Summary
Targeted therapeutic approaches have seen tremendous advances in the last decade, for good reason. Specifically intervening with a disease-causing gene can revert the deleterious phenotype while eliminating the toxicity often associated with broad-spectrum agents. Unfortunately, because these selective agents hit one target in a single location, acquired resistance is often high. An arguably better treatment approach includes coupling multiple targeted agents or using an agent that hits an individual target in several independent locations and/or alters multiple relevant targets in the disease-causing pathway(s), precisely the approach taken by George Calin, Anil Snood, and colleagues in their recent report aimed at identifying a better treatment option for ovarian cancer (1).
Because acquired resistance to a therapy, often brought on by a single mutation, can easily select for tumor cells that can evade the therapy, a second agent or agents that hit multiple tumor-addicted genes need to be evaluated and included in the therapy. Both of these characteristics are inherent to a class of endogenously expressed, small, non-coding RNAs termed microRNAs (miRNAs). Due to their some-what promiscuous behavior a single miRNA can bind to and modulate the expression of multiple target genes and in many instances the presence of numerous miRNA binding sites in the target ensures target gene repression. Nevertheless, because binding between the target and the mRNA is imperfect, repression is typically modest. Reducing the expression of one gene below its critical threshold is possible; however not always achievable with a miRNAs. For genes that require additional silencing or near-complete ablation small-interfering RNAs (siRNAs) are more effective. Unlike miRNAs, siRNAs bind with perfect complementarity in a very stringent manner to rigorously downregulate a single target. Yet, siRNAs are not endogenously encoded, which may contribute to off target effects and toxicity, and they only regulate a single gene increasing the chances of required resistance. One could imagine that in the right situation capitalizing on the benefits of a miRNA with that of an siRNA while circumventing their challenges may produce an enhanced therapeutic effect.
The study performed in the labs of Calin and Sood focused on identifying a superior treatment option for ovarian cancer through administering a combination of an siRNA that rigorously and specifically downregulates the EphA2 receptor with a miRNA that has a broader impact on targeting the Eph (erythropoietin-producing hepatocellular) receptor family and other potential targets (Figure 1) (1). EphA2 is overexpressed in more than 75% of ovarian cancer cases (2) and in the absence of cell-to-cell contact, which results in inefficient interaction of EphA2 with its ligand on adjacent cells, sustained MAPK and RhoA signing occurs leading to tumor promotion (reviewed in (3)). Silencing EphA2 through a variety of mechanisms has been shown to slow ovarian cancer cell growth, as such the approach taken by Calin and Sood, once validated, may be useful for treating thousands of ovarian cancer cases as well as breast, prostate, lung and colon cancers, which also present with overexpression of EphA2. While targeting this pathway is not novel, the approach taken by Calin and Sood is. Prior to this study nobody had yet assessed the combined efficacy of directly silencing EphA2, using an siRNA, with indirectly silencing other critical pathways with a specific miRNA.
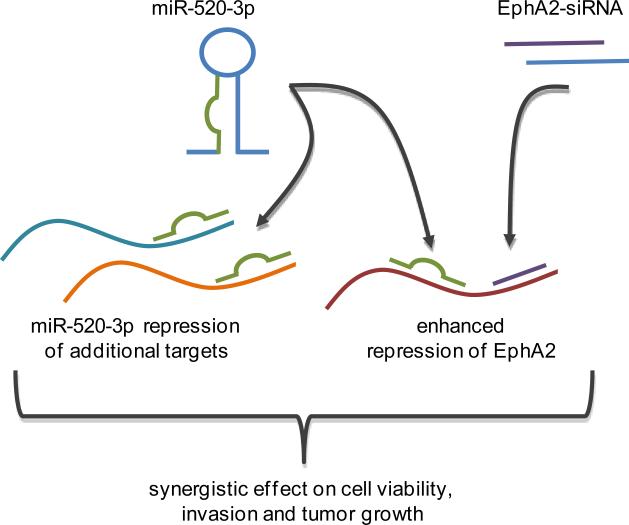
Figure 1.
A multi-RNA therapeutic approach to treating ovarian cancer. The study reported by Calin and Sood evaluated the efficacy of the EphA2-siRNA with miR-520-3p, which also targets EphA2 as well as other Eph-receptors. The utility of both small RNAs resulted in a synergistic response with respect to cell viability, invasion and tumor growth.
The group began by probing The Cancer Genome Atlas for a biologically relevant miRNA that was associated with response to therapy and overall survival of ovarian cancer, and was predicted to target EphA2. Their detailed investigation and validation study determined that high expression of the miRNA, miR-520-3p, was a favorable predictor for ovarian cancer patient survival. Furthermore, an inverse correlation of EphA2 and miR-520-3p was identified, and combining the expression levels of EphA2 and miR-520-3p enhanced the ability to predict patient survival. Functionally similar to the EphA2-siRNA, miR-520-3p inhibited migration, invasion, and tumor growth. This effect was dependent on the silencing of EphA2, which was identified as a direct target of miR-520-3p. It was also determined that miR-520-3p directly targets EphB2 and including the EphB2 expression data with the gene signatures of EphA2 and miR-520-3p further stratified the patient survival data.
Perhaps the most extraordinary part of the study was when the authors combined the two small RNAs into a single therapeutic and showed that the EphA2-siRNA, entering into clinical trial at MD Anderson (NCT01591356), and miR-520-3p synergized. The combination of these small RNAs enhanced repression of the EphA2 protein that translated into a synergistic effect on reducing cellular viability, decreasing cellular invasion, and inhibiting tumor growth in vivo. Other functional readouts were suggestive of an additive response; perhaps not surprising since there is overlap between the miRNA and the siRNA in their ability to target EphA2. Regardless, the long-term effects of using these two small RNAs are predicted to be greater than using either agent alone due to overcoming the potential acquired resistance of a monotherapy. Furthermore, modulating the expression of multiple Eph-receptors through miR-520-3p while strongly silencing the EphA2 receptor affords the cells limited compensatory pathways that it can use to evade this dual therapeutic approach (Figure 1).
These studies open the door to a multitude of potential therapeutic options. Using various agents in combination is not new, but as of yet the research to support the utility of small RNAs in combination is limited. Indeed individual siRNAs and more recently miRNAs and their antagonizing counterparts, antagomiRs, have entered into clinical trials (4-7). As researchers begin to understand the biological outcomes of silencing miRNAs or overexpressing siRNAs or miRNAs the ability to target relevant biological pathways in combination becomes an achievable reality. In the past many of these studies were bottlenecked at the level of delivering these small RNAs. However, the first miRNA is now in clinical trials (6) and as the anticipation of this trial is building scientist are beginning to evaluate the next generation of small RNA therapeutics: combining multiple RNAs into a single cocktail. It is expected that adding two or more miRNAs, antagomirs, or siRNAs, or a combination there of may have an enhanced effect if the agents are critically selected for based on the etiology of the disease, as performed by Calin and Sood.
References
- 1.Nishimura M, Jung E- J, Shah MY, Lu C, Spizzo R, Shimizu M, et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-13-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin. Ther. Targets. 2005;9:1179–87. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 3.Tandon M, Vemula SV, Mittal SK. Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets. 2011;15:31–51. doi: 10.1517/14728222.2011.538682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidson BL, McCray PB. Current prospects for RNA interference-based therapies. Nat. Rev. Genet. 2011;12:329–40. doi: 10.1038/nrg2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janssen HLA, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 6.Bouchie A. First microRNA mimic enters clinic. Nat Biotechnol. 2013;31:577. doi: 10.1038/nbt0713-577. [DOI] [PubMed] [Google Scholar]
- 7.Kasinski AL, Slack FJ. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nature Reviews Cancer. 2011;11:849–64. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]