Abstract
We recently demonstrated that axonal transport of adeno-associated virus (AAV) is serotype-dependent. Thus, AAV2 is anterogradely transported (e.g., from cell bodies to nerve terminals) in both rat and non-human primate (NHP) brain. In contrast, AAV6 is retrogradely transported from terminals to neuronal cells bodies in the rat brain. However, the directionality of axonal transport of AAV6 in the NHP brain has not been determined. In this study, two Cynomolgus macaques received an infusion of AAV6 harboring green fluorescent protein (GFP) into the striatum (caudate and putamen) by magnetic resonance (MR)-guided convection-enhanced delivery. One month after infusion, immunohistochemical staining of brain sections revealed a striatal GFP expression that corresponded well with MR signal observed during gene delivery. As shown previously in rats, GFP expression was detected throughout the prefrontal, frontal, and parietal cortex, as well as substantia nigra pars compacta and thalamus, indicating retrograde transport of the vector in NHP. AAV6-GFP preferentially transduced neurons, although a few astrocytes were also transduced. Transduction of non-neuronal cells in the brain was associated with upregulation of the major histocompatibility complex-II (MHC-II) and lymphocytic infiltration as previously observed with AAV1 and AAV9. This contrasts with highly specific neuronal transduction in the rat brain. Retrograde axonal transport of AAV6 from a single striatal infusion permits efficient transduction of cortical neurons in significant tissue volumes that otherwise would difficult to achieve.
Keywords: AAV6, MRI-guided, convection-enhanced delivery, axonal transport, Huntington’s disease
INTRODUCTION
Axonal transport of intact virions is a critical issue when designing a gene therapy for a particular central nervous system pathology, since it defines the brain areas that will be transduced and may predict off-target effects. Consequently, defining the directionality of axonal transport of vectors, such as adeno-associated virus (AAV), is essential. Retrograde transport requires uptake of vector by axonal projections within the infusion site and transport to the distally located soma where it transduces the host cell nucleus. In contrast, anterograde transport involves entry of vector into cell bodies at the infusion site. A subset of intact virions travel though axons to these neuronal terminals, to be released at the axon terminal, and become available to transduce neighboring cells1. We have consistently found that AAV serotype 2 (AAV2) is transported anterogradely in rat and non-human primate (NHP) brain2, 3,4. For instance, when infused into striatum, AAV2 transduces regions to which striatal neurons project like globus pallidus, substantia nigra pars reticulata and subthalamic nucleus 2. When delivered to the thalamus, AAV2 particles travel along thalamic axons to several cortical areas3. More recently, in order to elucidate the AAV serotype 6 (AAV6) vector behavior, we compared AAV6 to AAV2 in the rat brain 5. Parenchymal infusion of AAV6 harboring green fluorescent protein (GFP) into either striatum or thalamus revealed GFP staining in cell bodies of regions projecting to the infusion target nuclei, whereas, as expected, GFP expression in rats that received AAV2 was mainly found in areas to which either the striatum or the thalamus project. Although they exhibited a different directionality in axonal transport in rat brain, both AAV6 and AAV2 demonstrated a strongly neuronal tropism.
In our experience, however, it can be risky to extrapolate data obtained in rodents to the primate brain6. Thus, in the present study we investigated whether AAV6 is also transported retrogradely in the primate brain. We infused AAV6 into NHP brain parenchyma by convection-enhanced delivery (CED), a pressurized parenchymal infusion technique that allows therapeutic agents to be distributed throughout large volumes of brain 7, 8. Our group has optimized this delivery technique during the last decade primarily by co-infusing the AAV vector with a magnetic resonance imaging (MRI) contrast agent that allows real-time visualization of the infusion 9, 10. Pathologies such as lysosomal storage diseases, Parkinson’s disease, Alzheimer disease and Huntington’s disease (HD) could benefit from an AAV6-based gene therapy.
RESULTS
AAV6 distribution
We bilaterally infused AAV6-GFP into the striatum (caudate nucleus and putamen) of two Cynomolgus macaques by CED to ensure optimal distribution of the infusate7–10. In order to visualize the infusion in real time, AAV vector was co-infused with chelated gadolinium (Prohance, Bracco), a MR-compatible tracer. MRI contrast showed good coverage of both putamen (2–3 injection sites per hemisphere) and caudate nucleus in both animals (Fig. 1). No leakage outside the target nuclei was observed; however, some perivascular draining of the infusate through striatal large blood vessels was seen in MRI during the infusion (Fig. 1 black arrow). Slight reflux was observed after removing the cannulae. There were no adverse effects related to the infusion procedure or the vector post-operatively.
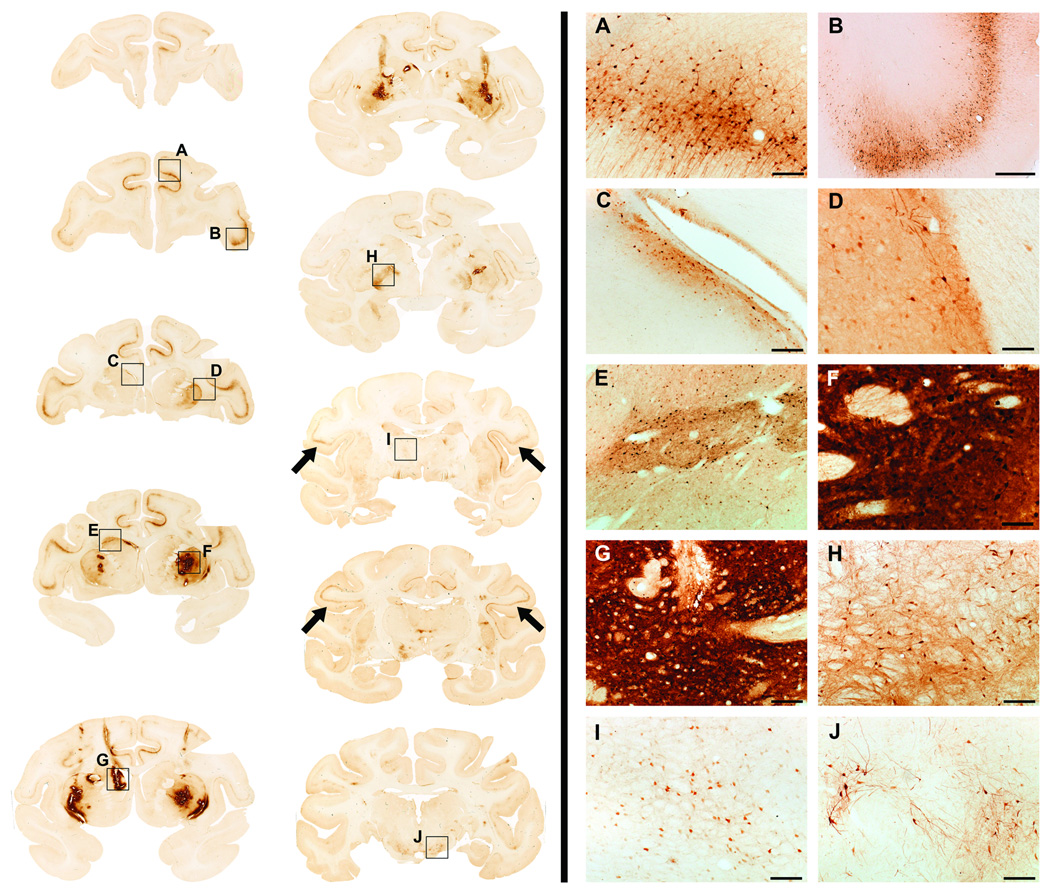
Figure 1. Convection-enhanced delivery of AAV6 into the primate striatum.
Panel shows some examples from the NHP1 of coronal magnetic resonance images (MRI) of infusion sites (A and E for left hemisphere, C and G for right hemosphere) and brain tissue sections, at coronal matching levels, stained for GFP (B and F). In all cases, post-mortem GFP staining matched MRI gadolinium signal observed during the infusion. Red arrows point to the bright gadolinium signal in MRI and their matching GFP staining in immunostaining images. Blue arrows indicate matching GFP staining and weaker gadolinium signals already fading that correspond to the first infusion sites. Black arrows in images B and E show some infusate following one of the lenticular blood vessels through the perivascular space. D and H high magnification images show the presence of both fibers and cell bodies expressing GFP around blood vessels (red asterisk).
Three to four weeks after infusion, animals were euthanized, brains harvested and histologically analyzed. Immunohistochemical staining against GFP revealed well-contained expression within both caudate nucleus and putamen for both animals (Figs. 1 & 2). Stained areas within these structures corresponded well with the MRI signal during infusion. GFP-transduced neurons and fibers were particularly abundant around blood vessels at the infusion sites11. Further immunohistochemical examination of GFP expression in the brain demonstrated GFP in cell bodies of the prefrontal, frontal and parietal cortex, as well as substantia nigra pars compacta neurons and thalamus, indicating retrograde transport of the vector (Fig. 2), since these brain areas project to the striatum. The lateral globus pallidus also contained GFP-expressing neuronal bodies, even though it does not have direct axons projecting to either the caudate nucleus or putamen. We believe that this may be an example of non-axonal transport via perivascular spread 11. Moreover, some GFP immunostained fibers could be found in the substantia nigra reticulata (data not shown). This staining represents merely cytoplasmic distribution of GFP protein within a transduced neuron12. It is rather the presence of transduced cell bodies in non-injected areas of the brain that is indicative of potential axonal transport of the vector. In all these areas, the morphology of the cells positively stained for GFP suggested they were mainly, but not exclusively, neurons.
Figure 2. GFP expression throughout the brain.
Left side of the panel shows brain coronal section stained against GFP in a representative animal. Note the presence of GFP-positive staining in both infusion sites [caudate nucleus (Cd; C, E and G) and putamen (Put; D and F)] as well as in distal regions such as the prefrontal cortex (PCx; A, B), parietal cortex (black arrows), external globus palidus (eGP; H), thalamus (Th, I) and substantia nigra pars compacta (SNpc; J).
Right side: High magnification images of the areas inside black squares are shown on the right side of the panel (A-J). Scale bars: A, C, G-J: 200 µm; B: 1 mm; D-F: 100 µm.
AAV6 tropism in NHP brain
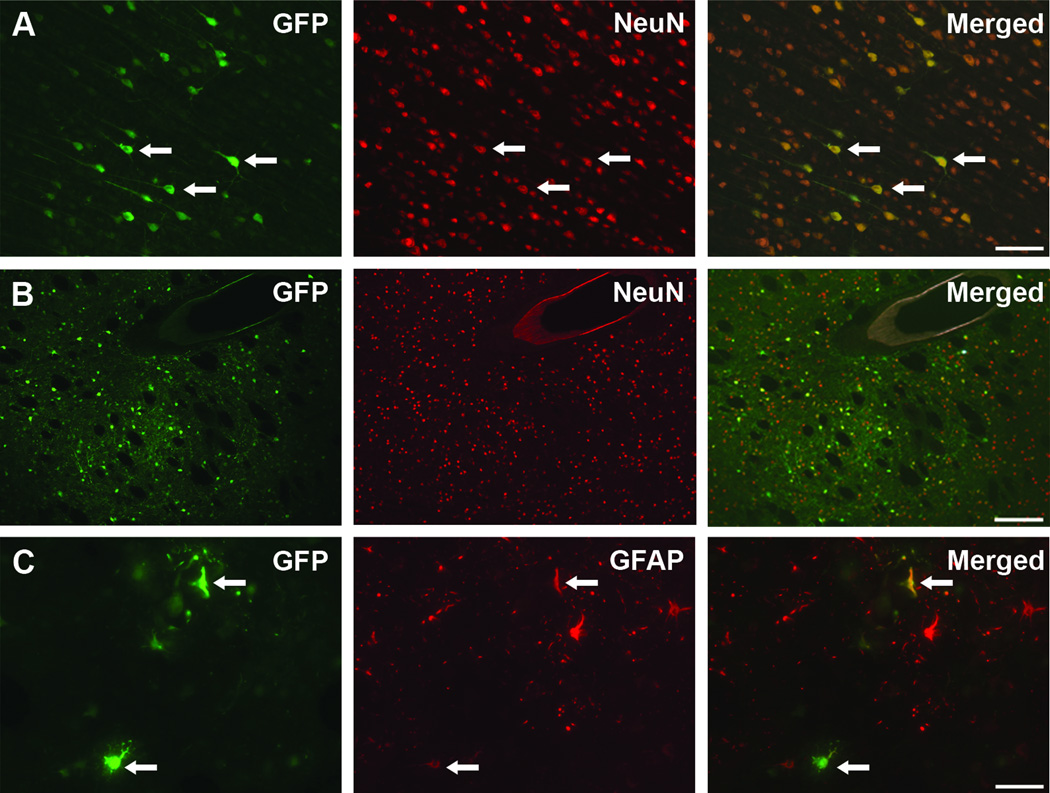
In order to establish the cellular tropism of AAV6-GFP vector after parenchymal infusion in NHP, we immunostained brain sections for the transgene and cell-specific markers such as NeuN for neurons and glial fibrillary acid protein (GFAP) for astrocytes. Double immunofluorescent staining revealed that the majority of the GFP-expressing cells were positive for NeuN neuronal marker (Fig. 3). GFP/NeuN positive cells were present both in the infusion nuclei (i.e., caudate nucleus and putamen; Fig. 3A) as well as in distal structures, such as prefrontal cortex (Fig. 3B), that project to the infusion areas, supporting the retrograde transport of the AAV6-GFP vector.
Figure 3. AAV6 cellular tropism analysis.
Immunofluorescence images of GFP-transduced cells (green) that also express NeuN neuronal marker (red) both in the prefrontal cortex (A) and at the infusion site in the putamen (B). Only very few GFP-positive cells were positive for the astrocytic marker GFAP (C), indicating that AAV6 primarily transduces neurons. Arrows point out double stained cells. GFAP: glial fibrillary acid protein. Scale bars: A: 100 µm; B: 200 µm; C: 50 µm.
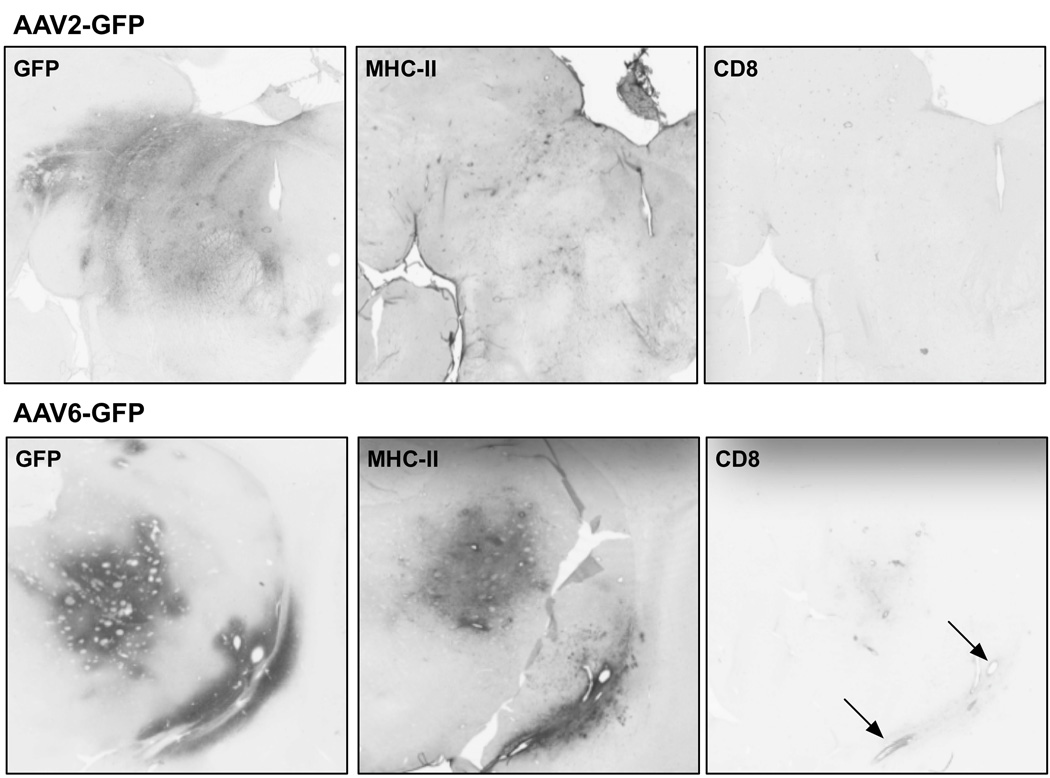
On the other hand, we also occasionally found GFP-stained cells that also expressed GFAP astrocytic marker (Fig. 3C). The few GFP/GFAP cells were located near the infusion site. Together with the large number of GFP-positive neurons (NeuN-positive cells) found, this finding supports the mainly neuronal tropism of AAV6 when infused in the NHP brain. Our previous demonstration that transduction of non-neuronal cells with AAV15 or AAV911 encoding a foreign protein like GFP engages the adaptive immune system raised concerns that even the modest astrocytic transduction observed with AAV6 in NHP brain might nevertheless trigger immune activation. As shown in Fig. 4, AAV6-mediated GFP expression in NHP putamen was associated with significant upregulation of the major histocompatibility complex-II (MHC-II) expressing cells (Fig. 4A), something we have never observed with AAV2-GFP. However, the degree of MHC-II upregulation at 21 days after vector infusion was much less than seen with AAV9-GFP at the same dose and post-infusion period consistent with the more robust astrocytic transduction seen with AAV9 previously described in rodents 11.
Figure 4. Induction of MHC-II expression by AAV6-GFP.
Scans of stained coronal sections of NHP brain at sites of infusion of AAV2-GFP (thalamus) or AAV6-GFP (putamen) stained for GFP, MHC-II and CD8. AAV2, because it transduces only neurons, does not elicit an immune response indicated by lack of MHC-II upregulation and absence of CD8-positive lymphocytes. In contrast, AAV6-GFP does trigger induction of MHC-II near the infusion site, presumably because it transduces non-neuronal cells in NHP. Also, some slight infiltration of CD8-positive lymphocytes is evident around blood vessels indicated by the arrows.
DISCUSSION
In the present study we report that AAV6 vector is transported retrogradely in the NHP brain, confirming findings in rodents13. Retrograde transport of intact virions consists of uptake of virions by axonal projections within the site of delivery and their transport to the distally located soma that is consequently transduced resulting in the presence of the transgenic protein throughout the neuron. Our data clearly show retrograde transport demonstrated by the presence of the transgenic protein in neuronal bodies of regions that send axonal projections to the striatum (i.e., prefrontal cortex, substantia nigra pars compacta, thalamus).
However, finding GFP-positive cell bodies in the globus pallidus was unexpected because this deep brain nucleus does not have axonal terminals in either the caudate nucleus or the striatum. During the infusion into the putamen, we observed that some of the AAV6-GFP infusate traveled rapidly through the perivascular space of some large blood vessels within the base of this structure. Probably this pathway facilitated the transport of the vector to adjacent structures such as the globus pallidus resulting in the transduction of those pallidal neurons11. Fibers containing GFP could be seen in the substantia nigra pars reticulata. This fact would fit better with an anterograde rather than retrograde transport of the vector. However, no GFP-positive cell bodies could be seen in the substantia nigra pars reticulata, a phenomenon that would rather describe an anterograde axonal transport. Since this structure receives axons from the striatum, those fibers probably belong to those striatal neurons transduced by AAV6-GFP at the infusion sites. Transgenic proteins are often present at high levels in the cytoplasm of transduced neurons and, therefore, neuronal fibers in distally innervated brain nuclei can be positive for the expressed protein. This phenomenon has previously been described12, 13 and it should not be confused with anterograde transport of the vector, since there were no GFP-positive cell bodies in the substantia nigra reticulata.
As already largely demonstrated for AAV2, AAV6 mainly transduces neurons when infused into the brain parenchyma of either rodents or NHP. In the two macaques of this study, few astrocytes expressed GFP and they were located close to the infusion sites. AAV6’s neuronal tropism is a very interesting feature, since it has been recently described that the expression of foreign transgenic proteins by glia after AAV infusion can result in a cell-mediated immune response, given that these cells are antigen-presenting cells in the brain14. AAV serotype 9 (AAV9) efficiently transduces both neurons and astrocytes. Thus, striatal infusion of AAV9 encoding non-self genes in the rat brain triggered an immune response that resulted in neuronal death within the transduced regions. Even though AAV6 in NHP brain still shows a strong preference for neurons as it does in rats, we wondered whether even the modest non-neuronal transduction seen in NHP was sufficient to trigger an immune response. To our surprise, AAV6 induced a brisk upregulation of MHC-II just as we saw with AAV1-hrGFP 6 and AAV9-GFP 14. As we have repeatedly emphasized, this response is driven by expression of GFP, a non-self, protein and is not directed at AAV6 itself. With that proviso, it appears that AAV6 evinces the same retrograde axonal transport properties in NHP as it does in rats 5.
Altogether, AAV6 raises as an adequate viral vector for neurological diseases. Although its ability to be retrogradely transport through axons maybe of great value for some of them, AAV6 is not recommended when aiming for local expression of the transgene. For instance, if AAV6 carrying a therapeutic transgene for Parkinson’s disease is delivered into the striatum the transgene would also be expressed in the cortex when the most affected area in this pathology is the substantia nigra pars compacta. Accordingly, among other pathologies, HD would be a disorder that could particularly benefit from an AAV6-based gene therapy. HD is an autosomal dominant neurodegenerative disease caused by a mutation in exon 1 of the gene encodinghuntingtin (Htt) protein15 that confers a gain of functional toxicity16. HD neuropathology is characterized by the atrophy of primarily the striatum, with massive degeneration of the striatal medium spiny neurons and cortex, although other brain regions (e.g., thalamus, hippocampus, and white matter) are also affected. Accordingly, most of the preclinical therapies being investigated aim to reduce the presence of mutant Htt protein in the striatum and the cortex17, 18. However, delivering a therapeutic agent to these regions remains a challenge, in particular to an area as extensive as the cortex. Thus, based on its ability to be retrogradely transported, AAV6 emerges as an appropriate candidate in order to achieve a wide distribution throughout the cortex. Our results in both rat and NHP demonstrate that infusion of AAV6 in the striatum yields transduction of striatal medium spiny neurons as well as transduction of cortical regions where cortico-striatal projections originate19, 20, and even regions secondarily affected in HD as thalamus. In addition, real-time monitoring of the infusion will allow us to ensure optimal coverage of the atrophic striatum in HD patients.
MATERIALS AND METHODS
Animals
Two adult female Cynomolgus macaques (Macaca fascicularis, ~3 kg, 5 years old) were included in this study. Experiments were performed according to National Institutes of Health guidelines and to protocols approved by the Institutional Animal Care and Use Committee at University of California San Francisco.
Vector production
GFP cDNA was cloned into an AAV6 shuttle plasmid, and a recombinant AAV6 containing GFP under the control of the chicken β-actin promoter was manufactured by the AAV Clinical Vector Core at Children’s Hospital of Philadelphia as previously described 21, 22. AAV6-GFP was purified from cell extracts by CsCl centrifugation and was concentrated to approximately 2.3 × 1013 vector genomes/mL.
Surgical procedure and vector infusion
NHP underwent stereotactic placement of skull-mounted, MR-compatible, temporary plastic plugs. NHP was placed supine in an MRI-compatible stereotactic frame. After craniectomy, the cannula trajectory guide devices were secured to the skull over both hemispheres. After placement of the plugs, the intubated animal was moved into the MRI suite and placed on inhaled isoflurane (1–3%). With the animal in the MR magnet, guides were filled with chelated gadolinium (MR-visible tracer, Prohance) prepared under sterile conditions and used to localize the plugs in the MR images in order to calculate the trajectory to the target structures inside the brain. Then, the NHP was moved into the bore and high-resolution anatomical MR scan was acquired for target identification and surgical planning. Once the target was selected, a custom-designed, ceramic, fused silica reflux-resistant cannula with a 3-mm stepped tip 9, 23 was used for the infusion. The cannula was attached to a 1-mL syringe mounted onto a MRI-compatible infusion pump (Harvard Bioscience, Holliston, MA). AAV6-GFP vector delivery was performed by CED. Briefly, infusion was initiated at 1 µL/min and, after visualizing fluid flow from the cannula tip when held at the height of the NHP head, the cannula was inserted through the plug and into the brain. When the depth stop encountered the top of the guide stem, it was secured with a locking screw. After visualization of gadolinium infusion at the cannula tip, the infusion rate was ramped up from an initial 1 µL/min to a maximum of 3 µL/min. Each NHP first received an infusion at 2 locations (pre- and post-commissural) in each putamen (bilateral) and then in one (left) or both caudate nuclei. The total infusion volume per hemisphere was 80 and 90 µL on left or right side respectively for one animal (NHP1, anterior putamen: 30 µL/left, 60 µL/right; posterior putamen: 9 µL/left1, 10 µL/left2, 29 µL/right; caudate nucleus: 30 µL/left) and 135 µL for the other (NHP2 anterior putamen: 40 µL/side; posterior putamen: 55 µL/side; caudate nucleus: 40 µL/side). Once the infusion ended, guide devices were removed from skull, animals were taken back to their home cages and monitored during recovery from anesthesia.
Tissue processing
Each NHP was perfused transcardially with PBS and PBS/4% paraformaldehyde 3–4 weeks after the infusion session. Sectioning and immunostaining of brain tissue was performed as previously described 24. Briefly, brains were harvested, sliced in 6-mm coronal sections in a brain matrix, post-fixed in PBS/4% paraformaldehyde and cryoprotected in 30% w/v sucrose. A sliding microtome was used to cut 40-µm serial sections for histological processing. For immunohistochemistry, sections were first washed in PBS followed by treatment with 1% H2O2/30% ethanol in PBS to block endogenous peroxidase. Sections were then incubated in Sniper® blocking solution (Biocare Medical, Concord, CA) followed by incubation with corresponding GFP primary antibody (rabbit anti-GFP, 1:1000, G10362 Molecular Probes) in Da Vinci Green® diluent (Biocare Medical) overnight at 4°C. Sections were rinsed in PBS/0.1% Tween20 (PBST) and incubated in Rabbit Mach 2 HRP Polymer (Biocare Medical) for 1 h, followed by several washes and colorimetric development with 3,3’-diaminobenzidine (DAB). Immunostained sections were mounted on slides and sealed with a toluene-based mounting media (Shandon-Mount, Thermo). GFP stained sections of one of the animals were counterstained with Nissl.
Double immunofluorescence staining was performed in order to characterize GFP-expressing cells. Sections containing striatal infusion site were washed in PBST and incubated overnight at ambient temperature with a mixture of GFP primary antibody (rabbit anti-GFP, 1:1000, G10362 Molecular Probes) and specific antibodies against either neurons (anti-NeuN, mouse monoclonal, 1:500, MAB377 Millipore) or astrocytes (mouse anti-glial fibrillary acid protein (GFAP), 1:500, MAB360 Dako). Next day, sections were rinsed in PBST and incubated for 2 h in a cocktail of the corresponding secondary antibodies conjugated to either Alexa 488 or 555 fluorophores (Molecular Probes). To detect MHC-II and CD8, sections were stained as described above with anti-MHC-II (monoclonal mouse anti-MHC-II, 1:300, M3887-30 US Biologicals) or with anti-CD8 (monoclonal mouse anti-CD8α, 1:100, MCA4609T AbD Serotec) as described 14. After PBS washes, tissue sections were incubated for 5 min in 0.3% Sudan Black B/70% ethanol solution in order to avoid autofluorescence due to lipofuscin.
ACKNOWLEDGEMENTS
This study was supported by a grant to K.S.B. from NIH-NINDS (R01NS073940-01).
Footnotes
CONFLICTS OF INTEREST
Authors have nothing to disclose.
REFERENCES
- 1.Hennig AK, Levy B, Ogilvie JM, Vogler CA, Galvin N, Bassnett S, et al. Intravitreal gene therapy reduces lysosomal storage in specific areas of the CNS in mucopolysaccharidosis VII mice. J Neurosci. 2003;23(8):3302–3307. doi: 10.1523/JNEUROSCI.23-08-03302.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ciesielska A, Mittermeyer G, Hadaczek P, Kells AP, Forsayeth J, Bankiewicz KS. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Molecular therapy : the journal of the American Society of Gene Therapy. 2011;19(5):922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kells AP, Hadaczek P, Yin D, Bringas J, Varenika V, Forsayeth J, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(7):2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Hum Gene Ther. 2006;17(3):291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 5.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20(3):348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadaczek P, Forsayeth J, Mirek H, Munson K, Bringas J, Pivirotto P, et al. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum Gene Ther. 2009;20(3):225–237. doi: 10.1089/hum.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bobo R, Laske D, Akbasak A, Morrison P, Dedrick R, Oldfield E. Convection-enhanced delivery of macromolecules in the brain. PNAS. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadaczek P, Kohutnicka M, Krauze MT, Bringas J, Pivirotto P, Cunningham J, et al. Convection-enhanced delivery of adeno-associated virus type 2 (AAV2) into the striatum and transport of AAV2 within monkey brain. Human gene therapy. 2006;17(3):291–302. doi: 10.1089/hum.2006.17.291. [DOI] [PubMed] [Google Scholar]
- 9.Fiandaca M, Forsayeth J, Dickinson P, Bankiewicz K. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5(1):123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salegio EA, Samaranch L, Kells AP, Forsayeth J, Bankiewicz K. Guided delivery of adeno-associated viral vectors into the primate brain. Advanced drug delivery reviews. 2012;64(7):598–604. doi: 10.1016/j.addr.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The "perivascular pump" driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14(1):69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793(1–2):169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salegio EA, Samaranch L, Kells AP, Mittermeyer G, San Sebastian W, Zhou S, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene therapy. 2012 doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciesielska A, Hadaczek P, Mittermeyer G, Zhou S, Wright JF, Bankiewicz KS, et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21(1):158–166. doi: 10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. The Huntington's Disease Collaborative Research Group. Cell. 1993;72(6):971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 16.Walker FO. Huntington's disease. Lancet. 2007;369(9557):218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 17.Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet neurology. 2011;10(1):83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 18.Vagner T, Young D, Mouravlev A. Nucleic Acid-Based Therapy Approaches for Huntington's Disease. Neurology research international. 2012;2012:358370. doi: 10.1155/2012/358370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kemp JM, Powell TP. The cortico-striate projection in the monkey. Brain. 1970;93(3):525–546. doi: 10.1093/brain/93.3.525. [DOI] [PubMed] [Google Scholar]
- 20.Selemon LD, Goldman-Rakic PS. Longitudinal topography and interdigitation of corticostriatal projections in the rhesus monkey. J Neurosci. 1985;5(3):776–794. doi: 10.1523/JNEUROSCI.05-03-00776.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita T, Elliger S, Elliiger C, Podsakoff G, Villarreal L, Kurtzman G, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5(7):938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 22.Wright J, Qu G, Tang C, Sommer J. Recombinant adeno-associated virus: formulation challenges and strategies for a gene therapy vector. Curr Opin Drug Discov Devel. 2003;6(2):174–178. [PubMed] [Google Scholar]
- 23.Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. Journal of neurosurgery. 2005;103(5):923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson RM, Kells AP, Rosenbluth KH, Salegio EA, Fiandaca MS, Larson PS, et al. Interventional MRI-guided Putaminal Delivery of AAV2-GDNF for a Planned Clinical Trial in Parkinson's Disease. Mol Ther. 2011 doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]