Abstract
Contrast agents are commonly used in combination with magnetic resonance imaging (MRI) to monitor the distribution of molecules in the brain. Recent experiments conducted in our laboratory have shown that co-infusion of recombinant Adeno-associated virus serotype 5 (rAAV5) and the MRI contrast agent gadoteridol (Gd) enhances vector transduction of in the rat striatum. The goal of this study was to determine whether gadoteridol may also be used as a tool to enhance transduction efficiency of rAAV1 and rAAV5 within the rat hippocampus. We show that Gd/rAAV1-GFP but not Gd/rAAV5-GFP co-infusion results in significantly higher distribution of the transgene both in the injected hemisphere as well as in the contralateral side and adjacent areas of cortex along the injection track. We also show that Gd/rAAV1-GFP co-infusion has no deleterious effect on hippocampal function as assessed by two tests of spatial memory formation. This work indicates that gadoteridol can be exploited as a method to increase transduction efficiency of AAV1 in the hippocampus for animal studies.
Keywords: gadoteridol, hippocampus, AAV, spatial memory
INTRODUCTION
AAV has proven to be successful for gene therapy in a number of animal models of disease, and is currently being used in human clinical trials for various conditions such as Leber congenital amaurosis, hemophilia, muscular dystrophy, Parkinson's disease, and Alzheimer's disease 1. rAAV2 has been used in all the human gene therapy trials for neurological disorders, yet its distribution in the brain is discrete 2-4. The development of methods to increase vector titer, and the characterization of novel serotypes has helped to significantly increase transduction efficiency of rAAV in various tissues5-7. A number of agents have been used to increase the transduction efficiency of several serotypes, including heparin and mannitol, or combinations of systemic mannitol and convection enhanced delivery (CED) 3, 4, 8-10. MRI contrast agents such as gadoteridol have been used to track the distribution of therapeutic drugs in real time, allowing visualization of the volume of spread across various structures6, 7. Studies have shown that agents like gadoteridol may be used to monitor the distribution of rAAV2 in primate models of gene therapy11. However, other studies have shown that gadoteridol monitored delivery of rAAV1 more accurately predicts distribution of this viral vector than monitored co-injection with rAAV212. These disparities may arise because distinct AAV serotypes interact differently with contrast agents (Osting et al. submitted).
We have previously shown that gadoteridol significantly increases the transduction efficiency of rAAV5 coding for green fluorescent protein (rAAV5-GFP) in in the rat striatum (Osting et al, submitted). Here we wanted to determine whether rAAV1-GFP and rAAV5-GFP transduction efficiency in the hippocampus could also be enhanced by co-infusion with gadoteridol. Studies have shown that infusion with gadoteridol can have particularly adverse effects in patients with pre-existing kidney disease 13. On the other hand, gadoteridol has been used in animal models and human studies without signs of toxicity6, 14. To discount any toxic effects of gadolinium infusion in the hippocampus we looked at any putative cytotoxic effects of Gd/rAAV co-infusion on performance on the Morris water maze and object location memory tasks. Here we show that rAAV1 but not rAAV5 transduction efficiency is significantly increased by co-infusion with gadoteridol, and that gadoteridol has no deleterious effects on behavior.
RESULTS
Gadoteridol Enhances rAAV1-GFP but not rAAV5-GFP distribution in the Rat Hippocampus
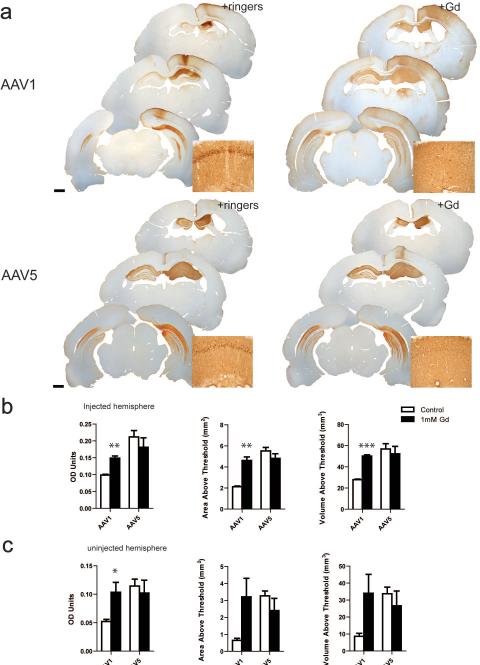
Rats were injected unilaterally with either rAAV1-GFP or rAAV5-GFP that was co-infused with gadoteridol or Lactated Ringer's solution as a control. Quantification of GFP-positive immunostaining was analyzed using densitometry analysis of each hippocampal hemisphere. Co-infusion of rAAV1-GFP with gadoteridol (Gd/rAAV1), significantly increased the distribution of GFP in the hippocampus relative to hemispheres co-injected with Lactated Ringer's solution (Ringers/rAAV1; Figure 1a-b). High magnification images show a more intense GFP immunoreactivity in the cell bodies in the pyramidal layer than in the axonal and dendritic projections, whereas Gd/AAV1 displays a more diffuse staining in the parenchima (Figure 1a Top panel insets). Mean optical density (OD) was 1.5-fold higher in Gd/rAAV1 hemispheres when compared to animals that had been injected with ringers/rAAV1 (Figure 1b, p= 0.0017). Similarly, mean area above threshold (AAT) was 2.2-fold higher in the hippocampal hemispheres of Gd/rAAV1 relative to ringers/rAAV1 (p=0.002). Finally, the volume of distribution (Vd) was also significantly higher in Gd/rAAV1 hemispheres (1.8-fold higher than ringers/rAAV1 ; p< 0.0001). We also found an increase in the distribution of GFP in the contralateral uninjected hemisphere of Gd/rAAV1 when compared to ringers/rAAV1 (Figure 1 a). We have previously found that both rAAV1 and rAAV5 transduce the contralateral hemisphere due to: 1) anterograde transport of the GFP protein to the uninjected side, 2) retrograde transport of the viral particles to the cell bodies of the contralateral hemisphere 15. It seems that gadoteridol enhances these two types of transport from the injected hemisphere (Figure 1 a and c). Animals injected with Gd/rAAV1 showed a statistically significant increase in OD in the uninjected hemisphere compared to those injected with Ringers/rAAV1 (2-fold, p=0.045). A trend towards an increase in the area above threshold and volume of distribution was also found in Gd/rAAV1 relative to ringers/rAAV1 non-injected hemispheres (Figure 1b; AAT, p=0.08, Vd, p= 0.086). Finally, gadoteridol also resulted in an increase in transduction in the injected cortical hemisphere, likely due to backflow from the infusion site (Figure 1a).
Figure 1. Gadoteridol increases the transduction efficiency of AAV1 but not AAV5.
(a) Representative sections illustrate the rostro-caudal extent of GFP expression in animals injected with rAAV1 (top panels) or AAV5 (bottom panels) co-infused with Lactated Ringer's solution (+Ringers) or gadoteridol (+Gd). Insets show magnification of dorsal area CA1. (b) Densitometry analysis of the injected hippocampal hemispheres. (c) Densitometry analysis of the contralateral uninjected hemisphere. Data represent the mean ± SEM (Gd/AAV1, n=3; ringers/rAAV1, n=3; Gd/AAV5, n=4; ringers/rAAV5, n=4). ***P<0.005, **P<0.005, *P<0.05. Significant indicators above histogram bars indicate comparison to Lactated Ringer's control. Scale bars = 1 mm.
In contrast to the results found with Gd/rAAV1, gadoteridol had no effect on any of the measures of distribution of AAV5 in the injected hemisphere. (Figure 1 a-b; Gd/rAAV5 vs Ringers/rAAV5: OD, p=0.42; AAT, p=0.272 Vd, p=0.63). Even though increased distribution of the transgene was not observed in the presence of Gd, Gd-AAV5 hippocampi display more diffuse staining of GFP in cell bodies, axons and dendrites when compared to Ringer/AAV5 (Figure 1a Top bottom panel insets). No differences were found in the distribution properties of uninjected hemispheres when rAAV5 was co-infused with either gadoteridol or Lactated Ringer's solution (Figure 1a-b OD, p=0.659; AAT, p=0.3270; Vd, p=0.4988).
In conclusion, gadoteridol co-infusion with rAAV1-GFP results in an increase in the distribution of the transgene in the injected hippocampus as well as in the contralateral hemisphere. On the other hand, this contrast agent did not affect the distribution of rAAV5-GFP.
Characterization of Cell Tropism and Tissue Health following Gadoteridol co-infusion
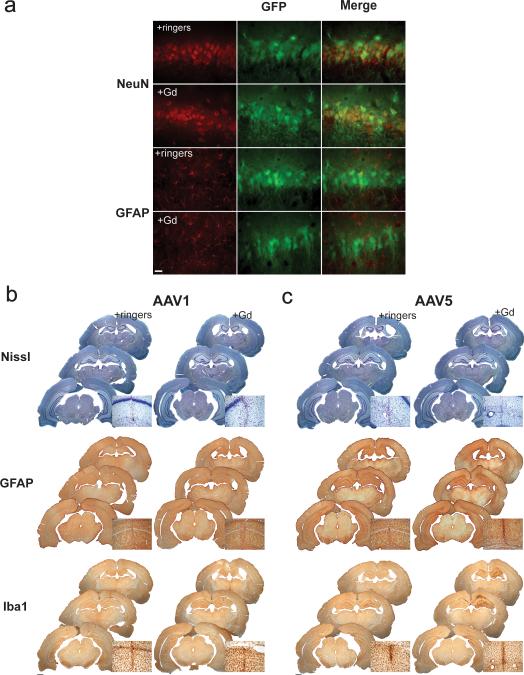
To determine whether the increased distribution of rAAV1 following gadoteridol co-infusion was due to a change in cell tropism, we performed immunohistochemistry on the tissue from the two experimental groups using antibodies against the neuronal marker NeuN, or the astrocytic marker GFAP. Both rAAV1 and rAAV5 have been shown to transduce mainly neurons in the rodent hippocampus 15. We found that the increased distribution of rAAV1-GFP gadoteridol co-infusion was not due to a change in cell tropism, since most of the cells expressing GFP were also NeuN immunopositive, whereas no GFP positive cells were GFAP positive (Figure 2a). Similar results were found with co-infusion of AAV5 and Gd (data not shown). These results demonstrate no change in tropism in the presence of Gd.
Figure 2. Gadoteridol co-infusion with AAV1 does not result in changes in tropism or adverse effects on tissue health.
(a) Labeling with NeuN and GFAP reveals that gadoteridol co-infusion does not affect AAV1 neuronal tropism. Scale bar= 25 μm. Nissl staining indicates there is no obvious cell damage when Gd is co-infused with either AAV1 (b) or AAV5 (c) (Top panels). GFAP immunostaining shows no inflammation outside the needle track (middle panel). Only co-infusion of Gd with AAV5 resulted in an increase Iba I immunoreactivity, indicating microglial activation in the injected hippocampi (c, bottom panel). Scale bars= 1 mm. (insets show magnification of the area around the needle track).
In order to analyze any inflammatory process resulting from gadoteridol and rAAV1or rAAV5 co-infusion, we looked at markers of cell damage and inflammation. No obvious morphological changes or tissue damage on the injected hippocampi were observed with Nissl staining . Only the area near the injection track signs of tissue damage, which was independent of treatement (Figure 2b top panel). Astrocytosis was examined by immunohistochemistry of glial fibrillary acidic protein (GFAP). We found no widespread inflammation in Gd/rAAV1 or Gd/rAAV5 injected hemispheres relative to Ringers/rAAV1 or Ringers/rAAV5 injected hemispheres (Figure 2b middle panel). Again, some minor astrocytosis was present the area around the needle track (which is common with intraparenchimal injections). Reactive microgliosis, determined by IbaI immunoreactivity, was present only in the hippocampal hemisphere of animals injected with Gd/rAAV5 (Figure 2b bottom panel). This apparent increase in Iba1 staining in Gd/rAAV5 hippocampi is not significantly different than Ringer/rAAV5 hippocampi in any of the measures of distribution of Iba1 in the injected hemisphere. (Gd/rAAV5 vs Ringers/rAAV5: OD, p=0.322; AAT, p=0.691 Vd, p=0.7157).
Co-Infusion of rAAV1-GFP and Gadoteridol does not Affect Behavior
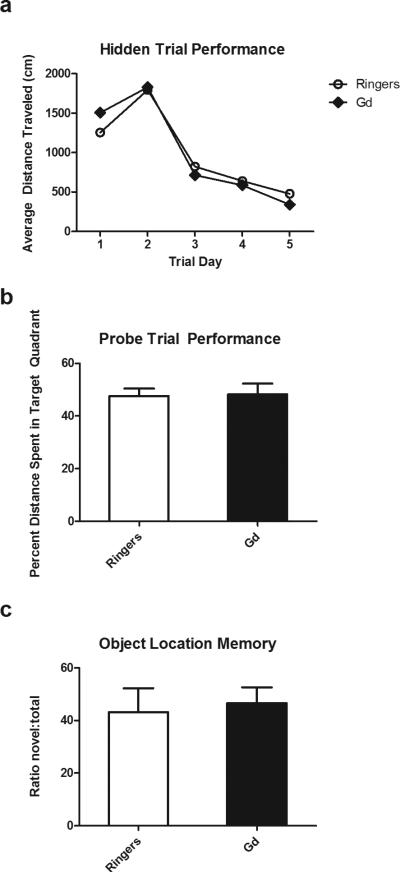
In order to show that gadoteridol can be used as a tool to safely enhance rAAV1 transduction efficiency in functional studies, we examined whether Gd/rAAV1 had any effects on hippocampal memory We have previously shown that rAAV-GFP hippocampal transduction does not adversely impact spatial memory in rodents 16. Animals were injected bilaterally with either rAAV1-GFP co-infused with gadoteridol or Lactated Ringer's as a control. Performance on the Morris water maze was unaffected by co-infusion with gadoteridol. Both experimental groups learned to find the location of the hidden platform at the same rate; the average distance traveled in hidden platform trials was not significantly different between groups (Fig. 3a; p=.96). In addition, there was no difference in the percent distance spent in the target quadrant between groups during the probe trial (Fig. 3b; p=.91). Performance in the object location memory task was also unchanged between groups, ringer/rAAV1 injected animals spending 43% and Gd/rAAV1 groups spending 46% of investigation time on the object in the novel location (Fig. 3c, p=.75). In conclusion, no statistically significant differences were found between groups injected with Gd/AAV1-GFP or ringers/AAV1-GFP in two independent tests of spatial memory. These results indicate that this contrast agent can be used to increase distribution of the viral vector in the rat hippocampus with no deleterious effects on hippocampal function.
Figure 3. Co-infusion of AAV1 and gadoteridol does not have any deleterious effects in hippampal behavior.
(a) Both Gd/AAV1-GFP and Ringers/AAV1 GFP animals show similar distance traveled to escape during the training trials in the Morris water maze (c) Animals spend a similar distance in the quadrant platform during probe trial portion of the MWM (e) There are no statistically significant differences in the performance of OLM Gd/AAV1 and Ringers/AAV1 animals in the OLM task. n= 7 Gd/AAV1; n= 7 Ringers/AAV1.
DISCUSSION
Results from our studies indicate that gadoteridol co-infusion significantly enhances the distribution of rAAV1 within the rat hippocampus. Furthermore, the gadoteridol enhanced the transduction of the viral vector to the contralateral, uninjected hemisphere, resulting in a more diffuse pattern of expression than in animals injected with rAAV1-GFP and Ringer's solution. On the other hand, the area and volume of distribution of rAAV5 was not affected by gadoteridol. This is in contrast with previous studies from our laboratory demonstrating a significant enhancement of transduction of rAAV5-GFP within the rat striatum following gadoteridol co-infusion (Osting et al., submitted). Another possible explanation could be that anatomical differences between the hippocampus and the striatum could impact the distribution of the viral vector in the presence of the contrast agent 17. An important difference between this study and the Osting et al. study is that we found reactive microgliosis in hippocampi that had been co-infused with rAAV5 and Gd, whereas we did not find any type of inflammation in Gd/rAAV5 injected animals in the striatum. Together these discrepancies suggest that conditions for co-infusing contrast agents and distinct AAV serotypes need to be determined empirically for different brain regions.
In order to use this co-infusion method as a tool to enhance viral vector distribution and transgene expression, one needs to demonstrate that gadoteridol does not have any deleterious effects on the functional outcome to be examined. Therefore, we also examined any putative effects of the contrast agent in two tests of hippocampal behavior. Results from Morris water maze and object location memory tests indicate co-infusion of rAAV1 with Gd had no deleterious effects on spatial memory and that this contrast agent does not impact hippocampal function. Therefore, co-infusion of this particular rAAV serotype with Gd presents a safe way to increase transduction efficiency of rAAV1 within the rat hippocampus to be used in functional studies. One pitfall of this approach is that the viral vector also distributes outside of the hippocampus proper resulting in transgene expression outside of the region of interest. Regardless, this tool can be used when larger areas of transgene expression are necessary. In many cases, intracerebral delivery of viral vectors requires injection in multiple sites. Our group and others have developed methods to optimize rAAV distribution in the central nervous system. Namely, co-infusion of heparin with rAAV2 18, co-infusion of rAAV with mannitol via intraparenchimal or systemic route 8, 9, 19, the use of CED 20,21, or a combination of mannitol and CED 10. Here we propose co-infusion of rAAV1 with gadoteridol as a safe method to enable targeting of larger brain regions with fewer injection sites and thus reduce surgery time, an important variable to consider especially when working with aged or fragile animal models of neurological disorders 22-24.
MATERIALS AND METHODS
Animals
Sprague-Dawley rats (3 -5 months) were housed in groups of 2-3 under 12/12 light/dark cycle and given access to food and water ad libitum. The protocols were approved by the University of Wisconsin-Madison Animal care and use advisory committee in accordance with guidelines established by the US Public Health Policy on Human care and use of laboratory animals.
Viral VectorProduction
rAAV expressing green fluorescent protein (GFP) construct has been described9. AAV1 and AAV5Viral vectors were produced and purified as previously described 5. Viral titers were determined by real-time PCR (AAV1-GFP= 5.9×1012vector genomes/ml (vg/ml); AAV5-GFP= 4.8×12vg/ml). Gadoteridol (Prohance: Brako Diagnostics, Princeton, NJ) was diluted to the working concentration in Lactated Ringer's solution: (mEq/L) Sodium 130 Potassium 4 Calcium 2.7 Chloride 109 Lactate 28 Osmolarity 273mOsmol/L pH 6.5 (Baxter Healthcare Corporation, Deerfield, IL).
Intracerebral Injections
All surgical procedures were performed as previously described 25. Unilateral or bilateral injections were made into the dorsal hippocampus using a stereotaxic frame (kopf Instruments Tujunga, CA). For unilateral injections, the right hemisphere received a mixture of 1μl gadoteridol and 1μl viral vector to a final 1mM concentration of Gd. Control animals received 1μl viral vector and 1μl Lactated Ringer's solution. Animals were infused a total of 2 ul at a rate of 0.5 μl/min with an infusion pump controlling the plunger on the Hamilton syringe precisely regulating the rate of injection. The needle was left in place for 1 min then lifted 3mm and left for another 4 minutes prior to withdrawal from the brain. Coordinates for hippocampus were AP = −3.5, Lat = ±2.5, DV = −2.6 from dura.
Immunohistochemistry
Three weeks after unilateral vector injection, or right after finishing behavioral experiments in bilaterally injected animals, subjects were anesthetized with Beuthanasia D (150 mg/kg i.p., with supplements if necessary) and perfused through the aorta with 4% formaldehyde (Sigma, St. Louis, MO) in 0.1 M phosphate buffer (PB). Cryoprotection took place in PB with 2% dimethylsulfoxide (DMSO) and a graded series of glycerol concentrations. The hemispheres were frozen with dry ice and sectioned in the coronal plane at 45 μm thickness, and sections were transferred to an ethylene glycol based storage solution and placed in the −20°C freezer until ready to use. All processing was at room temperature unless otherwise noted.
GFP and Iba 1 immunohistochemistry
All solutions were prepared with a buffer consisting of PBS with 2% bovine serum albumin (BSA; Calbiochem, La Jolla, CA) and 0.1% saponin for GFP or 0.3% Triton for Iba 1. All sections were washed, blocked in buffer with 20% normal serum for 45 minutes, incubated overnight in primary antiserum (GFP from Novus Biologicals, 1:30,000; Iba 1 from Wako Pure Chemical Industries, Ltd., 1:500) in 1% normal serum. After washes, sections were incubated in 1:300 biotinylated secondary IgG (Vector Laboratories) for 3 hours, followed by 1 hour in avidin-biotin complex (Standard Elite kit; Vector). Final visualization was with 0.04% 3,3’ –diaminobenzidine (DAB; from tablets; Sigma) and 0.01% H2O2 in PB, pH 7.4. Sections were mounted on subbed slides, dehydrated through graded concentrations of ethanol, cleared with Histo-Clear (National Diagnostics) and coverslipped with Eukitt.
GFAP immunocytochemistry
All solutions were prepared with a buffer consisting of PBS with 2% normal serum, 2% lysine and 0.2% Triton (blocking solution). Sections were washed, incubated overnight in blocking solution at 4°C. Next day, sections were incubated overnight at 4°C in primary antiserum (GFAP from DakoCytomation, 1:5000). Following washes, the sections were incubated in 1:200 biotinylated secondary IgG for 2 hours, followed by 1 hour in avidin-biotin complex (Standard Elite kit; Vector).
Nissl Staining
Sections were mounted on subbed slides, dehydrated through graded concentrations of ethanol, cleared with Histo-Clear, rehydrated, soaked in cresyl violet stain, dehydrated once again through graded concentrations of ethanol, cleared with Histo-Clear and coverslipped with Eukitt.
Quantification of striatal GFP immunoreactivity
Densitometry analysis was carried out using NIH ImageJ software 26. Images from 10-12 (30μm) coronal sections per rat 0.56 mm apart were captured using a Nikon Nikon E600W microscope equipped with a digital camera (Q Imaging Retiga 2000R; Nikon Instruments, Melville, NY). The threshold for each image was determined using the MaxEntropy function and the number of particles with an area between five and 75 square pixels were recorded. For optical density (OD) ImageJ was calibrated using a step tablet, grey scale values were converted to OD units using the Rodbard function, and the area in pixels above a threshold was recorded. Statistical analysis will be performed using Prism5 (Graphpad Software, Inc., La Jolla, CA) and shown as the mean ± SEM. Two tailed, unpaired t-tests were performed to determine statistical significance.
Fluorescent Immunohistochemistry
All solutions for GFAP fluorescent immunocytochemistry were prepared with a buffer consisting of PBS with 2% normal serum, 0.2% Triton X-100 and 2% lysine. Sections were washed, incubated overnight in buffer at 4°C, incubated overnight in GFAP (DakoCytomation, Glostrup, Denmark, 1:1000) primary antisera in buffer, washed, incubated in 1:1000 fluorescent secondary (Alexa Fluor 568 goat anti-rabbit IgG; Molecular Probes) for 3.5 hours and rinsed in PBS. Sections were mounted on subbed slides and coverslipped with ProLong Gold antifade reagent (Molecular Probes).
All solutions for NeuN fluorescent immunocytochemistry were prepared with a buffer consisiting of PBS with 0.1% saponin and 2% bovine serum albumin, blocked in the same buffer with 20% normal goat serum for 45 minutes, incubated overnight in primary antiserum to NeuN (EMD Millipore, Billerica, MA, 1:1000) with 0.1% normal goat serum, washed in buffer, incubated for 2 hours in 1:500 AlexaFluor 594 goat anti-mouse IgG (Molecular Probes) in PBS with 0.1% saponin and 2% BSA, rinsed with PBS and mounted with ProLong Gold antifade reagent.
Fluorescent microscopic imaging was performed with a digital camera (Q Imaging Retiga 2000R; Nikon Instruments, Melville, NY) on a Nikon E600W Eclipse epifluorescent microscope with the x60 planapochromatic objective and a standard FITC filter cube (FITC; EX 465-495 nm; DM 505 nm; BA 515-555 nm) ) and TRITC filter cube (TRITC; EX 528-553 nm; DM 565 nm; BA 600-660 nm). Fluorescent images were acquired at an initial 36-bit tone scale and saved as 8-bit files. Images were prepared for reproduction in Adobe Photoshop CS 3 (Mountain View, CA). Adjustments in the tone scale, contrast, and hue and subsequent sharpening with the unsharp mask algorithm were applied to the entire image.
Behavior
Three weeks post-injection, rats were subjected to the object location memory task. The protocol has previously been described in detail 25. Briefly, rats were trained on the locations of two identical objects for six minutes. Testing of object location memory occurred 24 hours after training. For testing, one of the objects was moved to a different location, and spatial memory was tested by comparing the time spent investigating the object in the novel location compared to the object in the familiar location. All trials on both the Training and Testing days were videotaped using Videotrack software by ViewPoint Life Sciences (Montreal, CANADA). Total amount of time spent exploring the novel and familiar objects was recorded for each animal. The relative exploration time (T, in seconds) was recorded for each object and expressed as a novelty score: (TNovel / [TNovel + TFamiliar] × 100). All handling and scoring of rats was done by an experimenter blind to the animal's treatment.
The Morris Water Maze task was performed two days after the OLM task. The protocol has been described in detail 27 with modifications. Animals were trained for one day in the visible version of the MWM to habituate the animals to the task and to test for swimming ability and visual acuity. This training consisted of four trials of 90 seconds each. On day two, the visible platform was replaced by a submerged, hidden one. Animals were trained for five days to find the hidden platform using spatial cues around the room. Each day consisted of four trials. The distance to find the platform was recorded as a measure of learning ability. At the end of last trial of day 5 (trial 20), the platform was removed and a probe trial was conducted to determine the total time and distance the animals spent in the quadrant that previously contained the platform as a test for memory.
All statistical analyses were performed using Prism 5 (Graphpad Software, Inc., La Jolla, CA). Two tailed, unpaired t-tests were performed to determine statistical significance in the OLM task. MWM acquisition data was analyzed using repeated measures ANOVA. For analysis of the probe trial percent distance in the platform quadrant, one way ANOVA was used. Figures show means ± SEM.
Acknowledgments
We would like to thank Jordan Wackett for technical assistance. This research was supported by funds from the Department of Neurology, University of Wisconsin-Madison to C.B. R.H. was supported by the University of Wisconsin Neuroscience Training Program Grant NIH/NIGMS T32GM007507 and a NSF Graduate Research Fellowship. LPS was supported by Universidad Nacional Autónoma de Mexico (UNAM), Doctorado en Ciencias Biomedicas, and program UNAM CONACYT, Beca Mixta No. 208245 scholarship program. We would also like to acknowledge the rat subjects who participated in this study.
Footnotes
Conflict of Interest: None of the authors have a conflict of interest.
REFERENCES
- 1.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene therapy. 2012;19(6):694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 2.Wass C, Denman-Brice A, Rios C, Light KR, Kolata S, Smith AM, et al. Covariation of learning and “reasoning” abilities in mice: evolutionary conservation of the operations of intelligence. Journal of experimental psychology. Animal behavior processes. 2012;38(2):109–24. doi: 10.1037/a0027355. [DOI] [PubMed] [Google Scholar]
- 3.Light KR, Grossman H, Kolata S, Wass C, Matzel LD. General learning ability regulates exploration through its influence on rate of habituation. Behav Brain Res. 2011;223(2):297–309. doi: 10.1016/j.bbr.2011.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Light KR, Kolata S, Hale G, Grossman H, Matzel LD. Up-regulation of exploratory tendencies does not enhance general learning abilities in juvenile or young-adult outbred mice. Neurobiol Learn Mem. 2008;90(2):317–29. doi: 10.1016/j.nlm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr., et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28(2):158–67. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 6.Song DK, Lonser RR. Convection-enhanced delivery for the treatment of pediatric neurologic disorders. Journal of child neurology. 2008;23(10):1231–7. doi: 10.1177/0883073808321064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagannathan J, Walbridge S, Butman JA, Oldfield EH, Lonser RR. Effect of ependymal and pial surfaces on convection-enhanced delivery. J Neurosurg. 2008;109(3):547–52. doi: 10.3171/JNS/2008/109/9/0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghodsi A, Stein C, Derksen T, Martins I, Anderson RD, Davidson BL. Systemic hyperosmolality improves beta-glucuronidase distribution and pathology in murine MPS VII brain following intraventricular gene transfer. Exp Neurol. 1999;160(1):109–16. doi: 10.1006/exnr.1999.7205. [DOI] [PubMed] [Google Scholar]
- 9.Burger C, Nguyen FN, Deng J, Mandel RJ. Systemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol Ther. 2005;11(2):327–31. doi: 10.1016/j.ymthe.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 10.Carty N, Lee D, Dickey C, Ceballos-Diaz C, Jansen-West K, Golde TE, et al. Convection-enhanced delivery and systemic mannitol increase gene product distribution of AAV vectors 5, 8, and 9 and increase gene product in the adult mouse brain. J Neurosci Methods. 2010;194(1):144–53. doi: 10.1016/j.jneumeth.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su X, Kells AP, Salegio EA, Richardson RM, Hadaczek P, Beyer J, et al. Real-time MR imaging with Gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol Ther. 2010;18(8):1490–5. doi: 10.1038/mt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiandaca MS, Varenika V, Eberling J, McKnight T, Bringas J, Pivirotto P, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2009;47(Suppl 2):T27–35. doi: 10.1016/j.neuroimage.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85(2):139–52. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Ding D, Kanaly CW, Cummings TJ, Herndon JE, 2nd, Raghavan R, Sampson JH. Long-term safety of combined intracerebral delivery of free gadolinium and targeted chemotherapeutic agent PRX321. Neurological research. 2010;32(8):810–5. doi: 10.1179/174367509X12581069052090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10(2):302–17. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Gerstein H, O'Riordan K, Osting S, Schwarz M, Burger C. Rescue of synaptic plasticity and spatial learning deficits in the hippocampus of Homer1 knockout mice by recombinant Adeno-associated viral gene delivery of Homer1c. Neurobiol Learn Mem. 2012;97(1):17–29. doi: 10.1016/j.nlm.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Astary GW, Kantorovich S, Carney PR, Mareci TH, Sarntinoranont M. Regional convection-enhanced delivery of gadolinium-labeled albumin in the rat hippocampus in vivo. J Neurosci Methods. 2010;187(1):129–37. doi: 10.1016/j.jneumeth.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen JB, Sanchez-Pernaute R, Cunningham J, Bankiewicz KS. Convection-enhanced delivery of AAV-2 combined with heparin increases TK gene transfer in the rat brain. Neuroreport. 2001;12(9):1961–4. doi: 10.1097/00001756-200107030-00037. [DOI] [PubMed] [Google Scholar]
- 19.Mastakov MY, Baer K, Xu R, Fitzsimons H, During MJ. Combined injection of rAAV with mannitol enhances gene expression in the rat brain. Mol Ther. 2001;3(2):225–32. doi: 10.1006/mthe.2001.0246. [DOI] [PubMed] [Google Scholar]
- 20.Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164(1):2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 21.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17(3):291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 22.Gerstein H, Lindstrom MJ, Burger C. Gene delivery of Homer1c rescues spatial learning in a rodent model of cognitive aging. Neurobiol Aging. 2013 doi: 10.1016/j.neurobiolaging.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sechtem U, Baer FM, Voth E, Theissen P, Schneider CA. Stress functional MRI: detection of ischemic heart disease and myocardial viability. J Magn Reson Imaging. 1999;10(5):667–75. doi: 10.1002/(sici)1522-2586(199911)10:5<667::aid-jmri9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.La Rosee K, Deutsch HJ, Schnabel P, Schneider CA, Burkhard-Meier C, Hopp HW. Thrombus formation after transcatheter closure of atrial septal defect. The American journal of cardiology. 1999;84(3):356–9. A9. doi: 10.1016/s0002-9149(99)00296-9. [DOI] [PubMed] [Google Scholar]
- 25.Austerberry CF, Snyder RO, Yao MC. Sequence microheterogeneity is generated at junctions of programmed DNA deletions in Tetrahymena thermophila. Nucleic acids research. 1989;17(18):7263–72. doi: 10.1093/nar/17.18.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature methods. 2012;9(7):671–5. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burger C, Cecilia Lopez M, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem. 2007;87(1):21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]