Highlights
-
•
Human bocavirus 1 (HBoV1) causes wheezing in young children with acute respiratory tract infections.
-
•
We tested two commercially available human airway epithelium (HAE) cultures, EpiAirway and MucilAir HAE, for HBoV1 infection.
-
•
Both HAE cultures fully support productive HBoV1 infection, and cause a significant airway epithelial damage.
-
•
We demonstrated, for the first time, the persistence of HBoV1 infection in EpiAirway HAE for as long as 50 days.
Keywords: Parvovirus, Human bocavirus
Abstract
Human bocavirus 1 (HBoV1), a human parvovirus, belongs to the genus Bocavirus of the Parvoviridae family. It causes wheezing in young children with acute respiratory tract infections. HBoV1 has been shown to infect polarized human airway epithelium (HAE) made in house, and induces airway epithelial damage. In this study, two commercially available HAE cultures, EpiAirway and MucilAir HAE, were examined for HBoV1 infection. Both HAE cultures support fully productive HBoV1 infection. Infected EpiAirway and MucilAir HAE cultures showed loss of cilia, disruption of the tight junction barrier, and a significant decrease in transepithelial electrical resistance. Notably, HBoV1 persistent infection was demonstrated by maintaining HBoV1-infected EpiAirway HAE for as long as 50 days. After 2 days post-infection, progeny virus was produced consistently daily at a level of over 2 × 108 viral genome copies per culture (0.6 cm2). This study is the first to use commercial sources of HAE cultures for HBoV1 infection. The availability of these cultures will enable a wide range of laboratories to study HBoV1 infection.
1. Introduction
Human bocavirus 1 (HBoV1) was discovered in 2005 by large scale sequencing in nasopharyngeal aspirates from young children (Allander et al., 2005). It is a respiratory virus associated with acute respiratory tract infections in young children (Allander et al., 2007, Brodzinski and Ruddy, 2009, Don et al., 2010, Garcia-Garcia et al., 2010, Gendrel et al., 2007, Jartti et al., 2011, Kahn, 2008, Proenca-Modena et al., 2011, Schildgen et al., 2008). Acute respiratory tract infection is one of the leading causes of hospitalization of young children in developed countries (Brodzinski and Ruddy, 2009, Lopez et al., 2006, Shay et al., 1999). HBoV1 infection shows frequently persistence and co-infections with other respiratory viruses (Jartti et al., 2011, Kesebir et al., 2006, Manning et al., 2006, Wang et al., 2010). However, acute HBoV1 infection, diagnosed by a high virus load in respiratory samples, viremia, detection of HBoV1-specific IgM, or an increase in the levels of HBoV1-specific IgG antibodies, and detection of HBoV1 mRNA in nasopharyngeal aspirates, results in respiratory illness (Allander et al., 2007, Christensen et al., 2010, Christensen et al., 2013, Deng et al., 2012, Don et al., 2010, Kantola et al., 2008, Nascimento-Carvalho et al., 2012, Soderlund-Venermo et al., 2009, Wang et al., 2010). Life-threatening HBoV1 infections in pediatric patients, which were associated with high virus loads or diagnostic HBoV1-specific antibodies (Edner et al., 2011, Korner et al., 2011, Ursic et al., 2011), have also been described. Moreover, a recent longitudinal study in children (from infants to puberty) documented a clear association between acute primary HBoV1 infection and respiratory symptoms (Meriluoto et al., 2012).
The virus was tentatively classified as a member of genus Bocavirus in the subfamily Parvovirinae of the Parvoviridae family (Tijssen et al., 2012). Parvoviruses are small, non-enveloped icosahedral viruses with linear single-stranded DNA genomes. The genus Bocavirus includes bovine parvovirus type 1 (BPV1) (Chen et al., 1986), minute virus of canines (MVC) (Sun et al., 2009), and other tentative members that are closely related to HBoV1, including porcine bocavirus (Zhai et al., 2010), gorilla bocavirus (Kapoor et al., 2010a) and HBoV genotypes 2–4 (Arthur et al., 2009, Kapoor et al., 2009, Kapoor et al., 2010b)
A pseudostratified and well-differentiated primary human airway epithelium (HAE) culture model has been used widely to infect respiratory RNA viruses from the apical surface, e.g., influenza viruses (Ilyushina et al., 2012, Matrosovich et al., 2004, Zeng et al., 2011), parainfluenza virus (Zhang et al., 2005, Zhang et al., 2011), respiratory syncytial virus (Villenave et al., 2012, Zhang et al., 2002), human coronaviruses (Pyrc et al., 2010, Sims et al., 2005, Wang et al., 2000), and human rhinovirus type C (Hao et al., 2012). Although respiratory DNA viruses have been reported to infect HAE, they infect HAE only from the basolateral surface, e.g., adenovirus (Zabner et al., 1997). In 2008, Dijkman et al. demonstrated that HBoV1 infects apically and replicates in HAE made in house (Dijkman et al., 2009). In 2012, an infectious clone of HBoV1 was established, which generates HBoV1 progeny virions from HEK293 cells transfected with this clone. Moreover, these HBoV1 virions infected HAE made in house productively, from the apical surface (Huang et al., 2012) as well as from the basolateral surface (Deng et al., 2013), which leads to airway epithelial damage.
In this report, two commercially available HAE cultures, i.e., EpiAirway and MucilAir HAE purchased from MatTek Co. (MA, USA) and Epithelix SàRL (Geneva, Switzerland), respectively, were tested for HBoV1 infection. Both HAE cultures can be infected by HBoV1 and caused hallmarks of airway epithelial damage.
2. Materials and methods
2.1. Polarized primary HAE cultures
EpiAirway HAE, which was purchased from MatTek (Ashland, MA, USA), was cultured in a Millicell insert of 0.6 cm2 (Millipore, Billerica, MA, USA). MucilAir HAE was obtained from Epithelix SàRL (Geneva, Switzerland), and was maintained in a Costar Transwell insert of 0.33 cm2 (Corning, NY, USA). Both HAE cultures were derived from healthy human primary tracheobronchial epithelial cells (derived from individual donors) cultured in an air-liquid interphase (ALI) with their respective property media.
2.2. Virus and infection
HBoV1 virions were collected from apical washes of HBoV1-infected primary B-HAE (Huang et al., 2012), and were used for infection as described previously (Deng et al., 2013).
For apical infection of EpiAirway HAE, HBoV1 virions were diluted in 200 μl of EpiAirway medium to achieve an MOI of 100 viral genomic copy numbers (gc)/cell, and were applied to the apical chamber. For apical infection of MucilAir HAE, HBoV1 was diluted in 100 μl of MucilAir medium to reach an MOI of 100 gc/cell. After incubation for 2 h, the apical chamber of the infected HAE ALI was washed three times with 400 μl and 200 μl of PBS for EpiAirway and MucilAir HAE, respectively. The cultures were maintained continuously at an ALI. To determine virus release kinetics, 200 μl and 150 μl aliquots of PBS, for EpiAirway and MucilAir HAE, respectively, were added to the apical chamber of the HAE culture at various time points, incubated for 15 min at 37 °C, and were harvested as apical washes. Meanwhile, an aliquot of 100 μl of medium from the basolateral chamber of the HAE culture was collected, followed by replacement with 100 μl of fresh medium. All the harvested aliquots were stored at 4 °C for quantification of viral DNA as gc.
For basolateral infection, HBoV1 virions were diluted in the ALI medium (1 ml and 0.5 ml for EpiAirway and MucilAir, respectively) in the basolateral chamber of the HAE cultures. The cultures were incubated at 37 °C and 5% CO2 for 2 h, and then the basolateral inoculums were removed and washed three times with PBS (1 ml and 0.5 ml for EpiAirway and MucilAir, respectively), followed by supplementation of fresh media. Progeny virion release was monitored daily by quantification of viral gc in samples collected from the basolateral chamber at a volume of 100 μl.
2.3. Real time quantitative PCR (qPCR) analysis of HBoV1 genome copy numbers
Aliquots of 100 μl of apical sample and 50 μl of basolateral sample, respectively, were incubated with 25 units of benzonase (Sigma, St. Louis, MO, USA) for 2 h at 37 °C. The treated samples were digested with 20 μl of proteinase K (15 mg/ml; Amresco, Solon, OH, USA) at 56 °C for 10 min. Viral DNA was extracted using the QIAamp blood mini kit (Qiagen, Valencia, CA, USA), and eluted in 100 μl and 50 μl of deionized H2O for apical and basolateral samples, respectively. The extracted DNA samples were quantified with respect to the numbers of HBoV1 gc by the qPCR method described previously (Deng et al., 2013, Huang et al., 2012, Lin et al., 2007).
2.4. Immunofluorescence analysis
Immunofluorescence analysis of HBoV1-infected HAE was performed as described previously (Deng et al., 2013, Huang et al., 2012). A rat anti-HBoV1 NS1 polyclonal antibody (Chen et al., 2010) was developed previously in-house (Chen et al., 2010). Anti-ZO-1 (Invitrogen, Grand Island, NY, USA) and anti-β-tubulin IV (Sigma, St. Louis, MO, USA) antibodies were used for detecting the tight junction and cilia, respectively.
2.5. Measurement of the transepithelial electrical resistance (TEER)
The TEER of both mock- and HBoV1-infected HAE cultures was measured using an epithelial volt-ohm meter (Millipore, Billerica, MA, USA) at the indicated days p.i. as described previously (Deng et al., 2013, Huang et al., 2012).
3. Results
3.1. HBoV1 infects EpiAirway HAE persistently
EpiAirway HAE was first infected with the apically washed HBoV1 virions at a multiplicity of infection (MOI) of 100 gc/cell. Progeny virions released from the apical surface at 1 × 106 gc/μl at 3 days post-infection (p.i.), and remained as a plateau along with the infection for 50 days (Fig. 1A). Virus was not detected in mock-infected EpiAirway HAE. Since 200 μl of phosphate buffered saline (pH 7.4; PBS) was used to wash the progeny virions from the apical surface every day, the total virus release from one HAE culture was approximately 2 × 108 gc per day. Virus was also released from the basolateral surface, but at a level of ∼1–2 log lower than that from the apical surface (Fig. 1A).
Fig. 1.
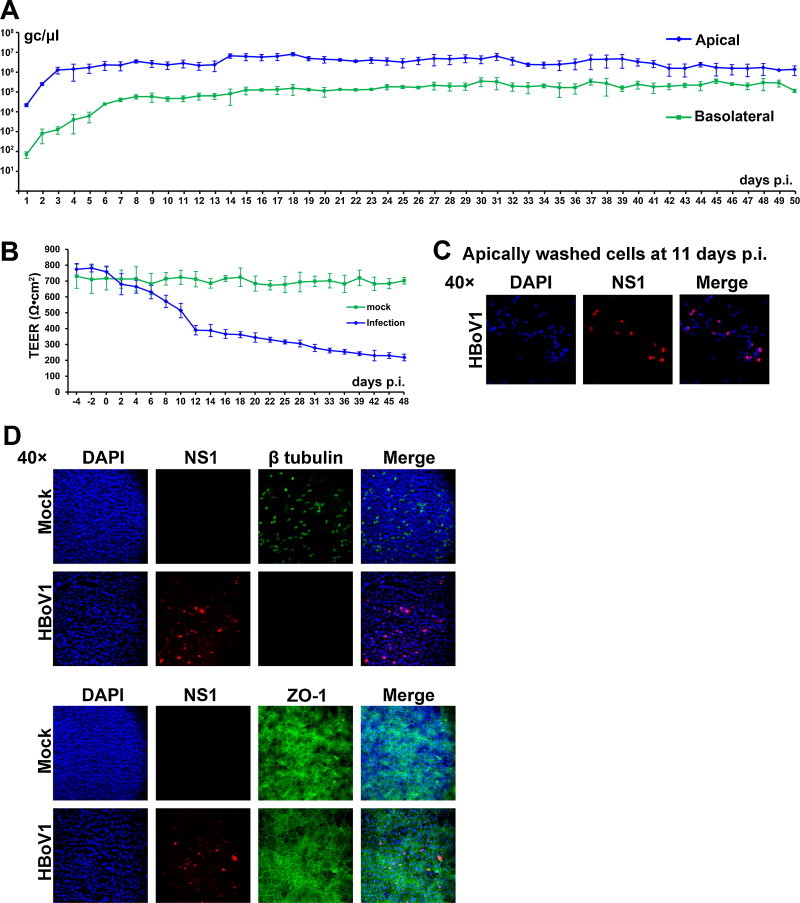
HBoV1 infects EpiAirway HAE productively and persistently. EpiAirway HAE was infected with the apically washed HBoV1 at an MOI of 100 gc/μl. (A) At the indicated days p.i., HBoV1 virions were collected from both the apical and basolateral chambers and quantified by qPCR. Averages and standard deviations of the viral gc/μl are shown. (B) The TEER of HBoV1-infected EpiAirway HAE was monitored at the indicated days p.i. Averages and standard deviations of the TEER are shown. (C) At 11 days p.i., the apical chambers of three infected EpiAirway HAE cultures were washed with 200 μl of PBS to collect the apically washed cells. A total of 600 μl of the sample was concentrated to 200 μl, which was then cytospun onto a slide followed by immunofluorescence analysis with an anti-HBoV1 NS1 antibody. Confocal images were taken with an Eclipse C1 Plus confocal microscope (Nikon, Melville, NY, USA) controlled by Nikon EZ-C1 software, at a magnification of 40× as indicated. (D) At 50 days p.i., insert membranes of the infected EpiAirway HAE were fixed and analyzed by an immunofluorescence assay with an anti-HBoV1 NS1 antibody with co-staining of anti-β-tubulin IV or anti-ZO1 antibody. Confocal images were taken at a magnification of 40×. Nuclei were stained with DAPI (blue).
Consistent with the virus release kinetics, the transepithelial electrical resistance (TEER) of HBoV1-infected HAE started to drop off at 4 days p.i., gradually lowering to a level of 390 Ω cm2 at 12 days p.i., 306 Ω cm2 at 28 days p.i, and to a level close to 200 Ω cm2 at 48 days p.i. (Fig. 1B). Compared with the mock-infected counterpart, the TEER decreased ∼3.5-fold by the end of infection (at 48 days p.i., Fig. 1B).
Of note, a few of the epithelial cells were washed off in the apical chamber of HBoV1-infected HAE, and many of which were NS1-positive (Fig. 1C), suggesting that HBoV1 infection induces epithelial cell death. By the end of the infection (at 50 days p.i.), β-tubulin IV (a marker of cilia (Matrosovich et al., 2004, Villenave et al., 2012)), the tight junction protein zona occludens-1 (ZO-1) (Gonzalez-Mariscal et al., 2003), as well as HBoV1 NS1 expression, was detected. Although only one third of the cells in the HBoV1-infected EpiAirway HAE expressed NS1, the infected HAE showed no expression of β-tubulin IV and a disassociation of the ZO-1 (Fig. 1D).
In addition, EpiAirway HAE was infected at the basolateral surface. A gradual virus release from both the apical and basolateral surface was observed from 1 to 11 days p.i. Virus release from apical infection reached a plateau (∼2 × 106 gc/μl) at 12 days p.i.; however, there was ∼1–1.5 log less virus released from the basolateral surface during infection between 10 and 26 days p.i. (Fig. 2A). Basolateral infection also caused significant epithelial damage represented by the gradual decrease in TEER (Fig. 2B), cilia loss and destruction of the tight junction (Fig. 2C).
Fig. 2.
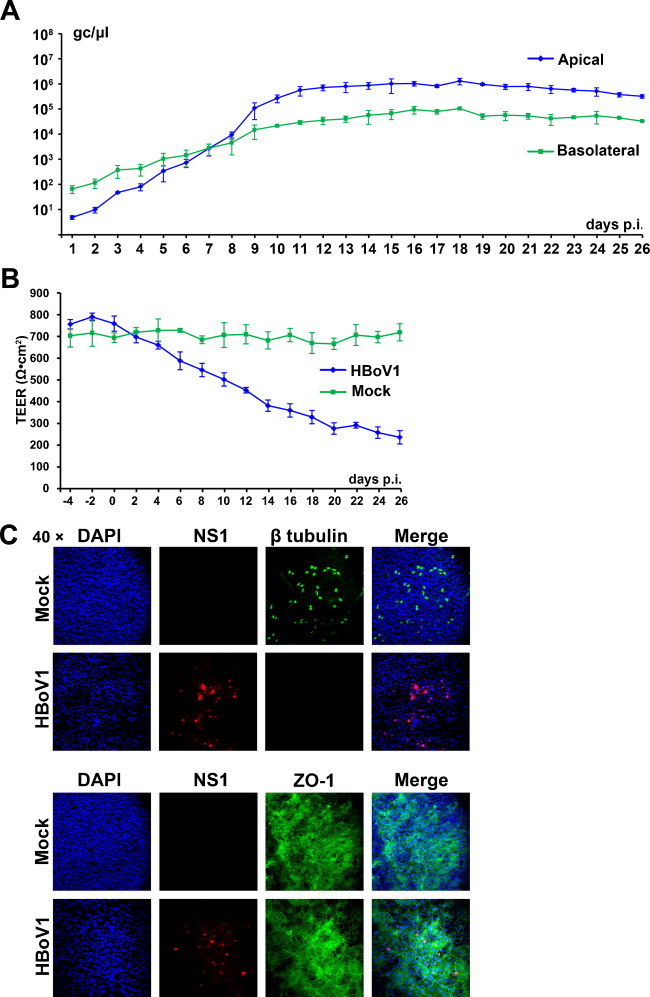
Analyses of EpiAirway HAE infected with HBoV1 basolaterally. EpiAirway HAE was infected with HBoV1 at an MOI of 100 gc/μl from the basolateral surface. (A) At the indicated days p.i., both apically washed and basolaterally collected samples were quantified by qPCR as gc/μl, which are plotted to the days p.i. and shown as averages and standard deviations. (B) The TEER of HBoV1-infected HAE was monitored at the indicated days p.i. Averages and standard deviations are shown. (C) At the end of the infection, insert membranes of the HAE cultures were fixed and stained with anti-HBoV1 NS1 and anti-β-tubulin IV or anti-ZO1 antibodies. Nuclei were stained with DAPI (blue). The cells were visualized by confocal microscopy at a magnification of 40×.
Taken together, these results suggest that HBoV1 infects EpiAirway HAE both apically and basolaterally, and that the infection is persistent and causes airway epithelial damage.
3.2. HBoV1 infects MucilAir HAE cultures
MucilAir HAE was infected with HBoV1 either apically or basolaterally at an MOI of 100 gc/cell. Virus production after infection was monitored by collecting samples from both the apical and basolateral chambers of the infected HAE.
During apical infection, progeny virions were released apically at a level of 2 × 104 gc/μl at 3 days p.i., reached a peak of 7 × 106 gc/μl at 9 days p.i., and gradually decreased to 2 × 105 gc/μl at 26 days p.i. (Fig. 3A). Virus was also released from the basolateral surface, but at a level of 1–2 log less than that from the apical surface (Fig. 3A). Mock-infected HAE did not release any virions from either the apical or basolateral surfaces. During basolateral infection, virions were released much more slowly in early infection (at 1–10 days p.i.), reached a peak (1 × 106 and 1 × 105 gc/μl, respectively, from the apical and basolateral surfaces) at 12–13 days p.i., and gradually decreased to levels of 8 × 104 and 5 × 103 gc/μl by the end of infection (Fig. 3B).
Fig. 3.
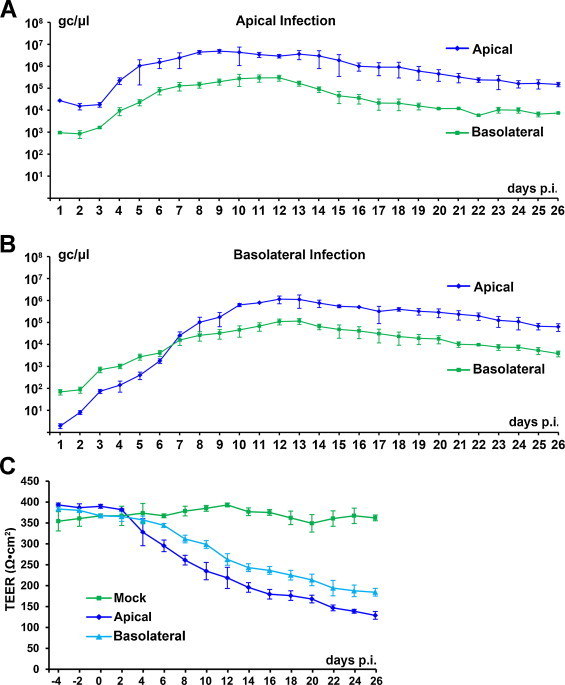
Kinetics of HBoV1-infected MucilAir HAE. MucilAir HAE was infected with HBoV1 from either the apical (A) or basolateral (B) surface at an MOI of 100 gc/μl. (A and B) At the indicated days p.i., progeny virions were collected from both the apical and basolateral chambers. HBoV1 virions were quantified by qPCR as viral gc/μl. Averages and standard deviations of the viral gc/μl are shown. (C) The TEER of HBoV1-infected HAE was monitored at the indicated days p.i. Averages and standard deviations of the detected TEER are shown.
The TEER was detected to monitor the epithelial barrier function of the HAE affected by HBoV1 infection. Although the TEER of the MucilAir HAE was initially low (300–400 Ω cm2), after HBoV1 infection, it decreased further by ∼2- to 3-fold, gradually to levels of 130 and 180 Ω cm2 during apical and basolateral infection, respectively, at 26 days p.i. (Fig. 3C), which is consistent with the virus release kinetics (Fig. 3A and B).
At 26 days p.i., al least one third of the cells in the infected HAE expressed HBoV1 NS1 (Fig. 4A). Either apically- or basolaterally infected MucilAir HAE lost cilia and the tight junction, as shown by staining with anti-β-tubulin IV (Fig. 4B) and anti-ZO1 (Fig. 4C) antibodies, respectively, and showed an obvious nucleus enlargement (Fig. 4B and C, DAPI), strongly suggesting airway epithelial damage.
Fig. 4.
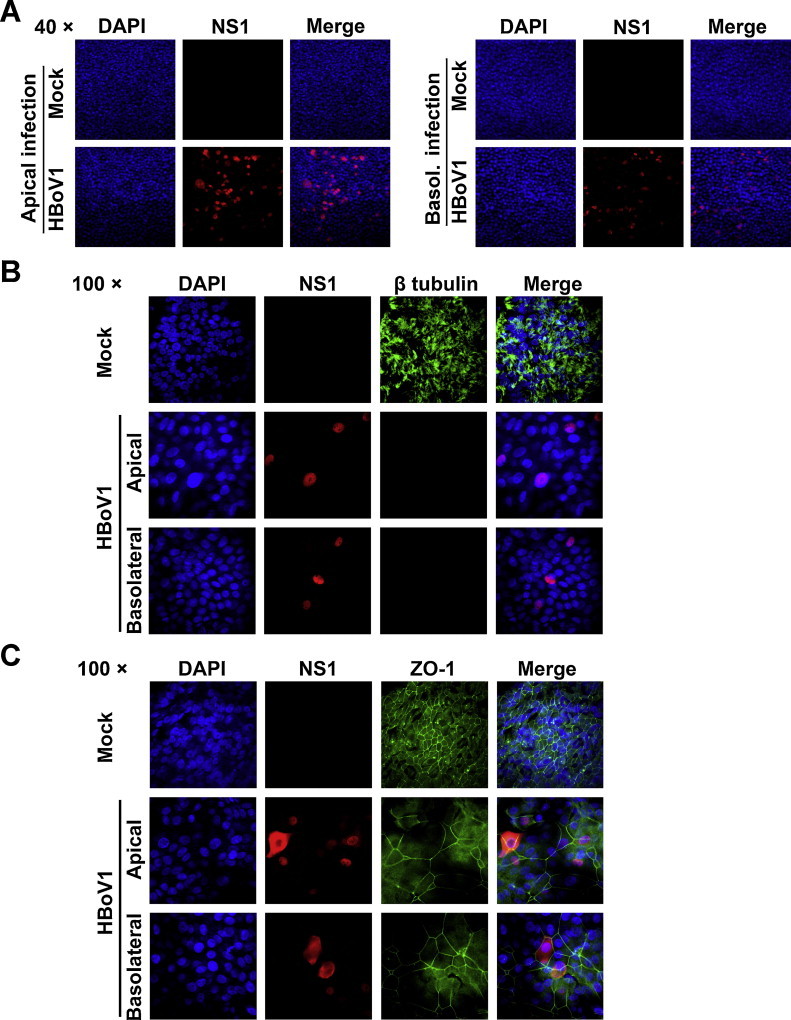
HBoV1 infection of MucilAir HAE causes airway epithelial damage. At the end of the infection (26 days p.i.), insert membranes of the infected MucilAir HAE cultures were fixed and stained with an anti-HBoV1 NS1 antibody (A), or co-stained with anti-HBoV1 NS1 and anti-β-tubulin IV antibody (B) and with anti-HBoV1 NS1 and anti-ZO-1 antibodies (C). Nuclei were stained with DAPI (blue). The stained cells were visualized by confocal microscopy at magnifications of 40× and 100×, as indicated.
4. Discussion
Pseudostratified and polarized HAE cultures have been reported for productive HBoV1 infection (Deng et al., 2013, Dijkman et al., 2009, Huang et al., 2012), but they were made in house, and thus were not available for most of the researchers in the field. In this report, HBoV1 infection was studied in polarized primary HAE cultures, MucilAir and EpiAirway HAE, purchased from MatTek Co. (Ashland, MA, USA) and Epithelix SàRL (Geneva, Switzerland), respectively. HBoV1 progeny virions were released from both infected MucilAir and EpiAirway HAE cultures, but are at an average of ∼1–2 × 108 gc per insert. These results suggest that the commercially available HAE cultures support productive HBoV1 infection. Thus, our study provides valuable information to culture HBoV1 and study HBoV1 infection in primary HAE.
Notably, HBoV1 infection can be persistent in EpiAirway HAE for at least 50 days p.i. Previous studies have identified the presence of episomal covalently closed circular (ccc) viral DNA in patients infected with HBoV1-3 (Kang et al., 2009, Kapoor et al., 2011, Windisch et al., 2013, Zhao et al., 2012). However, whether the cccDNA is the viral genomes persisting in HBoV1-infected EpiAirway HAE awaits further investigation, which will reveal mechanisms underlying HBoV1 persistent infection. The TEER of airway epithelium often indicates the extent of polarization and was used as a quality control of polarized HAE cultures from the manufacturers. Previously, primary B-HAE cultures made in house was used, which have a TEER of >1000 Ω cm2 (Huang et al., 2012). However, both MucilAir and EpiAirway HAE had a low TEER (400 and 800 Ω cm2, respectively) when we received them. Nevertheless, HBoV1 infection disrupts the polarity of both the MucilAir and EpiAirway HAE cultures infected with HBoV1. The TEER of the infected MucilAir and EpiAirway HAE decreased by 2- to 3-fold, similar to the decrease in TEER in HBoV1-infected HAE made in house (Deng et al., 2013). Notably, in the HAE cultures we tested, there was a higher expression level of cilia in MucilAir HAE than in EpiAirway HAE (Fig. 2, Fig. 4, compare the β-tubulin). Apparently, the high expression level of cilia did not contribute a high level of virus production from infected MucilAir HAE. Interestingly, by the end of infection, all the cilia in the infected HAE of both types were lost, although only one third of the cells showed NS1 expression, suggesting that HBoV1 infection may release cytotoxic cytokines into the cultures as do RNA respiratory viruses (Vareille et al., 2011), which warrants further investigation.
Because of the nature of the MucilAir and EpiAirway HAE insert membrane materials, further histology analyses of the airway epithelia were not successful in either frozen section or paraffin-embedded section (data not shown). However, for the MucilAir HAE, the polyethylene terephthalate (PET) membrane of the Transwell insert provides a better cell visualization that allowed us to take confocal images of directly fixed infected cells on the insert membrane at a high magnification (Fig. 4B and C), which show much clearer nucleus enlargement of the cells and destruction of the epithelial tight junction in the infected HAE. MucilAir HAE has been used to infect influenza virus (Brookes et al., 2011), respiratory syncytial virus and human rhinovirus (Huang et al., 2011), and EpiAirway HAE has been reported to infect influenza virus (Triana-Baltzer et al., 2010), parainfluenza virus (Moscona et al., 2010, Palermo et al., 2009, Palmer et al., 2012), human rhinovirus (Sharma et al., 2010), and respiratory syncytial virus (Donalisio et al., 2012). MucilAir HAE was cultured in a Transwell insert of 0.33 cm2 (Costar), while EpiAirway HAE was cultured in a Millicell insert of 0.6 cm2 (Millipore). Both HAE cultures are suitable model systems to study HBoV1 for many laboratories, which may lack experience to make primary HAE in house.
In conclusion, this study supports strongly that HBoV1 is a highly infectious respiratory virus. Two sources of HAE cultures, MucilAir and EpiAirway HAE, are commercially available for productive HBoV1 infection, which will facilitate the study of HBoV1 worldwide. More importantly, the airway barrier function was destroyed in both HBoV1-infected MucilAir and EpiAirway HAE. For the first time, HBoV1 infection was demonstrated to be persistent, for as long as 50 days p.i., in an in vitro model of human airway epithelia.
Acknowledgements
We thank Dr. Sarah Tague for help in taking confocal microscopy images and members of the Qiu lab for valuable discussion.
References
- Allander T., Jartti T., Gupta S., Niesters H.G., Lehtinen P., Osterback R., Vuorinen T., Waris M., Bjerkner A., Tiveljung-Lindell A., van den Hoogen B.G., Hyypia T., Ruuskanen O. Human bocavirus and acute wheezing in children. Clin. Infect. Dis. 2007;44:904–910. doi: 10.1086/512196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allander T., Tammi M.T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. U.S.A. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur J.L., Higgins G.D., Davidson G.P., Givney R.C., Ratcliff R.M. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009;5:e1000391. doi: 10.1371/journal.ppat.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodzinski H., Ruddy R.M. Review of new and newly discovered respiratory tract viruses in children. Pediatr. Emerg. Care. 2009;25:352–360. doi: 10.1097/PEC.0b013e3181a3497e. [DOI] [PubMed] [Google Scholar]
- Brookes D.W., Miah S., Lackenby A., Hartgroves L., Barclay W.S. Pandemic H1N1 2009 influenza virus with the H275Y oseltamivir resistance neuraminidase mutation shows a small compromise in enzyme activity and viral fitness. J. Antimicrob. Chemother. 2011;66:466–470. doi: 10.1093/jac/dkq486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A.Y., Cheng F., Lou S., Luo Y., Liu Z., Delwart E., Pintel D., Qiu J. Characterization of the gene expression profile of human bocavirus. Virology. 2010;403:145–154. doi: 10.1016/j.virol.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K.C., Shull B.C., Moses E.A., Lederman M., Stout E.R., Bates R.C. Complete nucleotide sequence and genome organization of bovine parvovirus. J. Virol. 1986;60:1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Døllner H., Shanke L.H., Krokstad S., Moe N., Nordbø S.A. Detection of spliced mRNA from human bocavirus 1 in clinical samples from children with respiratory tract infections. Emerg. Infect. Dis. 2013;19:574–580. doi: 10.3201/eid1904.121775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen A., Nordbo S.A., Krokstad S., Rognlien A.G., Dollner H. Human bocavirus in children: mono-detection, high viral load and viraemia are associated with respiratory tract infection. J. Clin. Virol. 2010;49:158–162. doi: 10.1016/j.jcv.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X., Yan Z., Luo Y., Xu J., Cheng Y., Li Y., Engelhardt J., Qiu J. In vitro modeling of human bocavirus 1 infection of polarized primary human airway epithelia. J. Virol. 2013;87:4097–4102. doi: 10.1128/JVI.03132-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y., Gu X., Zhao X., Luo J., Luo Z., Wang L., Fu Z., Yang X., Liu E. High viral load of human bocavirus correlates with duration of wheezing in children with severe lower respiratory tract infection. PLoS One. 2012;7:e34353. doi: 10.1371/journal.pone.0034353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkman R., Koekkoek S.M., Molenkamp R., Schildgen O., van der Hoek L. Human bocavirus can be cultured in differentiated human airway epithelial cells. J. Virol. 2009;83:7739–7748. doi: 10.1128/JVI.00614-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Don M., Soderlund-Venermo M., Valent F., Lahtinen A., Hedman L., Canciani M., Hedman K., Korppi M. Serologically verified human bocavirus pneumonia in children. Pediatr. Pulmonol. 2010;45:120–126. doi: 10.1002/ppul.21151. [DOI] [PubMed] [Google Scholar]
- Donalisio M., Rusnati M., Cagno V., Civra A., Bugatti A., Giuliani A., Pirri G., Volante M., Papotti M., Landolfo S., Lembo D. Inhibition of human respiratory syncytial virus infectivity by a dendrimeric heparan sulfate-binding peptide. Antimicrob. Agents Chemother. 2012;56:5278–5288. doi: 10.1128/AAC.00771-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edner N., Castillo-Rodas P., Falk L., Hedman K., Soderlund-Venermo M., Allander T. Life-threatening respiratory tract disease with human bocavirus-1 infection in a four-year-old child. J. Clin. Microbiol. 2011;50:531–532. doi: 10.1128/JCM.05706-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Garcia M.L., Calvo C., Falcon A., Pozo F., Perez-Brena P., De Cea J.M., Casas I. Role of emerging respiratory viruses in children with severe acute wheezing. Pediatr. Pulmonol. 2010;45:585–591. doi: 10.1002/ppul.21225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendrel D., Guedj R., Pons-Catalano C., Emirian A., Raymond J., Rozenberg F., Lebon P. Human bocavirus in children with acute asthma. Clin. Infect. Dis. 2007;45:404–405. doi: 10.1086/519505. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L., Betanzos A., Nava P., Jaramillo B.E. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Hao W., Bernard K., Patel N., Ulbrandt N., Feng H., Svabek C., Wilson S., Stracener C., Wang K., Suzich J., Blair W., Zhu Q. Infection and propagation of human rhinovirus C in human airway epithelial cells. J. Virol. 2012;86:13524–13532. doi: 10.1128/JVI.02094-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q., Deng X., Yan Z., Cheng F., Luo Y., Shen W., Lei-Butters D.C., Chen A.Y., Li Y., Tang L., Soderlund-Venermo M., Engelhardt J.F., Qiu J. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog. 2012;8:e1002899. doi: 10.1371/journal.ppat.1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Wiszniewski L., Constant S. The use of In Vitro 3D cell models in drug development for respiratory diseases. In: Kapetanoviæ I., editor. Drug Discovery and Development – Present and Future. InTech; Rijeka, Croatia: 2011. pp. 169–190. [Google Scholar]
- Ilyushina N.A., Bovin N.V., Webster R.G. Decreased neuraminidase activity is important for the adaptation of H5N1 influenza virus to human airway epithelium. J. Virol. 2012;86:4724–4733. doi: 10.1128/JVI.06774-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jartti T., Hedman K., Jartti L., Ruuskanen O., Allander T., Soderlund-Venermo M. Human bocavirus-the first 5 years. Rev. Med. Virol. 2011;22:46–64. doi: 10.1002/rmv.720. [DOI] [PubMed] [Google Scholar]
- Kahn J. Human bocavirus: clinical significance and implications. Curr. Opin. Pediatr. 2008;20:62–66. doi: 10.1097/MOP.0b013e3282f3f518. [DOI] [PubMed] [Google Scholar]
- Kang W., Wang L., Harrell H., Liu J., Thomas D.L., Mayfield T.L., Scotti M.M., Ye G.J., Veres G., Knop D.R. An efficient rHSV-based complementation system for the production of multiple rAAV vector serotypes. Gene Ther. 2009;16:229–239. doi: 10.1038/gt.2008.158. [DOI] [PubMed] [Google Scholar]
- Kantola K., Hedman L., Allander T., Jartti T., Lehtinen P., Ruuskanen O., Hedman K., Soderlund-Venermo M. Serodiagnosis of human bocavirus infection. Clin. Infect. Dis. 2008;46:540–546. doi: 10.1086/526532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Hornig M., Asokan A., Williams B., Henriquez J.A., Lipkin W.I. Bocavirus episome in infected human tissue contains non-identical termini. PLoS One. 2011;6:e21362. doi: 10.1371/journal.pone.0021362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Mehta N., Esper F., Poljsak-Prijatelj M., Quan P.L., Qaisar N., Delwart E., Lipkin W.I. Identification and characterization of a new bocavirus species in gorillas. PLoS One. 2010;5:e11948. doi: 10.1371/journal.pone.0011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Simmonds P., Slikas E., Li L., Bodhidatta L., Sethabutr O., Triki H., Bahri O., Oderinde B.S., Baba M.M., Bukbuk D.N., Besser J., Bartkus J., Delwart E. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J. Infect. Dis. 2010;201:1633–1643. doi: 10.1086/652416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Slikas E., Simmonds P., Chieochansin T., Naeem A., Shaukat S., Alam M.M., Sharif S., Angez M., Zaidi S., Delwart E. A newly identified bocavirus species in human stool. J. Infect. Dis. 2009;199:196–200. doi: 10.1086/595831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesebir D., Vazquez M., Weibel C., Shapiro E.D., Ferguson D., Landry M.L., Kahn J.S. Human bocavirus infection in young children in the United States: molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J. Infect. Dis. 2006;194:1276–1282. doi: 10.1086/508213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner R.W., Soderlund-Venermo M., van Koningsbruggen-Rietschel S., Kaiser R., Malecki M., Schildgen O. Severe human bocavirus infection, Germany. Emerg. Infect. Dis. 2011;17:2303–2305. doi: 10.3201/eid1712.110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F., Zeng A., Yang N., Lin H., Yang E., Wang S., Pintel D., Qiu J. Quantification of human bocavirus in lower respiratory tract infections in China. Infect. Agent Cancer. 2007;2:3. doi: 10.1186/1750-9378-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- Manning A., Russell V., Eastick K., Leadbetter G.H., Hallam N., Templeton K., Simmonds P. Epidemiological profile and clinical associations of human bocavirus and other human parvoviruses. J. Infect. Dis. 2006;194:1283–1290. doi: 10.1086/508219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matrosovich M.N., Matrosovich T.Y., Gray T., Roberts N.A., Klenk H.D. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. U.S.A. 2004;101:4620–4624. doi: 10.1073/pnas.0308001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriluoto M., Hedman L., Tanner L., Simell V., Makinen M., Simell S., Mykkanen J., Korpelainen J., Ruuskanen O., Ilonen J., Knip M., Simell O., Hedman K., Soderlund-Venermo M. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg. Infect. Dis. 2012;18:264–271. doi: 10.3201/eid1802.111293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscona A., Porotto M., Palmer S., Tai C., Aschenbrenner L., Triana-Baltzer G., Li Q.X., Wurtman D., Niewiesk S., Fang F. A recombinant sialidase fusion protein effectively inhibits human parainfluenza viral infection in vitro and in vivo. J. Infect. Dis. 2010;202:234–241. doi: 10.1086/653621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento-Carvalho C.M., Cardoso M.R., Meriluoto M., Kemppainen K., Kantola K., Ruuskanen O., Hedman K., Soderlund-Venermo M. Human bocavirus infection diagnosed serologically among children admitted to hospital with community-acquired pneumonia in a tropical region. J. Med. Virol. 2012;84:253–258. doi: 10.1002/jmv.22268. [DOI] [PubMed] [Google Scholar]
- Palermo L.M., Porotto M., Yokoyama C.C., Palmer S.G., Mungall B.A., Greengard O., Niewiesk S., Moscona A. Human parainfluenza virus infection of the airway epithelium: viral hemaglutinin-neuraminidase regulates fusion protein activation and modulates infectivity. J. Virol. 2009;83:6900–6908. doi: 10.1128/JVI.00475-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S.G., Porotto M., Palermo L.M., Cunha L.F., Greengard O., Moscona A. Adaptation of human parainfluenza virus to airway epithelium reveals fusion properties required for growth in host tissue. MBio. 2012;3:e00137–e212. doi: 10.1128/mBio.00137-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proenca-Modena J.L., Gagliardi T.B., Escremim de P.F., Iwamoto M.A., Criado M.F., Camara A.A., Acrani G.O., Cintra O.A., Cervi M.C., de Paula Arruda L.K., Arruda E. Detection of human bocavirus mRNA in respiratory secretions correlates with high viral load and concurrent diarrhea. PLoS One. 2011;6:e21083. doi: 10.1371/journal.pone.0021083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrc K., Sims A.C., Dijkman R., Jebbink M., Long C., Deming D., Donaldson E., Vabret A., Baric R., van der Hoek L., Pickles R. Culturing the unculturable: human coronavirus HKU1 infects, replicates, and produces progeny virions in human ciliated airway epithelial cell cultures. J. Virol. 2010;84:11255–11263. doi: 10.1128/JVI.00947-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildgen O., Muller A., Allander T., Mackay I.M., Volz S., Kupfer B., Simon A. Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin. Microbiol. Rev. 2008;21:291–304. doi: 10.1128/CMR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Schoop R., Hudson J.B. The efficacy of Echinacea in a 3-D tissue model of human airway epithelium. Phytother. Res. 2010;24:900–904. doi: 10.1002/ptr.3051. [DOI] [PubMed] [Google Scholar]
- Shay D.K., Holman R.C., Newman R.D., Liu L.L., Stout J.W., Anderson L.J. Bronchiolitis-associated hospitalizations among US children, 1980–1996. J. Am. Med. Assoc. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- Sims A.C., Baric R.S., Yount B., Burkett S.E., Collins P.L., Pickles R.J. Severe acute respiratory syndrome coronavirus infection of human ciliated airway epithelia: role of ciliated cells in viral spread in the conducting airways of the lungs. J. Virol. 2005;79:15511–15524. doi: 10.1128/JVI.79.24.15511-15524.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund-Venermo M., Lahtinen A., Jartti T., Hedman L., Kemppainen K., Lehtinen P., Allander T., Ruuskanen O., Hedman K. Clinical assessment and improved diagnosis of bocavirus-induced wheezing in children, Finland. Emerg. Infect. Dis. 2009;15:1423–1430. doi: 10.3201/eid1509.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Chen A.Y., Cheng F., Guan W., Johnson F.B., Qiu J. Molecular characterization of infectious clones of the minute virus of canines reveals unique features of bocaviruses. J. Virol. 2009;83:3956–3967. doi: 10.1128/JVI.02569-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijssen P., Agbandje-McKenna M., Almendral J.M., Bergoin M., Flegel T.W., Hedman K., Kleinschmidt J., Li Y., Pintel D.J., Tattersall P. Family Parvoviridae. In: King A.M., Lefkowitz E., Adams M.J., Carstens E.B., editors. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; 2012. pp. 405–425. [Google Scholar]
- Triana-Baltzer G.B., Babizki M., Chan M.C., Wong A.C., Aschenbrenner L.M., Campbell E.R., Li Q.X., Chan R.W., Peiris J.S., Nicholls J.M., Fang F. DAS181, a sialidase fusion protein, protects human airway epithelium against influenza virus infection: an in vitro pharmacodynamic analysis. J. Antimicrob. Chemother. 2010;65:275–284. doi: 10.1093/jac/dkp421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursic T., Steyer A., Kopriva S., Kalan G., Krivec U., Petrovec M. Human bocavirus as the cause of a life-threatening infection. J. Clin. Microbiol. 2011;49:1179–1181. doi: 10.1128/JCM.02362-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vareille M., Kieninger E., Edwards M.R., Regamey N. The airway epithelium: soldier in the fight against respiratory viruses. Clin. Microbiol. Rev. 2011;24:210–229. doi: 10.1128/CMR.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villenave R., Thavagnanam S., Sarlang S., Parker J., Douglas I., Skibinski G., Heaney L.G., McKaigue J.P., Coyle P.V., Shields M.D., Power U.F. In vitro modeling of respiratory syncytial virus infection of pediatric bronchial epithelium, the primary target of infection in vivo. Proc. Natl. Acad. Sci. U.S.A. 2012;109:5040–5045. doi: 10.1073/pnas.1110203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G., Deering C., Macke M., Shao J., Burns R., Blau D.M., Holmes K.V., Davidson B.L., Perlman S., McCray P.B., Jr. Human coronavirus 229E infects polarized airway epithelia from the apical surface. J. Virol. 2000;74:9234–9239. doi: 10.1128/jvi.74.19.9234-9239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Wang W., Yan H., Ren P., Zhang J., Shen J., Deubel V. Correlation between bocavirus infection and humoral response, and co-infection with other respiratory viruses in children with acute respiratory infection. J. Clin. Virol. 2010;47:148–155. doi: 10.1016/j.jcv.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windisch W., Schildgen V., Malecki M., Lenz J., Brockmann M., Karagiannidis C., Schildgen O. Detection of HBoV DNA in idiopathic lung fibrosis, Cologne, Germany. J. Clin. Virol. 2013;58:325–327. doi: 10.1016/j.jcv.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabner J., Freimuth P., Puga A., Fabrega A., Welsh M.J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J. Clin. Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H., Pappas C., Katz J.M., Tumpey T.M. The 2009 pandemic H1N1 and triple-reassortant swine H1N1 influenza viruses replicate efficiently but elicit an attenuated inflammatory response in polarized human bronchial epithelial cells. J. Virol. 2011;85:686–696. doi: 10.1128/JVI.01568-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S., Yue C., Wei Z., Long J., Ran D., Lin T., Deng Y., Huang L., Sun L., Zheng H., Gao F., Zheng H., Chen S., Yuan S. High prevalence of a novel porcine bocavirus in weanling piglets with respiratory tract symptoms in China. Arch. Virol. 2010;155:1313–1317. doi: 10.1007/s00705-010-0698-9. [DOI] [PubMed] [Google Scholar]
- Zhang L., Bukreyev A., Thompson C.I., Watson B., Peeples M.E., Collins P.L., Pickles R.J. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Collins P.L., Lamb R.A., Pickles R.J. Comparison of differing cytopathic effects in human airway epithelium of parainfluenza virus 5 (W3A), parainfluenza virus type 3, and respiratory syncytial virus. Virology. 2011;421:67–77. doi: 10.1016/j.virol.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Peeples M.E., Boucher R.C., Collins P.L., Pickles R.J. Respiratory syncytial virus infection of human airway epithelial cells is polarized, specific to ciliated cells, and without obvious cytopathology. J. Virol. 2002;76:5654–5666. doi: 10.1128/JVI.76.11.5654-5666.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zhao L., Sun Y., Qian Y., Liu L., Jia L., Zhang Y., Dong H. Detection of a bocavirus circular genome in fecal specimens from children with acute diarrhea in Beijing, China. PLoS One. 2012;7:e48980. doi: 10.1371/journal.pone.0048980. [DOI] [PMC free article] [PubMed] [Google Scholar]


