Abstract
Scope
Sulforaphane is a natural isothiocyanate in broccoli sprouts with cancer chemopreventive activity. This study is aimed to to use different methods to develop broccoli sprout preparations to compare their ability to deliver sulforaphane to the mice and to evaluate the kinetics and biodistribution of sulforaphane.
Methods and Results
The sulforaphane-enriched sprout preparation generated by two-step procedure (quick-steaming followed by myrosinase treatment) contained the highest level of sulforaphane, which was 11 and 5 times higher than the freeze-dried fresh broccoli sprouts and the quick-steamed, freeze-dried broccoli sprouts, respectively. After oral administration of 2.5 mg/g body weight of the broccoli sprout preparations, sulforaphane was quickly absorbed and distributed throughout the tissues. The sulforaphane-rich preparation resulted in the highest exposure, with peak plasma sulforaphane concentration of 337 ng/ml, which is 6.0 times and 2.6 times higher compared to the other two preparations. A whole body physiologically-based pharmacokinetic model (developed with ADAPT 5 software) suggests that distribution of sulforaphane is perfusion-limited in all organs.
Conclusion
This study provides a broccoli sprout preparation that can serve as a good source of sulforaphane, and the model can be utilized to guide the dose design for the use of broccoli sprout preparation in chemoprevention.
Keywords: Broccoli Sprout, Cancer Chemoprevention, Kinetics, Mouse, Sulforaphane
1 Introduction
Numerous studies continue to support that dietary intake of cruciferous vegetables, especially broccoli and broccoli sprouts, may reduce the risk of different types of malignancies [1]. The cancer chemopreventive properties of these vegetables have been primarily attributed to isothiocyanates that occur naturally as the glucosinolate precursors in the plant [1, 2]. In particular, sulforaphane, a member of the isothiocyanate family, has received extensive attention for its potent chemopreventive activity [3, 4]. Sulforaphane has been shown to be not only effective in preventing chemically induced cancers in animal models [4–7], but also inhibit the growth of established tumors [8, 9]. Early research focused on induction of Phase 2 enzymes and inhibition of Phase 1 enzymes by sulforaphane, which enhances the detoxification of carcinogens [1, 10]. Following studies suggest that sulforaphane offers protection against tumor development during the “post-initiation” phase by controlling cell proliferation, differentiation, apoptosis, cell cycle, angiogenesis and metastasis [1, 11]. During the past few years, sulforaphane has been shown to target cancer stem cells through direct or indirect influence on the self-renewal pathways of cancer stem cells in a number of studies [12–15].
Sulforaphane is converted from glucoraphanin, the major glucosinolate in broccoli and broccoli sprouts, by myrosinase, a β-thioglucosidase [4]. Broccoli sprouts contain approximately 20 times more glucoraphanin than mature broccoli, which represents 74% of all glucosinolates in the sprouts [16]. Myrosinase is physically separated from glucosinolates in the intact plant cells. Disruption of the plant during harvesting, processing, and chewing leads to loss of cellular compartmentalization and subsequent mixing of glucoraphanin and myrosinase to produce sulforaphane [17, 18]. Epithiospecifier protein (ESP), another protein naturally occurring in broccoli and broccoli sprouts, directs hydrolysis of glucoraphanin toward sulforaphane nitrile [19]. While sulforaphane has been shown to possess cancer chemopreventive properties, sulforaphane nitrile has not.
Glucoraphanin is abundant in broccoli and broccoli sprouts, however, the processing of these vegetables often results in decreased intake of sulforaphane. Cooking procedures that inactivate plant myrosinase and ESP were shown to significantly reduce the bioavailability of sulforaphane [20], although the conversion of glucoraphanin to sulforaphane can be also mediated by the microflora in human gastrointestinal tract [21]. In addition, sulforaphane is relatively thermo-labile [22, 23]. It was shown to rapidly degrade when the temperature was above 60 °C [23]. Glucoraphanin in broccoli sprouts, on the other hand, is relatively stable to heat. It was suggested that thermal degradation is unlikely to be a major cause of glucosinolate loss when cooking time is less than 10 min [24]. The loss of glucoraphanin during cooking is primarily due to the leaching into the cooking water [25].
Therefore, the two main objectives of this study are: (1) to use different methods to develop three broccoli sprout preparations and to compare their ability to deliver sulforaphane in vivo; and (2) to study the pharmacokinetics and for the first time to build a physiologically-based pharmacokinetic (PBPK) model of sulforaphane in mice after oral administration of the broccoli sprout preparations. This study will provide useful information to optimize dose regimen of broccoli sprout preparation for cancer chemoprevention studies in the mouse and help make predication for human studies in the future.
2 Materials and Methods
Chemicals
Sulforaphane and sulforaphane-GSH were purchased from LKT Laboratories (St. Paul, MN). Glucoraphanin was obtained from Cfm Oskar Tropitzsch (Marktredwitz, Germany). Sinigrin hydrate and myrosinase (thioglucosidase) were from Sigma-Aldrich (St. Louis, MO). Acetonitrile and methanol of HPLC-grade were purchased from Fisher Scientific (Pittsburgh, PA). Ammonium acetate (HPLC-grade) and formic acid (spectroscopic grade) were purchased from Sigma/Aldrich (MO).
Broccoli sprout preparations
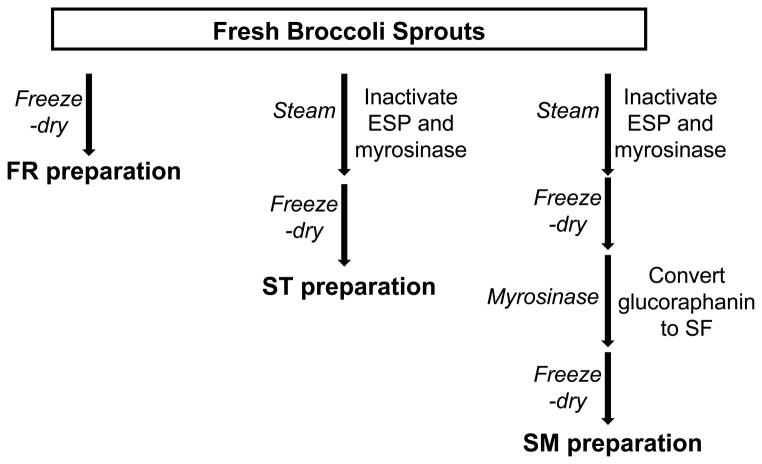
Broccoli sprouts were purchased from local Whole Foods Market (Ann Arbor, MI). The procedure of making broccoli sprout preparations is described below and illustrated in Figure 1. Fresh broccoli sprouts were quick-frozen in liquid nitrogen, and freeze-dried for 48 h. The dried sprouts were then ground into fine powder with pestle and mortar to get “fresh preparation” (FR preparation).
Figure 1.
Development of three broccoli sprout preparations.
To obtain “steamed preparation” (ST preparation), fresh broccoli sprouts were quick-steamed for 10 min over boiling water, frozen in liquid nitrogen, freeze-dried for 48 h, and then ground into fine powder. The length of steaming was determined based on several studies that had been done to characterize the thermal stability of myrosinase and ESP in intact cruciferous vegetables [26, 27]. The activity of broccoli myrosinase was shown to be reduced by more than 95% after a 10 min treatment at 70 °C [27]. The activity of ESP (which is more heat-labile than myrosinase) in broccoli and broccoli sprouts was completely eliminated after the vegetables had been heated at 70 °C for 5 min or 60 °C for 10 min [26].
The ST powder (3 g) was incubated with approximately 5 units of myrosinase in water at 37 °C for 4 h, with occasional stirring. The mixture was then frozen in liquid nitrogen and freeze-dried for three days to get SM preparation (“steamed preparation followed by myrosinase treatment”). The three preparations were stored at −80 °C until used.
Determination of glucoraphanin in broccoli sprout preparations
The procedure for measurement of glucoraphanin in broccoli sprout preparations was modified from previously reported method described by Tian et al. [28]. Briefly, 50 mg of sprout preparation was sonicated for 30 min at 70 °C in 1 ml of 70% aqueous methanol (1 mM sinigrin as internal standard). After cooling in an ice bath, the supernatant of the extracts was collected by centrifugation at 4,000 rpm for 10 min. The extraction procedure was repeated three times and the supernatants were combined to ensure the recovery. The 450 μl of each extract was transferred to a glass vial and dried under nitrogen gas. The dried samples were reconstituted in 500 μl water, filtered through 0.2 μm nylon filters, and diluted 1,000-fold in acetonitrile (containing 5 mM ammonium acetate) for analysis.
All mass spectra were obtained using a 3200 Q-Trap linear ion trap quadrupole mass spectrometer (Applied Biosystems, Carlsbad, CA) coupled to an Agilent 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA). HPLC separation was performed on a Luna 3-μm HILIC column (50 × 2.00 mm, 3 micron) (Phenomenex, Torrance, CA). Mobile phase A (acetonitrile/water 50%/40% containing 5 mM ammonium acetate) was first kept at 0% for 2.5 min, then increased linearly to 50% in 2.5 min, returned to 100% B (acetonitrile containing 5 mM ammonium acetate) and maintained for 5 min. The flow rate was 400 μl/min. Negative ion tandem mass spectrometry was conducted to detect glucoraphanin. The mass spectrometric conditions were as follows: source temperature, 700 °C; curtain gas (CUR), 20 psi; ionspray voltage (IS), 4500 V; desolvation gas temperature (TEM), 700 °C; ion source gas 1 (GS1), 40 psi; ion source gas 2 (GS2), 60 psi; collision gas (CAD), 6 psi; entrance potential (EP), 5 eV; collision energy (CE), 45 eV. The transitions 436.10 > 96.9 and 358.10 > 96.9 were used to detect glucoraphanin and sinigrin, respectively. A dwell time of 200 msec was used for each transition. Data acquisition and quantitation were performed using Analyst software version 1.4.2 (Applied Biosystems, Carlsbad, CA).
Determination of sulforaphane in broccoli sprout preparations
The procedure for measurement of sulforaphane in broccoli sprout preparations was modified from a previously reported method [29]. Briefly, 50 mg of sprout preparation was extracted with 1 ml of ethyl acetate (1 mM sinigrin as internal standard) by vortexing for 1 min and sonicating in ice bath for 10 min. After centrifugation at 4,000 rpm for 10 min, the organic layer was collected. The extraction procedure was repeated three times, the organic phases were combined and dried under nitrogen gas. The dried samples were reconstituted in 1 ml of a mixture of mobile phase A (water containing 5 mM ammonium acetate and 0.1% formic acid) and B (acetonitrile containing 0.1% ammonium acetate) (50:50), sonicated in ice bath for 10 min, filtered through 0.2 μm nylon filters, and diluted 1,000-fold for analysis.
HPLC separation was performed on an Agilent ZORBAX 5-μm extend-C18 column (50 × 2.1 mm) (Agilent Technologies, Santa Clara, CA). Mobile phase A (water containing 5 mM ammonium acetate and 0.1% formic acid) was first kept at 95% for 1 min, then decreased to 10% and maintained for 2 min, returned to 5% B (acetonitrile containing 0.1% ammonium acetate) and maintained for 3 min. The flow rate was 400 μl/min. Positive ion tandem mass spectrometry was conducted to detect sulforaphane. The mass spectrometric conditions were as follows: source temperature, 400 °C; curtain gas (CUR), 30 psi; ionspray voltage (IS), 5500 V; desolvation gas temperature (TEM), 400 °C; ion source gas 1 (GS1), 60 psi; ion source gas 2 (GS2), 40 psi; collision gas (CAD), high; entrance potential (EP), 4 eV; collision energy (CE), 15 eV. The transition 178 > 114 was used to detect sulforaphane, and a dwell time of 200 msec was used for the transition.
Collection of animal samples
All the animal experiments were performed under the guidelines of the University Committee on Use and Care of Animals (UCUCA) at University of Michigan. Female CD-1 mice (20–25 g in body weight) were purchased from Charles River Laboratories (Wilmington, MA), and randomly separated into groups (three mice per time point for each broccoli sprout preparation). Broccoli sprout preparations were suspended in PBS solution on ice, and administered to the mice through oral gavage (2.5 mg/g body weight). Blood samples were collected into heparinized tubes from each mouse by terminal cardiac puncture at pre-dose and 0.5, 1, 2, 4, 6, 8, 12, and 24 h post-dose. The plasma fraction was immediately separated by centrifugation at 2,000 g for 10 min at 4 °C and stored at 80 °C until analysis. Tissue samples, including heart, liver, kidney, lung, muscle, and mammary fatpad were immediately dissected, weighed, snap-frozen and ground with mortar and pestle in liquid nitrogen, and then stored at −80 °C until analysis.
To collect urine and feces, mice (n = 5/group) were placed in metabolic cages (Harvard Apparatus, South Natick, MA) and dosed with broccoli sprout preparations (2.5 mg/g body wt) via oral gavage. Pooled urine and feces samples were collected at pre-dose and 1, 2, 4, 6, 8, 12, and 24 h post-dose, and stored at −80 °C until analysis.
Determination of sulforaphane and sulforaphane-GSH in animal samples
Tissue samples and feces were homogenized using a tissue homogenizer (Tissuemiser Homogenizer, Fisher Scientific, Pittsburgh, PA). Plasma, tissue homogenate, urine, or feces homogenate (50 μl) was extracted with 400 μl methanol containing 0.5% formic acid (1 mM sinigrin as internal standard) and votexed for 4 min, as described in a previous report [30]. The supernatant was collected after centrifugation at 13,000 rpm for 5 min. The 450 μl of supernatant was transferred to a clean centrifuge tube and dried. The dried sample was reconstituted in 200 μl of mixture of mobile phase A (water containing 0.1% formic acid and 5 mM ammonium acetate) and B (acetonitrile containing 0.1% formic acid), mixed for 3 min, and centrifuged at 13,000 rpm for 5 min. The supernatant was transferred to HPLC vial for analysis. Similarly to above, an Applied Biosystems 3200 Q-Trap linear ion trap quadrupole mass spectrometer coupled to an Agilent 1200 Series HPLC system was used for the quantification of sulforaphane and sulforaphane-GSH. The transition 485.3 > 179 was used to detect sulforaphane-GSH.
Kinetic analysis and physiologically-based pharmacokinetic (PBPK) modeling
The plasma concentration data were analyzed by non-compartmental pharmacokinetic model using WinNonlin software (Pharsight Corp., Mountain View, CA). The pharmacokinetic parameters, Cmax (observed maximum concentration), Tmax (time of observed maximum concentration), AUC0-last (area under the concentration-time curve from time zero to the last time point), AUC0-∞ (area under the concentration-time curve from time zero to infinity) were calculated by the software.
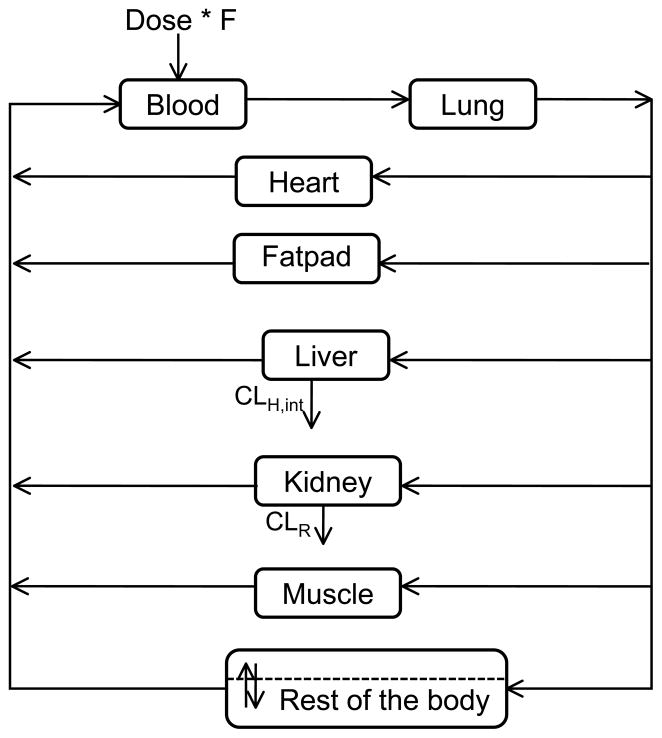
A whole body physiologically-based pharmacokinetic (PBPK) model was built with blood and tissue concentration-time data after oral administration of SM broccoli sprout preparation (2.5 mg/g body weight) using ADAPT 5 software [31]. In this model, the whole body of the mouse is composed of seven tissue compartments and one blood compartment; a “rest-of-body” compartment is incorporated to represent all the unsampled tissues (depicted in Figure 4). The amount of sulforaphane entering the blood was calculated with the bioavailability (F%) published by Lin et al. [32]. For each individual organ, as the equation shows, change in amount of drug in the tissue is equal to the difference between rate in and rate out.
Figure 4.
Whole body PBPK model for suforaphane following an oral dose. The whole body of the mouse is composed of seven tissue compartments and one blood compartment; a “rest-of-body” compartment is incorporated to represent all the unsampled tissues.
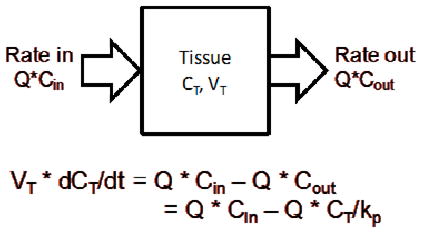
The physiological parameters were either obtained from literature [33–35] or determined by experiment and listed in Table 3. The parameters obtained from individual organ models were used as initial estimates for the whole body model. The initial estimates of partitioning coefficient were determined by the ratio of the area under concentration curves (AUC) between tissue and blood. The AUC values were calculated with noncompartmental analysis by WinNonlin software (Pharsight Corp., Mountain View, CA). All equations were solved simultaneously with the maximum likelihood estimator in the ADAPT 5 software [31].
Table 3.
Physiological parameters used in the whole body model in mice.
| Total Volume (ml) | Organ blood flow (% cardiac output) | |
|---|---|---|
| Whole body | 23.31a | — |
| Blood | 1.230b | 100 |
| Lung | 0.19a | 100 |
| Liver | 1.29a | 16.1b |
| Fatpad | 0.21a | 0.11d |
| Heart | 0.13a | 6.6b |
| Kidney | 0.35a | 9.1b |
| Muscle | 9.638b | 15.9b |
| Rest of the body | 10.272a | 52.2 |
determined by averaging the experimental data for each organ and assuming a density of 1g/ml except for bone (1.5 g/ml); average body weight = 25.1 g.
values obtained from Brown et al. (1997) [42].
values obtained from Baxter et al. (1994) [43].
values obtained from Wang et al. (1993) [44], cardiac output = 51.36 ml/min/100g body weight.
values obtained from Davies et al. (1993) [45].
Statistical analysis
Statistical differences were determined using two-tailed Student t-test. Data are presented as mean ± SD (n ≥ 3).
3 Results and Discussion
Development of broccoli sprout preparations
Although glucoraphanin is abundant in broccoli and broccoli sprouts, the processing of broccoli/broccoli sprouts often results in the loss of sulforaphane. This is attributed to several factors – the presence of epithiospecifier protein (ESP) in the plant, inactivation of plant myrosinase by cooking, the loss of glucoraphanin in the cooking water, and the low stability of sulforaphane at high temperature. ESP directs hydrolysis of glucoraphanin toward sulforaphane nitrile which does not have cancer chemopreventive properties [19]. However, cooking processes that inactivate plant ESP and myrosinase significantly reduce the bioavailability of sulforaphane up to 3-fold [20], indicating the incomplete conversion of glucoraphanin to sulforaphane by microflora in human gastrointestinal tract. In addition, glucoraphanin is easily leached into the cooking water during preparation [25]. Thus, steam cooking has minimal effects on glucosinolates of broccoli florets compared to high pressure boiling, conventional boiling, and microwave cooking [25]. Furthermore, sulforaphane once formed is relatively unstable at high temperature [22, 23].
Therefore, we developed three different broccoli sprout preparations and measured the content of sulforaphane and glucoraphanin in these preparations, as described in Materials and Methods and illustrated in Figure 1. They are freeze-dried fresh broccoli sprouts (FR preparation), quick-steamed, freeze-dried broccoli sprouts (ST preparation), and a sulforaphane-enriched SM preparation. We used a two-step method to generate the SM preparation. First, we quick-steamed broccoli sprouts to inactive myrosinase and ESP in the plant and thus minimize the hydrolysis of glucoraphanin. The length of steaming was determined based on several studies that had been done to characterize the thermal stability of myrosinase and ESP in intact cruciferous vegetables [26, 27]. Then we converted glucoraphanin to sulforaphane by adding back myrosinase. This product was then freeze-dried immediately to preserve the high level of sulforaphane.
The retention of sulforaphane and glucoraphanin in the broccoli sprout preparations was largely affected by the way the preparation was made (Table 1). ST preparation preserved the highest level of glucoraphanin, while SM preparation contained the largest amount of sulforaphane among all the three preparations. The glucoraphanin content of ST preparation (22.74 ± 1.33 mg/g dry wt.) was approximately 12- and 253-fold higher than that of FR (1.94 ± 0.10 mg/g dry wt.) and SM (0.09 ± 0.01 mg/g dry wt.), respectively. As expected, the glucoraphanin in SM preparation was converted to sulforaphane by incubation with myrosinase. The sulforaphane content of SM was 4.25 ± 0.15 mg/g dry wt., which is approximately 11 and 5 times higher than FR (0.39 ± 0.04 mg/g dry wt.) and ST (0.92 ± 0.05 mg/g dry wt.), respectively. Low content of both sulforaphane and glucoraphanin in FR preparation suggests that glucoraphanin might be converted to sulforaphane nitrile during the processing and storage. The low stability of sulforaphane might also contribute to this observation.
Table 1.
Glucoraphanin and SF content in three broccoli sprout preparations. Data are presented as mean ± SD (n = 3).
| Broccoli sprout preparations | Glucoraphanin (mg/g dry wt.) | SF (mg/g dry wt.) |
|---|---|---|
| Steamed + myrosinase (SM) | 0.09 ± 0.01 | 4.25 ± 0.15 |
| Steamed (ST) | 22.74 ± 1.33 | 0.92 ± 0.05 |
| Fresh (FR) | 1.94 ± 0.10 | 0.39 ± 0.04 |
Kinetic study of sulforaphane and sulforaphane-GSH after oral administration of the broccoli sprout preparations in mice
Next, we evaluated these broccoli sprout preparations for their ability to deliver sulforaphane in mouse plasma after oral administration and examined the in vivo pharmacokinetics and tissue distribution of sulforaphane. Each preparation was administered into CD-1 mice by oral gavage, and the plasma and tissue samples were collected at 0, 0.5, 1, 2, 4, 8, 12, 24 h. Previous studies have shown that after absorption sulforaphane is quickly conjugated to glutathione to form sulforaphane-GSH conjugates, which are the major means of transport of sulforaphane throughout the body [36], therefore, we also measured sulforaphane-GSH in plasma.
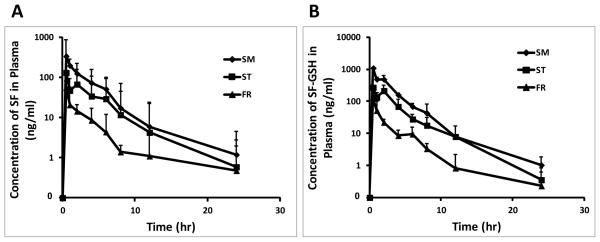
The plasma concentration-time profiles of sulforaphane and sulforaphane-GSH are shown in Figure 2. Oral administration of all three preparations led to an immediate increase in the levels of sulforaphane and sulforaphane-GSH in plasma, indicating a rapid oral absorption of sulforaphane. Sulforaphane was shown to efficiently and rapidly absorbed in human subjects given a single dose of broccoli extract, with peak plasma concentration occurring 1 h after feeding and a half life of 1.77 ± 0.13 h [1, 37, 38]. Similarly, in our study, following oral administration of broccoli sprout preparations, the greatest plasma concentration we measured occurred at 0.5 h.
Figure 2.
Plasma concentration profiles of SF (A), SF-GSH (B) after a single oral administration of 2.5 mg/g body weight of three different broccoli sprout preparations in mice (mean ± SD, n = 3). Blood samples were collected from each mouse by terminal cardiac puncture at pre-dose and 0.5, 1, 2, 4, 6, 8, 12, and 24 h post-dose, and the plasma fraction was immediately separated. The concentrations of SF and SF-GSH were determined by LC-MS/MS.
Notably, we observed a “secondary peak” at the time point of 2 h after oral administration of ST preparation, suggesting that it took approximately 2 h for the conversion of glucoraphanin to sulforaphane by gut microflora. Lai et al. reported that introduction of glucoraphanin (150μmol/kg body weight) directly into the cecum resulted in a significant rise of isothiocyanates (free sulforaphane plus glutathione metabolites) in the mesenteric plasma by 120 min [39]. Our observation of the secondary peak at 2 h is consistent with this finding [39].
A summary of pharmacokinetic parameters is listed in Table 2. The highest plasma concentration of sulforaphane was 6.0 and 2.6 times higher when SM preparation (337 ng/ml) was administered, compared to FR (56 ng/ml) and ST (130 ng/ml) preparations, respectively. The AUC0-last of SM preparation (856 ng*hr/ml) was 8.1 and 2.1 times higher, compared to FR (106 ng*hr/ml) and ST (399 ng*hr/ml) preparations, respectively. Similarly, the AUC0-∞ in mice after oral administration of SM preparation (864 ng*hr/ml) was 7.6 and 2.1 times higher, compared to FR (113 ng*hr/ml) and ST (402 ng*hr/ml) preparations, respectively. The AUC value reflects the overall exposure. Likewise, for sulforaphane-GSH conjugate, the Cmax of SM group (1090 ng/ml) was 8.3 and 4.0 times higher than the Cmax of FR (132 ng/ml) and ST (272 ng/ml) groups. The AUC in mice after oral administration of SM preparation (AUC0-last: 2290 ng*hr/ml; AUC0-∞: 2290 ng*hr/ml) was 11.9 and 2.6 times higher, in comparison to FR (AUC0-last: 191 ng*hr/ml; AUC0-∞: 193 ng*hr/ml) and ST (AUC0-last: 866 ng*hr/ml; AUC0-∞: 867 ng*hr/ml) preparations, respectively. Collectively, these data demonstrate that SM preparation can deliver the highest amount of sulforaphane and sulforaphane-GSH into plasma.
Table 2.
Pharmacokinetic parameters of SF and SF-GSH after a single oral administration of 2.5 mg/g body weight of broccoli sprout preparations in mice.
| Broccoli sprout preparations | SF
|
SF-GSH
|
||||
|---|---|---|---|---|---|---|
| SM | ST | FR | SM | ST | FR | |
| AUC0-∞ (ng*hr/ml) | 864 | 402 | 113 | 2290 | 867 | 193 |
| AUC0-last (ng*hr/ml) | 856 | 399 | 106 | 2290 | 866 | 191 |
| Tmax (hr) | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Cmax (ng/ml) | 337 | 130 | 56 | 1090 | 272 | 132 |
| T1/2 (hr) | 4.35 | 3.82 | 6.9 | 2.81 | 2.85 | 4.54 |
Physiologically-based pharmacokinetic (PBPK) modeling
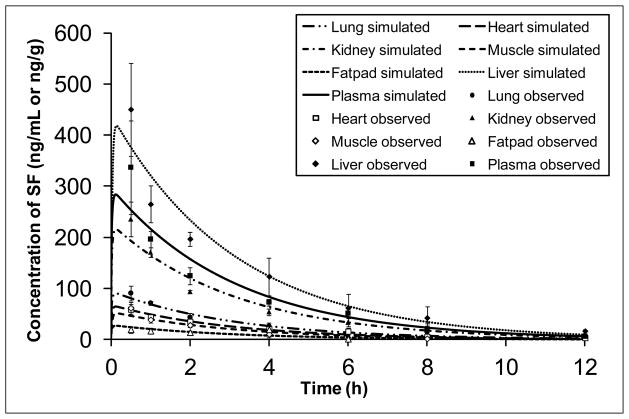
Given that the plasma levels of sulforaphane and sulforaphane-GSH were very low for FR preparation, we only evaluated tissue distribution for ST and SM groups. As shown in Figure 3, levels of sulforaphane in liver, kidney, lung, heart, muscle, and mammary fatpad tissues were highest at 0.5 h after administration of SM preparation, reaching 449.97 ± 90.45, 235.26 ± 33.84, 90.49 ± 14.34, 58.55 ± 10.73, 34.90 ± 11.47, and 19.40 ± 6.41 ng/g tissue, respectively, and decreased thereafter. Similarly, the greatest tissue concentrations of sulforaphane-GSH we measured occurred at 0.5 h (1857.97 ± 288.10, 51.85 ± 5.08, 536.30 ± 118.33, 190.76 ± 63.30, and 61.33 ± 2.16 ng/g tissue for liver, kidney, lung, heart, and muscle, respectively). Sulforaphane-GSH was under detection limit in mammary fatpad. Our data have shown that tissue levels of sulforaphane reached the highest at 0.5 h after dosing, indicating that sulforaphane was quickly distributed throughout the mouse body after consumption of broccoli sprout preparations, which is in agreement with previous studies [37].
Figure 3.
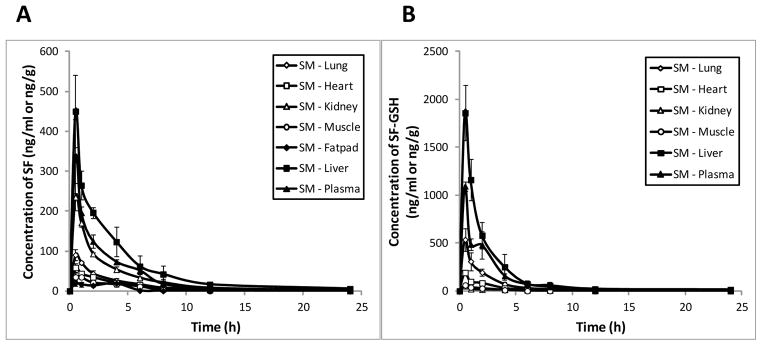
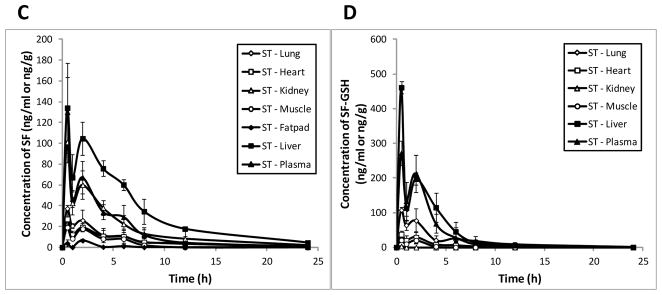
Tissue concentration profiles of SF and SF-GSH after a single oral administration of 2.5 mg/g body weight of SM (A & B) and ST (C & D) preparations in mice (mean ± SD, n = 3). Tissue samples, including heart, liver, kidney, lung, muscle, and mammary fatpad were collected at pre-dose and 0.5, 1, 2, 4, 6, 8, 12, and 24 h post-dose. The concentrations of SF and SF-GSH were determined by LC-MS/MS.
A PBPK model was developed to describe the concentration-time profiles in different tissues. PBPK models are mathematical models derived from the anatomical and physiological structure of the organism studied [40]. They are used to characterize the pharmacokinetics of drugs within the body in relation to blood flows, tissue volumes, routes of administration and other factors. PBPK modeling offers several advantages. It helps to gain insight of the pharmacokinetic behavior of the compound, in animals, in different organs that could otherwise never be assessed in humans [40, 41]. The whole body PBPK model was constructed as shown in Figure 4 to describe the sulforaphane disposition in plasma, lung, heart, kidney, muscle, fatpad and liver. Physiological parameters used in the model are reported in Table 3. They were either obtained from literature [33–35] or determined by experiment. The model simulated plasma and tissue concentration-time profiles of sulforaphane following a single dose of 2.5 mg/g body weight of SM broccoli sprout preparation in CD-1 mice were shown in Figure 5 (lines). The model depicted the observed data relatively well for all the organs studied. A perfusion rate-limited tissue model was adequate to describe the pharmacokinetics of sulforaphane for all the sampled organs, indicating that on entry with the blood circulation, the drug distributed freely and instantly across the membrane without diffusion barriers. After oral administration, sulforaphane was quickly absorbed and the estimated first-order absorption rate constant was 0.32 h−1. To the best knowledge of the authors, this is the first study to provide comprehensive pharmacokinetic data and build a PBPK model to describe the disposition of sulforaphane from broccoli sprouts preparation. This mouse model can be used to design dose regimen for efficacy study in mice and could be very helpful to determine efficacious dose in human after extrapolation.
Figure 5.
Plasma and tissue concentration-time profiles of sulforaphane following a single oral dose of 2.5 mg/g body weight of SM broccoli sprout preparations in mice. Data points represent observed concentrations (mean ± SD, n = 3) and lines are PBPK model-simulated profiles.
4 Concluding Remarks
In conclusion, as we hypothesized, the sulforaphane-rich broccoli sprout preparation (SM) generated by the two-step procedure contained the highest level of sulforaphane and produced the highest plasma concentration of sulforaphane. The freeze-dried broccoli sprouts with plant enzyme activities (FR) resulted in the lowest plasma concentration. The pharmacokinetic analysis and biodistribution indicate that sulforaphane was well and rapidly absorbed and distributed to various tissues following oral administration of our broccoli sprout preparations in the mice. The PBPK model for sulforaphane can be utilized to guide the dose regimen design for cancer chemoprevention efficacy studies in mice and could be very helpful to determine efficacious dose in humans after extrapolation.
Acknowledgments
This work was supported by NIH (RO1 CA120023, RO1 CA122906-01A1, and R21 CA143474) and University of Michigan Cancer Center Research Grant. We also thank Dr. Ken Riedl of the Nutrient and Phytochemical Shared Resource, The Ohio State University Comprehensive Cancer Center, for his valuable inputs on the LC-MS/MS analysis method.
Abbreviations
- SF
sulforaphane
- SF-GSH
sulforaphane glutathione conjugate
- ESP
epithiospecifier protein
- FR
fresh preaparation
- ST
steamed preparation
- SM
steamed preparation followed by myrosinase treatment
- PBPK
physiologically-based pharmacokinetic
Footnotes
Conflict of Interest
This work has no conflict of interest.
References
- 1.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conaway CC, Wang CX, Pittman B, Yang YM, et al. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- 4.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94:10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fahey JW, Haristoy X, Dolan PM, Kensler TW, et al. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci U S A. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Kensler TW, Cho CG, Posner GH, Talalay P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornyl isothiocyanates. Proc Natl Acad Sci U S A. 1994;91:3147–3150. doi: 10.1073/pnas.91.8.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung FL, Conaway CC, Rao CV, Reddy BS. Chemoprevention of colonic aberrant crypt foci in Fischer rats by sulforaphane and phenethyl isothiocyanate. Carcinogenesis. 2000;21:2287–2291. doi: 10.1093/carcin/21.12.2287. [DOI] [PubMed] [Google Scholar]
- 8.Singh AV, Xiao D, Lew KL, Dhir R, Singh SV. Sulforaphane induces caspase-mediated apoptosis in cultured PC-3 human prostate cancer cells and retards growth of PC-3 xenografts in vivo. Carcinogenesis. 2004;25:83–90. doi: 10.1093/carcin/bgg178. [DOI] [PubMed] [Google Scholar]
- 9.Jackson SJ, Singletary KW. Sulforaphane: a naturally occurring mammary carcinoma mitotic inhibitor, which disrupts tubulin polymerization. Carcinogenesis. 2004;25:219–227. doi: 10.1093/carcin/bgg192. [DOI] [PubMed] [Google Scholar]
- 10.Juge N, Mithen RF, Traka M. Molecular basis for chemoprevention by sulforaphane: a comprehensive review. Cell Mol Life Sci. 2007;64:1105–1127. doi: 10.1007/s00018-007-6484-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Tang L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin. 2007;28:1343–1354. doi: 10.1111/j.1745-7254.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang T, Korkaya H, Liu S, et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin Cancer Res. 2010;16:2580–2590. doi: 10.1158/1078-0432.CCR-09-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodova M, Fu J, Watkins DN, Srivastava RK, Shankar S. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS One. 2012;7:e46083. doi: 10.1371/journal.pone.0046083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li SH, Fu J, Watkins DN, Srivastava RK, Shankar S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol Cell Biochem. 2012;373:217–227. doi: 10.1007/s11010-012-1493-6. [DOI] [PubMed] [Google Scholar]
- 15.Kallifatidis G, Rausch V, Baumann B, Apel A, et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut. 2009;58:949–963. doi: 10.1136/gut.2008.149039. [DOI] [PubMed] [Google Scholar]
- 16.Shapiro TA, Fahey JW, Dinkova-Kostova AT, Holtzclaw WD, et al. Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study. Nutr Cancer. 2006;55:53–62. doi: 10.1207/s15327914nc5501_7. [DOI] [PubMed] [Google Scholar]
- 17.Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/s0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 18.Ratzka A, Vogel H, Kliebenstein DJ, Mitchell-Olds T, Kroymann J. Disarming the mustard oil bomb. Proc Natl Acad Sci U S A. 2002;99:11223–11228. doi: 10.1073/pnas.172112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matusheski NV, Swarup R, Juvik JA, Mithen R, et al. Epithiospecifier protein from broccoli (Brassica oleracea L. ssp. italica) inhibits formation of the anticancer agent sulforaphane. J Agric Food Chem. 2006;54:2069–2076. doi: 10.1021/jf0525277. [DOI] [PubMed] [Google Scholar]
- 20.Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, et al. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- 22.Jin Y, Wang M, Rosen RT, Ho CT. Thermal degradation of sulforaphane in aqueous solution. J Agric Food Chem. 1999;47:3121–3123. doi: 10.1021/jf990082e. [DOI] [PubMed] [Google Scholar]
- 23.Van Eylen D, Oey I, Hendrickx M, Van Loey A. Kinetics of the stability of broccoli (Brassica oleracea Cv. Italica) myrosinase and isothiocyanates in broccoli juice during pressure/temperature treatments. J Agric Food Chem. 2007;55:2163–2170. doi: 10.1021/jf062630b. [DOI] [PubMed] [Google Scholar]
- 24.Rosa EAS, Heaney RK, Fenwick GR, Portas CAM. Glucosinolates in crop plants. Hortic Rev. 1997;19:99–215. [Google Scholar]
- 25.vallejo F, tomas-Barberan FA, Garcia-Viguera C. Glucosinolates and vitamin C content in edible parts of broccoli florets after domestic cooking. Eur Food Res Technol. 2002;215:310–316. [Google Scholar]
- 26.Matusheski NV, Juvik JA, Jeffery EH. Heating decreases epithiospecifier protein activity and increases sulforaphane formation in broccoli. Phytochemistry. 2004;65:1273–1281. doi: 10.1016/j.phytochem.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Eylen DV, Oey I, Hendrickx M, Loey AV. Effects of pressure/temperature treatments on stability and activity of endogenous broccoli (Brassica oleracea L. cv. Italica) myrosinase and on cell permeability. Journal of food engineering. 2008;89:178–186. [Google Scholar]
- 28.Tian Q, Rosselot RA, Schwartz SJ. Quantitative determination of intact glucosinolates in broccoli, broccoli sprouts, Brussels sprouts, and cauliflower by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry. Anal Biochem. 2005;343:93–99. doi: 10.1016/j.ab.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal S, Winnik B, Buckley B, Mi L, et al. Simultaneous determination of sulforaphane and its major metabolites from biological matrices with liquid chromatography-tandem mass spectroscopy. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;840:99–107. doi: 10.1016/j.jchromb.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 30.Keum YS, Khor TO, Lin W, Shen G, et al. Pharmacokinetics and pharmacodynamics of broccoli sprouts on the suppression of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: implication of induction of Nrf2, HO-1 and apoptosis and the suppression of Akt-dependent kinase pathway. Pharm Res. 2009;26:2324–2331. doi: 10.1007/s11095-009-9948-5. [DOI] [PubMed] [Google Scholar]
- 31.D’Argenio DZ. ADAPT 5 User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Biomedical Simulations Resource; Los Angeles: 2009. [Google Scholar]
- 32.Lin W. Pharmaceutical Science. Rutgers, The State University of New Jersey; New Brunswick: 2009. p. 151. [Google Scholar]
- 33.Wang P, Ba ZF, Burkhardt J, Chaudry IH. Trauma-hemorrhage and resuscitation in the mouse: effects on cardiac output and organ blood flow. Am J Physiol. 1993;264:H1166–1173. doi: 10.1152/ajpheart.1993.264.4.H1166. [DOI] [PubMed] [Google Scholar]
- 34.Baxter LT, Zhu H, Mackensen DG, Jain RK. Physiologically based pharmacokinetic model for specific and nonspecific monoclonal antibodies and fragments in normal tissues and human tumor xenografts in nude mice. Cancer Res. 1994;54:1517–1528. [PubMed] [Google Scholar]
- 35.Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP. Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health. 1997;13:407–484. doi: 10.1177/074823379701300401. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen M, Klopping-Ketelaars IW, van den Berg R, Vaes WH. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J Agric Food Chem. 2008;56:10505–10509. doi: 10.1021/jf801989e. [DOI] [PubMed] [Google Scholar]
- 37.Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, et al. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- 38.Petri N, Tannergren C, Holst B, Mellon FA, et al. Absorption/metabolism of sulforaphane and quercetin, and regulation of phase II enzymes, in human jejunum in vivo. Drug Metab Dispos. 2003;31:805–813. doi: 10.1124/dmd.31.6.805. [DOI] [PubMed] [Google Scholar]
- 39.Lai RH, Miller MJ, Jeffery E. Glucoraphanin hydrolysis by microbiota in the rat cecum results in sulforaphane absorption. Food & function. 2010;1:161–166. doi: 10.1039/c0fo00110d. [DOI] [PubMed] [Google Scholar]
- 40.Nestorov I. Whole-body physiologically based pharmacokinetic models. Expert opinion on drug metabolism & toxicology. 2007;3:235–249. doi: 10.1517/17425255.3.2.235. [DOI] [PubMed] [Google Scholar]
- 41.Meno-Tetang GM, Li H, Mis S, Pyszczynski N, et al. Physiologically based pharmacokinetic modeling of FTY720 (2-amino-2[2-(-4-octylphenyl)ethyl]propane-1,3-diol hydrochloride) in rats after oral and intravenous doses. Drug Metab Dispos. 2006;34:1480–1487. doi: 10.1124/dmd.105.009001. [DOI] [PubMed] [Google Scholar]
- 42.Lee J, Son MJ, Woolard K, Donin NM, et al. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 2008;13:69–80. doi: 10.1016/j.ccr.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piccirillo SG, Reynolds BA, Zanetti N, Lamorte G, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 2006;444:761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 44.Lacy PD, Kennedy JT, Piccirillo JF. Validation of the Composite Laryngeal Recurrence Staging System. Cancer. 2004;101:761–767. doi: 10.1002/cncr.20438. [DOI] [PubMed] [Google Scholar]
- 45.Silber J, Lim DA, Petritsch C, Persson AI, et al. miR-124 and miR-137 inhibit proliferation of glioblastoma multiforme cells and induce differentiation of brain tumor stem cells. BMC medicine. 2008;6:14. doi: 10.1186/1741-7015-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]