Abstract
Citrullinemia type 1 (CTLN1) is an autosomal recessive disorder of metabolism caused by a deficiency of argininosuccinate synthetase. Despite optimal management, CTLN1 patients still suffer from lethal metabolic instability and experience life threatening episodes of acute hyperammonemia. A murine model of CTLN1 (fold/fold) that displays lethality within the first 21 days of life was used to determine the efficacy of adeno-associated viral (AAV) gene transfer as a potential therapy. An AAV serotype 8 (AAV8) vector was engineered to express the human ASS1 cDNA under the control of a liver-specific promoter (thyroxine binding globulin, TBG), AAV8-TBG-hASS1, and delivered to 7–10 day old mice via intraperitoneal injection. Greater than 95% of the mice were rescued from lethality and survival was extended beyond 100 days after receiving a single dose of vector. AAV8-TBG-hASS1 treatment resulted in liver specific expression of hASS1, increased ASS1 enzyme activity, reduction in plasma ammonia and citrulline concentrations, and significant phenotypic improvement of the fold/fold growth and skin phenotypes. These experiments highlight a gene transfer approach using AAV8 vector for liver targeted gene therapy that could serve as a treatment for CTLN1.
Keywords: urea cycle disorders, citrullinemia, AAV8, hyperammonemia
INTRODUCTION
Citrullinemia type I (CTLN1, OMIM 215700) is an autosomal recessive disorder and results from a deficiency of argininosuccinate synthetase (ASS1), the third enzyme in the urea cycle. The incidence of CTLN1 is estimated to be 1 in 57,0001. This urea cycle disorder is characterized by intermittent hyperammonemia and persistent citrullinemia. The clinical spectrum of CTLN1 ranges from neonatal hyperammonemia and death to milder late-onset forms 2. Due to its relatively recent addition to the newborn screening panel, patients with citrullinemia will be identified early, allowing immediate implementation of treatment. However, despite this early identification of disease and treatment, some patients may progress. The untreated mortality rate in untreated classical CTLN1 is 100%, with most deaths occurring before 17 days of life 3.
Currently the main treatment for patients with CTLN1 involves restriction of dietary protein intake, combined with the addition of the urea cycle intermediate arginine. Liver transplantation has been performed in an attempt to improve metabolic stability through the provision of organ-specific enzymatic activity 4. Although this approach has been effective, and even curative, for other metabolic disorders, the clinical utility of solid organ transplantation as a standard treatment in all UCD is unclear. While liver transplantation has been successful in lowering hyperammonemia and citrulline in CTLN1, extrahepatic effects can still be seen in persistent renal ASS1 deficiency necessitating continued arginine supplementation 5. In addition, extrahepatic effects of ASS1 deficiency post transplantation such as NO generation remain to be explored 6, 7. Overall, the number of cases of liver transplantation for CTLN1 is small, and long-term follow up is not available making it difficult for meaningful conclusions to be drawn regarding the efficacy of this treatment 8–10.
A bovine model of CTLN1 has been described 11; however, the logistics of generating large numbers of animals for studies and the feasibility of genetic manipulation is difficult in a large animal model. The first mouse model of CTLN1 was generated by a targeted disruption of the ASS1 gene and termed the ASS1 KO mouse 12. Homozygous mutants develop grossly elevated plasma citrulline levels and hyperammonemia, resulting in death by 48 hours. This life span can be extended up to 6 days with dietary treatment, arginine supplementation and nitrogen scavenging with sodium benzoate. This marginally improved survival occurs in the absence of weight gain.
Recently, two independent hypomorphic recessive mutations at the mouse ASS1 locus, barthez (bar) and follicular dystrophy (fold), have been reported 7. The fold allele harbors a T389I substitution in exon 15 leading to an unstable protein structure with normal ASS1 mRNA and protein levels. Unlike the ASS1 KO model, fold mice survive up to 3 weeks or longer, have 5–10% enzyme activity and display clinical and biochemical parameters similar to CTLN1. In addition, these mice display significant brain abnormalities including defects in neuronal migration and reduced generation of nitric oxide. Since the average length of time to diagnosis of CTLN1 in patients can be up to 1 week or longer (McGuire et al., submitted) the homozygous fold mouse is the ideal candidate model for liver targeted gene therapy for the correction of ASS1 deficiency outside the 24–48 hour time period.
The efficacy of gene delivery has been demonstrated in the ASS1 KO mouse using an E1 deleted adenoviral vector, which carried the ASS1 cDNA driven by a ubiquitous CMV promoter 13. Although the adenoviral treated mice did exhibit a modest increase in survival and metabolic improvement, mice treated twice with the adenovirus and supplemented with sodium benzoate survived on average, 40 days. Recent successes in the spf-ash mouse, a model of ornithine transcarbamylase deficiency, using adeno-associated viral vectors capable of long-term transgene expression suggests that gene therapy for CTLN1 could be advanced to the clinic 14–16.
Herein, we describe the therapeutic efficacy of a liver targeted single-strand AAV8 vector as a new gene therapy treatment for CTLN1 with the potential for translation to the clinic. The AAV8 treated homozygous fold mice are rescued from lethality, display reduced circulating metabolites, and increased hepatic ASS enzyme activity. However, liver directed gene therapy did not fully correct the biochemical phenotype of systemic ASS1 deficiency as arginine levels plummeted in treated fold/fold mice due to persistent a renal deficiency. Our results provide the first evidence of the utility of systemic gene delivery for citrullinemia using AAV8.
RESULTS AND DISCUSSSION
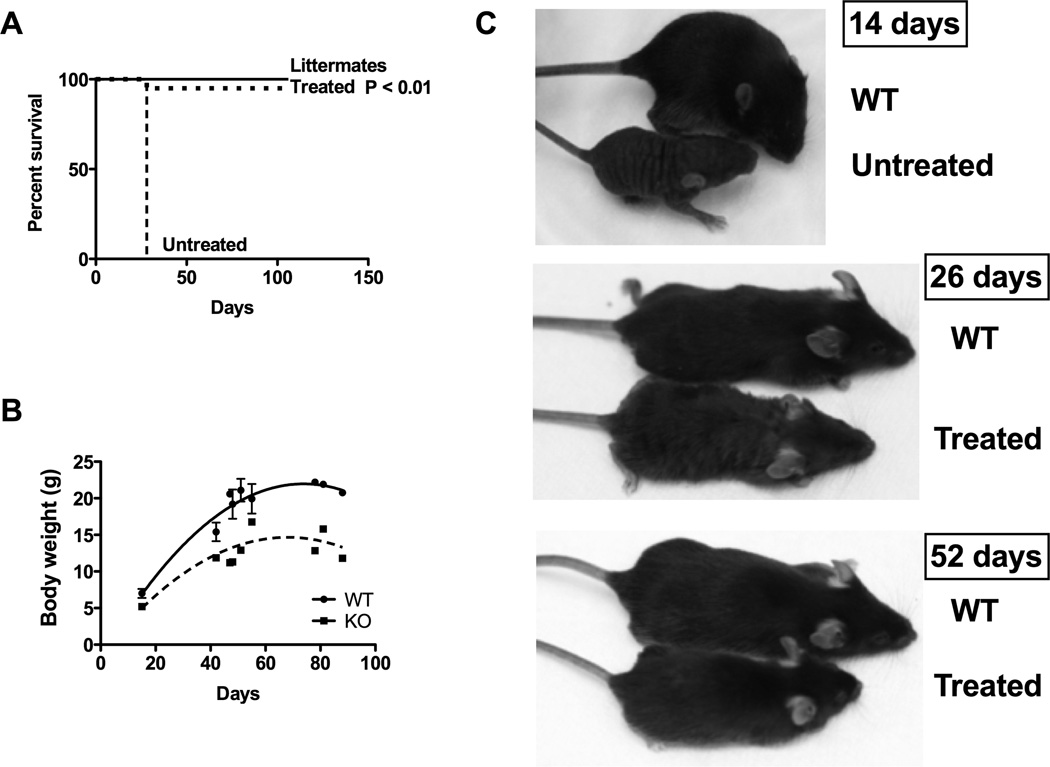
Unlike the adenovirus that used a ubiquitous CMV promoter for correction of the ASS1 KO mouse 13, our AAV vector utilized a TBG promoter to direct ASS1 expression to the liver. A total of 20 ASS1fold/fold (fold/fold) mice received an intraperitoneal injection of 1×1010 genome copies (GC) of AAV8 carrying the human ASS1 cDNA (AAV8-hASS1) under the control of the liver specific TBG promoter at 7–10 days of life. This time point was chosen due to the ease of phenotypic identification of animals (i.e. fur abnormalities) and agreement with time to diagnosis in CTLN1. The AAV8 dose was based on previous descriptions of gene therapy mediated rescue of murine models of methylmalonic and propionic acidemia 17, 18. All WT mice survived for the duration of study for up to 106 days (N=25). Untreated fold/fold mice (N=6) could be recognized at 7–10 days of life but were not recovered at weaning at 3–4 weeks, consistent with the original description of this model. Nearly all (N=19) of the treated fold/fold mice (95%) survived the early lethality period (<28 days) up to 106 days (P < 0.01, Figure 1A), with the exception of a single treated fold/fold mouse that died at 28 days. AAV8 gene therapy significantly prolonged survival when compared to the ASS1 mouse 13, likely due to the ability of AAV vectors to provide long-term transgene expression in comparison to E1 deleted adenoviral vectors 19.
Figure 1.
Survival, weight, length and coat in fold/fold treated with liver targeted gene therapy. Fold/fold mice received 1×1010 GC/mouse at 7–10 days of life. (A) Survival in untreated fold/fold (N=6), treated fold/fold (N=20) and WT littermates (N=25). (B) Cross-sectional analysis of weights in fold/fold (N=19) and WT littermates (N=20). (C) Length and coat texture in untreated fold/fold, treated fold/fold and WT littermates.
Fold/fold mice were not distinguishable from fold/+ or WT littermates at birth. As untreated fold/fold mice develop over time, a growth disparity becomes apparent in the rate of weight gain when compared to WT littermates. In a cross sectional analysis of weights, fold/fold mice treated with AAV8-hASS1 followed a distinct growth curve (Figure 1B) similar to treated ASS1 KO mice 13, albeit with greater weight gain. At ~ 80–90 days, fold/fold mice (mean = 13.5 g, SD = 2.1) weighed approximately 40% less than littermate controls (mean = 21.6 g, SD = 0.7). In addition to weight, overall length was also decreased compared to WT littermates (Figure 1C). Fold/fold was initially described for its abnormal patchy hair pattern (fold – follicular dystrophy) 7. With hepatic ASS1 correction, coat texture and fullness was still patchy at 26 days but significantly improved by 52 days.
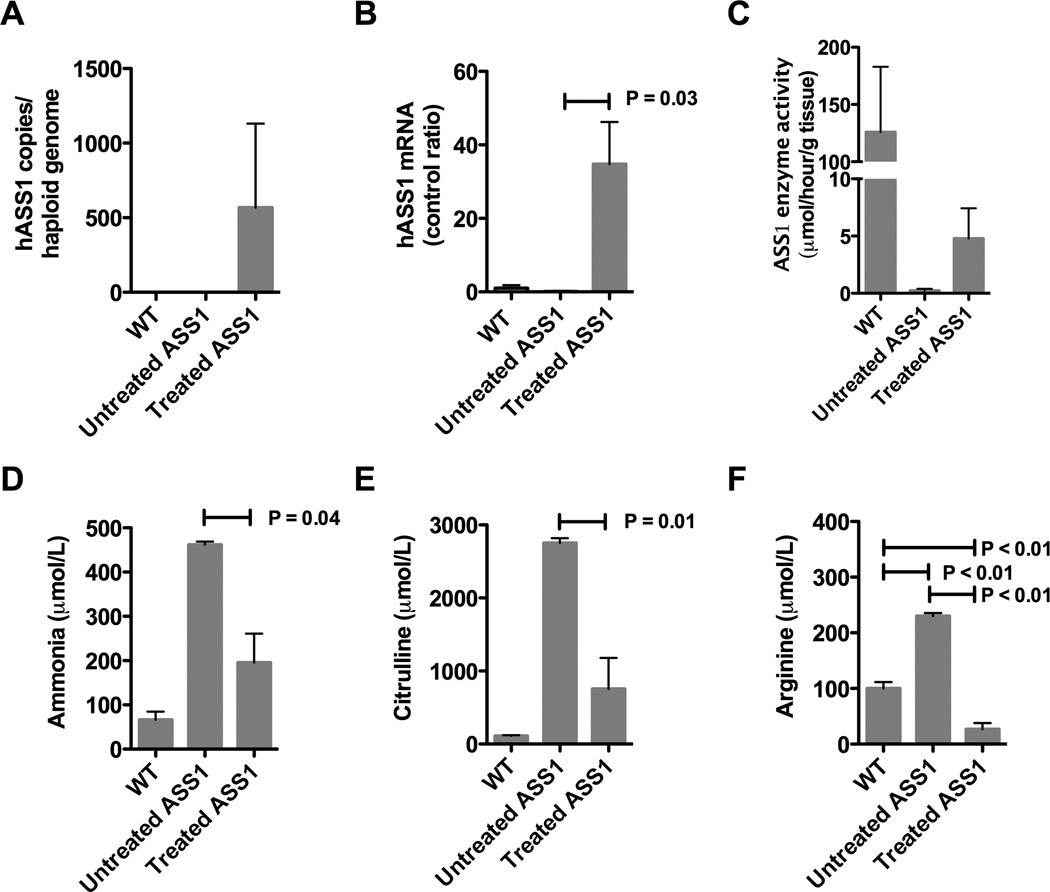
With improvement in survival of treated fold/fold mice, we next looked at the liver 30 days post treatment to examine whether hASS1 mRNA was expressed and if hepatic ASS1 enzyme activity was increased. Using quantitative PCR, AAV-hASS1 vector was detectable on average at 567 copies/haploid genome in the treated animals, but was undetectable in WT and untreated animals (Figure 2A). Similar to the vector copy number, treated fold/fold demonstrated ASS1 mRNA levels 35X (SD = 25.6) above WT baseline in most cases (Figure 2B). With the presence of vector and mRNA expression, hepatic enzyme activity was detectable (mean = 5.0, SD = 6.0) in some treated fold/fold at 30 days post injection, however this finding was not significant (P = 0.51, Figure 2C). Since pieces of liver were used for the various assays, these results suggest that liver correction was not uniform.
Figure 2.
Hepatic correction in treated fold/fold 1 month post gene therapy. (A) Quantitative PCR detection of vector copy number/haploid genome in WT (N=3), untreated (N=3) and treated fold/fold (N=6). Data is represented as a ratio to WT levels (control ratio). (B) RT-qPCR detection of hASS1 mRNA in WT (N=3) and treated fold/fold (N=3). (C) Enzyme activity in WT (N=3), untreated fold/fold (N=3) and treated fold/fold (N=5) 1 month post gene therapy. (D) Plasma ammonia was measured in WT littermates (N=4), untreated fold/fold (N=3), and treated fold/fold (N=7). (E) Plasma citrulline was measured in WT littermates (N=4), untreated fold/fold (N=3), and treated fold/fold (N=4). (F) Plasma arginine was measured in WT littermates (N=4), untreated fold/fold (N=3), and treated fold/fold (N=4). Hatched bars indicate p < 0.05
With longer survival in treated fold/fold, we next measured plasma ammonia and amino acids to assess biochemical improvement (Figure 2D-F). Similar to the ASS1 KO mouse 13, the average plasma ammonia and citrulline levels were grossly elevated in untreated fold/fold. Blood samples taken approximately 1 month after treatment with gene therapy in fold/fold showed a reduction in plasma ammonia levels by > 50% in treated animals (mean = 195.4 µmol/L, SD = 173.2, P = 0.03, Figure 2D).
As with ammonia, plasma citrulline levels were also reduced (Figure 2E). At baseline, plasma citrulline was grossly elevated (mean = 2754 µmol/L, SD = 111.9) in untreated fold/fold when compared to WT mice (mean = 110.9 µmol/L, SD = 22.5). Post gene therapy, plasma citrulline levels were reduced by 73% (mean = 756.3 µmol/L, SD = 844.0, P = 0.01) when compared to untreated fold/fold, although these levels still remained elevated above WT. Similar to the ASS1 KO mouse 13, the average plasma citrulline levels remained elevated in fold/fold. This variability may be due to stochastic effects due to injection, transduction or expression of the human mRNA.
The kidney plays an important role in the maintenance of plasma arginine levels by converting citrulline to arginine through ASS1 and ASL. This is known as the intestinal- renal axis1, 20. Patients with CTLN1 may display arginine deficiency on plasma amino acid profiles due to renal ASS1 enzyme deficiency. In addition, arginine supplementation is often required after liver transplantation for CTLN1 5. Surprisingly, pretreatment plasma arginine levels in fold/fold were two times greater than WT (mean = 230.0 µmol/L, SD = 9.6, P < 0.01, Figure 2F). Original descriptions of the fold/fold model reported mean plasma arginine levels of 171 µmol/L, SD = 68, which can overlap with WT mice 7. We hypothesized that plasma arginine in fold/fold may be related to the large citrulline pool being fed through the hypomorphic ASS enzyme in the kidney, however, these data reflect a specific point in time and may not reflect overall arginine status. More importantly, with liver-targeted gene therapy, fold/fold plasma arginine levels plummeted on average to less than half of WT (mean = 26.8 µmol/L, SD = 22.0, P < 0.01) consistent with persistent renal ASS enzyme deficiency seen post liver transplantation 5. Regarding the ASS1 KO post gene therapy, marginal improvement was seen in plasma arginine 13. This discrepancy in plasma arginine levels between the treated fold/fold and ASS1 KO levels is likely related to the vectors used. In the ASS1 KO, the adenoviral vector had a ubiquitous promoter, which may transduce the kidney and result in an improved intestinal-renal axis.
In conclusion, these studies are the first to establish the efficacy of liver directed gene therapy in the rescue of argininosuccinate synthetase deficiency using AAV8. Improvements in biochemical parameters and survival are clearly demonstrated. In addition, the importance of extrahepatic ASS1 deficiency in the maintenance of plasma arginine levels is suggested by the metabolic parameters observed in the treated fold/fold mice.
METHODS
Murine model of CTLN1
The fold mutation was initially described at the Jackson Laboratory in a production colony of P/J mice. Congenic (N4) B6Ei.P-fold/J (stock number 006449) mice were purchased from The Jackson Laboratory (Bar Harbor, ME); herein referred to as fold. Fold mice display elevated plasma ammonia and massively elevated plasma citrulline concentrations. Wild-type (WT) littermate animals were used as controls throughout.
AAV8 construction, production, and delivery
The expression vector, pENN-AAV-TBG-PI-RBG (PennVector P1015) was obtained from the University of Pennsylvania Vector Core. This vector contains transcriptional control elements from the thyroid binding globulin (TBG) promoter, cloning sites for the insertion of a complementary DNA, and the rabbit β-globin polyA signal. Terminal repeats from AAV serotype 2 flank the expression cassette. The human ASS1 cDNA was isolated from a human liver cDNA library by RT-PCR using ASS1 specific primers (listed below) and was sequence verified. The ASS1 cDNA was then cloned into pENN-AAV-TBG-PI-RBG. This newly created vector AAV-TBG-PI-ASS1-RBG was packaged into AAV8, purified by cesium chloride centrifugation, and titered by qPCR as previously described 21. Animal studies were reviewed and approved by the National Human Genome Research Institute Animal Care and Use Committee. Viral particles were diluted to a total volume of 100 microliters with phosphate-buffered saline immediately before injection and 1×1010 genome copies (GC) were administered via intraperitoneal injection at 7–10 days of age.
ASS1-Koz-XhoI CTCGAGgccaccATGTCCAGCAAAGGCTCCGTG
ASS1-Stop-MluI acgcgtCGGGTCTATTTGGCAGTGAC
Quantitatification of hepatic ASS1 mRNA expression
Total RNA was extracted from the liver using RNeasy Mini Kit (Qiagen, Valencia, CA), and DNase digested was preformed using DNA-free (Ambion, Austin, TX). Reverse transcription was preformed using Applied Biosystems High capacity cDNA Transcription Kit. Quantitative real-time PCR was subsequently preformed on the cDNA with TaqMan gene expression assays [murine β-actin (Mm00607939_s1) and murine Ass1 (Hs01597989_g1) from Applied Biosystems, Foster City, CA]. Samples were analyzed in an Applied Biosystems 7500 fast real-time PCR system, in accordance with the manufacturer's protocol. All samples were analyzed in triplicate.
Vector genome copy number
Genome copy (GC) number was measured by quantitative real-time PCR analysis. A standard curve was prepared, using serial dilutions of the AAV plasmid carrying AAS1 cDNA. Genomic DNA was extracted from murine liver samples and murine genomic DNA was used to determine the vector genome copy number per mouse haploid genome.
Metabolic studies
Plasma was isolated from blood collected by retro-orbital bleeding. The samples were immediately centrifuged, and the plasma was removed and stored at −80 °C for later analysis. Plasma citrulline and arginine were analyzed by ion exchange chromatography (Biochrom 30, Holliston, MA). ASS1 activity was determined using a clinically available enzyme assay which measures the conversion of 14C-aspartate into argininosuccinic acid22 (Clinical Biochemical Genetics, Baylor College of Medicine, Houston, TX).
Statistical analyses
In all instances, P values were considered significant if the value was <0.05. Kaplan-Meier survival curves were used to compare groups on the basis of treatment with or without liver targeted gene replacement therapy. The weights between treated and untreated mice, and differences in metabolite and enzyme levels were assessed using a two-sided, two-tailed unpaired Student's t-test.
ACKNOWLEDGEMENTS
For this work, R.J.C., T.N.T., C.P.V., and P.M. were supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. Thanks to the National Human Genome Research Institute mouse core for mouse care and technical assistance.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Brosnan ME, Brosnan JT. Renal arginine metabolism. J Nutr. 2004;134(10 Suppl):2791S–2795S. doi: 10.1093/jn/134.10.2791S. discussion 2796S-2797S. [DOI] [PubMed] [Google Scholar]
- 2.Dhanakoti SN, Brosnan JT, Brosnan ME, Herzberg GR. Net renal arginine flux in rats is not affected by dietary arginine or dietary protein intake. J Nutr. 1992;122(5):1127–1134. doi: 10.1093/jn/122.5.1127. [DOI] [PubMed] [Google Scholar]
- 3.Thoene J. GeneReviews. Seattle: University of Washington; 2011. Citrullinemia Type I. [Google Scholar]
- 4.Saudubray JM, Touati G, Delonlay P, Jouvet P, Narcy C, Laurent J, et al. Liver transplantation in urea cycle disorders. Eur J Pediatr. 1999;158(Suppl 2):S55–S59. doi: 10.1007/pl00014323. [DOI] [PubMed] [Google Scholar]
- 5.Rabier D, Narcy C, Bardet J, Parvy P, Saudubray JM, Kamoun P. Arginine remains an essential amino acid after liver transplantation in urea cycle enzyme deficiencies. J Inherit Metab Dis. 1991;14(3):277–280. doi: 10.1007/BF01811681. [DOI] [PubMed] [Google Scholar]
- 6.Pita AM, Fernandez-Bustos A, Rodes M, Arranz JA, Fisac C, Virgili N, et al. Orotic aciduria and plasma urea cycle-related amino acid alterations in short bowel syndrome, evoked by an arginine-free diet. J. PEN J Parenter Enteral Nutr. 2004;28(5):315–323. doi: 10.1177/0148607104028005315. [DOI] [PubMed] [Google Scholar]
- 7.Perez CJ, Jaubert J, Guenet JL, Bamhart KF, Ross-Inta CM, Quintanilla VC, et al. Two Hypomorphic Alleles of Mouse Ass1 as a New Animal Model of Citrullinemia Type I and Other Hyperammonemic Syndromes. American Journal of Pathology. 2010;177(4):1958–1968. doi: 10.2353/ajpath.2010.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim IK, Niemi AK, Krueger C, Bonham CA, Concepcion W, Cowan TM, et al. Liver transplantation for urea cycle disorders in pediatric patients: a single-center experience. Pediatr Transplant. 2013;17(2):158–167. doi: 10.1111/petr.12041. [DOI] [PubMed] [Google Scholar]
- 9.Leonard JV, McKiernan PJ. The role of liver transplantation in urea cycle disorders. Mol Genet Metab. 2004;81(Suppl 1):S74–S78. doi: 10.1016/j.ymgme.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 10.Morioka D, Kasahara M, Takada Y, Shirouzu Y, Taira K, Sakamoto S, et al. Current role of liver transplantation for the treatment of urea cycle disorders: a review of the worldwide English literature and 13 cases at Kyoto University. Liver Transpl. 2005;11(11):1332–1342. doi: 10.1002/lt.20587. [DOI] [PubMed] [Google Scholar]
- 11.Harper PAW, Healy PJ, Dennis JA, Obrien JJ, Rayward DH. Citrullinemia as a Cause of Neurological Disease in Neonatal Friesian Calves. Aust Vet J. 1986;63(11):378–379. doi: 10.1111/j.1751-0813.1986.tb02907.x. [DOI] [PubMed] [Google Scholar]
- 12.Patejunas G, Bradley A, Beaudet AL, O'Brien WE. Generation of a mouse model for citrullinemia by targeted disruption of the argininosuccinate synthetase gene. Somat Cell Mol Genet. 1994;20(1):55–60. doi: 10.1007/BF02257486. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Whiteman B, Jerebtsova M, Batshaw ML. Correction of argininosuccinate synthetase (AS) deficiency in a murine model of citrullinemia with recombinant adenovirus carrying human AS cDNA. Gene Ther. 2000;7(20):1777–1782. doi: 10.1038/sj.gt.3301303. [DOI] [PubMed] [Google Scholar]
- 14.Cunningham SC, Spinoulas A, Carpenter KH, Wilcken B, Kuchel PW, Alexander IE. AAV2/8-mediated correction of OTC deficiency is robust in adult but not neonatal Spf(ash) mice. Mol Ther. 2009;17(8):1340–1346. doi: 10.1038/mt.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moscioni D, Morizono H, McCarter RJ, Stern A, Cabrera-Luque J, Hoang A, et al. Long-term correction of ammonia metabolism and prolonged survival in ornithine transcarbamylase-deficient mice following liver-directed treatment with adeno-associated viral vectors. Mol Ther. 2006;14(1):25–33. doi: 10.1016/j.ymthe.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Morizono H, Lin J, Bell P, Jones D, McMenamin D, et al. Preclinical evaluation of a clinical candidate AAV8 vector for ornithine transcarbamylase (OTC) deficiency reveals functional enzyme from each persisting vector genome. Mol Genet Metab. 2012;105(2):203–211. doi: 10.1016/j.ymgme.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandler RJ, Chandrasekaran S, Carrillo-Carrasco N, Senac JS, Hofherr SE, Barry MA, et al. Adeno-associated virus serotype 8 gene transfer rescues a neonatal lethal murine model of propionic acidemia. Hum Gene Ther. 2011;22(4):477–481. doi: 10.1089/hum.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chandler RJ, Venditti CP. Pre-clinical efficacy and dosing of an AAV8 vector expressing human methylmalonyl-CoA mutase in a murine model of methylmalonic acidemia (MMA) Mol Genet Metab. 2012;107(3):617–619. doi: 10.1016/j.ymgme.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer DJ, Ng P. Helper-dependent adenoviral vectors for gene therapy. Hum Gene Ther. 2005;16(1):1–16. doi: 10.1089/hum.2005.16.1. [DOI] [PubMed] [Google Scholar]
- 20.Wakabayashi Y, Yamada E, Yoshida T, Takahashi H. Arginine becomes an essential amino acid after massive resection of rat small intestine. J Biol Chem. 1994;269(51):32667–32671. [PubMed] [Google Scholar]
- 21.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratner S. A radiochemical assay for argininosuccinate synthetase with [U-14C]aspartate. Anal Biochem. 1983;135(2):479–488. doi: 10.1016/0003-2697(83)90716-9. [DOI] [PubMed] [Google Scholar]