi: 10.1111/j.1365-2567.2004.01899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma Y, Kosaka K, Kashimata M. Phospholipase D-dependent and -independent p38MAPK activation pathways are required for superoxide production and chemotactic induction, respectively, in rat neutrophils stimulated by fMLP. Eur J Pharmacol. 2007;568:260–268. doi: 10.1016/j.ejphar.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Cheng TJ, Lin YL, Chiang AS, Lai YK. Association of protein phosphatase 2A with its substrate vimentin intermediate filaments in 9L rat brain tumor cells. J Cell Biochem. 2000;79:126–138. [PubMed] [Google Scholar]
- Chou YH, Bischoff JR, Beach D, Goldman RD. Intermediate filament reorganization during mitosis is mediated by p34cdc2 phosphorylation of vimentin. Cell. 1990;62:1063–1071. doi: 10.1016/0092-8674(90)90384-q. [DOI] [PubMed] [Google Scholar]
- Chou YH, Ngai KL, Goldman R. The regulation of intermediate filament reorganization in mitosis. p34cdc2 phosphorylates vimentin at a unique N-terminal site. J Biol Chem. 1991;266:7325–7328. [PubMed] [Google Scholar]
- Chou YH, Flitney FW, Chang L, Mendez M, Grin B, Goldman RD. The motility and dynamic properties of intermediate filaments and their constituent proteins. Exp Cell Res. 2007;313:2236–2243. doi: 10.1016/j.yexcr.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994;79:679–694. doi: 10.1016/0092-8674(94)90553-3. [DOI] [PubMed] [Google Scholar]
- Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol. 2001;2:749–759. doi: 10.1038/35096019. [DOI] [PubMed] [Google Scholar]
- Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber D, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111:1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- Eriksson JE, Opal P, Goldman RD. Intermediate filament dynamics. Curr Opin Cell Biol. 1992;4:99–104. doi: 10.1016/0955-0674(92)90065-k. [DOI] [PubMed] [Google Scholar]
- Ernens I, Rouy D, Velot E, Devaux Y, Wagner DR. Adenosine inhibits matrix metalloproteinase-9 secretion by neutrophils: Implication of A2a receptor and cAMP/ PKA/Ca2+ pathway. Circ Res. 2006;99:590–597. doi: 10.1161/01.RES.0000241428.82502.d4. [DOI] [PubMed] [Google Scholar]
- Ferry X, Eichwald V, Daeffler L, Landry Y. Activation of betagamma subunits of G(i2) and G(i3) proteins by basic secretagogues induces exocytosis through phospholipase Cbeta and arachidonate release through phospholipase Cgamma in mast cells. J Immunol. 2001;167:4805–4813. doi: 10.4049/jimmunol.167.9.4805. [DOI] [PubMed] [Google Scholar]
- Fletcher AI, Shuang R, Giovannucci DR, Zhang L, Bittner MA, Stuenkel EL. Regulation of exocytosis by cyclin-dependent kinase 5 via phosphorylation of Munc18. J Biol Chem. 1999;274:4027–4035. doi: 10.1074/jbc.274.7.4027. [DOI] [PubMed] [Google Scholar]
- Fu AK, Fu WY, Ng AK, Chien WW, Ng YP, Wang JH, Ip NY. Cyclin-dependent kinase 5 phosphorylates signal transducer and activator of transcription 3 and regulates its transcriptional activity. Proc Natl Acad Sci USA. 2004;101:6728–6733. doi: 10.1073/pnas.0307606100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Negash S, Guo HT, Ledee D, Wang HS, Zelenka P. CDK5 regulates cell adhesion and migration in corneal epithelial cells. Mol Cancer Res. 2002;1:12–24. [PubMed] [Google Scholar]
- Gao CY, Stepp MA, Fariss R, Zelenka P. Cdk5 regulates activation and localization of Src during corneal epithelial wound closure. J Cell Sci. 2004;117:4089–4098. doi: 10.1242/jcs.01271. [DOI] [PubMed] [Google Scholar]
- Gaudreault E, Thompson C, Stankova J, Rola-Pleszczynski M. Involvement of BLT1 endocytosis and Yes kinase activation in leukotriene B4-induced neutrophil degranulation. J Immunol. 2005;174:3617–3625. doi: 10.4049/jimmunol.174.6.3617. [DOI] [PubMed] [Google Scholar]
- Inada H, Togashi H, Nakamura Y, Kaibuchi K, Nagata K, Inagaki M. Balance between activities of Rho kinase and type 1 protein phosphatase modulates turnover of phosphorylation and dynamics of desmin/vimentin filaments. J Biol Chem. 1999;274:34932–34939. doi: 10.1074/jbc.274.49.34932. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Nishi Y, Nishizawa K, Matsuyama M, Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987;328:649–652. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Matsuoka Y, Tsujimura K, Ando S, Tokui T, Takahashi T, Inagaki N. Dynamic property of intermediate filaments: Regulation by phosphorylation. BioEssays. 1996;18:481–487. [Google Scholar]
- Izawa I, Inagaki M. Regulatory mechanisms and functions of intermediate filaments: A study using site- and phosphorylation state-specific antibodies. Cancer Sci. 2006;97:167–174. doi: 10.1111/j.1349-7006.2006.00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Rosales JL, Tang D, Wang JH. Interaction of cyclin-dependent kinase 5 (Cdk5) and neuronal Cdk5 activator in bovine brain. J Biol Chem. 1996;271:1538–1543. doi: 10.1074/jbc.271.3.1538. [DOI] [PubMed] [Google Scholar]
- Lee KY, Qi Z, Yu YP, Wang JH. Neuronal Cdc2-like kinases: Neuron-specific forms of Cdk5. Int J Biochem Cell Biol. 1997;29:951–958. doi: 10.1016/s1357-2725(97)00048-4. [DOI] [PubMed] [Google Scholar]
- Lee JS, Zhang MH, Yun EK, Geum D, Kim K, Kim TH, Lim YS, Seo JS. Heat shock protein 27 interacts with vimentin and prevents insolubilization of vimentin subunits induced by cadmium. Exp Mol Med. 2005;37:427–435. doi: 10.1038/emm.2005.53. [DOI] [PubMed] [Google Scholar]
- Leitch AE, Riley NA, Sheldrake T, Festa M, Fox S, Duffin R, Haslett C, Rossi AG. The cyclin-dependent kinase inhibitor R-roscovitine down-regulates Mcl-1 to override pro-inflammatory signalling and drive neutrophil apoptosis. Eur J Immunol. 2010;40:1127–1138. doi: 10.1002/eji.200939664. [DOI] [PubMed] [Google Scholar]
- Lew J, Wang JH. Neuronal cdc2-like kinase. Trends Biochem Sci. 1995;20:33–37. doi: 10.1016/s0968-0004(00)88948-3. [DOI] [PubMed] [Google Scholar]
- Matsubara M, Kusubata M, Ishiguro K, Uchida T, Titani K, Taniguchi H. Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J Biol Chem. 1996;271:21108–21113. doi: 10.1074/jbc.271.35.21108. [DOI] [PubMed] [Google Scholar]
- Meshki J, Tuluc F, Bredetean O, Ding Z, Kunapuli SP. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: Role for the P2Y2 receptor. Am J Physiol Cell Physiol. 2004;286:C264–C271. doi: 10.1152/ajpcell.00287.2003. [DOI] [PubMed] [Google Scholar]
- Mor-Vaknin N, Punturieri A, Sitwala K, Markovitz DM. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: Challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–162. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- Nusse O, Serrander L, Lew DP, Krause KH. Ca2+-induced exocytosis in individual human neutrophils: High- and low-affinity granule populations and submaximal responses. EMBO J. 1998;17:1279–1288. doi: 10.1093/emboj/17.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryzwansky KB, Merricks EP. Chemotactic peptide-induced changes of intermediate filament organization in neutrophils during granule secretion: Role of cyclic guanosine monophosphate. Mol Biol Cell. 1998;9:2933–2947. doi: 10.1091/mbc.9.10.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanar JL. Vimentin in cultured chromaffin cells: An immunofluorescent, biochemical and functional study. Cell Physiol Biochem. 2000;10:91–98. doi: 10.1159/000016338. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Ernst JD. Calcium-dependent neutrophil secretion: Characterization and regulation by annexins. J Immunol. 1997;159:6195–6202. [PubMed] [Google Scholar]
- Rosales JL, Ernst JD. GTP-dependent permeabilized neutrophil secretion requires a freely diffusible cytosolic protein. J Cell Biochem. 2000;80:37–45. doi: 10.1002/1097-4644(20010101)80:1<37::aid-jcb40>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Lee KY. Extraneuronal roles of cyclin-dependent kinase 5. BioEssays. 2006;28:1023–1034. doi: 10.1002/bies.20473. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Ernst JD, Hallows J, Lee KY. GTP-dependent secretion from neutrophils is regulated by Cdk5. J Biol Chem. 2004a;279:53932–53936. doi: 10.1074/jbc.M408467200. [DOI] [PubMed] [Google Scholar]
- Rosales JL, Lee BC, Modarressi M, Sarker KP, Lee KY, Jeong YG, Oko R. Outer dense fibers serve as a functional target for Cdk5.p35 in the developing sperm tail. J Biol Chem. 2004b;279:1224–1232. doi: 10.1074/jbc.M310867200. [DOI] [PubMed] [Google Scholar]
- Rossi AG, Sawatzky DA, Walker A, Ward C, Sheldrake TA, Riley NA, Caldicott A, Martinez-Losa M, Walker TR, Duffin R, Gray M, Crescenzi E, Martin MC, Brady HJ, Savill JS, Dransfield I, Haslett C. Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med. 2006;12:1056–1064. doi: 10.1038/nm1468. [DOI] [PubMed] [Google Scholar]
- Sahlgren CM, Mikhailov A, Vaittinen S, Pallari HM, Kalimo H, Pant HC, Eriksson JE. Cdk5 regulates the organization of Nestin and its association with p35. Mol Cell Biol. 2003;23:5090–5106. doi: 10.1128/MCB.23.14.5090-5106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuang R, Zhang L, Fletcher A, Groblewski GE, Pevsner J, Stuenkel EL. Regulation of Munc-18/syntaxin 1A interaction by cyclin-dependent kinase 5 in nerve endings. J Biol Chem. 1998;273:4957–4966. doi: 10.1074/jbc.273.9.4957. [DOI] [PubMed] [Google Scholar]
- Styers ML, Salazar G, Love R, Peden AA, Kowalczyk AP, Faundez V. The endolysosomal sorting machinery interacts with the intermediate filament cytoskeleton. Mol Biol Cell. 2004;15:5369–5382. doi: 10.1091/mbc.E04-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Yeung J, Lee KY, Matsushita M, Matsui H, Tomizawa K, Hatase O, Wang JH. An isoform of the neuronal cyclin-dependent kinase 5 (Cdk5) activator. J Biol Chem. 1995;270:26897–26903. doi: 10.1074/jbc.270.45.26897. [DOI] [PubMed] [Google Scholar]
- Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J. 2005;388:773–783. doi: 10.1042/BJ20050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao JX, Ip W. Site-specific antibodies block kinase A phosphorylation of desmin in vitro and inhibit incorporation of myoblasts into myotubes. Cell Motil Cytoskeleton. 1991;19:109–120. doi: 10.1002/cm.970190206. [DOI] [PubMed] [Google Scholar]
- Wei S, Liu JH, Epling-Burnette PK, Gamero AM, Ussery D, Pearson EW, Elkabani ME, Diaz JI, Djeu JY. Critical role of Lyn kinase in inhibition of neutrophil apoptosis by granulocyte-macrophage colony-stimulating factor. J Immunol. 1996;157:5155–5162. [PubMed] [Google Scholar]
- Whyte MK, Meagher LC, MacDermot J, Haslett C. Impairment of function in aging neutrophils is associated with apoptosis. J Immunol. 1993;150:5124–5134. [PubMed] [Google Scholar]
- Wyatt TA, Pryzwansky KB, Lincoln TM. KT5823 activates human neutrophils and fails to inhibit cGMP-dependent protein kinase phosphorylation of vimentin. Res Commun Chem Pathol Pharmacol. 1991;74:3–14. [PubMed] [Google Scholar]