Abstract
Background
Hypertrophic cardiomyopathy (HCM) is the most common monogenic cardiac disorder encountered in the clinic. Data relative to the electrophysiologic characteristics and pharmacologic responsiveness of human tissues and cells isolated from patients with hypertrophic cardiomyopathy are rare. As a consequence, cellular mechanisms underlying arrhythmogenicity are poorly understood.
Methods
Cardiomyocytes were enzymatically dissociated from a septal myectomy surgically removed from a patient with obstructive hypertrophic cardiomyopathy. Sharp microelectrodes and patch-clamp techniques were used to evaluate action potential and sodium channel current (INa) characteristics.
Results
Action potential morphology recorded was that typical of an M cell, but with a longer than normal duration (APD) and a relatively steep APD-rate relationship. APD at all rates was significantly reduced following exposure to ranolazine (10 μM). Whole cell patch clamp recording yielded robust peak INa and large late INa (1.1% of peak INa vs. 0.1–0.2% in healthy controls). A large window current was observed as well. Ranolazine (10 μM) shifted steady-state V0.5 of inactivation by − 8 mV, reduced late INa by 82% and significantly diminished the window current.
Conclusion
Our results indicate the presence of cells with M cell characteristics in the septum of the human heart, as has previously been described in the canine heart. They also point to an ameliorative effect of ranolazine to reduce augmented late INa and thus to reduce the prolonged APD in the setting of HCM. These results suggest a potential therapeutic role for ranolazine in HCM.
Keywords: M Cell, Ranolazine, Sodium Channel Current, Arrhythmia, Action potential
Introduction
Hypertrophic cardiomyopathy (HCM), with a prevalence of 1:500, is the most common monogenic cardiac disorder encountered in the clinic.1 HCM is also the most common cause of arrhythmic sudden cardiac death in young athletes.2
Its high prevalence notwithstanding, it is poorly understood from the standpoint of cellular mechanisms underlying arrhythmogenicity and diastolic dysfunction. Data relative to the electrophysiologic characteristics and pharmacologic responsiveness of human tissues and cells isolated from patients with hypertrophic cardiomyopathy are rare. HCM also lacks a disease-specific pharmacological treatment.3
Here we report action potential (AP) characteristics of myocytes enzymatically dissociated from a septal myectomy surgically removed from a patient with obstructive hypertrophic cardiomyopathy, together with characteristics of sodium channel current (INa) and its response to the late INa blocker ranolazine.
Methods
The patient, a 48-y/o male, presented with a systolic murmur and concentric left ventricular hypertrophy with a significant gradient of 39 mmHg. A septal myectomy and a mitral valve replacement, necessitated due to mitral stenosis, were performed. The procedure was done at the Mohawk Valley Heart Institute, Utica, NY, USA, with ethical committee approval for tissue retrieval. The sample was obtained from the left ventricular septal wall.
Cardiomyocytes were obtained by standard enzymatic dissociation methods.4 Transmembrane APs were recorded at 36.5°C from single cardiomyocytes using high resistance microelectrodes (DC resistance=40 to 50 MΩ) filled with 2.7 mol/L KCl. Macroscopic peak and late INa were recorded at room temperature from single cardiomyocytes using the whole cell patch-clamp technique. I–V relationship, steady-state activation and inactivation and window currents were determined as previously described.5–7
Late INa was evaluated using a square depolarization pulse to −20 mV for 300 ms from at holding potential of −120 mV, applied once every 10 seconds. Late INa is presented as a fraction of peak INa. The effect of ranolazine to inhibit late INa was evaluated at a concentration of 10 μM. Recordings in the presence of the drug were performed >3 minutes after exposure to the drug as well as >5 minutes after washout.
Statistics
Data are expressed as mean±SEM. Statistical analysis was performed using Student paired t test or ANOVA, as appropriate. p<0.05 was considered to be statistically significant.
Results
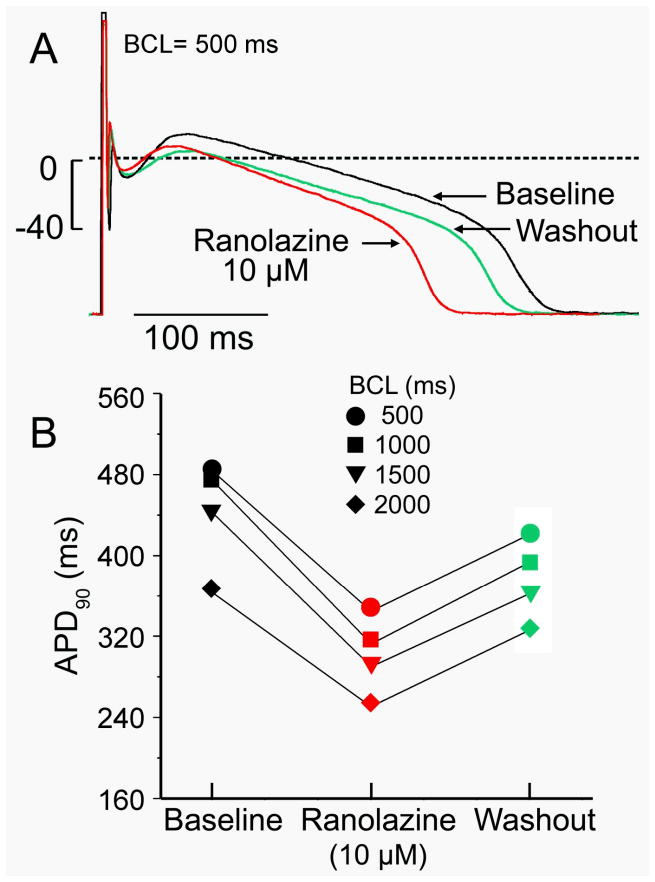
Action potential (AP) morphology recorded using sharp microelectrodes was that typical of an M cell (Figure 1A), although with a longer duration at baseline (450 ms at 1 Hz) than typically recorded in human M cells.8, 9 The prolonged AP duration (APD) is consistent with the prolonged QT/QTc interval recorded from this patient (476/455 ms) and HCM patients in general.10 APD-rate relationship is relatively steep (Figure 1B), consistent with what is reported in experimental models of HCM.11 APD at all rates was significantly reduced following exposure to ranolazine (10 μM).
Figure 1. Rate-dependence of action potential duration (APD) of a cardiomyocyte isolated from a left ventricular septal myectomy from a patient with hypertrophic cardiomyopathy and the effect of ranolazine.
A: Action potential recorded using high resistance microelectrode at body temperature in the absence (black) and presence of 10 μM ranolazine and after 10 min of washout. B: Rate-dependence of APD in the absence and presence of 10 μM ranolazine and after 10 min of washout. Basic cycle length (BCL). n=1
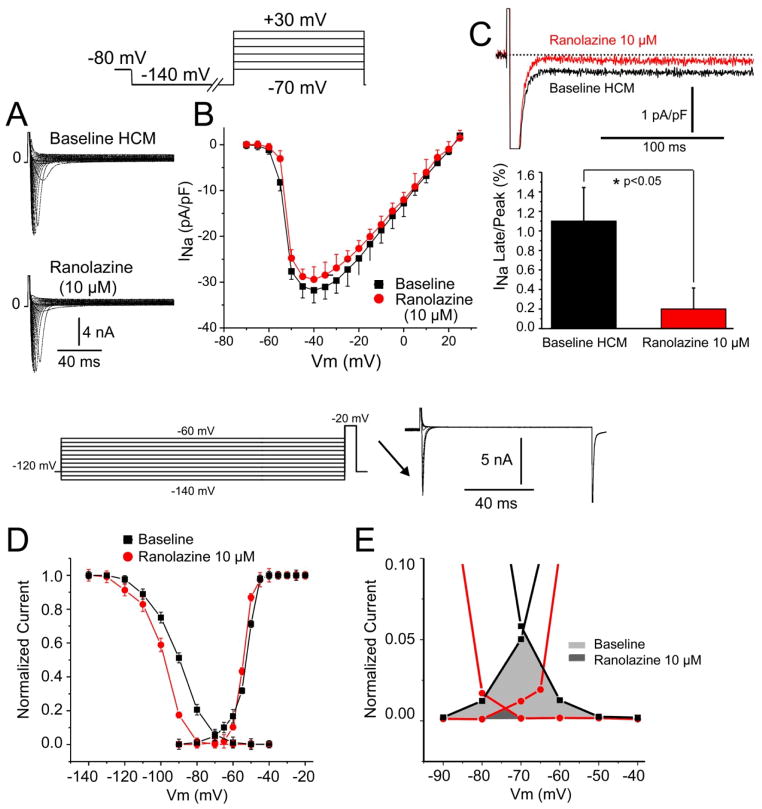
Whole cell patch clamp recording yielded robust peak INa (Figures 2A and B) and a relatively large late INa as well as window current (Figures 2C and). Steady-state inactivation was shifted to more negative potentials (−8 mV) by ranolazine resulting in a marked reduction of window current (Figures 2D and E). Late Na+ channel current (1.1% of peak INa ) was larger than typically observed in normal myocardium (0.1–0.2%)12, 13 and was reduced by 82% by 10 uM ranolazine (Figure 2C). The ranolazine-induced reduction in late INa and window current likely contribute to the abbreviation of APD.
Figure 2. Characteristics of peak and late sodium channel current (INa) and the effect of ranolazine.
A: Macroscopic INa recorded under control condition and the presences of ranolazine (10 μM) using whole cell patch-clamp technique and the protocol shown in the inset. B: Current-voltage relationship of INa (n=3). C: Ranolazine reduced late INa (as % of peak) by 82% (p<0.05, n=3). D: Steady state activation and inactivation curves recorded under control conditions and after ranolazine (10 μM). Ranolazine shifts half-inactivation voltage (V0. 5) by −8 mV (from −90.1 ± 0.9 to −98.2 ± 0.8; n=3; p<0.05). E: Region of overlap of activation and inactivation curves amplified showing window sodium current under control conditions (light shaded area) and in the presence of 10 μM ranolazine (dark shaded area). Hypertrophic Cardiomyopathy (HCM).
Discussion
Our findings indicate the presence of cells with M cells characteristics in the septum of the human heart, as has been described in the canine heart,14 and point to an ameliorative effect of ranolazine to reduce augmented late INa and thus to reduce the prolonged APD in the setting of HCM.15 Our finding suggest that augmented late INa is a major contributor to the prolonged APD of the septal cardiomyocytes isolated from the HCM patient and that inhibition of this current using ranolazine may be of therapeutic value.
Our findings are consistent with those of Coppini and coworkers15 showing that the phenotype of isolated cardiomyocytes derived from HCM patients differs significantly from that of controls. These authors reported that enhanced CaMKII activity slowed ICa inactivation and increased late INa thus contributing to APD prolongation and related arrhythmias. Their data also suggested that by altering the function of EC-coupling proteins, CaMKII might also contribute to the altered Ca2+-transient kinetics and elevation of diastolic [Ca2+]i, which are responsible for the development of delayed afterdepolarizations (DAD). As in our study, ranolazine, at therapeutic concentrations, was shown to be capable of partially reversing the HCM-related cellular abnormalities via inhibition of late INa, but with negligible effects in myocytes isolated from control hearts.
The effect of ranolazine to block late INa and to abbreviate APD in HCM cardiomyocytes is also expected to reduce diastolic [Ca2+]i and thus to suppress DADs and ameliorate diastolic dysfunction.15, 16
Acknowledgments
FUNDING SOURCES
This work was supported by NIH grant HL47678 (CA), NYSTEM grant #C026424 (CA) and New York, Florida, Massachusetts, Connecticut and Rhode Island Freemasons.
Footnotes
DISCLOSURE STATEMENT
The authors have no financial or other considerations to disclose.
Reference List
- 1.Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. doi: 10.1016/S0140-6736(12)60397-3. [DOI] [PubMed] [Google Scholar]
- 2.Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:1064–1075. doi: 10.1056/NEJMra022783. [DOI] [PubMed] [Google Scholar]
- 3.Spoladore R, Maron MS, D’Amato R, Camici PG, Olivotto I. Pharmacological treatment options for hypertrophic cardiomyopathy: high time for evidence. Eur Heart J. 2012;33:1724–1733. doi: 10.1093/eurheartj/ehs150. [DOI] [PubMed] [Google Scholar]
- 4.Scamps F, Carmeliet E. Delayed K+ current and external K+ in single cardiac Purkinje cells. Am J Physiol. 1989;257:C1086–C1092. doi: 10.1152/ajpcell.1989.257.6.C1086. [DOI] [PubMed] [Google Scholar]
- 5.Barajas-Martinez H, Haufe V, Chamberland C, Blais Roy MJ, Fecteau MH, Cordeiro JM, Dumaine R. Larger dispersion of INa in female dog ventricle as a mechanism for gender-specific incidence of cardiac arrhythmias. Cardiovasc Res. 2009;81:82–89. doi: 10.1093/cvr/cvn255. [DOI] [PubMed] [Google Scholar]
- 6.Zygmunt AC, Eddlestone GT, Thomas GP, Nesterenko VV, Antzelevitch C. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am J Physiol. 2001;281:H689–H697. doi: 10.1152/ajpheart.2001.281.2.H689. [DOI] [PubMed] [Google Scholar]
- 7.Zygmunt AC, Nesterenko VV, Rajamani S, Hu D, Barajas-Martinez H, Belardinelli L, Antzelevitch C. Mechanisms of atrial-selective block of sodium channel by ranolazine I. Experimental analysis of the use-dependent block. Am J Physiol Heart Circ Physiol. 2011;301:H1606–H1614. doi: 10.1152/ajpheart.00242.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lou Q, Fedorov VV, Glukhov AV, Moazami N, Fast VG, Efimov IR. Transmural heterogeneity and remodeling of ventricular excitation-contraction coupling in human heart failure. Circulation. 2011;123:1881–1890. doi: 10.1161/CIRCULATIONAHA.110.989707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drouin E, Charpentier F, Gauthier C, Laurent K, Le Marec H. Electrophysiological characteristics of cells spanning the left ventricular wall of human heart: Evidence for the presence of M cells. J Am Coll Cardiol. 1995;26:185–192. doi: 10.1016/0735-1097(95)00167-x. [DOI] [PubMed] [Google Scholar]
- 10.Badran HM, Elnoamany MF, Soltan G, Ezat M, Elsedi M, Abdelfatah RA, Yacoub M. Relationship of mechanical dyssynchrony to QT interval prolongation in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2012;13:423–432. doi: 10.1093/ejechocard/jer290. [DOI] [PubMed] [Google Scholar]
- 11.Antoons G, Oros A, Beekman JDM, Engelen MA, Houtman MJC, Belardinelli L, Stengl M, et al. Late Na+ current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55:801–809. doi: 10.1016/j.jacc.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 12.Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, Kamp TJ, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. 2005;38:475–483. doi: 10.1016/j.yjmcc.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Maltsev VA, Undrovinas AI. A multi-modal composition of the late Na+ current in human ventricular cardiomyocytes. Cardiovasc Res. 2006;69:116–127. doi: 10.1016/j.cardiores.2005.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sicouri S, Glass A, Ferreiro M, Antzelevitch C. Transseptal dispersion of repolarization and its role in the development of torsade de pointes arrhythmias. J Cardiovasc Electrophysiol. 2010;21:441–447. doi: 10.1111/j.1540-8167.2009.01641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coppini R, Ferrantini C, Yao L, Fan P, Del LM, Stillitano F, Sartiani L, et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation. 2013;127:575–584. doi: 10.1161/CIRCULATIONAHA.112.134932. [DOI] [PubMed] [Google Scholar]
- 16.Maier LS, Layug B, Karwatowska-Prokopczuk E, Belardinelli L, Lee S, Sander JLC, Wachter R, Edelmann F, Hasenfuss G, Jacobshagen C. RAnoLazIne for the treatment of Diastolic Heart Failure in patients with preserved ejection fraction: The RALI-DHF proof-of-concept study. JACC Heart Failure. 2013 doi: 10.1016/j.jchf.2012.12.002. In press. [DOI] [PubMed] [Google Scholar]