Abstract
Robert Weiss (1973) conceptualized loneliness as perceived social isolation, which he described as a gnawing, chronic disease without redeeming features. On the scale of everyday life, it is understandable how something as personally aversive as loneliness could be regarded as a blight on human existence. However, evolutionary time and evolutionary forces operate at such a different scale of organization than we experience in everyday life that personal experience is not sufficient to understand the role of loneliness in human existence. Research over the past decade suggests a very different view of loneliness than suggested by personal experience, one in which loneliness serves a variety of adaptive functions in specific habitats. We review evidence on the heritability of loneliness and outline an evolutionary theory of loneliness, with an emphasis on its potential adaptive value in an evolutionary timescale.
Introduction
Solitude expresses the glory of being alone, whereas loneliness expresses the pain of feeling alone (Tillich, 1959). In Weiss’ (1973) seminal work, loneliness was conceptualized as “perceived social isolation,” a state Weiss described as a gnawing, chronic disease without redeeming features. Early in our history as a species, we survived and prospered by banding together—in couples, in families, in tribes—to provide mutual protection and assistance. The pain of loneliness served to prompt us to renew the connections we needed to insure survival and to promote social trust, cohesiveness, and collective action. Loneliness may feel like it has no redeeming features, but it may have evolved as an aversive state that, like hunger, thirst, and pain, promotes behavior change to increase the likelihood of the survival of one’s genes. Our aim here is to review evidence that loneliness has a significant heritable component, and we propose several mechanisms that may contribute to this heritability.
The Heritability of Loneliness
Heritability refers to the proportion of the individual differences in a trait – also referred to as a phenotype- in a specific population that is attributable to genetic variation between the individuals. Heritability is sometimes confused with genetic determinism, but these are not equivalent concepts. For instance, the development of the human heart is genetically determined but the heritability of a person having a heart is zero. This is because there is no individual variability in a person having a heart. Conversely, a phenotype may have a heritability of 100% (e.g. phenylketonuria (PKU) in our current environment) but its treatment still can be successful in all cases through environmental manipulation (diet).
The heritability of a trait is a population parameter that is estimated for a particular population in a given environment (Boomsma, Busjahn, & Peltonen, 2002). Heritability can differ by environment, age or sex or other modifiers, and genetic contributions to variables such as loneliness can accrue over time, or the expression of genetic factors may change across the life span, so that the heritability of loneliness may be different in adults than in children. Moreover, because heritability refers to the proportion of phenotypic variation that is attributable to individual differences in genes, heritability can also be influenced by increases or decreases in the influence of environmental factors across age or habitats.
McGuire and Clifford (2000) were the first to investigate the heritability of loneliness. In their first study, children aged 9, 10, 11, and 12 years from the Colorado Adoption Project completed an 8-item loneliness scale. The resemblance between 69 biological sibling pairs (who share genes and family environment) and 64 unrelated pairs (who share environment but not genes) was contrasted. In a second study, 22 monozygotic (MZ, genetically identical or nearly identical) twins, 40 dizygotic (DZ) twins, and 80 full-siblings 8–14 years of age completed a 16-item scale to assess loneliness in relation to their schoolmates. Results revealed a significant genetic (h2 =55% and 48%, respectively, in Studies 1 & 2) contribution to individual differences in loneliness. However, because of small sample sizes models that contributed the resemblance between children to shared environment rather than to shared genes were hard to distinguish on statistical grounds.
Using data from the Netherlands Twin Register, Bartels, Cacioppo, Hudziak, and Boomsma (2008; Boomsma, Cacioppo, Muthen, Asparouhov, & Clark, 2007) extended this early work in larger samples to better distinguish between genetic and environmental sources of variation, especially shared environmental factors, which refer to all non-genetic factors that make children who grow up in the same family more phenotypically similar. The sample consisted of 7,995 Dutch twin pairs sampled at ages 7, 10, and 12 and included both same-sex and opposite-sex twin pairs to test for sex differences in the magnitude of genetic and environmental influences. Loneliness was assessed by summing two items from the Child Behavior Checklist. Average scores of loneliness over ages 7, 10, and 12 were 46% heritable-similar to McGuire and Clifford’s estimate- with a significant contribution also of shared environmental influences (12%). Results from the genetic longitudinal analyses were more interesting: heritability changed across childhood and was estimated to be 60% at age 7, 54% at age 10, and only 17% at age 12. A parallel increase in influences of shared family environment was observed, explaining 4% of the variance at age 7, 12% at age 10 and 41% at age 12. The remaining variance was explained by relatively stable influences of non-shared environmental factors. As it would be expected from the heritability estimates, the stability of loneliness in children was high, with correlations across age in the range of .35 to .78. This phenotypic stability is caused by genetic influences, and shared and non-shared environmental influences.
What might be responsible for the drop in heritability seen in 12 year olds? One possibility is the onset of puberty, a largely genetically programmed change in gonadal steroids that children undergo at around the same age. With the change in sex hormones that wash over 12 year olds comes a change in how children perceive and seek to relate to one another. That is, this biological change leads children to look to their social environments to reshape their peer relationships, with the result being that their satisfaction with peer relationships at the onset of puberty largely being a function of environmental rather than genetic variance. This notion implies that the heritability of loneliness would increase again by adulthood. A study from Norway in over 1,300 twin pairs aged 12–18 years observed a heritability of 44% based on self-ratings on the UCLA loneliness scale (5 item version). Interestingly, heritability increased when ratings from parents (both fathers and mothers) were analyzed. The estimate of 44% is remarkably close to the estimate reported by Boomsma et al. (2005) for loneliness in 8,387 young adult and adult Dutch twins. In this study, a measure of loneliness was developed based on factor analyses of items of the YASR (Achenbach, 1990). The estimate of genetic contributions to variation in loneliness in adults was 48%. Similar to the heritability estimates found in children, and adolescents there was no evidence for sex differences in genetic architecture. Unlike the studies of children, however, shared environmental factors did not account for significant phenotypic variation in loneliness in the Dutch adult sample. A gender difference in prevalence was found, but the results of Boomsma et al. (2005) suggest that gender differences are environmentally (e.g., social norms, cultural influences) rather than genetically determined – a conclusion that may help explain the inconsistencies in gender differences in prevalence that has been observed across studies (e.g., see Hawkley et al., 2009).
To address concerns that heritability estimates for loneliness from twin studies might not be generalized to the general population, Distel et al. (2010) examined the genetic architecture of loneliness in an extended twin-family design including 8,683 twins, 917 spouses of twins and siblings and parents from 3,911 families. The presence of assortative (non-random) mating, genetic non-additivity, vertical cultural transmission, genotype-environment (GE) correlation and interaction were modeled. Results indicated the presence of positive assortative mating for loneliness – people who are similar in their trait loneliness tend to mate. Distel et al. (2010) also confirmed that loneliness is moderately heritable, but interestingly found a significant contribution of non-additive genetic variation. No evidence was found for vertical cultural transmission, which suggests that parents may pass on genes for loneliness, and all transmission is genetic. With respect to demographic characteristics, results indicated that marriage, having offspring, more years of education, and a higher number of siblings are associated with lower levels of loneliness. Interestingly, these effects tended to be stronger for men than women. There was little evidence of changes in genetic architecture as a function of these characteristics, however. Together, the architecture of loneliness points to non-additive genetic influences, suggesting it may be a trait that was not neutral to selection in our evolutionary past.
These studies also indicate that there are environmental influences on the phenotypic variation in loneliness found in populations. For instance, freshmen who leave family and friends behind often feel increased social isolation when they arrive at college even though they are surrounded by large numbers of other young adults (e.g., Cutrona, 1982; Russell, Peplau, & Cutrona, 1980). Lower levels of loneliness are associated with marriage (Hawkley, Browne, & Cacioppo, 2005; Pinquart & Sőrensen, 2003), higher education (Savikko, Routasalo, Tilvis, Strandberg, & Pitkala, 2005), and higher income (Andersson, 1998; Savikko, et al., 2005), whereas higher levels of loneliness are associated with living alone (Routasalo, Savikko, Tilvis, Strandberg, & Pitkala, 2006), infrequent contact with friends and family (Bondevik & Skogstad, 1998; Hawkley et al., 2005; Mullins & Dugan, 1990), dissatisfaction with living circumstances (Hector-Taylor & Adams, 1996), physical health symptoms (Hawkley, Hughes, Waite, Masi, Thisted, & Cacioppo, 2008), disabilities (Hughes, Hawkley, Waite, Masi, Thisted, & Cacioppo,, 2010), chronic work and/or social stress (Hawkley et al., 2008), small social network (Hawkley et al., 2005; Mullins & Dugan, 1990), lack of a spousal confidant (Hawkley et al., 2008), marital or family conflict (Jones, 1992; Segrin, 1999), poor quality social relationships (Hawkley et al., 2008; Mullins & Dugan, 1990; Routasalo, et al., 2006), and divorce and widowhood (Dugan & Kivett, 1994; Dykstra & de Jong, 1999; Holmen, Ericsson, Andersson, & Winblad, 1992; Samuelsson, Andersson, & Hagberg, 1998).
Comparative Evidence
For members of social species, life on the social perimeter is aversive and unsafe. The presence of connections among conspecifics is the defining characteristic of social species, and the absence of these connections (i.e., social isolation) threatens the health, life, and genetic legacy of members of many different social species. For instance, social isolation has been shown to decrease the lifespan of the fruit fly, Drosophilia melanogaster (Ruan & Wu, 2008); promote the development of obesity and Type 2 diabetes in mice (Nonogaki, Nozue, & Oka, 2007); exacerbate the infarct size and edema and decrease post-stroke survival rate following experimentally induced stroke in mice (Karelina et al., 2009); delay the positive effects of running on adult neurogenesis in rats (Stranahan, Khalil, & Gould, 2006); decrease neuronal connectivity and plasticity in rats (Silva-Gomez, Rojas, Juarez, & Flores,, 2003; Day-Wilson, Jones, Southam, Cilia, & Totterdell, 2006); lower levels of brain-derived neurotrophic factor in rats (Scaccianoce, Del Bianco, Paolone, Caprioli, Modafferi, Nencini, & Badiani, 2006); alter prefrontal cortex function and myelination that do not recover with reintroduction into a social environment in mice (Makinodan, Rosen, Ito, & Corfas, 2012); increase the activation of the sympathetic adreno-medullary response to acute stressors in rats (Dronjak, Gavrilovic, Filipovic, & Radojcic, 2004); decrease the expression of genes regulating glucocorticoid response in the frontal cortex of piglets (Poletto, Steibel, Siegford, & Zanella, 2006); decrease open field activity (i.e., increases predator evasion), increase basal cortisol concentrations, and decrease lymphocyte proliferation to mitogens in pigs (Kanitz, Tuchscherer, Puppe, Tuchschere, & Stabenow, 2004); increase morning rises in cortisol in squirrel monkeys (Lyons, Ha, & Levine, 1995); and elevate 24 hr urinary catecholamines and oxidative stress in the Watanabe Heritable Hyperlipidemic rabbit (Nation, Gonzales, Mendez, Zaias, Szeto, Brooks, et al., 2008). Together, these experimental studies suggest that social isolation increases chronic sympathetic tonus, oxidative stress, and HPA activation while decreasing inflammatory control, immunity, and the expression of genes regulating glucocorticoid responses. The effects of perceived isolation in humans share much in common with the effects of experimental manipulations of isolation in nonhuman social species: increased threat surveillance, tonic sympathetic tonus, and HPA activation, and decreased inflammatory control, immunity, sleep salubrity, executive functioning, and expression of genes regulating glucocorticoid responses (cf. Cacioppo, Hawkley, Norman, & Berntson, 2011 for a review).
The physiological effects of loneliness are clearly deleterious for the individual in contemporary times, in which life expectancy now extends well into the eighth decade of life. However, many of these behavioral and biological costs (e.g., hypertension, dementia) are incurred in older adulthood, whereas the benefits of an aversive signal associated with social isolation (increased threat surveillance, increased focus on self-preservation, increased motivation to reconnect) are realized across the lifespan. Across much of human history, people did not live long enough for the cognitive and physiological deficits to be of much consequence for the individual or for the population, whereas the benefits increased their likelihood of survival.
Possible Evolutionary Mechanisms for Loneliness
The heritability and comparative studies raise the question of how loneliness might have evolved. Evolution operates over generations on phenotypes expressed in specific habitats. Evolutionary mechanisms have been proposed for various phenotypes that are related to loneliness, including depression/depressive symptomatology (Allen & Badcock, 2003; Andrews & Thompson, 2009; Keller & Neese, 2005) and self-esteem (Leary & Downs, 1995; Kirkpatrick & Ellis, 2001) to the need to belong (Baumeister & Leary, 1995), but these phenotypes are functionally (e.g., Adam, Hawkley, Kudielka, & Cacioppo, 2006; Cacioppo, Hawkley, & Thisted, 2010; Cole, et al., 2007, 2011; Hawkley, Masi, Berry, & Cacioppo, 2006) and stochastically (e.g., Cacioppo, Hawkley, Ernst, et al., 2006) distinguishable from loneliness. Loneliness, for instance, makes people feel not only unhappy but also unsafe. Experimental manipulations of loneliness increase depressive symptomatology, shyness, anxiety, and fear of negative evaluation, and decreases self esteem, social skills, and overall mood (Cacioppo, Hawkley, Ernst, et al., 2006). Longitudinal studies have shown that loneliness predicts increases in depressive symptomatology above and beyond what can be explained by basal levels of depressive symptomatology (Cacioppo et al., 2006; Heikkinen & Kauppinen, 2004; Wei, Russell, & Zakalik, 2005) and beyond what is predicted by associated psychosocial variables such as objective stress, perceived stress, social network size, neuroticism, and social support (Cacioppo et al., 2010).
An experience sampling study, in which participants were beeped randomly nine times per day for seven days, confirmed that the social interactions of lonely, in contrast to nonlonely individuals were more negative and less satisfying, and such interactions contributed subsequently to more negative moods and interactions (Hawkley, Preacher, & Cacioppo, 2007). A subsequent social network analysis of residents in Framingham, Massachusetts, further revealed that loneliness was contagious and led to a person being moved over time to the periphery of the social network – an effect that could not be explained in terms of depressive symptomatology (Cacioppo, Fowler, & Christakis, 2009).
The early and extended dependence on caregivers and the limited physical endowments across the lifespan, together, place humans at risk when they are isolated. In this context, it may be adaptive to have evolved an aversive signal that draws attention to the prospect that our social connection to others is at risk or absent and that motivates us to ensure or replace the safe, collaborative social surround we need to ensure a genetic legacy (Cacioppo et al., 2006). Hunger, thirst, and pain, for instance, have evolved to prompt an organism to change its behavior in a way that protects the individual and promotes the likelihood his or her genes will make their way into the gene pool. We have proposed that the awareness of loneliness evolved to serve as a signal that one’s connections to others are frayed or broken and to motivate the repair and maintenance of the connections to others that are needed for our health and well being as well as for the survival of our genes (Cacioppo et al., 2006). That is, just as physical pain is an aversive signal that evolved to motivate one to take action that minimizes damage to one’s physical body, loneliness is an aversive state that motivates us to take action that minimizes damage to one’s social body (cf. Cacioppo, Fowler, & Christakis, 2009).
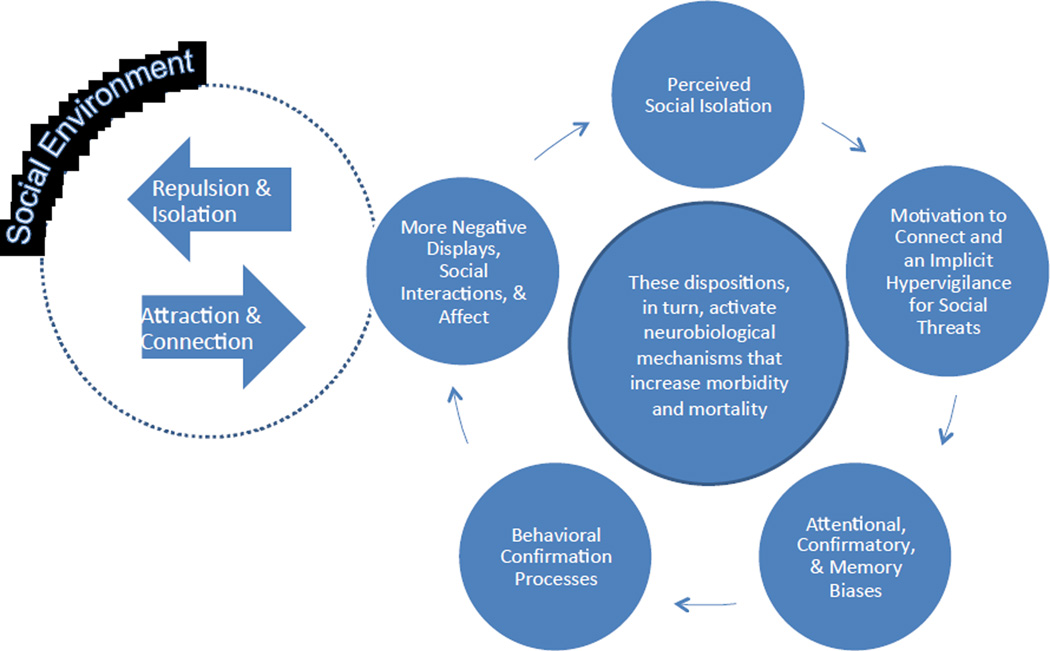
Humans are capable of duplicity and changing alliances, so being with others is not sufficient to ensure one is embedded in a safe social surround, especially in vulnerable times such as when one is asleep (Cacioppo, Hawkley, Berntson, et al., 2002). Accordingly, research has shown that it is the quality, not the quantity of one’s social connections that predicts loneliness (i.e., perceived social isolation) across a lifetime (Cacioppo, Ernst, Burleson, et al., 2000; Wheeler, Reis, & Nezlek, 1983). In non-human social species, being shunned or ostracized is associated with high rates of mortality (Williams, 2003). In human beings, finding oneself uncertain that one can confide in, depend on, or trust others is not only an unhappy social environment, it can also be a profoundly unsafe social environment. Others with whom one once cooperated can no longer be counted on for cooperation, and strangers cannot be assumed to be friends rather than foes. Thus, we have posited that the perceived social isolation that motivates one to attend to and to seek connection with others also leads to the expression of other features on which natural selection may operate (see Figure 1).
Figure 1.
The effects of loneliness on human cognition. From Cacioppo, J. T., & Hawkley, L. C. (2009). Perceived social isolation. Trends in Cognitive Sciences,13, 447–454.
First, loneliness increases explicit attention to social stimuli and increases implicit attention to social threats have a counterpart in hunger. Hunger increases one’s attention to and motivation to find food. Not everything that appears edible is safe to eat by humans, however. Over an evolutionary timescale, our taste buds have developed to be much more sensitive to bitter (e.g., concentrations of 1:2,000,000) than to sweet (e.g., concentrations of 1:200). Poisons tend to have a bitter taste, so this difference in sensitivity has evolved to protect the individual from dangers that arise as a result of the drive to find food. Given it is more costly to fall victim to a fatal assault than to forego a friendship, becoming more sensitive to social threats may be beneficial, especially in environments populated by dangerous foes.
The effects of loneliness on the motivation to connect and on implicit hypervigilance for social threats serve to increase the salience of social cues, produce a confirmatory bias toward seeing social dangers, and create negative memory biases for social information. For instance, lonely individuals not only tend to form more negative social impressions of others, but their memory for their interactions with others grows more negative over time (e.g., Duck, Pond, & Leatham, 1994). When an individual’s negative social expectations elicit behaviors from others that validate these expectations, the expectations are buttressed and increase the likelihood of the individual behaving in ways that push away the very people to whom he or she most wants to be close to better fulfill their social needs (Downey & Romero-Canyas, 2005; Murray, Bellavia, Rose, & Griffin, 2003; Rotenberg, 1994). Consequently, lonely individuals may view themselves to be passive victims in their social world, but they are active contributors through their self-protective interactions with others (Cacioppo & Hawkley, 2005). These are largely counterproductive effects of loneliness when viewed within a personal timescale. For instance, the effects of loneliness on social cognition and self-preservation may produce paradoxically self-defeating behavior in safe and embracing social environments but these effects on social cognition may benefit the individual in hostile and duplicitous environments.
Lonely people are viewed more negatively—in terms of their psychosocial functioning and in terms of their interpersonal attraction or acceptance—than are nonlonely people (Lau & Gruen, 1992; Rotenberg & Kmill, 1992). Once people in a lonely person’s social environment form a negative impression, their behaviors toward that individual can reinforce his or her negative social expectancies (Rotenberg, Gruman, & Ariganello, 2002), promote hostile or antagonistic behavior, and sustain the lonely individual’s isolated existence. For instance, Rotenberg, et al., 2002 found that individuals rated opposite-gender partners who they expected to be lonely as less sociable, and behaved toward them in a less sociable manner than they did toward partners they expected to be nonlonely. The opposite social forces appear to preserve the superior life of individuals very low in loneliness, in that they are perceived and treated more positively and are more likely to be given a benefit of the doubt in uncertain or ambiguous situations (see Figure 1).
Evidence from behavioral and functional magnetic resonance imaging (fMRI) studies also supports the notion that loneliness increases attention to negative social stimuli (e.g., social threats). Using a modified emotional Stroop task, lonely participants, relative to nonlonely participants, showed greater Stroop interference specifically for negative social relative to negative non-social words (Shintel, Cacioppo, & Nusbaum, 2006). No differences between lonely and non-lonely participants were found in Stroop interference for positive social relative to positive non-social words. Stroop interference is used to gauge the implicit processing of stimuli, so these results suggest that loneliness is associated with a heightened accessibility of negative social information. Similarly, Yamada and Decety (2009) investigated the effects of subliminal priming on the detection of painful facial expressions. Using signal detection analyses, they found that, although the pain was more easily detected in dislikable than likable faces overall, lonely individuals were more sensitive (d’) to the presence of pain in dislikable faces than were non-lonely individuals.
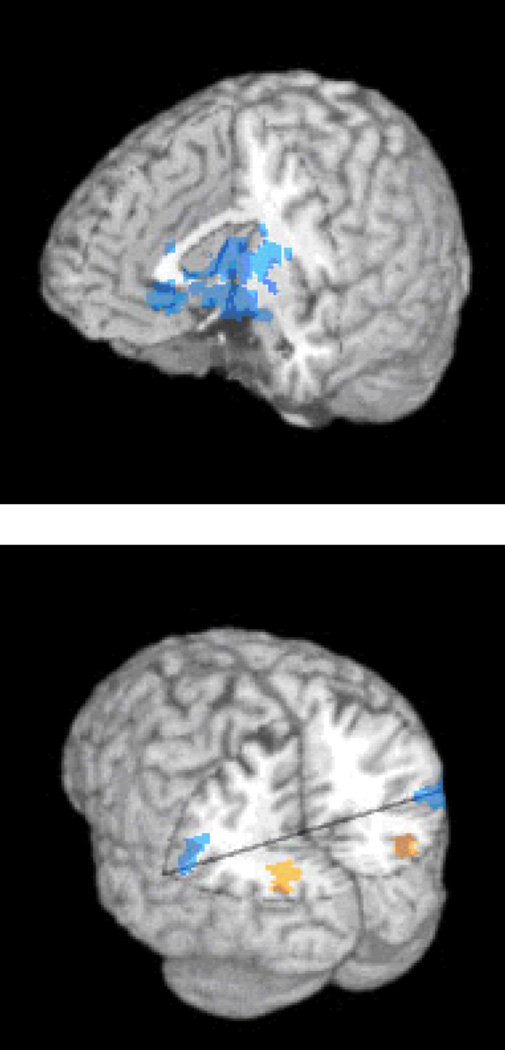
We have also found differences in the pattern of regional brain activation produced by lonely and non-lonely individuals when thinking about people (Cacioppo, Norris, Decety, Monteleone, & Nusbaum, 2009). Activation of the visual cortex to the presentation of unpleasant social, in contrast to nonsocial, pictures was directly related to the loneliness of the participant, indicative of greater visual attention to the negative social stimuli (see Figure 2). These results are consistent with the behavioral data indicating that loneliness is related to an attentional bias for negative social stimuli.
Figure 2.
Top panel. A cluster of voxels centered in the ventral striatum, but extending to the amygdala and portions of the anterior thalamus, showed an inverse relationship between loneliness and activation in the Pleasant Social – Pleasant Nonsocial contrast. Bottom Panel. Clusters of voxels in the left and right visual cortices exhibited a positive relationship between loneliness and activation in the Unpleasant Social – Unpleasant Nonsocial contrast; whereas clusters of voxels in the left and right TPJ exhibited a negative relationship between loneliness and activation in the Unpleasant Social – Unpleasant Nonsocial contrast. Cacioppo, J. T., Norris, C. J., Decety, J., Monteleone, G., & Nusbaum, H. (2009). In the eye of the beholder: Individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience, 21, 83–92.
A possible casualty of loneliness and the priming of social threats is that lonely individuals may be more likely to focus on their personal needs and self-preservation in negative circumstances. To examine this possibility, activation in the temporo-parietal junction (TPJ) -- a region that has been found previously to be activated in theory of mind tasks and in tasks in which individuals take the perspective of another, was also examined (Cacioppo et al., 2009). Consistent with this reasoning, greater TPJ activation was observed when nonlonely (than lonely) participants viewed unpleasant pictures of people versus objects, consistent with the notion that they are more likely to reflect spontaneously on the perspective of distressed others.
Recent research suggests that loneliness modulates the rudimentary reward system, as well. The ventral striatum, a key component of the mesolimbic dopamine system, is rich in dopaminergic neurons and is critical in reward processing and learning (Delgado, Miller, Inati, & Phelps, 2005; O'Doherty, 2004). The ventral striatum is activated by primary rewards such as stimulant drugs (Leyton, 2007), abstinence-induced cravings for primary rewards (Wang, Faith, Patterson, et al., 2007), and secondary rewards such as money (Seymour, Daw, Dayan, Singer, & Dolan, 2007). Evidence that social reward also activate the ventral striatum has begun to accumulate in studies of romantic love (Aron, Fisher, Mashek, Strong, Li, & Brown, 2005), social cooperation (Rilling, Gutman, Zeh, Pagnoni, Berns, & Kilts, 2002), social comparison (Fliessbach, Weber, Trautner, et al., 2007), and punitive altruism (De Quervain, Fischbacher, Treyer, et al., 2004). We investigated how an individual’s loneliness was related to the differential activation of the ventral striatum to pleasant social versus matched nonsocial images (Cacioppo et al., 2009). As depicted in Figure 2, lonely individuals showed weaker activation of the ventral striatum to pleasant pictures of people than of equally pleasant pictures of objects, whereas nonlonely individuals showed stronger activation of the ventral striatum to pleasant pictures of people than of objects.
There is a synergism between loneliness and affect, as well. For instance, experimental and longitudinal research has shown that loneliness increases depressive symptomatology (Cacioppo et al., 2006, 2010). We have posited that loneliness has evolved to increase depressive symptomatology for two reasons. First, this lessens the likelihood individuals will try to force one’s way back into a group. Second and more importantly, by acting on depressive symptomatology, loneliness increases the likelihood that an individual who feels socially isolated will evince sad facial and postural displays and audible crying, which function as a call for others to come to their aid and serve as a supportive connection (Allen & Badcock, 2003). Whether this passive mechanism for broaching a social divide succeeds and benefits the individual again depends on the social environment, such as the likelihood that a caring friend will see and be willing and able to respond to the distress cues.
Loneliness has yet another cognitive consequence – it diminishes self-regulation. Social isolation in nonhuman animals has been found to increase the likelihood of a prepotent response (cf. Cacioppo & Hawkley, 2009b). Early evidence from young adults who performed a dichotic listening task suggested the same effect occurred in humans as a function of loneliness (Cacioppo, et al., 2000). Specifically, participants were asked to identify the consonant-vowel pair presented in the left or right ear. Typically, performance shows a right-ear advantage and performance is better for the ear to which participants have been instructed to attend. In this task, then, the prepotent response is a right-ear advantage. Lonely and nonlonely individuals showed an equivalent right ear advantage under the no-instruction condition and an equivalent attentional shift to the right ear when instructed to attend to the consonant-vowels presented in the right ear. However, lonely participants showed a weaker left-ear advantage (the non-prepotent response) when instructed to attend to this ear than did nonlonely participants, thereby demonstrating a stronger influence of the prepotent response when the non-prepotent response was required.
Poorer self-regulation when feeling isolated is not limited to attentional control. In cross-sectional and longitudinal research, lonely individuals have been found to have lower odds of engaging in regular exercise than nonlonely individuals, and the poorer emotional regulation of individuals when they felt lonely mediated the effect (Hawkley, Thisted, & Cacioppo, 2009). Experimental manipulations that lead people to believe they face a future of social isolation also decrease self-regulation. In an illustrative study, Baumeister and colleagues (Baumeister & DeWall, 2005) had the participants complete two questionnaires: an introversion/extraversion test and a personality inventory. Participants then were randomly assigned to receive no feedback (Control Group) or to receive feedback to induce feelings of a future of social isolation (Future Alone), social connection (Future Belonging), or general misfortune (Misfortune Control Group). Results revealed that the Future Alone group performed significantly worse than the other groups on the General Mental Ability Test of the Graduate Record Exam. Bad news itself was not enough to cause the disruption, only bad news about social connection.
In subsequent variations on this experimental paradigm, randomly assigned participants to the Future Alone Group, relative to the other groups, performed similarly on a rote memorization task but attempted the fewest problems and made the most mistakes on a logical reasoning task (Baumeister, DeWall, Ciarocco, & Twenge, 2005), consumed more delicious but unhealthy foods (Baumeister et al., 2005), and were more aggressive toward others (Twenge, Baumeister, Tice, & Stucke, 2001). A perceived future of social isolation, then, did not impair routine mental ability, only the higher order cognitive and self-regulatory processes that are characteristic of executive functioning. A brain scan conducted while participants performed moderately difficult math problems revealed that the brains of the future socially isolated participants were less active in the areas involved in the “executive control” of attention (Campbell, Krusemark, Dyckman, et al., 2006). When one considers how unpleasant perceived social isolation is, it is perhaps understandable why individuals dealing with the misery of loneliness are less likely to deny themselves guilty pleasures or require of themselves the discipline and effort required to maintain good executive functioning.
In sum, loneliness can have beneficial and deleterious effects within a personal lifetime, with the balance between the two influenced to some extent by features of the environment. The remarkable fact may not be that loneliness can have positive and negative effects on an individual, but that most people do not feel lonely most of the time. The search for social connection may be slowed by the self-preservational biases outlined in Figure 1 but, ultimately, the motive to repair or replace one’s frayed social connections is likely to succeed (Masi, Chen, Hawkley, & Cacioppo, 2011).
Multi-level models of evolution conceptualize fit in terms of embedded structures, genes within cells, cells within organ systems, organ systems within individuals, individuals within groups, groups within populations (Caporael & Brewer, 1991; Wilson, Krueger, Arnold, et al., 2007). The effects of loneliness on the hypothalamic pituitary adrenocortical axis (Cacioppo & Hawkley, 2009b; Hawkley & Cacioppo, 2009), for instance, serve to position individuals to respond to threats, with few long-term health consequences until the relatively recent expansion in the human lifespan.
Natural selection operates on phenotypes. It therefore can be useful to articulate the full phenotype of loneliness and to consider its adaptive function within an evolutionary timescale. Specifically, an explication of the phenotype of loneliness and a description of the adaptive function each aspect of this phenotype might be serving may help us understand the processes that operate between individuals and contribute to genetic perpetuation across generations.
The phenotypic expression of loneliness has been described as a strong sense of social pain, emptiness, isolation, sadness for lack of confidants, unimportance and worthlessness (Weiss, 1973). Moving beyond phenomenology, however, psychometric analyses reveal three basic dimensions underlying loneliness, reflecting the degree of isolation (or connection) in three related but separable domains: intimate attachments, face-to-face relations, and social identities (Hawkley et al., 2005, 2012). These three dimensions are correlated but separable and robust. For instance, this factor structure has been identified in men as well as women; African Americans, Euro-Americans, and Latino-Americans; and in young adults as well as older adults in the United States (Hawkley et al., 2005) and in China (Hawkley et al., 2012). If these different aspects of the phenotype have evolved, what is the adaptive function served by each across generations? Evolution provides more than a historical perspective, it is a continuous process that may shed light on who we are as a species and why certain behavioral tendencies operate as they do.
The first factor is intimate isolation/connection, or what Weiss (1973) termed emotional loneliness, and it refers to the perceived presence/absence of someone in your life who serves as a nurturing confidant, someone who affirms your value as a person. In a population-based study of middle-age and older adults, the best predictor of intimate isolation (net the other two factors that were identified) was marital status: participants who were married were, on average, lower in intimate isolation than were participants who were unmarried (Hawkley et al., 2005).
This facet of the phenotype of loneliness, with its emphasis on emotional aspects of loneliness and intimate connectedness, may be based on heritable differences in sensitivity to the pain of social disconnection (Cacioppo & Hawkley, 2009a). These individual differences can serve an important evolutionary function. Individuals who are relatively insensitive to the pain of social disconnection may be more likely to serve as explorers but their insensitivity to social connection may not compel them return to share their discoveries. Individuals who are sensitive to the pain of social connection, in contrast, may be more likely to remain in or return to the group and contribute to the protection and maintenance of the group, but they may be less likely to make solitary journeys that reveal new territories, threats, or opportunities. Both types of predispositions can be important. A population consisting only of explorers may be characterized by sufficiently weak forces holding the group together that the group would splinter when pitted against oppositional forces. A population consisting only of people who are susceptible to loneliness, on the other hand, might be at risk for insecurities of other kinds, such as starvation or predation as a result of a slow rate of exploration and discovery. Note that the benefits of the population having individual differences in sensitivity to disconnection would accrue even if the group as a whole tended to peripheralize individuals who manifested loneliness. Most individuals who feel lonely resolve their loneliness before it becomes an extreme condition, and even those who fail to resolve loneliness before it becomes severe nevertheless tend to form new connections given sufficient time (Masi et al., 2011). These new connections may be with others who share a sense of isolation, which through the process of assortive mating contributes to the continuation of heritable individual differences in loneliness at the level of the population (Distel et al., 2010).
What evidence is there that the heritability of loneliness reflects, at least in part, individual differences in sensitivity to social disconnection? Relevant evidence is available from perhaps a surprising source: Myron Hofer’s (2009) research on the selective breeding of rats. A well-characterized response to maternal separation is the separation cry. In the rat, the separation cry is in the ultrasonic range (40–45 kHz). These ultrasonic vocalizations (USVs) to isolation are attenuated in a dose-related fashion by anxiolytics that act on benzodiazepine and serotonin receptors and are exaggerated by anxiogenics such as the benzodiazepine receptor partial inverse agonist FG7142 (e.g., Brunelli & Hofer, 2001). Neuroanatomical studies reviewed by Hofer (2009) point to the periaquaductal grey area as a neural substrate for these USVs in rats and the hypothalamus, amygdala, thalamus, and hippocampus and cingulate cortex as the neural substrates for isolation calls in primates. As Hofer notes:
The evolution of such a response is clarified by the finding that infant rat USV is a powerful stimulus for the lactating rat, capable of causing her to interrupt an ongoing nursing bout, initiate searching outside the nest, and direct her search toward the source of the calls… The mother’s retrieval response to the pup’s vocal signals then results in renewed contact between pup and mother. This contact, in turn, quiets the pup (Hofer, 2009, p. 20).
Hofer uses the concept of attachment to describe the emotional expression represented by the USVs and the reestablishment of the social bond by the maternal search for, renewed contact with, and comfort response of the rat pup.
Although the infant USV helps guide the mother to the infant, these USVs can also lead predators to the infant. USVs may be beneficial or deleterious depending on the presence of predators in the environment. As a consequence, no single level of intensity of USVs to isolation is universally best, and heritable individual differences in this predisposition exist in the population. Some rat pups, who might be characterized as sensitive to separation, cry frequently (albeit ultrasonically) when isolated, whereas others are less sensitive to separation and show less distress when isolated. Hofer and colleagues selectively bred adult rats that, as rat pups, showed either high or relatively low rates of USVs to separation (Brunelli & Hofer, 2007). After 25 generations of selective breeding, differences behavior between the two lines of rats were reminiscent of some of the differences observed in humans who are high or low in loneliness: the high, relative to low, USV line showed more distress to isolation as an infant; greater latency to play as an adolescent; and greater depression-like behaviors, greater anxiety-like behaviors, greater latency to social interactions, greater startle, and diminished learning as an adult. That is, the high USV line was anxious and passive, whereas the low USV line was exploratory, active, and aggressive (Hofer, 2009).
This work, then, is consistent with a link between loneliness and attachment processes and points to a specific aspect of the phenotype (sensitivity to isolation) and evolutionary mechanisms for this phenotype. Note, however, that in this context the mother-infant attachment builds on heritable differences in sensitivity to isolation rather than the pain of isolation resulting from poor infant attachment behaviors by the mother. The forces of attachment are not limited to mother-infant relations, either, as paternal attachment (Bowen & Miller, 1980) and romantic attachment between partners (Howard, 2010) are observed in humans, as well.
The second factor is relational isolation/connection, or what Weiss (1973) termed social loneliness, and refers to the perceived presence/absence of quality friendships or family connections. The best predictor of relational isolation was the frequency of contact with friends and family: participants who had frequent contact with friends and family were lower in relational isolation even after statistically controlling for the two other loneliness factors (Hawkley et al., 2005). This factor is found in women as well as men, but it appears to be more heavily weighted in women than in men such that it plays a larger role in influencing degree of loneliness in women than in men.
The relational connectedness aspect of the phenotype of loneliness may have a different evolutionary basis than the intimate connectedness aspect. For instance, loneliness not only serves to signal the prospect that our social connection is at risk or absent and to motivate us to repair or restore the safe, collaborative social surround we need to ensure a genetic legacy, but it may also provide incentives to become more compassionate and empathic members of our social species (cf. time-out, shunning, ostracism). That is, loneliness serves as a punishment for selfish behavior and a negative reinforcer for more socially positive behaviors. The general principle is that when an individual in a social setting is made to feel isolated, he or she is compelled to change his or her behavior toward others.
Consider the contemporary practice of time-out. When a child acts selfishly or socially inappropriately, a common practice is to isolate the child from the others for approximately one minute per year of age. This enforced social isolation is typically aversive for the child and may be associated with displays of sadness or general negative affect, which we noted above serves as an appeal for connection and support by anyone in the vicinity. Upon reintegration to the group following the time-out, the child tends to act in a more empathetic, less narcissistic fashion – that is, the existence of the aversive state of loneliness can contribute to our socialization and culture.
Although time-out is a recent innovation, it is illustrative of a more general principle in which an individual in a social setting who is made to feel isolated is compelled to change his or her behavior toward others. The “silent treatment” between close social others, workplace expulsion, teasing, being excluded or ignored in social circumstances, and countless other passive or active rejection behaviors, whether enacted for virtuous or malicious reasons, are potent elicitors of social pain (Williams, Suchy, & Rau, 2009) and can also serve to promote other-oriented social motives. Evidence of shunning and ostracism can be found in nonhuman social species as well as in various cultures across human history. Ostracism in most social species is associated with an early death (Williams, 2001). In humans, the chronic feeling of social isolation, even when the person remains among the protective embrace of others, is associated with significant adverse mental and physical outcomes (Hawkley & Cacioppo, 2009; Patterson & Veenstra, 2010). Because humans need to be able to work together to survive and prosper, the effects of time-out, shunning, and related methods of interpersonal rejection on people’s social skills (e.g., reduced selfishness, increased perspective taking and empathy) may ultimately benefit the individual and contribute to social adhesion and resilience.
Humans are especially adept in observational learning – extracting information about the environment based on their observations of the costs and benefits of those with whom they are connected or about whom they care. Social learning, in turn, promotes the development of common knowledge and practices, that is culture, adding to the centripetal forces banding individuals together to form adaptable, cooperative groups (Rendell, Boyd, Cownden, et al., 2010). The effect of loneliness on people’s attention to interpersonal information may, therefore, have the additional benefit of promoting social learning.
But isn’t selfishness the law of our genes? The notion of “selfish genes” (and, by extension, selfish organisms) was popularized in Richard Dawkins’ 1976 book by that title. Not long afterwards, an article appeared in Science that presented evidence that the most vicious members of a warlike tribe in South America had the most wives and children. The underlying notion was one of (genetic) survival of the fittest: Those warriors who were particularly vicious were more likely to contribute their genes to the gene pool. Methodological objections have left this an open question, however, and new evidence now exists that calls this interpretation into question. Beckerman and colleagues (Beckerman, Erickson, Yost, et al., 2009) found that the most aggressive warriors may have more children but they have lower indices of reproductive success than their milder brethren in part perhaps because the most aggressive warriors and their offspring are also more likely to be the targets of revenge killings. These data are consistent with the notion that the content of the human gene pool is influenced by interpersonal connections and the reproductive success of one’s offspring, not simply to one’s own short-term reproductive success.
The third factor is collective isolation/connection, an aspect that Weiss (1973) did not identify in his qualitative studies. Collective isolation refers to the perceived presence/absence of a meaningful connection with a group or social entity beyond the level of individuals (e.g., school, team, nation). The best predictor found in middle-age and older adults for collective isolation was the number of voluntary groups to which participants belonged: the more voluntary associations to which participants belonged, the lower their collective isolation, again even after statistically controlling for the two other factors (Hawkley et al., 2005). This factor is also found in women as well as men, but it is more heavily weighted in men than in women.
Given humans are adapted for group living, an aspect of the phenotype of loneliness that represents superorganismal structures, collective identities, and ingroup biases may also have evolved due to their unique adaptive function in certain habitats. For instance, warfare among ancestral hunter-gatherers appears to have contributed to selection for human social behaviors, especially altruistic behaviors (Bowles, 2009; LeVine & Campbell, 1972; Wilson, et al., 2007). De Waal and Pollick (2005) argued that generally in the animal kingdom if males compete, coalitions tend to form. Competition between groups promotes hierarchies and cooperation within groups, an effect demonstrated in the classic study of boys at summer camps by Sherif, Harvey, White, Hood, and Sherif (1961) and in recent research on chimpanzees:
Chimpanzee males conduct warfare against neighboring groups and do so in a highly consolidated fashion; if they did not, then foreign groups would invade their territories. We see how competition and cooperation are not mutually exclusive aspects of society, be it human or ape (de Waal & Pollick, 2005, p. 40).
Recent anthropological investigations of Ardipithecus ramidus (who lived approximately 4.4 million years ago) has led to the conclusion that certain adaptations exhibited by this early hominin species (i.e., diminution of male canine size, upright walking, and absence of ovulatory signaling) reduced intra-sexual conflict among males and fostered pair-bonding and greater male parental investment (de Waal & Pollick, 2005; Lovejoy, 2009). In the human pair bond and nuclear family, the members are part of a larger collective within which the nuclear family is fully integrated. The human pair bond may have evolved hand in hand with the group (de Waal & Pollick, 2005). The notion is that heterosexual pair-bonding in humans raises the certitude of paternity, thereby increasing the parental investment in dependent offspring. In addition, it lessens rivalry for sexual partners among males, with the consequent reduction in sexual rivalry lessening the competition among males, promoting cooperation in hunting and warfare, and altering the organization and operation of the population to make it more resilient. As de Waal and Pollick (2005) note: “Human families are part and parcel of the society … In evolutionary terms, there first must have been the larger society, within which developed the human family” (p. 34).
Social hierarchies in the Macaque are rigid and matriarchal. In chimpanzees and human beings, in contrast, social hierarchies reflect alliances and coalitions based on cooperative agreements. Typically, physical might and recognizing the shifting status of friends and foes may not be sufficient to achieve and maintain a position of status and power in these hierarchies. One also needs to be able to represent and navigate complex social hierarchies, social norms, and cultural developments; subjugate self interests to the interests of the group in exchange for long-term benefits; and recruit support to sanction individuals who violate social norms – that is, to be perceived as serving the interests of the group or at least much of the group. Moreover, individual reciprocity promotes cooperative arrangements between individuals; network reciprocity promotes cooperation and prosocial behavior at the level of the group. When prosocial behavior and cooperation can be assumed based on group membership rather than personal friendship, the transaction costs of interactions within the group can be reduced significantly, and a strong connection to the group benefits the individual members and increases the ability of the group to adapt to new challenges and to martial an effective defense.
In sum, research on social dilemmas has shown that people are more likely to act for the common good rather than selfishly when a collective social identity is available (Kramer & Brewer, 1984; Swann, Gomez, Dovidio, Hart, & Jetten, 2010). When people perceive themselves to be part of a valued group (collective connection), they are also more inclined to agree with other group members, even on beliefs that may seem irrational, than when they think of themselves as unique individuals. This tendency can result in poorer decisions in some circumstances, but the cooperation and collective investments it promotes can also be beneficial in other circumstances. The emergence of a collective connectedness factor underlying loneliness, therefore, suggests that we may have evolved the capacity for and motivation to form relationships not only with other individuals but also with groups (e.g., a Chicago Cubs or Boston Red Sox fan), with the consequence being the promotion of cooperation in adverse conditions (e.g., competition, hunting, warfare). The identification with and investments in the group, in turn, may increase the likelihood of the continuity of the group, its members, and their individual genetic legacy (Brewer, 2004).
Conclusion
Natural selection operates across generations, and it is a process that continues. The capacity for feeling loneliness, when viewed from an evolutionary perspective as an adaptive biological capacity, is not so much about a dysfunctional property of humankind that produces personal misery as it is about promoting an individual’s genetic legacy. We have argued that loneliness may have deleterious consequences for an individual in industrialized societies in which the expected lifespan is nearly eight decades long, but it is a heritable individual difference that evolved because of the important functions it serves.
Brewer (2004) notes that: “If humans are adapted to live in groups and depend on group effectiveness for survival, then our motivational systems should be tuned to the requirements for group effectiveness” (p. 111). Loneliness may feel like a painfully miserable, hopeless, and unwanted state, but different aspects of this motivational phenotype may each have an important adaptive value (and a distinct evolutionary origin) for a complex social species such as our own where our genetic survival depends on caregiving, trust and cooperation, and group living, especially when alliances and allegiances can change dramatically as situations shift. If this reasoning is correct, then loneliness may well be a polygenic trait subject to epigenetic influences.
Acknowledgments
This work was supported by National Institute of Aging Grant No. RO1AG033590.
Contributor Information
John T. Cacioppo, University of Chicago
Stephanie Cacioppo, University of Chicago.
Dorret I. Boomsma, Free University Amsterdam
References
- Achenbach TM. The Young Adult Self-Report. Burlington, VT: Department of Psychiatry, University of Vermont; 1990. [Google Scholar]
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience - cortisol associations in a population-based sample of older adults. Proceedings of the National Academy of Science USA. 2006;103(45):17058–17063. doi: 10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen NB, Badcock PBT. The Social Risk Hypothesis of Depressed Mood: Evolutionary, Psychosocial, and Neurobiological Perspectives. Psychological Bulletin. 2003;129(6):887–913. doi: 10.1037/0033-2909.129.6.887. [DOI] [PubMed] [Google Scholar]
- Andrews PW, Thompson JA. The bright side of being blue: Depression as an adaptation for analyzing complex problems. Psychological Review. 2009;116:620–654. doi: 10.1037/a0016242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson L. Loneliness research and interventions: a review of the literature. Aging & Mental Health. 1998;2(4):264–274. [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. 10.1152/jn.00838.2004 [doi] [DOI] [PubMed] [Google Scholar]
- Bartels M, Cacioppo JT, Hudziak JJ, Boomsma DI. Genetic and environmental contributions to stability in loneliness throughout childhood. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):385–391. doi: 10.1002/ajmg.b.30608. [doi] [DOI] [PubMed] [Google Scholar]
- Baumeister R, DeWall N. The inner dimension of social exclusion: Intelligent thought and self-regulation among rejected persons. In: Williams JPFKD, von Hippel W, editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York: Psychology Press; 2005. pp. 53–76. [Google Scholar]
- Baumeister RF, DeWall CN, Ciarocco NJ, Twenge JM. Social exclusion impairs self-regulation. Journal of Personality and Social Psychology. 2005;88(4):589–604. doi: 10.1037/0022-3514.88.4.589. 10.1037/0022-3514.88.4.589 [doi] [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Leary MR. The Need to Belong: Desire for Interpersonal Attach- ment as a Fundamental Human Motivation. Psychological Bulletin. 1995;117:497–529. [PubMed] [Google Scholar]
- Beckerman S, Erickson PI, Yost J, Regalado J, Jaramillo L, Sparks C, et al. Life histories, blood revenge, and reproductive success among the Waorani of Ecuador. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(20):8134–8139. doi: 10.1073/pnas.0901431106. 10.1073/pnas.0901431106 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondevik M, Skogstad A. The oldest old, ADL, social network, and loneliness. Western Journal of Nursing Research. 1998;20(3):325–343. doi: 10.1177/019394599802000305. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3(11):872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Willemsen G, Dolan C, Hawkley L, Cacioppo J. Genetic and environmental contributions to loneliness in adults: The Netherlands twin register Study. Behavior Genetics. 2005;35(6):745–752. doi: 10.1007/s10519-005-6040-8. [DOI] [PubMed] [Google Scholar]
- Boomsma DI, Cacioppo JT, Muthen B, Asparouhov T, Clark S. Longitudinal genetic analysis for loneliness in Dutch twins. Twin Res Hum Genet. 2007;10(2):267–273. doi: 10.1375/twin.10.2.267. [doi] [DOI] [PubMed] [Google Scholar]
- Bowen SM, Miller BC. Paternal attachment behavior as related to presence at delivery and parenthood classes: A pilot study. Nursing Research. 1980;29:307–311. [PubMed] [Google Scholar]
- Bowles S. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science. 2009;324(5932):1293–1298. doi: 10.1126/science.1168112. [DOI] [PubMed] [Google Scholar]
- Brewer MB. Taking the Social Origins of Human Nature Seriously: Toward a More Imperialist Social Psychology. Personality & Social Psychology Review (Lawrence Erlbaum Associates) 2004;8(2):107–113. doi: 10.1207/s15327957pspr0802_3. [DOI] [PubMed] [Google Scholar]
- Brunelli SA, Hofer MA. Selective breeding for an infantile phenotype (isolation calling): A window on developmental processes. In: Blass, editor. Handbook of Behavioral Neurobiology. New York, NY: Plenum Press; 2001. pp. 433–482. [Google Scholar]
- Brunelli SA, Hofer MA. Selective breeding for infant rat separation-induced ultrasonic vocalizations: Developmental precursors of passive and active coping styles. Behavioural brain research. 2007;182(2):193. doi: 10.1016/j.bbr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Ernst JM, Burleson MH, McClintock MK, Malarkey WB, Hawkley LC, et al. Lonely traits and concomitant physiological processes: the MacArthur social neuroscience studies. International Journal of Psychophysiology. 2000;35(2–3):143–154. doi: 10.1016/s0167-8760(99)00049-5. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Fowler JH, Christakis NA. Alone in the crowd: The structure and spread of loneliness in a large social network. Journal of Personality and Social Psychology. 2009;97(6):977–991. doi: 10.1037/a0016076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC. People thinking about people: The vicious cycle of being a social outcast in one's own mind. In: Williams KD, Forgas JP, von Hippel W, editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York: Psychology Press; 2005. pp. 91–108. [Google Scholar]
- Cacioppo JT, Hawkley LC. Handbook of individual differences in social behavior. New York: Guilford; 2009a. Loneliness; pp. 227–240. [Google Scholar]
- Cacioppo JT, Hawkley LC. Perceived social isolation and cognition. Trends in Cognitive Sciences. 2009b;13(10):447–454. doi: 10.1016/j.tics.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Berntson GG, Ernst JM, Gibbs AC, Stickgold R, et al. Do lonely days invade the nights? Potential social modulation of sleep efficiency. Psychological Science. 2002;13(4):384–387. doi: 10.1111/1467-9280.00469. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, et al. Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality. 2006;40(6):1054–1085. [Google Scholar]
- Cacioppo JT, Hawkley LC, Norman GJ, Berntson GG. Social isolation. Annals of the New York Academy of Sciences. 2011;1231:17–22. doi: 10.1111/j.1749-6632.2011.06028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Thisted RA. Perceived social isolation makes me sad: Five year cross-lagged analysis of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations study. Psychology and Aging. 2010;25(2):453–463. doi: 10.1037/a0017216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Norris CJ, Decety J, Monteleone G, Nusbaum H. In the Eye of the Beholder: Individual Differences in Perceived Social Isolation Predict Regional Brain Activation to Social Stimuli. Journal of Cognitive Neuroscience. 2009;21(1):83–92. doi: 10.1162/jocn.2009.21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WK, Krusemark EA, Dyckman KA, Brunell AB, McDowell JE, Twenge JM, et al. A magnetoencephalography investigation of neural correlates for social exclusion and self-control. Soc Neurosci. 2006;1(2):124–134. doi: 10.1080/17470910601035160. [DOI] [PubMed] [Google Scholar]
- Caporael LR, Brewer MB. Reviving Evolutionary Psychology: Biology Meets Society. Journal of Social Issues. 1991;47(3):187–195. [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Sung CY, Rose RM, Cacioppo JT. Social regulation of gene expression in human leukocytes. Genome Biology. 2007;8(9):R189.181–R189.113. doi: 10.1186/gb-2007-8-9-r189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108(7):3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrona CE. Transition to college: Loneliness and the process of social adjustment. In: Peplau LA, Perlman D, editors. Loneliness: A Sourcebook of Current Theory, Research, and Therapy. New York: John Wiley & Sons; 1982. pp. 291–309. [Google Scholar]
- Dawkins R. The Selfish Gene. New York: Oxford University Press; 1976. [Google Scholar]
- Day-Wilson KM, Jones DN, Southam E, Cilia J, Totterdell S. Medial prefrontal cortex volume loss in rats with isolation rearing-induced deficits in prepulse inhibition of acoustic startle. Neuroscience. 2006;141(3):1113–1121. doi: 10.1016/j.neuroscience.2006.04.048. [DOI] [PubMed] [Google Scholar]
- De Quervain DJF, Fischbacher U, Treyer V, Schellhammer M, Schnyder U, Buck A, et al. The Neural Basis of Altruistic Punishment. Science. 2004;305(5688):1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- de Waal FBM, Pollick AS. The biology of family values: Reproductive strategies of our fellow primates. In: Tipton SM, Witte J, editors. Family Transformed: Religion, Values, and Society in American Life. Washington, D.C.: Georgetown University Press; 2005. pp. 34–51. [Google Scholar]
- Delgado MR, Miller MM, Inati S, Phelps EA. An fMRI study of reward-related probability learning. Neuroimage. 2005;24(3):862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Distel MA, Rebollo-Mesa I, Abdellaoui A, Derom CA, Willemsen G, Cacioppo JT, et al. Familial Resemblance for Loneliness. Behavior Genetics. 2010;40(4):480–494. doi: 10.1007/s10519-010-9341-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey GD, Romero-Canyas R. Rejection sensitivity as a predictor of affective and behavioral responses to interpersonal stress: A defensive motivational system. In: Williams KD, Forgas JP, von Hippel W, editors. The social outcast: Ostracism, social exclusion, rejection, and bullying. New York: The Psychology Press; 2005. [Google Scholar]
- Dronjak S, Gavrilovic L, Filipovic D, Radojcic MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiology and Behavior. 2004;81(3):409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Duck S, Pond K, Leatham G. Loneliness and the evaluation of relational events. Journal of Social and Personal Relationships. 1994;11(2):253. [Google Scholar]
- Dugan E, Kivett VR. The importance of emotional and social isolation to loneliness among very old rural adults. Gerontologist. 1994;34(3):340–346. doi: 10.1093/geront/34.3.340. [DOI] [PubMed] [Google Scholar]
- Dykstra PA, de Jong GJ. Differential indicators of loneliness among elderly. The importance of type of partner relationship, partner history, health, socioeconomic status and social relations. Tijdschrift Voor Gerontologie En Geriatrie. 1999;30(5):212–225. [PubMed] [Google Scholar]
- Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, et al. Social Comparison Affects Reward-Related Brain Activity in the Human Ventral Striatum. Science. 2007;318(5854):1305–1308. doi: 10.1126/science.1145876. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Berntson GG, Engeland CG, Marucha PT, Masi CM, Cacioppo JT. Stress, aging, and resilience: Can accrued wear and tear be slowed? Canadian Psychology-Psychologie Canadienne. 2005;46(3):115–125. [Google Scholar]
- Hawkley LC, Browne MW, Cacioppo JT. How can I connect with thee? Let me count the ways. Psychological Science. 2005;16(10):798–804. doi: 10.1111/j.1467-9280.2005.01617.x. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Encyclopedia of Human Relationships. Thousand Oaks, CA: Sage Press; 2009. Loneliness. [Google Scholar]
- Hawkley LC, Gu Y, Luo Y-J, Cacioppo JT. The mental representation of social connections: Generalizability extended to Beijing adults. PLoS ONE. 2012;7(9):e44065. doi: 10.1371/journal.pone.0044065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Hughes ME, Waite LJ, Masi CM, Thisted RA, Cacioppo JT. From social structure factors to perceptions of relationship quality and loneliness: The Chicago Health, Aging, and Social Relations Study. Journal of Gerontology: Social Sciences. 2008;63B:S375–S384. doi: 10.1093/geronb/63.6.s375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkley LC, Masi CM, Berry JD, Cacioppo JT. Loneliness is a unique predictor of age-related differences in systolic blood pressure. Psychology and Aging. 2006;21(1):152–164. doi: 10.1037/0882-7974.21.1.152. [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Preacher KJ, Cacioppo JT. Multilevel modeling of social interactions and mood in lonely and socially connected individuals: The MacArthur social neuroscience studies. In: Ong AD, van Dulmen M, editors. Oxford Handbook of Methods in Positive Psychology. New York: Oxford University Press; 2007. pp. 559–575. [Google Scholar]
- Hawkley LC, Thisted RA, Cacioppo JT. Loneliness predicts reduced physical activity: Cross-sectional & longitudinal analyses. Health Psychology. 2009;28(3):354–363. doi: 10.1037/a0014400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector-Taylor L, Adams P. State versus trait loneliness in elderly New Zealanders. Psychological Reports. 1996;78(3 Pt 2):1329–1330. doi: 10.2466/pr0.1996.78.3c.1329. [DOI] [PubMed] [Google Scholar]
- Heikkinen R-L, Kauppinen M. Depressive symptoms in late life: a 10-year follow-up. Archives of gerontology and geriatrics. 2004;38(3):239–250. doi: 10.1016/j.archger.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Hofer MA. Handbook of neuroscience for the behavioral sciences. Hoboken: Wiley; 2009. Developmental neuroscience; pp. 12–31. [Google Scholar]
- Holmen K, Ericsson K, Andersson L, Winblad B. Subjective loneliness--a comparison between elderly and relatives. Vard i Norden. 1992;12(2):9–13. doi: 10.1177/010740839201200203. [DOI] [PubMed] [Google Scholar]
- Howard KS. Paternal attachment, parenting beliefs and children's attachment. Early Child Development and Care. 2010;180:157–171. [Google Scholar]
- Hughes ME, Hawkley LC, Waite LJ, Masi CM, Thisted RA, Cacioppo JT. Social characteristics and loneliness: The Health and Retirements Study. 2010 [Google Scholar]
- Jones DC. Parental Divorce, Family Conflict and Friendship Networks. Journal of Social and Personal Relationships. 1992;9(2):219–235. [Google Scholar]
- Kanitz E, Tuchscherer M, Puppe B, Tuchscherer A, Stabenow B. Consequences of repeated early isolation in domestic piglets (Sus scrofa) on their behavioural, neuroendocrine, and immunological responses. Brain, Behavior, and Immunity. 2004;18(1):35–45. doi: 10.1016/s0889-1591(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009;106(14):5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MC, Nesse RM. Is low mood an adaptation? Evidence for subtypes with symptoms that match precipitants. Journal of Affective Disorders. 2005;86:27–35. doi: 10.1016/j.jad.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LA, Ellis BJ. An evolutionary-psychological approach to self-esteem: Multiple domains and multiple functions. In: Fletcher GJO, Clark MS, editors. Blackwell handbook of social psychology: Interpersonal processes. Oxford, United Kingdom: Blackwell; 2001. pp. 411–436. [Google Scholar]
- Kramer RM, Brewer MB. Effects of group identity on resource use in a simulated commons dilemma. Journal of Personality and Social Psychology. 1984;46(5):1044–1057. doi: 10.1037//0022-3514.46.5.1044. [DOI] [PubMed] [Google Scholar]
- Lau S, Gruen GE. The Social Stigma of Loneliness: Effect of Target Person's and Perceiver's Sex. Personality and Social Psychology Bulletin. 1992;18(2):182–189. [Google Scholar]
- Leary MM, Downs DL. Interpersonal functions of the self-esteem motive: The self-esteem system as a sociometer. In: Kermis MH, editor. Efficacy, agency, and self-esteem. New York: Plenum; 1995. pp. 123–144. [Google Scholar]
- LeVine RA, Campbell DT. Ethnocentrism : Theories of conflict, ethnic attitudes, and group behavior. New York (osv.): John Wiley; 1972. [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(8):1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Lovejoy CO. Reexamining Human Origins in Light of Ardipithecus ramidus. Science. 2009;326(5949):74–748. [PubMed] [Google Scholar]
- Lyons DM, Ha CM, Levine S. Social effects and circadian rhythms in squirrel monkey pituitary-adrenal activity. Hormones and Behavior. 1995;29(2):177–190. doi: 10.1006/hbeh.1995.1013. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337(6100):1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi CM, Chen HY, Hawkley LC, Cacioppo JT. A meta-analysis of interventions to reduce loneliness. Personality and Social Psychology Review. 2011;15:219–266. doi: 10.1177/1088868310377394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire S, Clifford J. Genetic and Environmental Contributions to Loneliness in Children. Psychological Science. 2000;11(6):487–491. doi: 10.1111/1467-9280.00293. [DOI] [PubMed] [Google Scholar]
- Mullins LC, Dugan E. The influence of depression, and family and friendship relations, on residents' loneliness in congregate housing. Gerontologist. 1990;30(3):377–384. doi: 10.1093/geront/30.3.377. [DOI] [PubMed] [Google Scholar]
- Murray SL, Bellavia GM, Rose P, Griffin DW. Once hurt, twice hurtful: How perceived regard regulates daily marital interactions. Journal of Personality and Social Psychology. 2003;84(1):126–147. [PubMed] [Google Scholar]
- Nation DA, Gonzales JA, Mendez AJ, Zaias J, Szeto A, Brooks LG, et al. The effect of social environment on markers of vascular oxidative stress and inflammation in the Watanabe heritable hyperlipidemic rabbit. Psychosom Med. 2008;70(3):269–275. doi: 10.1097/PSY.0b013e3181646753. [DOI] [PubMed] [Google Scholar]
- Nonogaki K, Nozue K, Oka Y. Social Isolation Affects the Development of Obesity and Type 2 Diabetes in Mice. Endocrinology. 2007;148(10):4658–4666. doi: 10.1210/en.2007-0296. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Patterson AC, Veenstra G. Loneliness and risk of mortality: A longitudinal investigation in Alameda County, California. Social Science and Medicine. 2010;71(1):181–186. doi: 10.1016/j.socscimed.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Pinquart M, Sőrensen S. Risk factors for loneliness in adulthood and old age—A meta-analysis. In: Shohov SP, editor. Advances in Psychology Research. Vol. 19. Hauppauge, NY: Nova Science Publishers, Inc; 2003. pp. 111–143. [Google Scholar]
- Poletto R, Steibel JP, Siegford JM, Zanella AJ. Effects of early weaning and social isolation on the expression of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase 1 and 2 mRNAs in the frontal cortex and hippocampus of piglets. Brain Research. 2006;1067(1):36–42. doi: 10.1016/j.brainres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Rendell L, Boyd R, Cownden D, Enquist M, Eriksson K, Feldman MW, et al. Why Copy Others? Insights from the Social Learning Strategies Tournament. Science. 2010;328(5975):208–213. doi: 10.1126/science.1184719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A Neural Basis for Social Cooperation. Neuron. 2002;35:395–504. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Rotenberg Loneliness and interpersonal trust. Journal of Social and Clinical Psychology. 1994;13(2):152–173. [Google Scholar]
- Rotenberg, Gruman JA, Ariganello M. Behavioral Confirmation of the Loneliness Stereotype. Basic & Applied Social Psychology. 2002;24(2):81–89. [Google Scholar]
- Rotenberg, Kmill J. Perception of Lonely and Non-Lonely Persons as a Function of Individual Differences in Loneliness. Journal of Social and Personal Relationships. 1992;9(2):325–330. [Google Scholar]
- Routasalo PE, Savikko N, Tilvis RS, Strandberg TE, Pitkala KH. Social contacts and their relationship to loneliness among aged people - a population-based study. Gerontology. 2006;52(3):181–187. doi: 10.1159/000091828. [DOI] [PubMed] [Google Scholar]
- Ruan H, Wu CF. Social interaction-mediated lifespan extension of Drosophila Cu/Zn superoxide dismutase mutants. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(21):7506–7510. doi: 10.1073/pnas.0711127105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D, Peplau LA, Cutrona CE. The revised UCLA Loneliness Scale: concurrent and discriminant validity evidence. J Pers Soc Psychol. 1980;39(3):472–480. doi: 10.1037//0022-3514.39.3.472. [DOI] [PubMed] [Google Scholar]
- Samuelsson G, Andersson L, Hagberg B. Loneliness in relation to social, psychological and medical variables over a 13-year period: A study of the elderly in a Swedish rural district. Journal of Mental Health and Aging. 1998;4:361–378. 4, 361–378. [Google Scholar]
- Savikko N, Routasalo P, Tilvis RS, Strandberg TE, Pitkala KH. Predictors and subjective causes of loneliness in an aged population. Archives of gerontology and geriatrics. 2005;41(3):223–233. doi: 10.1016/j.archger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Scaccianoce S, Del Bianco P, Paolone G, Caprioli D, Modafferi AM, Nencini P, Badiani A. Social isolation selectively reduces hippocampal brain-derived neurotrophic factor without altering plasma corticosterone. Behav Brain Res. 2006;168(2):323–325. doi: 10.1016/j.bbr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Segrin C. Social skills, stressful events, and the development of psychosocial problems. Journal of Social & Clinical Psychology. 1999;18:14–34. [Google Scholar]
- Seymour B, Daw N, Dayan P, Singer T, Dolan R. Differential encoding of losses and gains in the human striatum. Journal of Neuroscience. 2007;27(18):4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif M, Harvey OJ, White BJ, Hood WR, Sherif GW. Intergroup conflict and cooperation; the Robbers Cave experiment. Norman: University Book Exchange; 1961. [Google Scholar]
- Shintel H, Cacioppo JT, Nusbaum H. Accentuate the negative, eliminate the positive? Individual differences in attentional bias to positive and negative information; Paper presented at the 47th Annual Meeting of the Psychonomic Society; Houston, Texas. 2006. [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983(1–2):128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nature Neuroscience. 2006;9(4):526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann WB, Gomez A, Dovidio JF, Hart S, Jetten J. Dying and Killing for One's Group. Psychological Science. 2010 doi: 10.1177/0956797610376656. [DOI] [PubMed] [Google Scholar]
- Tillich P. The eternal now. In: Feifel H, editor. The meaning of death. New York: McGraw–Hill; 1959. pp. 30–38. [Google Scholar]
- Twenge JM, Baumeister RF, Tice DM, Stucke TS. If you can't join them, beat them: Effects of social exclusion on aggressive behavior. Journal of Personality and Social Psychology. 2001;81(6):1058–1069. doi: 10.1037//0022-3514.81.6.1058. [DOI] [PubMed] [Google Scholar]
- Wang Z, Faith M, Patterson F, Tang K, Kerrin K, Wileyto EP, et al. Neural Substrates of Abstinence-Induced Cigarette Cravings in Chronic Smokers. J. Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M, Russell DW, Zakalik RA. Adult attachment, social self-efficacy, self-disclosure, loneliness, and subsequent depression for freshman college students: A longitudinal study. Journal of Counseling Psychology. 2005;52(4):602–614. [Google Scholar]
- Weiss RS. Loneliness: The experience of emotional and social isolation. Cambridge, MA: MIT Press; 1973. [Google Scholar]
- Wheeler L, Reis H, Nezlek J. Loneliness, social interaction, and sex roles. J Pers Soc Psychol. 1983;45(4):943–953. doi: 10.1037//0022-3514.45.4.943. [DOI] [PubMed] [Google Scholar]
- Williams DR. The Health of Men: Structured Inequalities and Opportunities. American Journal of Public Health. 2003;93(5):724–731. doi: 10.2105/ajph.93.5.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Ostracism: The power of silence. New York: Guilford Press; 2001. [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Annals of Behavioral Medicine. 2009;37(2):126–140. doi: 10.1007/s12160-009-9100-0. [doi] [DOI] [PubMed] [Google Scholar]
- Wilson RS, Krueger KR, Arnold SE, Schneider JA, Kelly JF, Barnes LL, et al. Loneliness and risk of Alzheimer disease. Archives of General Psychiatry. 2007;64(2):234–240. doi: 10.1001/archpsyc.64.2.234. [DOI] [PubMed] [Google Scholar]
- Yamada M, Decety J. Unconscious affective processing and empathy: An investigation of subliminal priming on the detection of painful facial expressions. Pain. 2009;143(1–2):71–75. doi: 10.1016/j.pain.2009.01.028. [DOI] [PubMed] [Google Scholar]