Abstract
Cadmium (Cd) is a highly toxic heavy metal for both plants and animals. The presence of Cd in agricultural soils is of great concern regarding its transfer in the soil-plant system. This study investigated the transfer of Cd (exogenous salts) from a wide range of Chinese soils to corn grain (Zhengdan 958). Through multiple stepwise regressions, prediction models were developed, with the combination of Cd bioconcentration factor (BCF) of Zhengdan 958 and soil pH, organic matter (OM) content, and cation exchange capacity (CEC). Moreover, these prediction models from Zhengdan 958 were applied to other non-model corn species through cross-species extrapolation approach. The results showed that the pH of the soil was the most important factor that controlled Cd uptake and lower pH was more favorable for Cd bioaccumulation in corn grain. There was no significant difference among three prediction models in the different Cd levels. When the prediction models were applied to other non-model corn species, the ratio ranges between the predicted BCF values and the measured BCF values were within an interval of 2 folds and close to the solid line of 1∶1 relationship. Furthermore, these prediction models also reduced the measured BCF intra-species variability for all non-model corn species. Therefore, the prediction models established in this study can be applied to other non-model corn species and be useful for predicting the Cd bioconcentration in corn grain and assessing the ecological risk of Cd in different soils.
Introduction
Trace metal elements accumulation in crop of agricultural soils is of extensive concern due to its potential risks to human health and detrimental effects on soil ecosystems [1]–[4]. Cadmium (Cd) is one of the most dangerous trace elements because excessive dietary intake of Cd and its accumulation in human organs over a lifetime can lead to kidney malfunction [5]–[8].
Numerous studies have demonstrated that a number of factors affect Cd bioavailability in soils, including soil pH, organic matter (OM), cation exchange capacity (CEC), cultivars of crop plants, plant age, and so on [9]–[13]. Among these soil properties, the pH plays the most important role in determining metal speciation, due to its strong effects on solubility and speciation of metals in the soil and solution [14]–[15]. Dramatic increases in Cd desorption from soil constituents and its dissolution ability in solution have been observed with the decreasing soil pH [16]–[17]. The bioavailability of Cd in soil also increases when soil pH decreases [18]–[23]. Apart from soil pH, OM in soil is another important factor affecting heavy metal availability. It was reported that heavy metal adsorption onto soil constituents declined when organic matter content in soils decreased [24]–[25], and that the ability of plants to Cd uptake decreased with an increase in soil organic matter [26]–[27].
The influence of soil factors derived from the soil types on plant uptake of heavy metals and the difficulties in assessing the ecological risk of heavy metals in complex soil types have urged the research on prediction models that can predict trace element transfer to plant [28]–[30]. These models provide great opportunities to carry out ecological risk assessments and establish soil quality criteria for heavy metals [31]–[32]. However, the influences of soil factors may result in variability of ecological risk assessments in different soils and of the soil quality criteria for heavy metals [33]. Therefore, when the soil environmental quality standard is established based on the species sensitivity distributions (SSD), which are routinely used in ecological risk assessment procedures [34]–[35], the toxicology data from the soils with different properties need to be normalized using toxicity prediction models to eliminate the influence of soil factors [36]. The normalization of toxicology data can also improve the accuracy of the sensitivity distribution of species and environmental quality standard values [37]. Ideally, separate species-specific models should be developed for each single species. However, this is unrealistic due to the expenses and efforts involved. Thus, for practical reason, the cross-species extrapolation of biological toxicity model was applied in some cases of pollutants risk assessment. For example, Deleebeeck et al. [38]–[39] have applied biotic bigand model (BLM) from O. mykiss to other fish species and from D. magna to other cladocera species. Schlekat et al. [33] applied chronic nickel BLMs developed for the cladocera such as Daphnia magna and Ceriodaphnia dubia to predict chronic toxicity of nickel to three other invertebrates including snail (Lymnaea stagnalis), insect (Chironomus tentans), and rotifer (Brachionus calyciflorus). However, little effort has been devoted to establishing models to describe the relationship between Cd uptake of corn grain and soil properties. In addition, the cross-species extrapolation approach has rarely been applied to terrestrial ecosystem. The objectives of this study are: 1) to develop the models for predicting Cd accumulation in corn grain on seventeen soils with greatly different properties, 2) to assess the feasibility of applying these models to other non-model corn species, and 3) to investigate their accuracy in predicting accumulation of Cd in non-model corn species.
Materials and Methods
None of these 17 soil samples were collected from national parks or other protected areas. It is confirmed that no specific permissions were required for the soils sampling activities in the 17 locations in China. It is also can be confirmed that the field studies did not involve endangered or protected species. No tested corn species are under first- or second-class state protection, and they are not listed in the Inventory of Rare and Endangered Plants of China (http://zrbhq.forestry.gov.cn/portal/zrbh/s/3053/content-457748.html), or the Key Protected Inventory of Wild Plants of China (http://zrbhq.forestry.gov.cn/uploadfile/zrbh/2010-10/file/2010-10-14-bb296addeaa047798d6b6c476aaa1da9.doc). These corn species were used for only scientific research as permitted by the Ministry of Agriculture of China.
Soil samples
A set of 17 soils covering a wide range of soil properties was collected from typical locations in China. In each sample location, the soils were collected from the top 20 cm of the soil profile. For analysis of their phys-chemical characteristics, the soils were air dried and sorted to pass a 2-mm sieve. Soil pH was measured in deionized water (soil:solution ratio, 1∶5) [40]. Cation exchange capacity (CEC) was determined by the unbuffered silver-thiourea method [41]. OM was measured by dry combustion [42]. The total phosphorus in soils (TP) was measured by colorimetric method [43]. The total nitrogen in soils was determined by the Kjeldahl's method [44]. The background Cd content in the soils was determined by aqua regia (1∶3 fresh mixture of concentrated HNO3 and HCl) digestion [45]. The selected properties of the 17 soil samples were shown in Table 1.
Table 1. Selected soil properties of the 17 soils used in the cadmium phytotoxicity test.
| Soil NO. | Location* | pH | OM (g.kg−1) | CEC (cmol. kg−1) | TN (g.kg−1) | TP (mg.kg−1) | TK (g.kg−1) | Background Cd (mg.kg−1) |
| S1 | Hunan | 4.90 | 15.52 | 10.85 | 1.14 | 468.5 | 15.26 | 0.18 |
| S2 | Chongqing | 5.74 | 17.48 | 21.34 | 1.00 | 547.8 | 22.61 | 0.18 |
| S3 | Liaoning | 5.74 | 25.84 | 12.19 | 1.00 | 726.6 | 23.94 | 0.16 |
| S4 | Yunnan | 5.92 | 34.26 | 11.10 | 2.01 | 811.2 | 4.77 | 0.26 |
| S5 | Jiangxi | 6.01 | 11.69 | 8.70 | 0.51 | 518.0 | 9.96 | 0.14 |
| S6 | Anhui | 6.25 | 20.04 | 19.08 | 0.99 | 347.1 | 15.41 | 0.17 |
| S7 | Heilongjiang | 6.27 | 35.69 | 28.59 | 1.74 | 481.1 | 24.7 | 0.18 |
| S8 | Jilin | 6.82 | 32.85 | 31.11 | 1.75 | 349.2 | 24.58 | 0.17 |
| S9 | Jiangsu | 6.93 | 47.69 | 26.20 | 2.44 | 689.2 | 21.03 | 0.15 |
| S10 | Shaanxi | 7.90 | 16.49 | 22.37 | 1.36 | 984.0 | 24.37 | 0.24 |
| S11 | Hebei | 7.98 | 8.57 | 8.12 | 0.68 | 529.9 | 24.22 | 0.19 |
| S12 | Henan | 8.07 | 17.79 | 16.01 | 1.07 | 746.1 | 19.86 | 0.19 |
| S13 | Xinjiang | 8.12 | 19.43 | 25.25 | 1.32 | 780.0 | 25.49 | 0.16 |
| S14 | Shanxi | 8.24 | 23.17 | 16.80 | 1.13 | 950.7 | 23.70 | 0.20 |
| S15 | Tianjin | 8.29 | 22.02 | 24.67 | 1.42 | 916.3 | 24.63 | 0.19 |
| S16 | Shandong | 8.65 | 11.84 | 13.09 | 0.93 | 965.6 | 21.37 | 0.18 |
| S17 | Neimeng | 8.80 | 16.30 | 11.61 | 0.96 | 376.3 | 26.40 | 0.21 |
Soil sample locations are listed in the order of increasing pH.
Experimental design
Bioaccumulation factors for Cd by corn with one species from seventeen soils
According to Grade Two Standard for Cd in the Soil Environmental Quality Standards of China (GB15618–1995), the tested levels of Cd in soils were shown in Table 2. To obtain the tested levels, a certain amount of exogenous Cd (3[CdSO4].8H2O) was added to the 8 kg air-dried soils. All the soils were thoroughly mixed and placed into pots. Then the soils were moistened with deionized water to the 60% of field moisture capacity. Each tested level of Cd was carried out in triplicate. The soils containing Cd at different levels were covered by plastic film with several pores. For Cd aging, all the pots were kept for 3 months at a greenhouse at the temperature of 25±3°C during the daytime and 20±3°C at night with a natural light photoperiod. During the period of aging, water contents of each soil sample were retained by adding deionized water every 3 days. After 3 months' aging, uniformed seeds of corn (Zea may L. cv. Zhengdan 958) were sown in each soil containing Cd.
Table 2. Soil Environmental Quality Standards of China (GB15618–1995) and the content of exogenous Cd (mg.kg−1).
| pH | <6.5 | 6.5∼7.5 | >7.5 |
| Grade Two Standard | 0.3 | 0.3 | 0.6 |
| Low Cd | 0.3 | 0.3 | 0.6 |
| High Cd | 0.6 | 0.6 | 1.0 |
Bioaccumulation factors for Cd of eight corn species from three soils
Three soils with typical properties sampled from Jiangxi, Shaanxi, and Shanxi provinces were selected for this experiment. The tested levels of Cd in soils and exogenous Cd addition were the same as those in the experiment of bioaccumulation factors for Cd by corn from different soils. Triplicate was conducted on each tested level of Cd. After three months' aging of soils, the seeds of corn (cv. Jingketian 183, Chuandan 30, Liaodan 565, Tunyu 88, Zhongdan 808 and Nongda 84) were surface sterilized with 1% (v/v) NaOCl, rinsed, and soaked in distilled water for 24 h at 33°C in the dark. Then these seeds were sown in each soil containing Cd. All these pots were placed in the greenhouse at the temperature of 25±3°C during the daytime and 20±3°C at night with a natural light photoperiod.
Experimental soil sampling and analysis
After three months' aging, soils in the pots of the above two experiments were sampled, air-dried, passed through a <0.20 mm sieve, and then thoroughly mixed. 0.5 g of the soil sample was weighed and added to digestion tubes containing 9 ml HNO3 and 3 ml HF. The corn grains were harvested through being oven-dried at 105°C for 30 minutes and kept at 70°C until the weight of the grains was stable. The samples (0.5 g) were digested in 6 ml concentrated HNO3 (70% w/v) and 3 ml H2O2 in a CEM Mars X microwave oven (CEM Mars) at a pressure of 3.1 MPa. The Cd concentrations in the digestion solution from soil and corn grain samples were measured by inductively coupled plasma-mass spectrometry (ICP-MS, Agilent 7500a, Agilent Technologies Co. Ltd., USA).
Data analysis
The bioconcentration factor (BCF) was calculated as the ratio of the content of Cd in the corn grain to that in the soil (Equation 1) [46]–[47].
| (1) |
Where C grain is the Cd concentration in the corn grain and Csoil is Cd concentration in the soil.
SPSS 16.0 for Windows® 107 (SPSS Inc, Chicago, IL, USA) was used for the regression analysis and statistical analysis of the significant differences. Origin 8.0 (OriginLab Co., Northampton, MA, USA) was employed for figure rendering.
Model
The prediction models for Cd transfer were established through multiple stepwise regression of the Cd BCF from Zhengdan 958 in a wide range of soil properties. These models are based on equation 2.
| (2) |
Where Log[BCF], log[OM], and log[CEC] were the logarithm base 10 of the BCF values, the content of organic matter (g.kg−1) in soils, and the cation exchange capacity (cmol.kg−1) of soils, respectively. The slope of soil property parameters such as a, b and c indicate the impacts of soil properties on heavy metal. The intercept k is the intrinsic sensitivity that characterizes the species absorbing Cd.
Cross-species extrapolation
In the process of cross-species extrapolation, an interim alternative was to assume that interactions among Cd and pH, OM, CEC were the same among related species. In other words, the models stability constants including a, b, c were assumed the same among related species, and the only difference between related species was assumed to be their intrinsic sensitivity (k) [33]. The variation of intrinsic sensitivities within a species among plants reflects residual variation [36]. According to the minimum squared error between predicted BCF value and the measured BCF value 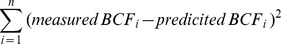 , the intercept (k) for different models corresponding to various species were obtained through Excel Solver for linear optimization [32].
, the intercept (k) for different models corresponding to various species were obtained through Excel Solver for linear optimization [32].
The accuracy of the model predictions was evaluated by comparing the measured BCF of the other non-model corn species with the predicted BCF from the model. The predictions for other non-model corn species were calculated with the prediction model developed for Zhengdan 958 [48].
Analysis of the reduction of intra-species variability
The Cd BCF values of non-model corn species were normalized to the specific soil conditions by means of the obtained model for Zhengdan 958 and equation 3.
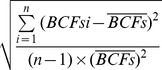 |
(3) |
Where the BCF of i-th conditions for specific corn specie was normalized to specific soil conditions. BCFsi is the BCF of i-th conditions for specific corn species.  is the mean of n BCFs. n is the number of different conditions for specific corn species.
is the mean of n BCFs. n is the number of different conditions for specific corn species.
The BCFs that Cd for different corn species was normalized by the prediction models to a set soil condition should be equal. Therefore, the decrease of intra-species variability indicates that normalization processing eliminated soil properties to a certain extent.
Results
Major factors affecting accumulation of Cd in corn grain in different soils
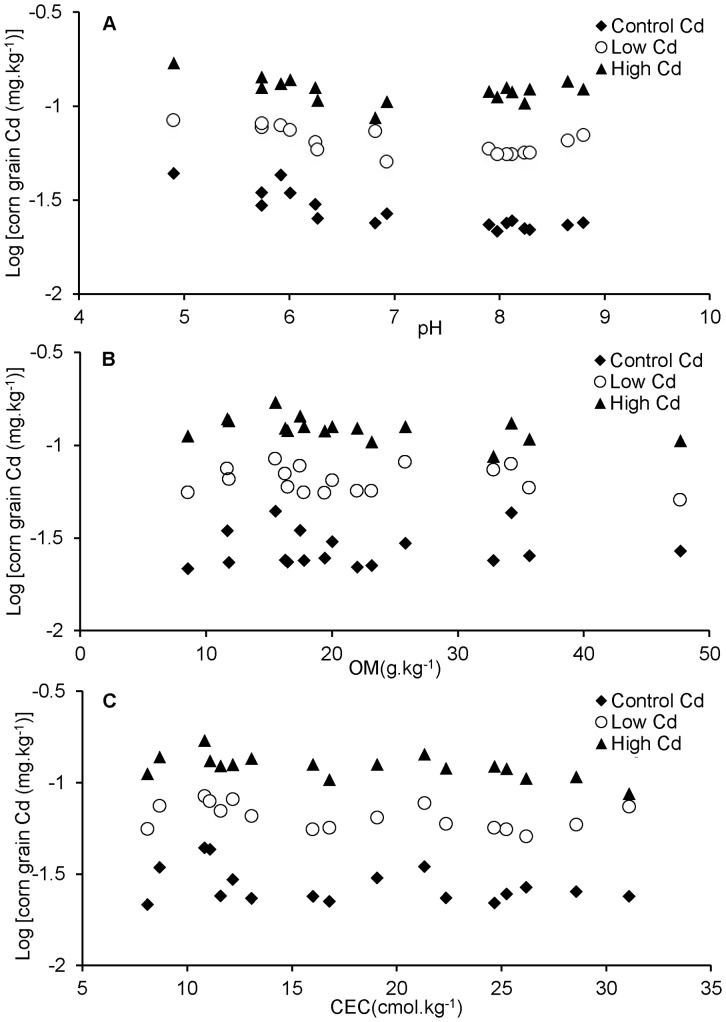
Simple relationships between log [corn grain Cd] at different Cd levels and soil pH, OM and CEC are shown in Figure 1. At the treatment of low concentration Cd, the Cd content in maize grain decreased with an increase of pH. Similar trends were found in the treatments of control and high concentration Cd. The significantly negative correlations were observed between Cd accumulation in corn grain and soil pH in the treatments with control Cd, low concentration Cd, and high concentration Cd (P<0.001). There was no significant correlation between the log [corn grain Cd] and soil OM and CEC. These results indicate that soil pH is the main factor that controls the accumulation of Cd in corn grain.
Figure 1. Relationships between Cd in corn grain and (A) soil pH, (B) organic matter (OM) and (C) cation exchange capacity (CEC).
Effects of different soil types on Cd transfer in soil-corn system
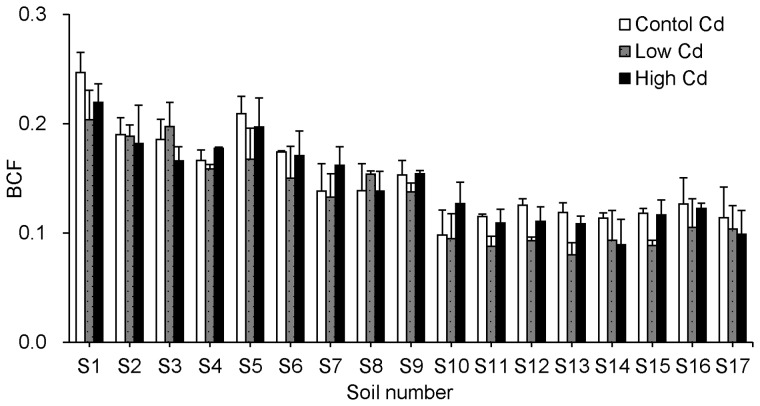
As shown in Figure 2, the BCF values from cultivar Zhengdan 958 with different treatments were significantly different in 17 soils with a wide range of soil properties in China. In general, the variation tendency of BCF values from cultivar Zhengdan 958 was alike over all soil samples from S1 to S17 at different treatments. These BCF values decreased with the increase of soil pH. The BCF values detected in S1 to S9 (pH from 4.90 to 6.93) were significantly higher than those in S10 to S17 (pH from 7.90 to 8.80). In the control soil, the maximum value for BCF (0.24) was achieved in S1 (pH 4.90), and the minimum value for BCF (0.09) in S10. The maximum value was 2.67 folds as high as the minimum value. At the treatment of low Cd, the maximum BCF (0.20) was observed in S1, and the minimum value for BCF (0.08) was found in S13. The maximum value was 2.50 folds as high as the minimum. At the treatment of high Cd, the highest BCF (0.22) values among the 17 soils were once again obtained in S1, and the minimum value for BCF (0.10) was in S17. The maximum was 2.22 folds as high as the minimum. These results indicate that Cd under acidic soil conditions is more highly bioavailable, leading to increased absorption by plants.
Figure 2. Effects of soil types on the transfer factor based on different Cd treatments in soils.
Prediction models
The prediction models established by stepwise regression are shown in Table 3. A significantly negative correlation existed between the Log[BCF] and soil pH in the Cd treatments including control, low Cd, and high Cd with the R2 values ranging from 0.713–0.811 (P<0.001). All models displayed the same trend in which Log[BCF] of Cd in corn grain was negatively related to soil pH. There was no significant difference among three prediction models at the different Cd levels.
Table 3. Prediction models for the different Cd levels.
| Model No. | Cadmium levels | Prediction models | R2 | P |
| Model 1 | Control Cd | Log[BCF] = −0.081pH-0.254 | 0.728 | <0.001 |
| Model 2 | Low Cd | Log[BCF] = −0.104pH-0.170 | 0.811 | <0.001 |
| Model 3 | High Cd | Log[BCF] = −0.079pH-0.280 | 0.713 | <0.001 |
Cross-species extrapolation
The intercept (k) for different corn species deduced by three different models are shown in Table 4. The intercept (k) means the sensitivity of corn species to Cd accumulation. No significant differences were found to exist either among the k values of all tested non-model corn species deduced by the same model (model 1, 2, or 3) or among those in each of the tested non-model corn species deduced by different models (model 1, 2, and 3).
Table 4. Intrinsic sensitivity (k) for non-model species fitted by models from cultivar Zhengdan 958.
| Intrinsic sensitivities (k) | ||||||
| Prediction Model | Jingketian 183 | Chuandan 30 | Liaodan 565 | Tunyu 88 | Zhongdan 808 | Nongda 84 |
| Model 1 | −0.284 | −0.284 | −0.339 | −0.418 | −0.324 | −0.317 |
| Model 2 | −0.128 | −0.123 | −0.175 | −0.254 | −0.164 | −0.158 |
| Model 3 | −0.302 | −0.299 | −0.353 | −0.432 | −0.338 | −0.331 |
On the basis of the k values, the BCF values of non-model corn species were predicted by different models developed from cultivar Zhengdan958. The relationship between the predicted BCF values and the measured BCF values of non-model corn species was shown in Figure 3. The ratio between the predicted BCF values and the measured BCF values was within 2 folds interval and close to the solid line of 1∶1 relationship, indicating that these models from cultivar Zhengdan 958 can be applied to predict the Cd BCF of non-model corn species.
Figure 3. Relativity between measured and predicted BCF values for Cd in non-model corn grains.
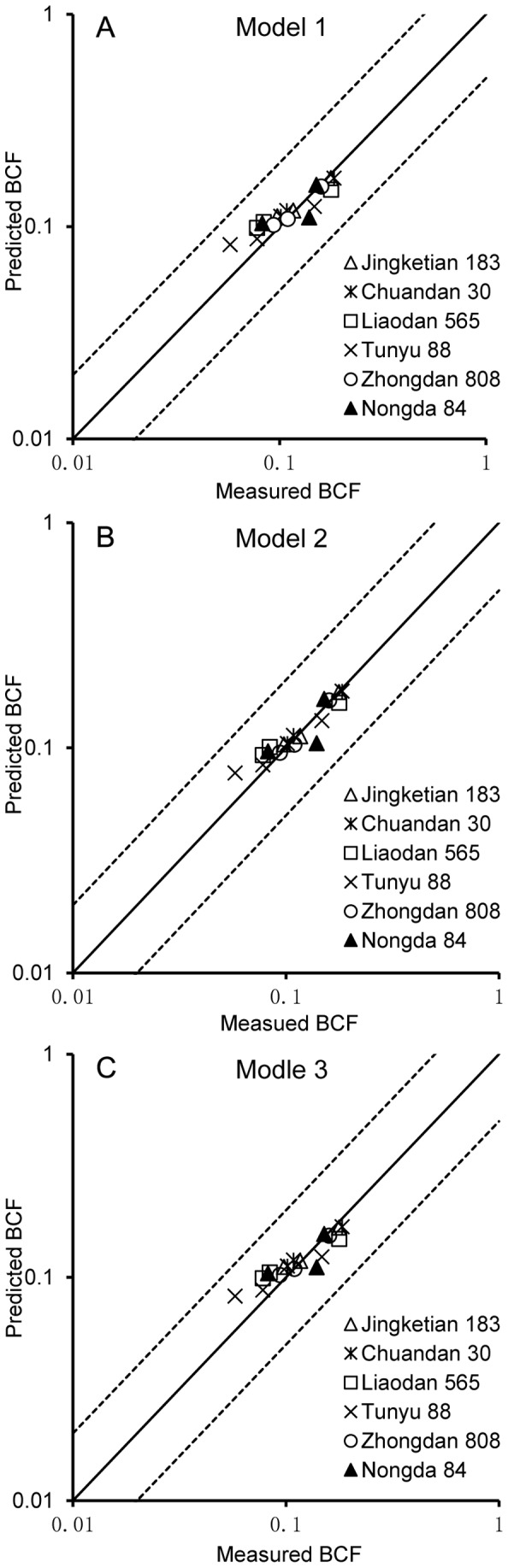
The predicted BCF values in Figure 3 were estimated by (A) model 1, (B) model 2, and (C) model 3 in Table 3. The solid line represents a 1∶1 relationship; the dashed lines indicate a 2-fold prediction interval between the predicted and measured values.
Reduction of intra-species variability
Figure 4 shows the intra-species variability of Cd BCF of non-model species which were normalized with the models listed in Table 3. The prediction models can reduce the measured BCF intra-species variability for all non-model corn species. The intra-species variability of Cd BCF fitted by model 2 was found much lower than that by model 1 and model 3 except for cultivar Nongda 84. Interestingly, the intra-species variability of Cd BCF for cultivar Nongda 84 normalized with model 1 was much lower than that with model 2 and model 3. No significant differences existed in the intra-species variability of cultivars Chuandan 30, Liaodan 565 and Tunyu 88 which were normalized with model 1 and model 3. Since all models are effective in reducing the uncertainty caused by the soil property differences, they all can be used to establish the soil Cd SSD curves and provide an ecological benchmark in China.
Figure 4. Intra-species variability of Cd BCF.
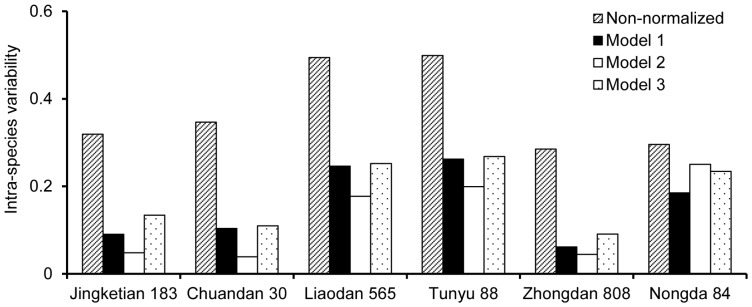
The data from all non-model corn species were normalized with the models listed in Table 3.
Discussion
The bioavailable concentration of Cd in soil is more important than its total concentration in terms of its uptake and accumulation in plants [49]. In this study, we found that Cd uptake into corn grain increased with an increase of Cd concentration in soil. However, the BCF values tended to be similar when the concentration of Cd in soil was changed responding to the same soil. These findings were in agreement with previous studies [28]–[30], [50]–[51].
We also found that the soil pH was the major factor influencing the bioavailability of Cd in soil. This may be attributed to Cd uptake by plants from soils through diverse reactions such as absorption, ionic exchange, redox reactions, and precipitation-dissolution etc. [52]. These reactions are mainly affected by soil pH. The pH effects also include its influence on the solution activity of Cd and the distribution of Cd between the soil phase and the solution phase [53]–[54]. The increased sorption of Cd at high pH values could reduce the solution concentration, which tends to decrease the bioavailable concentration of Cd in soil. In this study, the BCF values observed in acidic soils (S1 to S9) were higher than those in alkaline soils (S10 to S17). These results suggest that Cd is much more bioavailable and leads to increased absorption by plants under acidic conditions [55]–[57]. No significant differences were observed among three prediction models at the different Cd levels. There were no significant differences among Cd BCF values of corn grain at different Cd levels, either. These results were consistent with the findings of Rezvani et al. [50].
More importantly, we confirmed that the Cd BCF prediction models from cultivar Zhengdan 958 could be used to predict Cd BCF of other non-model corn species according to the soil pH values through cross-species extrapolation approach. Although many prediction models were developed to describe the relationship between concentration of heavy metals in plants and soil properties in terrestrial ecosystems, these models were only suitable for single species [31]–[32], [58]–[60]. Only a few studies were carried out to develop the prediction models for one species in aquatic ecosystems and subsequently used to normalize ecotoxicity data for other non-model species [33], [38], [39].
In order to further validate the feasibility of the prediction modes developed in the present study for other non-model plant species, the measured Cd BCF values of wheat grain from soils were selected from the paper of Jamali et al. [61], and shown in Table 5. Models presented in Table 3 were applied to these non-model wheat species. The relativity between measured and predicted BCF values for Cd in non-model wheat grains is shown in Figure 5. The ratios between all of the predicted BCF values and the measured BCF values were also within 2 folds interval and close to 1∶1 relationship. In addition, the intra-species variability of Cd BCF of all non-model wheat species was decreased except for Abadgar (Figure 6). This indicates that the models from cultivar Zhendan 958 can be applied not only to non-model corn species but also to non-model wheat species.
Table 5. Soil properties and BCF values for Cd in non-model wheat grain [61].
| BCF | |||||||
| Cultivation soil | pH | OM(%) | CEC(mequiv./100g) | Anomol | Tj-83 | Abadgar | Mehran |
| Soil | 7.53 | 75.2 | 15.0 | 0.241 | 0.332 | 0.266 | 0.417 |
| Sewage | 7.70 | 87.6 | 20.8 | 0.179 | 0.311 | 0.287 | 0.375 |
Figure 5. Relativity between measured and predicted BCF values for Cd in non-model wheat grains.
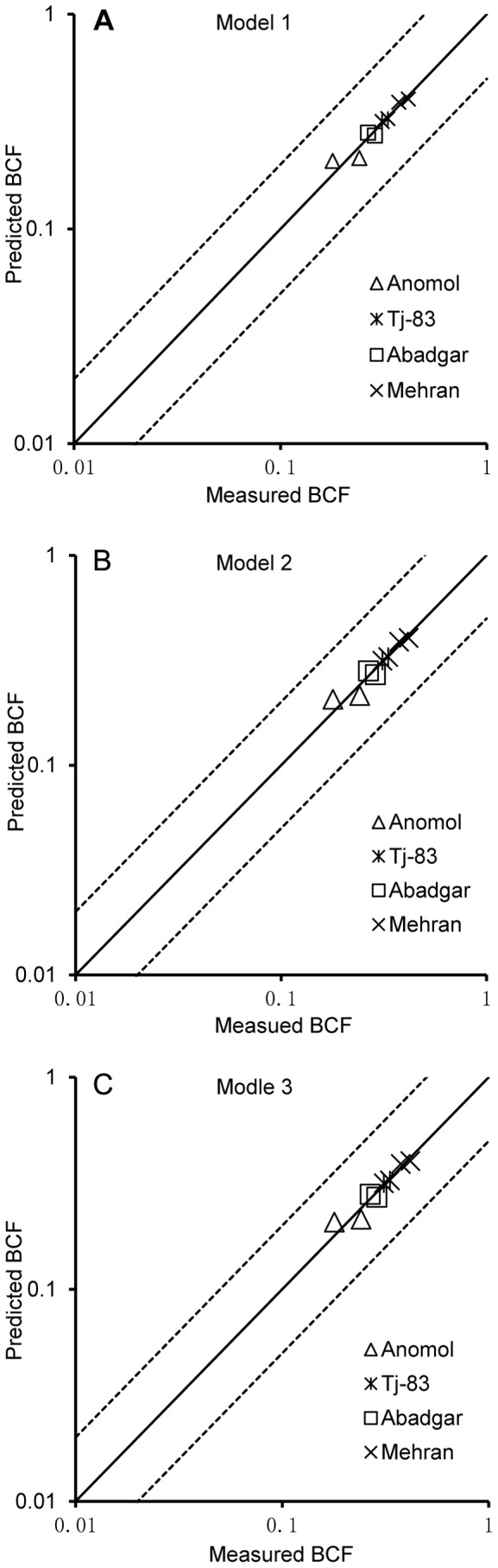
The predicted BCF values in Figure 5 were estimated by (A) model 1, (B) model 2, and (C) model 3 in Table 3. The solid line represents a 1∶1 relationship; the dashed lines indicate a 2-fold prediction interval between the predicted and measured values.
Figure 6. Intra-species variability of BCF for Cd.
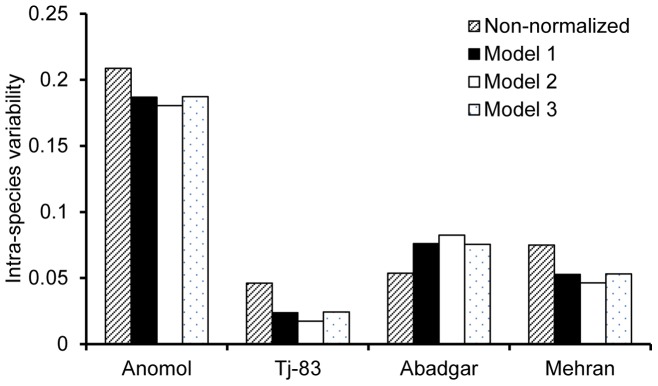
The data from all non-model wheat species were normalized with the models listed in Table 3.
Conclusions
The soil pH was the most important factor that can control Cd uptake and lower pH was more favorable for Cd bioaccumulation in corn grain. The BCF of Cd in corn grain can be predicted by the prediction models such as equation 4. There was no significant difference among three prediction models in the different Cd sources. These prediction models could be applied to other non-model corn species,
| (4) |
Acknowledgments
We are indebted to Prof. Yibing Ma and Dr. Xiaoqing Wang for their help in the data processing and model derivation.
Funding Statement
This work was jointly supported by the Special Fund of Public Industry in China (Agriculture, project No. 200903015; website: http://www.most.gov.cn/) and the Natural Science Foundation of Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences (Project No.2013-9; website:http://www.iarrp.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McLaughlin MJ, Singh BR (1999) Cadmium in soil and plants: a global perspective. Kluwer Academic Publishers, Dordrecht, 13–21.
- 2. Li ZJ, Xu JM, Tang CX, Wu JJ, Muhammad A, et al. (2006) Application of 16S rDNA-PCR amplification and DGGE fingerprinting for detection of shift in microbial community diversity in Cu-, Zn-, and Cd- contaminated paddy soils. Chemosphere 26: 1374–1380. [DOI] [PubMed] [Google Scholar]
- 3. Zhang CB, Huang LN, Luan TG, Jin J, Lan CY (2006) Structure and function of microbial community during the early stages of revegetation near Shaoguan Pb/Zn Smelter, Guangdong, P.R. China. Geoderma 136: 555–565. [Google Scholar]
- 4. Chien CC, Kuo YM, Chen CC, Hung CW, Yeh CH, et al. (2008) Microbial diversity of soil bacteria in agricultural field contaminated with heavy metals. Journal of environmental sciences 20: 359–363. [DOI] [PubMed] [Google Scholar]
- 5. Tudoreanu L, Phillips CJC (2004) Empirical models of cadmium accumulation in maize, rye grass and soya bean plants. J Sci Food Agric 84: 845–852. [Google Scholar]
- 6. Mushtakova VM, Fomina VA, Rogovin VV (2005) Toxic effect of heavy metals on human blood neutrophils. Biol Bull 32: 276–278. [PubMed] [Google Scholar]
- 7. Huang ML, Zhou SL, Sun B, Zhao QG (2008) Heavy metals in wheat grain: Assessment of potential health risk for inhabitants in Kunshan, China. Science of the Total Environment 405: 54–61. [DOI] [PubMed] [Google Scholar]
- 8. Hu JL, Wu FY, Wu SC, Sun XL, Lin XG, et al. (2013) Phytoavailability and phytovariety codetermine the bioaccumulation risk of heavy metal from soils, focusing on Cd-contaminated vegetable farms around the Pearl River Delta, China. Ecotoxicology and Environmental Safety 91: 18–24. [DOI] [PubMed] [Google Scholar]
- 9. Harter RD, Naidu R (2001) Anassessment of environmental and solution parameter impact on trace-metal sorption by soil. Soil Sci Soc Am J 65: 597–612. [Google Scholar]
- 10. Jung MC (2008) Heavy metal concentration in soils and factors affecting metal uptake by plants in the vicinity of a Korean Cu-W mine. Sensors 8: 2413–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang J, Zhang CB, Jin ZX (2009) The distribution and phytoavailability of heavy metal fractions in rhizosphere soils of paulowniu fortune (seem) Hems near a Pb/Zn smelter in Guangdong, PR China. Geodema 148: 299–306. [Google Scholar]
- 12. Chapman EEV, Dave G, Murimboh JD (2013) A review of metal (Pb and Zn) sensitive and pH tolerant bioassay organisms for risk screening of metal-contaminated acidic soils. Environmental Pollution 179: 326–342. [DOI] [PubMed] [Google Scholar]
- 13. Barančíková G, Madaras M, Rybár O (2004) Crop contamination by selected trace elements. Journal of Soils and Sediments 4: 37–42. [Google Scholar]
- 14. Mühlbachová G, Simon T, Pechová M (2005) The availability of Cd, Pb and Zn and their relationships with soil pH and microbial biomass in soils amended by natural clinoptilolite. Plant, Soil and Environment 51: 26–33. [Google Scholar]
- 15. Zhao KL, Liu XM, Xu JM, Selim HM (2010) Heavy metal contaminations in a soil-rice system: identification of spatial dependence in relation to soil properties of paddy fields. Journal of Hazardous Materials 181: 778–787. [DOI] [PubMed] [Google Scholar]
- 16. Sukreeyapongse O, Holm PE, Strobel BW, Magid SJ, Panichsakpatana S, et al. (2002) pH-dependent release of cadmium, copper, and lead from natural and sludge-amended soils. Journal of Environmental Quality 31: 1901–1909. [DOI] [PubMed] [Google Scholar]
- 17. Bang J, Hesterberg D (2004) Dissolution of trace element contaminants from two coastal plain soils as affected by pH. Journal of Environmental Quality 33: 891–901. [DOI] [PubMed] [Google Scholar]
- 18. Badawy SH, Helal MID, Chaudri AM, Lawlor K, McGrath SP (2002) Soil solidphase controls lead activity in soil solution. Journal of Environmental Quality 31: 162–167. [DOI] [PubMed] [Google Scholar]
- 19. Wang AS, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant and Soil 281: 325–337. [Google Scholar]
- 20. Du Laing G, Rinklebe J, Vandecasteele B, Meers E, Tack FMG (2009) Heavy metal mobility and availability in estuarine and riverine floodplain soils and sediments: a review. Science of the Total Environment 407: 3972–3985. [DOI] [PubMed] [Google Scholar]
- 21. Kirkham MB (2006) Cadmiumin plantson polluted soils: Effect of soil factors, hyperaccumulation and amendments. Geoderma 137: 19–32. [Google Scholar]
- 22. Kim KR, Owens G, Naidu R (2009) Heavy metal distribution, bioaccessibility and phytoavailability in long-term contaminated soils from Lake Macquarie, Australia. Aust J Soil Res 47: 166–176. [Google Scholar]
- 23. Zeng F, Ali S, Zhang H, Quyang Y, Qiu B, et al. (2001) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environmental pollution 159: 84–91. [DOI] [PubMed] [Google Scholar]
- 24. Hettiarachchi GM, Ryan JA, Chaney RL, La Fleur CM (2003) Sorption and desorption of cadmium by different fractions of biosolids-amended soils. Journal of Environmental Quality 32: 1684–1693. [DOI] [PubMed] [Google Scholar]
- 25. Antoniadis V, Robinson JS, Alloway BJ (2008) Effects of short-term pH fluctuations on cadmium, nickel, lead, and zinc availability to ryegrass in a sewage sludge-amended field. Chemosphere 71: 759–764. [DOI] [PubMed] [Google Scholar]
- 26. Eriksson JE (1988) The effects of clay, organic matter and time on adsorption and plant uptake of cadmium added to the soil. Water Air Soil Pollut 40: 359–373. [Google Scholar]
- 27. Pinto AP, Mota AM, De Varennes A, Pinto FC (2004) Influence of organic matter on the uptake of cadmium, zinc, copper and iron by sorghum plants. Science of the total environment (326) 239–247. [DOI] [PubMed] [Google Scholar]
- 28. Brown SL, Chaney RL, Angle JS, Ryan JA (1998) The phytoavailability of cadmium to lettuce in long-term biosolids-amended soils. J Environ Qual 27: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 29. Kim SJ, Chang AC, Page AL, Warneke JE (1988) Relative concentrations of cadmium and zinc in tissue of selected food plants grown on sludge-treated soils. Journal of Environmental Quality 17: 568–573. [Google Scholar]
- 30. Ge Y, Murray P, Hendershot WH (2000) Trace metal speciation and bioavailability in urban soils. Environ Pollut 107: 137–144. [DOI] [PubMed] [Google Scholar]
- 31. Li Q, Xu XH, Zuo YB (2009) Effects of biosolid on the accumulation and transfer of trace elements in soil-wheat/maize system. Journal of agro-environment science 28: 2042–2049. [Google Scholar]
- 32.Wang XQ (2012) Ecological Thresholds for Copper and Nickel in Chinese Agricultural Soils, Beijing, China Mining University.
- 33. Schlekat CE, Genderen EV, UGent KDS, Antunes PMC, Rogevich EC, et al. (2010) Cross-species extrapolation of chronic nickel Biotic Ligand Models. Science of the Total Environment 408: 6148–6157. [DOI] [PubMed] [Google Scholar]
- 34. Solomon KR, Baker DB, Richards RP, Dixon KR, Klaine SJ, et al. (1996) Ecological risk assessment of Atrazine in North American surface waters. Environmental Toxicology and Chemistry 15: 31–76. [DOI] [PubMed] [Google Scholar]
- 35. Steen RJCA, Leonards PEG, Brinkman UAT, Barcelo D, Tronczynski J, et al. (1999) Ecological risk assessment of agrochemicals in European estuaries. Environmental Toxicology and Chemistry 18: 1574–1581. [Google Scholar]
- 36. Van Sprang PA, Verdonck FAM, Van Assche F, Regoli L, De Schamphelaere KAC (2009) Environmental risk assessment of zinc in European freshwaters: a critical appraisal. Sci Tot Environ 407: 5373–5391. [DOI] [PubMed] [Google Scholar]
- 37. Allard P, Fairbrother A, Hope BK, Hull RN, Johnson MS, et al. (2010) Recommendations for the development and application of wildlife toxicity reference values. Intergrated environmental assessment and management 6: 28–37. [DOI] [PubMed] [Google Scholar]
- 38. Deleebeeck NME, De Schamphelaere KAC, Janssen CR (2007a) A bioavailability model predicting the toxicity of nickel to rainbow trout (Oncorhynchus mykiss) and fathead minnow (Pimephales promelas) in synthetic and natural waters. Ecotoxicol Environ Saf 67: 1–13. [DOI] [PubMed] [Google Scholar]
- 39. Deleebeeck NME, Muyssen BTA, De Laender F, Janssen CR, De Schamphelaere KAC (2007b) Comparison of nickel toxicity to cladocerans in soft versus hard surface waters. Aquat Toxicol 84: 223–35. [DOI] [PubMed] [Google Scholar]
- 40.Sparks DL, Page AL, Helmke PA, Loeppert RH, Soltanpour PN, et al.. (1996) Methods of Soil Anlysis. Part 3. Chemical Methods. Soil Science Society of America, Inc, Madison. WI.
- 41. Dohrmann R (2006) Cation exchange capacity methodology II: A modified silver–thiourea method. Applied clay science 34: 38–46. [Google Scholar]
- 42. Ball DF (1964) Loss-on-ignition as an estimate of organic matter and organic carbon in noncalcareous soils. Journal of Soil Science 15: 84–92. [Google Scholar]
- 43. Bray RH, Kurtz LT (1945) Determination of total, organic, and available forms of phosphorus in soils. Soil science 59: 39–46. [Google Scholar]
- 44.Bremner JM (1996) Nitrogen total. Methods of soil analysis. Part 3: Chemical methods, American Society of Agronomy-Soil Science Society of America, 1996, 1085–1121.
- 45. Zarcinas BA, McLaughlin MJ, Smart MK (1996) The effect of acid digestion technique on the performance of nebulisation systems used in inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal 27: 1331–1354. [Google Scholar]
- 46. Baker AJM, Reeves RD, Hajar ASM (1994) Heavy metal accumulation and tolerance in British populations of metallophyte Thlaspi caerulescens. New Phytol 127: 61–68. [DOI] [PubMed] [Google Scholar]
- 47. Raskin I, Kumar PBAN, Dushenkov S, Salt DE (1994) Bioconcentration of heavy metals by plants. Curr Opin Biotechnol 5: 285–290. [Google Scholar]
- 48. De Schamphelaere KAC, Heijerick D, Janssen C (2006) Cross-phylum comparison of a chronic biotic ligand model to predict chronic toxicity of copper to a freshwater rotifer, Brachionus calyciflorus (Pallas). Ecotoxicol Environ Saf 63: 189–195. [DOI] [PubMed] [Google Scholar]
- 49. Sarwar N, Saifullah, Malhi SS, Zia MH, Naeem A, et al. (2010) Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric 90: 925–937. [DOI] [PubMed] [Google Scholar]
- 50. Rezvani M, Zaefarian F (2011) Bioaccumulation and translocation factors of cadmium and lead in Aeluropus littoralis. Australian Journal Agricutural Engineering 2: 114–119. [Google Scholar]
- 51. Mertens J, Luyssaert S, Verheyen K (2005) Use and abuse of trace metal concentrations in plants tissue for biomonitoring and phytoextraction. Environ Pollut 138: 1–4. [DOI] [PubMed] [Google Scholar]
- 52. Smical AI, Hotea V, Oros V, Juhasz J, Pop I (2008) Studies on transfer and bioaccumulation of heavy metals from soil into lettuce. Environmental Engineering and Management Journal 7: 609–615. [Google Scholar]
- 53. Christensen TH (1984) Cadmium soil sorption at low concentrations: I. Effect of time, cadmium load, pH, and calcium. Water. Air. Soil Pollut 21: 105–114. [Google Scholar]
- 54. Naidu R, Bolan NS, Kookana RS, Tiller KG (1994) Ionic-strength and pH effects on the sorption of cadmium and the surface charge of soils. Euroean Journal of Soil Science 45: 419–429. [Google Scholar]
- 55. Hinesly TD, Redborg KE, Pietz RI, Ziegler EL (1984) Cadmium and zinc uptake by corn (Zea mays L.) with repeated applications of sewage sludge. J Agric Food Chem 32: 155–163. [Google Scholar]
- 56. Castilho PD, Chardon WJ (1995) Uptake of soil cadmium by three field crops and its prediction by a pH-dependent Freundlich sorption model. Plant Soil 171: 263–266. [Google Scholar]
- 57. Adams ML, Zhao FJ, McGrath SP, Nicholsonb FA, Chambersb BJ (2004) Predicting Cadmium Concentrations in Wheat and Barley Grain Using Soil Properties. Journal of Environmental Quality 33: 532–541. [DOI] [PubMed] [Google Scholar]
- 58. Hao XZ, Zhou DM, Li DD (2012) Growth, cadmium and zinc accumulation of ornamental sunflower (Helianthus annuus L.) in contaminated soil with different amendments. Pedosphere 22: 631–639. [Google Scholar]
- 59. McBride MB (2002) Cadmium uptake by crops estimated from soil total Cd and pH. Soil Sci. 167: 62–67. [Google Scholar]
- 60. Wang XP, Shan XQ, Zhang SZ, Wen B (2004) A model for evaluation of the phytoavailability of trace elements to vegetables under the field conditions. Chemosphere. 55: 811–822. [DOI] [PubMed] [Google Scholar]
- 61. Jamali MK, Kazi TG, Arain MB, Afridi HI, Jalbani N, et al. (2009) Heavy metal accumulation in different varieties of wheat (Triticum aestivum L.) grown in soil amended with domestic sewage sludge. Journal of Hazardous Materials 164: 1386–1391. [DOI] [PubMed] [Google Scholar]