Abstract
Objectives
This short report relied on multi-year data from the National Alzheimer’s Coordinating Center-Uniform Data Set (NACC-UDS) to examine whether significant changes occurred in functional status, neuropsychiatric symptom, and depressive symptoms in the years prior to receiving an Alzheimer’s disease (AD) diagnosis.
Methods
The secondary analysis utilized a retrospective cohort design. The NACC-UDS is a publicly accessible, longitudinal database that includes standardized data on neuropsychiatric symptoms, functional status, and depressive symptoms for Alzheimer’s Disease Center (ADC) participants in the United States based on their annual visits from 2005 to 2011. ADC participants were considered diagnosed with AD if a follow-up data form indicated an affirmative response to whether the ADC participant had "Probable AD (NINCDS/ADRDA)" or "Possible AD (NINCDS/ADRDA)." This yielded an analytic sample of 2,478 individuals (139 with an eventual Probable AD diagnosis, 109 individuals with an eventual Possible AD diagnosis, and 2,230 without any AD diagnosis) representing a total of 11,358 visits/points of data.
Results
Multi-level linear models revealed significant decreases (p < .05) in functional status prior to a Probable or Possible AD diagnosis and significant increases in depressive symptoms prior to a Probable AD diagnosis.
Discussion
Changes in functional and depressive symptoms were partly independent of cognitive decline. The longitudinal results lend additional support to conceptual and empirical models of pre-diagnosis declines in AD.
Keywords: Alzheimer’s disease, Screening and Diagnosis, Psychological and Behavioral Symptoms, Depression, Functional Status
There is a growing consensus among practitioners and researchers that effective treatment of Alzheimer’s disease (AD) should occur earlier in the course of the disease trajectory (Alzheimer's Association, 2011; Assistant Secretary for Planning and Evaluation, 2012), but such treatment is not yet available. Several descriptive efforts have examined decline or stability in key indices of dementia disease progression before an AD diagnosis. For example, one study found that decreases in cognitive functioning among older persons were not due to chronological age but instead preclinical dementia (Sliwinski, Hofer, & Hall, 2003). Other studies reported rates of decreases in global cognitive functioning as well as declines in episodic memory, perceptual speed, and executive function in the years prior to formal diagnosis of AD. These findings suggest that what was once thought of as age-related decline in memory loss may be more attributable to pre-clinical sympomatology consistent with dementia (Bäckman, Jones, Berger, Laukka, & Small, 2005; Bäckman, 2008; Chen et al., 2001; Sliwinski et al., 2003). Pre-clinical changes in other domains associated with the progression of AD, such as functional status (degree of independence in completing daily activities such as writing checks, shopping alone, preparing a balanced meal, or remembering appointments; see Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982), depressive symptoms (e.g., feelings of emptiness, boredom, helplessness, worthlessness; see Yesavage et al., 1982), or neuropsychiatric symptoms (e.g., delusions, hallucinations, agitation/aggression, anxiety, night-time behaviors; see Cummings et al., 1994) are less clear. While several cross-sectional studies have examined the prevalence of depressive and neuropsychiatric symptoms for individuals with mild cognitive impairment (e.g., Chan, Kasper, Black, & Rabins, 2003; Lykestos, Lopez, Jones, Fitzpatrick, Breitner, & DeKosky, 2002), current research has not investigated how changes occur in these or other key domains in the months and years prior to an AD diagnosis.
The purpose of this short report was to ascertain whether significant changes occurred in functional status, neuropsychiatric symptoms, and depressive symptoms prior to an AD diagnosis. Insights from this analysis may inform the need to: a) recognize changes in functional status, depressive symptoms, and neuropsychiatric symptoms prior to an AD diagnosis; and b) develop more comprehensive support strategies for individuals who are subject to these pre-diagnostic declines.
Methods
Procedure
The National Alzheimer’s Coordinating Center (NACC) (Morris et al., 2006) serves as the primary repository and data hub of all 31 of the National Institute on Aging Alzheimer's Disease Centers (ADCs). The ADCs are located throughout the United States with many located in university medical centers in urban areas (Beach, Monsell, Phillips, & Kukull, 2012). The NACC Uniform Data Survey (or NACC-UDS) is a publicly accessible longitudinal database that includes standardized cognitive, behavioral, and functional data for each ADC participant based on their annual visits. The procedure of pre-mortem AD diagnoses generally adhere to standardized clinical criteria including those outlined by a recent National Institute on Aging workgroup (McKhann et al., 2011). For more detail on the construction of the NACC-UDS, please see Morris and colleagues (2006) as well as http://www.alz.washington.edu/WEB/researcher-home.html.
Alzheimer’s Disease Centers operate as tertiary referral centers (Beach et al., 2012). ADC participants are largely recruited from the clinical practices of neurologists although community-based recruitment also occurs. Two types of individuals were included in the NACC-UDS database. One consists of those who were referred to ADCs by community clinics or neurologists; these individuals likely already received clinical assessments that identified possible dementia. The second include “cognitively normal” controls; these individuals were explicitly recruited by ADCs to create a prospective cohort of adults to track age-related changes in cognition and other variables and also to examine transitions to dementia.
For the present study we identified a cohort that initially did not have dementia; this cohort included ADC participants with annual longitudinal data from 2005–2011 prior to a Probable or Possible AD diagnosis as well as those who never received an AD diagnosis. Participants had to have 3 or more total annual ADC visits in order to allow for the multi-level trajectory models described below. These criteria yielded an analytic sample of 2,478 individuals (139 with an eventual Probable AD diagnosis, 109 individuals with an eventual Possible AD diagnosis, and 2,230 without any AD diagnosis) representing a total of 11,358 visits/points of data.
Measures
Functional status
The 10-item Functional Activities Questionnaire (FAQ) is utilized in the NACC-UDS to measure functional status in ADC participants at each visit (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982). The FAQ was completed by clinicians based on informant report for the ADC participant. Specifically, ADC participants were rated as 3 = “dependent;” 2 = “requires assistance;” 1 = “has difficulty but does by self;” and 0 = “normal/never did the activity but could do now.” Sample items include “Heating water, making a cup of coffee, turning off stove after use;” “Preparing a balanced meal;” and “Traveling out of neighborhood, driving, arranging to take buses.” The FAQ has demonstrated reliability alpha coefficients above .80 as well as construct validity in predicting cognitive status and dementia severity in prior research (Pfeffer, Kurosaki, Harrah, Chance, & Filos, 1982).
Neuropsychiatric symptoms
At each annual visit ADC clinicians administer the Neuropsychiatry Inventory Questionnaire (NPI-Q), an inventory that measures the severity and frequency of a range of dementia-related psychiatric symptoms and behavioral disturbances (delusions, hallucinations, agitation/aggression, dysphoria, anxiety, euphoria, apathy, disinhibition, irritability, motor disturbance, nighttime behaviors, appetite/eating; Cummings et al., 1994). Prior research has found that the overall internal consistency of the NPI-Q was .88, and the various symptoms assessed on the NPI-Q have shown significant correlations with the Mini-Mental Status Examination (Cummings et al., 1994).
Depressive symptoms
The presence (0 = “no;” 1 = “yes”) of depressive symptoms on the part of ADC participants is measured and summed with the 15-item Geriatric Depression Scale (GDS; Yesavage et al., 1982). The GDS is completed by ADC clinicians based on participant response. The GDS has shown high reliability (α =.94) as well as high sensitivity and specificity when compared to diagnostic criteria of depression in older adults, including older persons with memory loss (Yesavage et al., 1982).
Demographics, health history, and dementia severity
Sociodemographic information on the part of NACC-UDS participants as well as presence of chronic health conditions were assessed at the initial visit (see Table 1). The widely-used and validated Mini-Mental Status Exam (MMSE) is also administered to ADC participants at each visit to measure severity of cognitive impairment (Folstein, Folstein, & McHugh, 1975). The MMSE has shown strong test-retest reliability (.85–.95), inter-rater reliability (.85–.95), and correlates well with other cognitive screening tests (r = .70–.90) (Langley, 2000).
Table 1.
Sociodemographic and Background Characteristics (N = 2,478).
| Variable | No AD Diagnosis (n = 2230) |
Probable AD (n = 139) |
Possible AD (n = 109) |
|---|---|---|---|
| Age*** |
M = 73.1 SD = 9.1 |
M = 81.2 SD = 8.5 |
M = 79.7 SD = 7.7 |
| Years of education |
M = 16.0 SD = 6.0 |
M = 15.9 SD = 2.7 |
M = 15.7 SD = 2.9 |
| Female* | n = 1488 (66.7%) | n = 83 (59.7%) | n = 61 (56.0%) |
| Caucasian* | n = 1897 (85.1%) | n = 129 (92.8%) | n = 102 (93.6%) |
| Living with spouse or partner | n = 1291 (35.3%) | n = 75 (36.0%) | n = 61 (38.5%) |
| Lives independently*** | n = 2203 (98.8%) | n = 131 (94.2%) | n = 104 (95.4%) |
| Single family residence*** | n = 2006 (90.0%) | n = 111 (79.9%) | n = 88 (80.7%) |
| Married | n = 1291 (57.9%) | n = 75 (54.0%) | n = 63 (57.8%) |
| Ever had cardiovascular disease* | n = 104 (4.7%) | n = 11 (7.9%) | n = 6 (5.5%) |
| Ever had stroke*** | n = 41 (1.8%) | n = 9 (6.5%) | n = 8 (7.3%) |
| Ever had depression in last 2 years*** | n = 292 (13.1%) | n = 20 (14.4%) | n = 23 (21.1%) |
| Mini-Mental Status Examination score* |
M = 29.0 SD = 1.5 |
M = 28.7 SD = 1.4 |
M = 28.7 SD = 1.1 |
NOTE
p < .001
p < .01
p < .05
Analysis Plan
A multilevel change model was used to estimate longitudinal trajectories and to test for statistically significant (p < .05) changes in depressive, functional, and neuropsychiatric symptoms while controlling for key sociodemographic variables, health conditions, and cognitive impairment. In these models, person-specific, linear rates of change over time were estimated at the individual level and modeled as effects of between-subjects predictors at a second, higher-order level (Singer & Willett, 2003). In order to compare effects across different symptom trajectories, all symptoms (functional status, neuropsychiatric symptoms, and depressive symptoms) were standardized by subtracting the mean from assessment 1 from each person’s score at all assessments and dividing these quotients by the assessment 1 standard deviation. Time-varying predictors included the overall assessment number and the assessment number before diagnosis. These two time-varying predictors tested the overall linear rate of change and whether this linear rate of change increased (or decreased) prior to diagnosis, respectively. In addition, the MMSE score was standardized based on the visit 1 mean and standard deviation, and the standardized MMSE score was entered as a time-varying predictor to adjust for cognitive impairment at each wave. Time-invariant (level 2) predictors included demographic covariates and an indicator for whether the case was one that did or did not receive a Probable or Possible AD diagnosis. A dementia case by assessment number interaction effect was also included that tested whether the linear rate of change over time before a Probable or Possible AD diagnosis varied from those who did not receive an AD diagnosis.
We included persons with Possible and Probable AD diagnoses (which are routinely used to clinically diagnose persons with AD at ADCs) as well as controls. We were interested in determining whether statistically significant changes occurred prior to a Possible and Probable AD diagnosis. The inclusion of controls allowed us to statistically compare changes among those with an eventual AD diagnosis to changes among those without an AD diagnosis; among the latter we presumed there would be no significant change in functional status, neuropsychiatric symptoms, and depressive symptoms. Comparing trajectories between those who receive an eventual Possible or Probable AD diagnosis helped to more fully describe whether functional, depressive, or neuropsychiatric changes were similar in the years prior to receiving these accepted clinical AD diagnoses.
All models were estimated using the restricted maximum likelihood method as conducted by SAS PROC MIXED (Littell, Milliken, Stroup, Wolfinger, & Schabenberger, 2006).
Results
Demographics, health conditions, and MMSE
As shown in Table 1, several demographics and health conditions significantly varied at baseline. Individuals in the Probable or Possible AD groups were older than those in the control group (p < .001). Individuals in the Probable and Possible AD groups were less likely to be women and more likely to be Caucasian (p < .05). More controls lived independently and in a single family residence when compared to those with a Probable or Possible AD diagnosis (p < .001). Controls were also less likely to have ever had a heart attack, a stroke, or depression in the last 2 years (p < .05 to p < .001). Controls also had less cognitive impairment at baseline (via higher MMSE scores) than those with a Probable or Possible AD diagnosis (p < .05).
Trajectory analysis
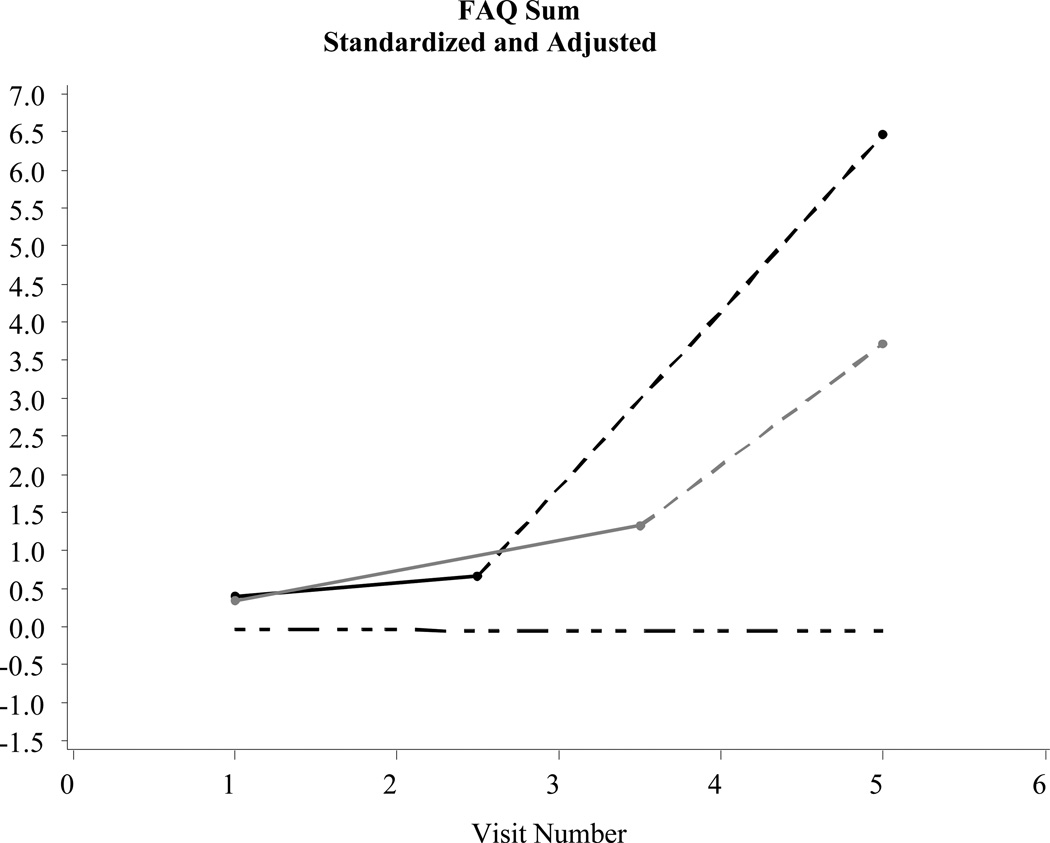
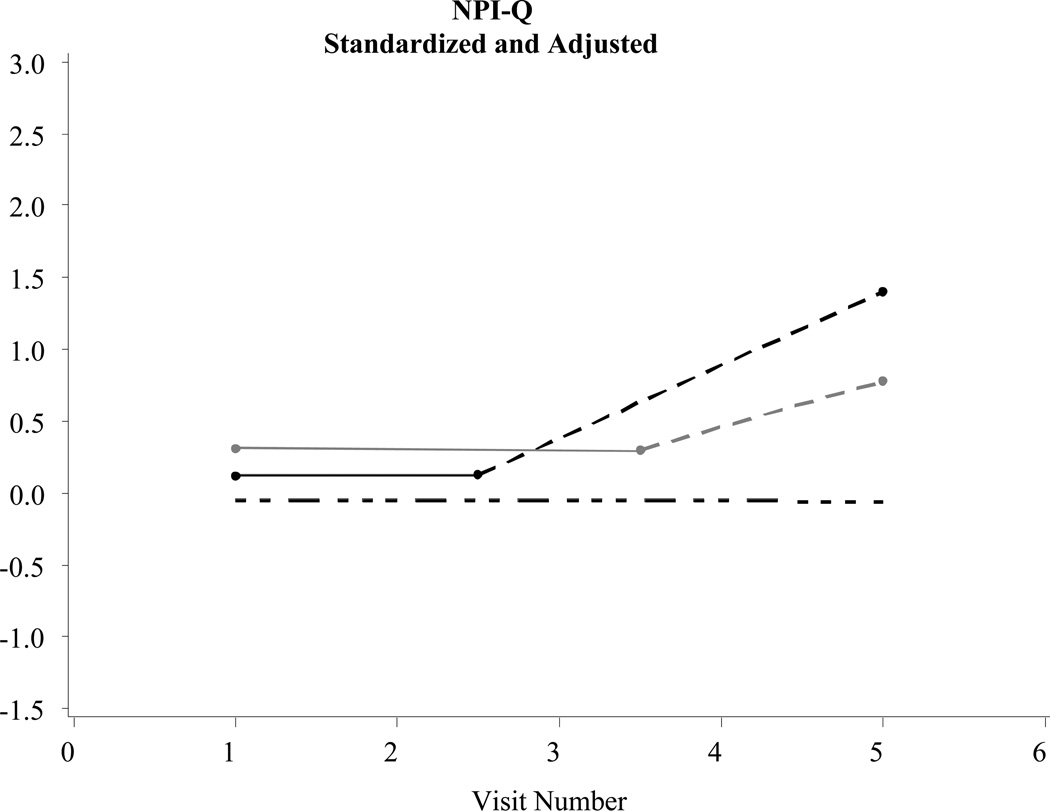
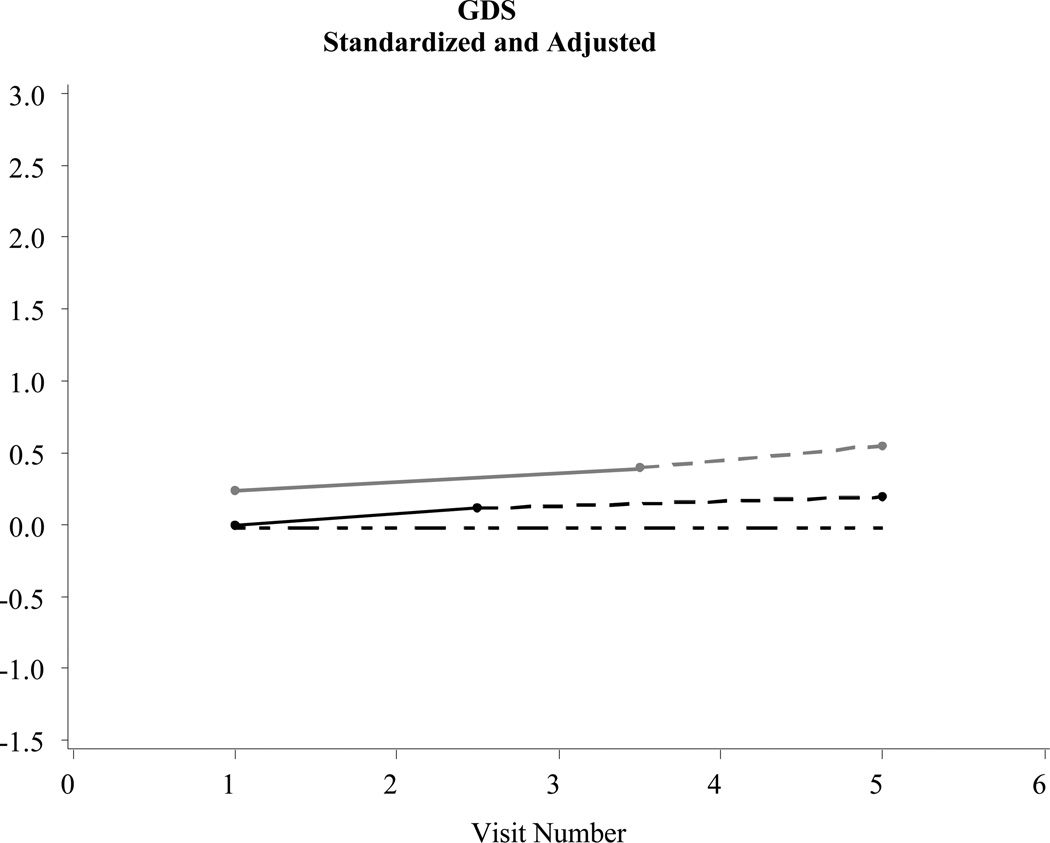
Table 2 describes changes in several parameters central to the purpose of this study: Rate of decline pre-diagnosis: Probable AD*controls and the Rate of decline pre-diagnosis: Possible AD*controls. These parameters tracked statistical changes in functional status, depressive symptoms, and neuropsychiatric symptoms in the years prior to a Probable or Possible AD diagnosis when compared to controls. Post-diagnosis data in Table 2 and Figures 1–3 are shown to simply provide a fuller longitudinal context to the results, rather than to support a formal pre/post comparison.
Table 2.
Parameter Estimates, AD Trajectory Analysis Prior to Diagnosis (N = 2,478)
| NPI-Q | GDS | FAQ | |
|---|---|---|---|
| Effect | Estimate | Estimate | Estimate |
| Intercept | −.27* | −1.23*** | −.63*** |
| Overall rate of change for controls | .00 | .00 | .−01 |
| Post-diagnosis change: Possible AD | .33** | .04 | 1.20** |
| Post-diagnosis change: Probable AD | .51*** | −.05 | 2.13*** |
| Baseline difference: Probable AD group vs. controls | .17 | −.07 | .29* |
| Baseline difference: Possible AD group vs. controls | .37*** | .20 | −.02 |
| Rate of decline pre-diagnosis: Probable AD*controls | .00 | .08** | .19*** |
| Rate of decline pre-diagnosis: Possible AD*controls | .00 | .06 | .40*** |
| MMSE (time varying) | −.03** | −.02* | −.09*** |
| Age | .00 | .01*** | .01*** |
| Male | .13*** | −.01 | .05 |
| Widowed | .02 | −.09** | .04 |
| Divorced | .05 | .12** | .07 |
| Separated | .90*** | .26 | .05 |
| Never married | −.04 | .18* | −.07 |
| Living with caregiver | .14 | .17* | .09 |
| Other living arrangement | −.03 | .30* | .00 |
| Ever had cardiovascular disease | .00 | .03 | −.02 |
| Ever had a stroke | .12 | .28*** | .43*** |
| Depression in the last 2 years | .53*** | .60*** | .23*** |
NOTE
p < .05
p < .01
p < .001
NPI-Q = Neuropsychiatric Inventory; FAQ = Functional Assessment Questionnaire; GDS = Geriatric Depression Scale; MMSE = Mini-Mental Status Examination
Figure 1.
Examples of significant changes before a diagnosis of Alzheimer’s disease: Functional Assessment Questionnaire (FAQ) (N = 2,463)
Figure 3.
Examples of significant changes before a diagnosis of Alzheimer’s disease: Neuropsychiatric Inventory Questionnaire (NPI-Q) (N = 2,463)
Statistically significant (p < .05) declines in functional status were evident in the intervals prior to a Probable and/or Possible AD diagnosis when compared to controls. In addition, statistically significant increases in depressive symptoms were evident in the intervals prior to a Probable AD diagnosis when compared to controls. Changes in neuropsychiatric symptoms prior to diagnosis were not statistically different between those with Probable/Possible AD or controls (see the trajectory model in Figure 3).
Discussion
The multi-year progression of AD necessitates the trajectory-focused analysis approach adopted here. We used MMSE as a time-varying covariate to determine if changes in depressive symptoms, functional status, and neuropsychiatric symptoms were partly independent of cognitive decline. These results demonstrate rates of change leading up to the AD diagnosis event for functional, depressive, and neuropsychiatric symptoms in order to extend current findings on patterns of pre-dementia declines in cognition which are well-established. Various health transitions occur during AD disease progression (such as diagnosis) that may alter the rate and shape of functional status and depressive symptom trajectories. This multi-level analysis of the NACC-UDS demonstrates that functional dependence and depressive symptoms worsen in the years prior to a Probable or Possible AD diagnosis. When compared to controls, persons with AD indicated significant declines in functional status, a dimension that serves as a marker for AD progression. Those who eventually received a Probable AD diagnosis were also more likely to indicate increases in depressive symptoms in the years leading up to this event.
Increases in depressive symptoms imply the self-recognition older adults may have related to their memory loss prior to a formal AD diagnosis. It is important to note that we adjusted for changes in cognitive impairment, and thus changes in depressive symptoms were independent of at least some dementia-related increases in cognitive impairment prior to AD diagnosis. As several studies have noted, the emergence of depressive symptoms in early or prodromal AD may be due to underlying neurological changes (Aznar & Knudsen, 2011; Carpenter et al., 2008; Simard, Hudon, & van Reekum, 2009). The stigma, denial, and social pressures that older adults may experience related to dementia symptoms (Clare, 2002; Macquarrie, 2005) also appear associated with depression in the early stages of AD. Declines in functional status in the months and years prior to a formal AD diagnosis suggest subtle changes in individuals’ independence and ability to carry out daily activities. Overall, the trajectory analysis indicates that prior to an AD diagnosis, changes in functional status and depressive symptoms do occur, but whether these changes are clinically significant enough to warrant more extensive diagnostic procedures by healthcare providers is not clear. Conceptual models of the AD disease trajectory emphasize that pre-mortem AD diagnosis often occurs late in the neurological process of the disease as it is reliant on the presentation of cognitive decline (Jack et al., 2010). Although the current analysis suggests declines in functional status and increases in depressive symptoms leading up to the AD diagnosis, more work is required to ascertain whether such changes are not only statistically significant, but also clinically observable.
There are several limitations to this trajectory analysis. Many consider the diagnostic and treatment services offered by ADCs as the “gold standard,” and thus the findings here may not be representative of how AD diagnosis is conducted in routine practice. The timing of diagnosis may also not be generalizable. Although ADCs are located in all major regions of the U.S. and have emphasized recruitment of ethnically and racially diverse participants, ADC participants are not representative of the population of older persons in the U.S. with or without AD. In addition, the sample was limited to those who made three or more ADC visits in order to allow for the trajectory analysis. There are also methods that can track the trajectory of cognitive change related to the onset of dementia other than the analytic approach chosen here (Hall, Ying, Kuo, & Lipton, 2003; Jacqmin-Gadda, Commenges, & Dartigues, 2006). The NACC-UDS does not include detailed data on caregivers, which could have had an influence on some of the trajectories reported here. The lack of item-information on the publicly available NACC-UDS data measures is an additional limitation. For these reasons, analyses of NACC-UDS measures’ subdimensions, such as those on the NPI-Q, were not possible. Given the potential influence of an AD diagnosis on ADC participants’ anxiety, this would have been an important psychological/mental health dimension to consider. Moreover, the GDS relies on self-report. Although the GDS has been used reliably in those with mild and moderate dementia (see Grann, 2000), completion of the GDS among those with more severe symptomatology is less likely. This issue may potentially threaten the validity of the GDS in this sample.
Increases in depressive symptoms and functional impairment occur in the months leading up to AD diagnoses. The intersection of increased depressive symptoms, functional dependence, and initial AD diagnosis clearly demonstrates that progression to AD occurs not only in a purely cognitive context (Bäckman et al., 2005; Bäckman, 2008; Chen et al., 2001; Sliwinski et al., 2003), but in a psychological/emotional one as well. Developing necessary psychosocial support (either through more sensitive case/care management protocols or therapeutic models; see Gaugler et al., 2011; Logsdon et al., 2010; Sørensen, Waldorff, & Waldemar, 2008; Watkins, Cheston, Jones, & Gilliard, 2006), education/information resources for individuals, families, and care providers (Milne, Hamilton-West, & Hatzidimitriadou, 2005; Wilkinson & Milne, 2003), and rehabilitative services are appropriate for individuals during the diagnosis event itself. The empirical findings also suggest that supports available for individuals with AD at the time of diagnosis could be brought to bear earlier for those who develop functional dependence and depressive symptoms in advance of AD diagnoses.
Figure 2.
Examples of significant changes before a diagnosis of Alzheimer’s disease: Geriatric Depression Scale (GDS) (N = 2,463)
Acknowledgments
This research was funded by Eli Lilly and Company. The NACC database is funded by National Institute on Aging Grant U01 AG016976.
Contributor Information
Joseph E. Gaugler, School of Nursing, Center on Aging, University of Minnesota-Twin Cities
Martha Hovater, Department of Biostatistics, School of Public Health, University of Alabama-Birmingham.
David L. Roth, Center on Aging and Health, Johns Hopkins University
Joseph A. Johnston, Global Health Outcomes, Eli Lilly and Company
Robert L. Kane, Division of Health Policy and Management, Center on Aging, University of Minnesota-Twin Cities
Khaled Sarsour, Department of Epidemiology, Genentech Incorporated. During the time this study was conducted, Dr. Sarsour was an employee of Eli Lilly and Company.
References
- Alzheimer's Association. 2011 Alzheimer's disease facts and figures. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2011;7(2):208–244. doi: 10.1016/j.jalz.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Assistant Secretary for Planning and Evaluation. Draft National Plan to address Alzheimer's disease. Bethesda, MD: Department of Health and Human Services; 2012. [Google Scholar]
- Aznar S, Knudsen GM. Depression and Alzheimer's disease: Is stress the initiating factor in a common neuropathological cascade? Journal of Alzheimer's Disease. 2011;23(2):177–193. doi: 10.3233/JAD-2010-100390. [DOI] [PubMed] [Google Scholar]
- Bäckman L. Memory and cognition in preclinical dementia: What we know and what we do not know. Canadian Journal of Psychiatry. 2008;53(6):354–360. doi: 10.1177/070674370805300604. [DOI] [PubMed] [Google Scholar]
- Bäckman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer's disease: A meta-analysis. Neuropsychology. 2005;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- Carpenter BD, Xiong C, Porensky EK, Lee MM, Brown PJ, Coats M, Morris JC. Reaction to a dementia diagnosis in individuals with Alzheimer's disease and mild cognitive impairment. Journal of the American Geriatrics Society. 2008;56(3):405–412. doi: 10.1111/j.1532-5415.2007.01600.x. [DOI] [PubMed] [Google Scholar]
- Chan DC, Kasper JD, Black BS, Rabins PV. Prevalence and correlates of behavioral and psychiatric symptoms in community-dwelling elders with dementia or mild cognitive impairment: The Memory and Medical Care Study. International Journal of Geriatric Psychiatry. 2003;18(2):174–182. doi: 10.1002/gps.781. [DOI] [PubMed] [Google Scholar]
- Chen P, Ratcliff G, Belle SH, Cauley JA, DeKosky ST, Ganguli M. Patterns of cognitive decline in presymptomatic Alzheimer disease: A prospective community study. Archives of General Psychiatry. 2001;58(9):853–858. doi: 10.1001/archpsyc.58.9.853. [DOI] [PubMed] [Google Scholar]
- Clare L. We'll fight it as long as we can: Coping with the onset of Alzheimer's disease. Aging & Mental Health. 2002;6(2):139–148. doi: 10.1080/13607860220126826. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gaugler JE, Gallagher-Winker K, Kehrberg K, Lunde AM, Marsolek CM, Ringham K, Barclay M. The Memory Club: Providing support to persons with early-stage dementia and their care partners. American Journal of Alzheimer's Disease and Other Dementias. 2011;26(3):218–226. doi: 10.1177/1533317511399570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grann JD. Assessment of emotions in older adults: Mood disorders, anxiety, psychological well-being, and hope. In: Kane RL, Kane RA, editors. Assessing older persons: Measures, meanings, and practical applications. New York: Oxford University Press; 2000. pp. 129–169. [Google Scholar]
- Hall CB, Ying J, Kuo L, Lipton RB. Bayesian and profile likelihood change point methods for modeling cognitive function over time. Computational Statistics and Data Analysis. 2003;42:91–109. [Google Scholar]
- Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurology. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacqmin-Gadda H, Commenges D, Dartigues JF. Random change point model for joint modeling of cognitive decline and dementia. Biometrics. 2006;62(1):254–260. doi: 10.1111/j.1541-0420.2005.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley L. Cognitive assessment of older adults. In: Kane RL, Kane RA, editors. Assessing older persons: Measures, meanings, and practical applications. New York: Oxford University Press; 2000. pp. 65–128. [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for mixed models. 2nd ed. Cary, NC: SAS Institute; 2006. [Google Scholar]
- Logsdon RG, Pike KC, McCurry SM, Hunter P, Maher J, Snyder L, Teri L. Early-stage memory loss support groups: Outcomes from a randomized controlled clinical trial. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences. 2010;65(6):691–697. doi: 10.1093/geronb/gbq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. JAMA: The Journal of the American Medical Association. 2002;288(12):1475–1483. doi: 10.1001/jama.288.12.1475. [DOI] [PubMed] [Google Scholar]
- Macquarrie CR. Experiences in early stage Alzheimer's disease: Understanding the paradox of acceptance and denial. Aging & Mental Health. 2005;9(5):430–441. doi: 10.1080/13607860500142853. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne AJ, Hamilton-West K, Hatzidimitriadou E. GP attitudes to early diagnosis of dementia: Evidence of improvement. Aging & Mental Health. 2005;9(5):449–455. doi: 10.1080/13607860500142762. [DOI] [PubMed] [Google Scholar]
- Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Kukull WA. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Disease and Associated Disorders. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. Journal of Gerontology. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- Simard M, Hudon C, van Reekum R. Psychological distress and risk for dementia. Current Psychiatry Reports. 2009;11(1):41–47. doi: 10.1007/s11920-009-0007-z. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York, New York: Oxford University Press; 2003. [Google Scholar]
- Sliwinski MJ, Hofer SM, Hall C. Correlated and coupled cognitive change in older adults with and without preclinical dementia. Psychology and Aging. 2003;18(4):672–683. doi: 10.1037/0882-7974.18.4.672. [DOI] [PubMed] [Google Scholar]
- Sørensen LV, Waldorff FB, Waldemar G. Early counselling and support for patients with mild Alzheimer's disease and their caregivers: A qualitative study on outcome. Aging & Mental Health. 2008;12(4):444–450. doi: 10.1080/13607860802224342. [DOI] [PubMed] [Google Scholar]
- Watkins R, Cheston R, Jones K, Gilliard J. ‘Coming out’ with Alzheimer's disease: Changes in awareness during a psychotherapy group for people with dementia. Aging & Mental Health. 2006;10(2):166–176. doi: 10.1080/13607860500312209. [DOI] [PubMed] [Google Scholar]
- Wilkinson H, Milne AJ. Sharing a diagnosis of dementia--learning from the patient perspective. Aging & Mental Health. 2003;7(4):300–307. doi: 10.1080/1360786031000120705. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. Journal of Psychiatric Research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]