Abstract
Minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) of various antimicrobial agents were measured against 12 strains of Streptococcus pyogenes isolated from children with invasive infections between 2003 and 2012. The patients ranged in age from 1 day to 15 years, with patients younger than 5 years, including three neonates, accounting for a half of the patients. The disease was sepsis in four patients, skin and soft tissue infection in three patients, retropharyngeal abscess in two patients, pneumonia plus sepsis in one patient, empyema in one patient, and pyogenic arthritis in one patient. One patient with sepsis died, while cure without sequelae was achieved in all the remaining patients. When classified by type, emm1 (six strains) was the most prevalent type, followed by emm12 (two strains). The MIC90/MBC90 values were 0.015/0.015 μg/mL for penicillin G, 0.03/0.03 μg/mL for ampicillin, 0.015/0.03 μg/mL for cefotaxime, 0.03/0.03 μg/mL for ceftriaxone, 0.008/0.008 μg/mL for panipenem, 0.008/0.008 μg/mL for meropenem, and ≤0.004/≤0.004 μg/mL for doripenem, indicating the superior antimicrobial activities of carbapenem.
Keywords: Streptococcus pyogenes, Susceptibility, Emm type, Bacteremia, Penicillin, Carbapenem
Introduction
Streptococcus pyogenes is the major causative agent for pharyngitis and tonsillitis, but may also cause, although relatively rarely, invasive infections such as meningitis, sepsis, and necrotizing fasciitis. For such infections, treatment with antimicrobial agents exhibiting superior activity against S. pyogenes must be initiated immediately. Penicillin agents are representative drugs used as the agents of first choice for infections caused by hemolytic streptococci. However, many reports on the sensitivity of S. pyogenes to antimicrobial agents pertain to strains isolated from patients with pharyngitis or tonsillitis, and there are very few reports focusing on strains isolated from patients with invasive infections. Moreover, most of the drugs evaluated in comparison with the penicillins in these reports are oral antimicrobial agents, and there are no reports on the efficacy of intravenous antimicrobial agents, carbapenems in particular, used for the treatment of invasive infections.
In order to select appropriate antimicrobial agents for the treatment of hemolytic streptococcal infections associated with serious symptoms, the minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) of various antimicrobial agents were examined for S. pyogenes strains isolated from children with invasive infections such as sepsis. Although many reports state that severe infection is associated with specific emm types, no results are available for children in Japan. Therefore, we evaluated this association.
Subjects and method
A total of 12 strains of S. pyogenes isolated from children who were examined for invasive infections at pediatric departments in medical institutions in Hokkaido, including our hospital, during the 10-year period between July 2003 and June 2012 were included for this study. Severe infection with S. pyogenes was defined as: (1) detection of S. pyogenes from originally sterile sites such as blood, cerebrospinal fluid, and puncture fluid and (2) while S. pyogenes was detected from non-sterile sites such as the skin and pharynx, no other bacteria could be assumed to be the cause and, furthermore, the pathology is not inconsistent with infection due to S. pyogenes. Patients considered to have invasive infections were those with serious symptoms that were almost life-threatening, those requiring a surgical procedure such as drainage and/or debridement, and those requiring hospitalization for 2 weeks or more.
The MIC values of seven drugs (penicillin G, ampicillin, cefotaxime, ceftriaxone, panipenem, meropenem, doripenem) were measured by the broth microdilution method [1]. For determination of the MBC, a culture suspension of 10 μL was collected from the plate on which the MIC was measured and applied to non-selective culture media in wells containing the drug at a concentration equal to or greater than the MIC. After aerobic cultivation at 35°C for 20–24 h, the number of colonies were counted, and the concentration that caused a decrease in the volume of the growing bacteria by 99.9 % or more was determined as the MBC [2].
The Laboratory of Molecular Epidemiology for Infectious Agents, Kitasato Institute of Life Sciences, was requested to perform a determination of the emm types. At the laboratory, M protein-coding genes were amplified by PCR and the base sequence at the 5′ terminal end of the amplified product was determined; thereafter, that sequence of 300 bp was transmitted to the CDC Reference Center and the types were determined by matching with the CDC database [3].
Results
Table 1 shows a list of patients with invasive infections caused by S. pyogenes. The patients ranged widely in age from 1 day to 15 years, with patients younger than 5 years, including three neonates, accounting for half of all the patients. None of the patients had any underlying diseases. The disease was sepsis in four patients, skin and soft tissue infection in three patients, retropharyngeal abscess in two patients, pneumonia plus sepsis in one patient, empyema in one patient, and pyogenic arthritis in one patient. None of the patients had concurrent toxic shock syndrome. One patient (a 15-year-old adolescent girl) with pneumonia plus sepsis died of shock. The most prevalent emm type was emm1 (six strains; 50.0 %), followed by emm12 (two strains; 16.7 %), while emm3, emm4, emm6, and emm28 were recognized in one strain each.
Table 1.
Characteristics of children with severe infection due to Streptococcus pyogenes
| Case number | Age | Type of emm | Diagnosis | Isolated | Antibiotic therapy | Prognosis |
|---|---|---|---|---|---|---|
| 1 | 1 day | 6 | Pyothorax | Pleural fluid | ABPC 11 days | Cure |
| 2 | 4 days | 1 | Sepsis | Blood | ABPC 9 days | Cure |
| 3 | 7 days | 1 | Sepsis | Blood | ABPC 11 days | Cure |
| 4 | 1 year | 12 | Cellulitis | Skin swab | ABPC 7 days | Cure |
| 5 | 3 years | 12 | Sepsis | Blood | ABPC 14 days | Cure |
| 6 | 3 years | 1 | Sepsis | Blood | ABPC 14 days | Cure |
| 7 | 5 years | 28 | Arthritis | Synovial fluid | CEZ 7 days | Cure |
| 8a | 5 years | 4 | Retropharyngeal abscess | Pharyngeal swab | IPM/CS 2 days | Cure |
| 9 | 7 years | 3 | Cellulitis | Skin exudate | IPM/CS 7 days | Cure |
| 10 | 11 years | 1 | Erysipelas | Pharyngeal swab | ABPC 7 days | Cure |
| 11 | 11 years | 1 | Retropharyngeal abscess | Pharyngeal swab | ABPC 10 days | Cure |
| 12b | 15 years | 1 | Sepsis + pneumonia | Blood | PAPM/BP one shot | Dead |
ABPC ampicillin, CEZ cefazolin, IPM/CS imipenem/cilastatin sodium, PAPM/BP panipenem/betamipron
aPatient was transferred to another hospital for operation
bPatient was transported in a state of shock in the evening by ambulance and was transferred to an advanced emergency medical care center after receiving basic life support and a single dose of PAPM/BP, but died the following morning. The patient had no underlying disease associated with increased susceptibility to infection
Figures 1 and 2 show the distributions of the MICs and MBCs for each drug, and Table 2 shows the MIC90 and MBC90 values. The drug with the most potent antibacterial activity was doripenem, with MICs and MBCs of 0.004 μg/mL or less for all the strains. This drug was followed in antibacterial potency by panipenem, the MIC and MBC of which were determined to be 0.004 μg/mL or less and 0.008 μg/mL for six strains each. For meropenem, both the MIC and MBC were 0.008 μg/mL for all strains. The drugs showing divergent values of the MIC and MBC were: penicillin G for five strains, ampicillin for three strains, cefotaxime for five strains, and ceftriaxone for eight strains. A comparison of the sensitivities to these four drugs revealed that penicillin G had the highest activity, followed by cefotaxime, ampicillin and ceftriaxone, in that order. There was no association between emm type and drug sensitivity.
Fig. 1.
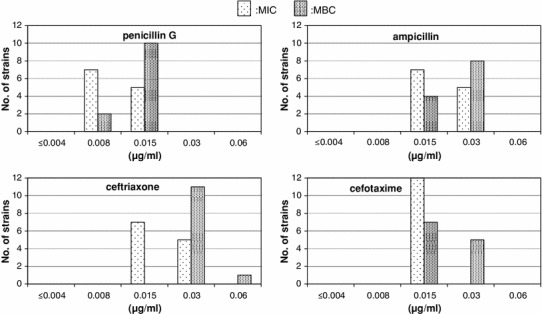
Susceptibilities of Streptococcus pyogenes isolates to penicillins and cephems
Fig. 2.

Susceptibilities of S. pyogenes isolates to carbapenems
Table 2.
MIC and MBC of S. pyogenes
| MIC range | MIC90 | MBC range | MBC90 | |
|---|---|---|---|---|
| Penicillin G | 0.008 ~ 0.015 | 0.015 | 0.008 ~ 0.015 | 0.015 |
| Ampicillin | 0.015 ~ 0.03 | 0.03 | 0.015 ~ 0.03 | 0.03 |
| Cefotaxime | 0.015 ~ 0.03 | 0.015 | 0.015 ~ 0.03 | 0.03 |
| Ceftriaxone | 0.015 ~ 0.03 | 0.03 | 0.03 ~ 0.06 | 0.03 |
| Meropenem | 0.008 | 0.008 | 0.008 | 0.008 |
| Panipenem | ≤0.004 ~ 0.008 | 0.008 | ≤0.004 ~ 0.008 | 0.008 |
| Doripenem | ≤0.004 | ≤0.004 | ≤0.004 | ≤0.004 |
Discussion
Invasive Group A streptococcal infections include sepsis, empyema, bone and joint infections, necrotizing fasciitis, and streptococcal toxic shock syndrome. According to a number of reports, the estimated incidence of invasive group A streptococcal infection in children in developed countries is 1–3 per 100 000 children, and the mortality is 5–20 % [4]. While among all age groups, the incidence is the highest in elderly people, children younger than 5 years of age are the next most commonly affected age group [5].
Among reports on invasive group A streptococcal infections in children, Mulla [6] from the United States reported the outcomes of 25 children ranging in age from 3 weeks to 17 years with hemolytic streptococcal infections, who were examined between 1996 and 2000. In regard to the patients’ age, six children were younger than 1 year old and eight children were 1–4 years of age, with children younger than 5 years accounting for more than half of the cases. With regard to the presence of invasive disease, 18 patients had bacteremia and three had necrotizing fasciitis. The mortality was 4.4 %. Henriet et al. [7] from France reported that, among 28 children examined between 2000 and 2007, 15 had joint infections, seven had soft tissue infections, three had pneumonia, and three had toxic shock syndrome, and the median age was 2.9 years.
Wajima et al. [3]. analyzed the emm types of S. pyogenes isolated from patients with invasive group A streptococcal infections diagnosed at medical institutions in Japan between 2003 and 2006. Among the 74 strains obtained, many were isolated from patients aged 60–80 years, while only 11 strains (14.9 %) were isolated from patients younger than 20 years of age. Among the 74 strains, the most prevalent type was emm1 (29 strains; 39.2 %), followed by emm49 (eight strains; 10.8 %) and emm12 and emm28 (five strains each; 6.7 %). The emm1 type was the most prevalent, which was consistent with the results of the present study. In regard to results reported from Europe and the United States, according to one report, out of 247 strains, including those isolated from pediatric patients in Spain between 1998 and 2009, the most prevalent emm type was emm1 (60 strains; 24.3 %), followed by emm3 (21 strains; 8.5 %) and emm4 (14 strains; 5.7 %); these results indicate a high prevalence of the emm1 type [8]. In Germany, evaluation of 586 strains revealed that the most prevalent emm type was emm1 (179 strains; 30.5 %), accounting for nearly one-third of all the strains, followed by emm28 (107 strains; 18.3 %) and emm3 (56 strains; 9.6 %) [9]. Similarly, emm1 was also the most commonly identified type according to other reports, indicating that invasive infections are strongly associated with the emm1 type [7, 10]. According to recent studies [11, 12], emm12 is the most common among the emm types of S. pyogenes isolated from pharyngitis. However, the frequencies of emm1 were about half of those in severe infections, showing differences in distributions of emm types between pharyngitis and severe infections.
While there are many reports [7, 9, 13, 14] examining the associations between emm types and pathogenic factors, such as toxin production and super-antigens, why emm type 1 is commonly detected in severe infections has not been elucidated.
Among the drugs used in the drug sensitivity testing, doripenem exhibited the highest activity, with an MIC of 0.004 μg/mL against all strains. The MIC90 values of panipenem and meropenem were 0.008 μg/mL, indicating the superior antibacterial activity of these drugs as compared to that of other agents (MIC90: 0.015–0.03 μg/mL). The MIC and MBC of carbapenem agents were consistent for all strains, whereas those of the penicillin agents and cephem agents were not quite so consistent. No reports have focused on S. pyogenes exhibiting resistance to penicillin agents, although there are occasional reports of tolerance [15–17]; it is considered that, due to the divergence between MIC and MBC, the strain requires more time to be killed as compared to other strains, or there is some kind of mechanism against killing in the bacteria. This phenomenon is considered to be one of the reasons for the recurrence of pharyngitis and tonsillitis caused by S. pyogenes, even after the administration of effective antimicrobial agents [18, 19]. Moreover, It is also considered to be one reason why not a few patients treated with penicillin alone have poor outcomes in association with the “inoculum effect,” a decrease in drug susceptibility when the bacterial load is large in patients with severe infections [20].
While there are no unified criteria for tolerance, it is said that the MIC/MBC ratio is equal to or greater than 16 or 32 [19, 21]. In this study, the maximum MIC/MBC ratio was twofold, which did not fulfill the definition of tolerance; however, this phenomenon seems to require attention.
Penicillin agents are considered as the drugs of first choice for invasive streptococcal infections, and concurrent use of clindamycin is recommended, with the expectation of its special antimicrobial activities, such as tissue permeability, inhibition of toxin production, and promotion of phagocytic activity. Carbapenem agents have lower MBC than penicillin agents and, moreover, penicillin is highly effective in the log phase while its effect decreases in a steady state [20]. However, there are results, albeit in vitro, raising the possibility of high efficacy in the early stationary phase. It is thus considered to be appropriate for the treatment of severe S. pyogenes infection [22]. As there is no other study comparing the sensitivities of S. pyogenes to penicillin agents, carbapenem agents, and cephem agents, this awaits further basic and clinical investigations.
Acknowledgments
I deeply thank Prof. Kimiko Ubukata and co-workers at the Laboratory of Molecular Epidemiology for Infectious Agents, Kitasato Institute for Life Science, Kitasato University, for analysis of the emm type of S. pyogenes. This work was supported by grants for a ‘Research Project for Emerging and Re-emerging Infectious Diseases’ (H-22-013) from the Japanese Ministry of Health, Labour and Welfare.
Conflict of interest
None.
References
- 1.Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard M07–A8. 8. Wayne: CLSI; 2009. [Google Scholar]
- 2.National Committee for Clinical Laboratory Standards . Methods for determining bactericidal activity of antimicrobial agents; Approved guideline. M26-A. Wayne: NCCLS; 1999. [Google Scholar]
- 3.Wajima T, Murayama SY, Sunaoshi K, Nakayama E, Sunakawa K, Ubukata K. Distribution of emm type and antibiotic susceptibility of group A streptococci causing invasive and noninvasive disease. J Med Microbiol. 2008;57:1383–1388. doi: 10.1099/jmm.0.2008/002642-0. [DOI] [PubMed] [Google Scholar]
- 4.Steer AC, Lamagni T, Curtis N, Carapetis JR. Invasive group a streptococcal disease: epidemiology, pathogenesis and management. Drugs. 2012;72:1213–1227. doi: 10.2165/11634180-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steer AC, Jenney A, Kado J, Good MF, Batzloff M, Waqatakirewa L, et al. Prospective surveillance of invasive group a streptococcal disease, Fiji, 2005–2007. Emerg Infect Dis. 2009;15:216–222. doi: 10.3201/eid1502.080558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mulla ZD. Clinical and epidemiologic features of invasive group A streptococcal infections in children. Pediatr Int. 2007;49:355–358. doi: 10.1111/j.1442-200X.2007.02378.x. [DOI] [PubMed] [Google Scholar]
- 7.Henriet S, Kaguelidou F, Bidet P, Lorrot M, De Lauzanne A, Dauger S, et al. Invasive group A streptococcal infection in children: clinical manifestations and molecular characterization in a French pediatric tertiary care center. Eur J Clin Microbiol Infect Dis. 2010;29:341–346. doi: 10.1007/s10096-009-0854-x. [DOI] [PubMed] [Google Scholar]
- 8.Montes M, Ardanuy C, Tamayo E, Domènech A, Liñares J, Pérez-Trallero E. Epidemiological and molecular analysis of Streptococcus pyogenes isolates causing invasive disease in Spain (1998–2009): comparison with non-invasive isolates. Eur J Clin Microbiol Infect Dis. 2011;30:1295–1302. doi: 10.1007/s10096-011-1226-x. [DOI] [PubMed] [Google Scholar]
- 9.Imöhl M, Reinert RR, Ocklenburg C, van der Linden M. Epidemiology of invasive Streptococcus pyogenes disease in Germany during 2003–2007. FEMS Immunol Med Microbiol. 2010;58:389–396. doi: 10.1111/j.1574-695X.2010.00652.x. [DOI] [PubMed] [Google Scholar]
- 10.Siljander T, Lyytikäinen O, Vähäkuopus S, Snellman M, Jalava J, Vuopio J. Epidemiology, outcome and emm types of invasive group A streptococcal infections in Finland. Eur J Clin Microbiol Infect Dis. 2010;29:1229–1235. doi: 10.1007/s10096-010-0989-9. [DOI] [PubMed] [Google Scholar]
- 11.Shulman ST, Tanz RR, Kabat W, Kabat K, Cederlund E, Patel D, et al. Group A streptococcal pharyngitis serotype surveillance in North America, 2000–2002. Clin Infect Dis. 2004;39:325–332. doi: 10.1086/421949. [DOI] [PubMed] [Google Scholar]
- 12.Shea PR, Ewbank AL, Gonzalez-Lugo JH, Martagon-Rosado AJ, Martinez-Gutierrez JC, Rehman HA, et al. Group A Streptococcus emm gene types in pharyngeal isolates, Ontario, Canada, 2002–2010. Emerg Infect Dis. 2011;17:2010–2017. doi: 10.3201/eid1711.110159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatellier S, Ihendyane N, Kansal RG, Khambaty F, Basma H, Norrby-Teglund A, et al. Genetic relatedness and superantigen expression in group A streptococcus serotype M1 isolates from patients with severe and nonsevere invasive diseases. Infect Immun. 2000;68:3523–3534. doi: 10.1128/IAI.68.6.3523-3534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang Y, Liu X, Chang H, Ji L, Huang G, Fu Z, et al. Epidemiological and molecular characteristics of clinical isolates of Streptococcus pyogenes collected between 2005 and 2008 from Chinese children. J Med Microbiol. 2012;61(Pt 7):975–983. doi: 10.1099/jmm.0.042309-0. [DOI] [PubMed] [Google Scholar]
- 15.Krasinski K, Hanna B, LaRussa P, Desiderio D. Penicillin tolerant group A streptococci. Diagn Microbiol Infect Dis. 1986;4:291–297. doi: 10.1016/0732-8893(86)90069-6. [DOI] [PubMed] [Google Scholar]
- 16.Van Asselt GJ, Van Boven CP. Guidelines for detection of penicillin tolerance in Streptococcus pyogenes by MIC-MBC method. Adv Exp Med Biol. 1997;418:451–452. doi: 10.1007/978-1-4899-1825-3_107. [DOI] [PubMed] [Google Scholar]
- 17.Steininger C, Allerberger F, Gnaiger E. Clinical significance of inhibition kinetics for Streptococcus pyogenes in response to penicillin. J Antimicrob Chemother. 2002;50:517–523. doi: 10.1093/jac/dkf174. [DOI] [PubMed] [Google Scholar]
- 18.Kim KS, Kaplan EL. Association of penicillin tolerance with failure to eradicate group A streptococci from patients with pharyngitis. J Pediatr. 1985;107(5):681–684. doi: 10.1016/S0022-3476(85)80392-9. [DOI] [PubMed] [Google Scholar]
- 19.Dagan R, Ferne M. Association of penicillin-tolerant streptococci with epidemics of streptococcal pharyngitis in closed communities. Eur J Clin Microbiol Infect Dis. 1989;8:629–631. doi: 10.1007/BF01968144. [DOI] [PubMed] [Google Scholar]
- 20.Eagle H. Experimental approach to the problem of treatment failure with penicillin. I. Group A streptococcal infection in mice. Am J Med. 1952;13:389–399. doi: 10.1016/0002-9343(52)90293-3. [DOI] [PubMed] [Google Scholar]
- 21.EUCAST Definitive Document E. Def 1.2, May 2000: Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID). Clin Microbiol Infect 2000;6:503–8. [DOI] [PubMed]
- 22.Nishino T, Otsuki M, Izawa M. In vivo and in vitro antibacterial activity of doripenem. Jpn J Chemotherapy. 2005;53(1):32–46. [Google Scholar]


