Abstract
Autoimmune diseases are associated with significant morbidity and mortality, afflicting about 5% of the population of the United States. They encompass a wide range of disorders that affect all organs of the human body and have a predilection for women. In the past, autoimmune pathogenesis was not thought to be a major mechanism for cardiovascular disorders, and potential relationships remain understudied. However, accumulating evidence suggests that a number of vascular and cardiac conditions are autoimmune-mediated. Recent studies indicate that autoantibodies play an important role in the development of cardiac arrhythmias, including atrial fibrillation, modulation of autonomic influences on heart rate and rhythm, conduction system abnormalities, and ventricular arrhythmias. This manuscript will review the current evidence for the role of autoantibodies in the development of cardiac arrhythmias.
Keywords: Autoantibodies, cardiac arrhythmias, atrial fibrillation, complete heart block, sudden cardiac death, cardiomyopathy
AUTOIMMUNITY AND AUTOANTIBODIES
Autoimmunity underlies a wide range of disorders, including multisystem diseases such as systemic lupus erythematosus, and organ-specific diseases such as type 1 diabetes mellitus and Graves’ disease 1. The cause of autoimmune disease is poorly understood, but the presence of autoantibodies was long considered a sine qua non of the condition. However, a low level of natural autoantibodies exists in healthy people that is considered physiologic. These natural autoantibodies fulfill diverse housekeeping and surveillance immunological functions, including early innate immune protection, removal of endogenous apoptotic cellular debris, and protection against atherogenesis, cancer, and autoimmune diseases 2, 3.
Specificity, antigenic diversity, and self tolerance are the hallmarks of the adaptive immune system, a vital defense mechanism that involves the interplay of T cells and B cells to produce antibodies against pathogens 4. Production of pathogen-specific antibodies is an elaborate process that is coupled with a host of tolerance mechanisms to avoid self-destruction. When the mechanisms of physiologic tolerance become deranged, autoimmune diseases may ensue (Figure 1). This can occur when autoreactive B cells escape clonal deletion, inhibition by IL-6 and CD40L, receptor editing, anergy, or any of the other intrinsic and extrinsic regulatory mechanisms that normally protect against autoimmunity (Figure 1). Likewise, self-reactive T cells may escape deletion and inactivation, becoming threats to tolerance when they are released into the periphery.
Figure 1.
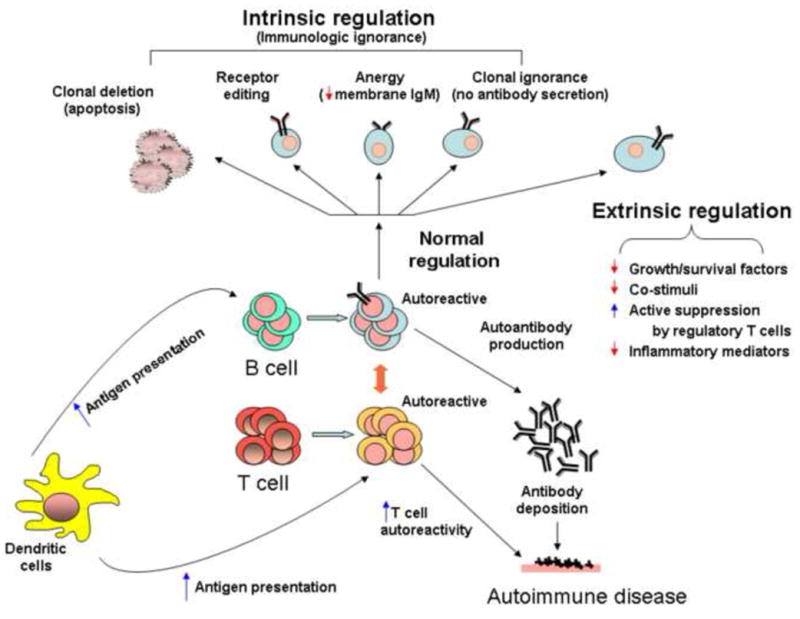
Immunologic tolerance and autoimmune diseases. An illustration showing the normal intrinsic and extrinsic mechanisms of immunologic tolerance. Loss of tolerance results in inappropriate production of autoantibodies and in autoimmune diseases.
AUTOIMMUNE MECHANISMS OF CARDIAC ARRHYTHMIAS
Contrary to past assumptions, emerging evidence indicates that autoantibodies are involved in the development of many cardiovascular disorders 5, 6. Patients with multi-organ autoimmune diseases are at increased risk for developing cardiovascular diseases 7, with increased cardiovascular mortality, reduced cardiac function, and advanced vascular pathology.
In patients with known autoimmune diseases, abnormal electrocardiographic findings are common. For example, a study of 50 patients with progressive systemic sclerosis found that 62% showed serious abnormalities on 24-hour ambulatory electrocardiographic recordings, including supraventricular tachycardia, conduction disturbances, coupled ventricular extrasystoles, and ventricular tachycardia 8. In this review, we will focus on autoantibodies as a direct cause of bradyarrhythmias, conduction system abnormalities, supraventricular tachyarrhythmias, and ventricular tachyarrhythmias (Figure 2).
Figure 2.
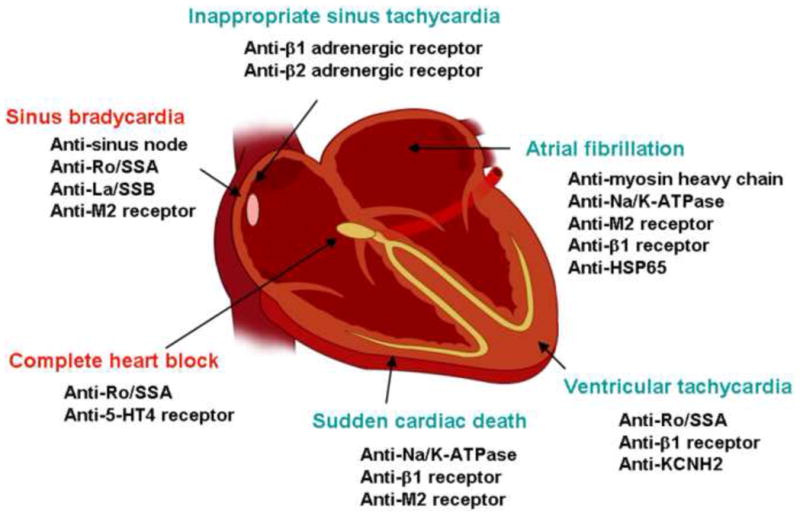
Autoantibodies and cardiac arrhythmias. Autoantibodies known to contribute to the development of bradyarrhythmias (red) and tachyarrhythmias (green) are illustrated here.
BRADYARRHYTHMIAS AND CONDUCTION ABNORMALITIES
Sinus Bradycardia
The role of autoantibodies in causing sinus node dysfunction was first demonstrated in 1986 when Maisch et al. found that antibodies against the human sinus node were present in 29% of 45 patients with sick sinus syndrome and in 24% of 17 patients with bradyarrhythmia 9. Since then, more evidence has emerged supporting the hypothesis that specific antibodies may render sinus nodal function abnormal.
Sinus bradycardia has been reported in a number of fetuses and newborns of women who are positive for anti-Ro/SSA antibodies 9. A review of the medical records from the Research Registry for Neonatal Lupus included 187 children with congenital heart block whose mothers had anti-Ro/SSA-La/SSB antibodies. Sinus bradycardia (<100 bpm) was present in three (3.8%) of 78 fetuses for whom atrial rates were recorded by echocardiogram 10. Autopsy studies in these children showed hypoplastic or absent sinus nodes and the presence of extensive fibrosis 10.
Animal studies lend support to the hypothesis that disease-specific antibodies are responsible for sinus node dysfunction. Passive transfer of purified human IgG containing anti-Ro/SSA and anti-La/SSB antibodies from mothers of children with congenital heart block into timed pregnant mice at 11 days of gestation produced significant sinus bradycardia (56% reduction in sinus rate) in 70% of the newborn mice 8, 10, 11. When Langendorff-perfused rabbit hearts were perfused with IgG containing anti-Ro/SSA and anti-La/SSB antibodies (positive IgG), optical action potential recordings showed that the hearts underwent sinus bradycardia before the development of atrioventricular block 8, 10, 11. Patch clamp recordings of isolated rabbit sinus nodal cells showed significant inhibition of ICa,L and ICa,T but not If or IK upon acute exposure to positive IgG 12 (Figure 3). Current clamp recordings of these cells showed significant slowing of beat frequencies with a decrease in action potential amplitude and a reduction in the slope of phase 4 12. The molecular mechanism underlying sinus bradycardia is thought to be direct binding of autoantibodies to the α1D calcium channel protein, which constitutes the ICa,L in fetal sinus nodal cells and modulates depolarization of the sinus node 13. The inhibitory effects of the positive IgG on ICa,L can be rescued by BayK8644, an activator of ICa,L 13.
Figure 3.
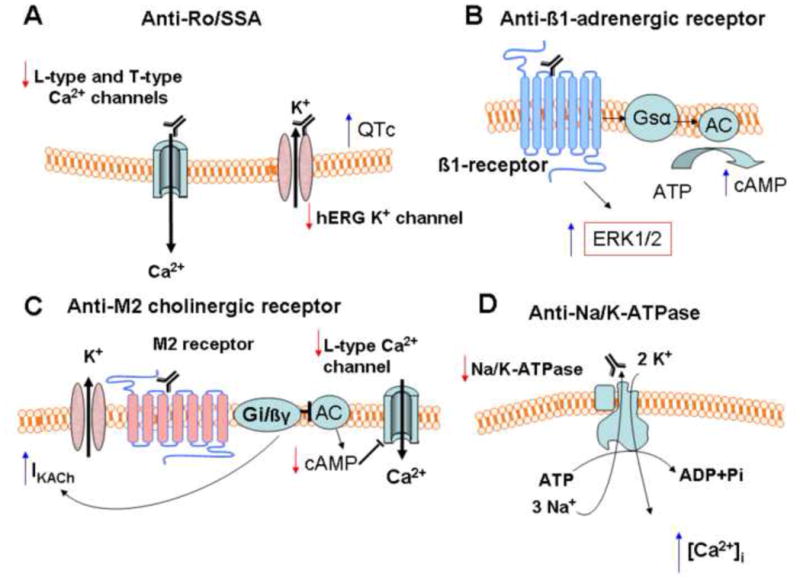
Mechanisms of autoantibody-induced cellular events that lead to the development of cardiac arrhythmias. A Anti-Ro/SSA antibodies. B Anti-β1-adrenergic receptor antibodies. C Anit-M2 muscarinic cholinergic receptor antibodies. D Anti-Na/K-ATPase antibodies.
Meanwhile, in patients with idiopathic dilated cardiomyopathy and Chagas’ disease, it has been demonstrated that agonist-like autoantibodies directed against the muscarinic M2 receptor are related to sinus node dysfunction 14. In Langendorff-perfused rabbit hearts, acute exposure to IgG-rich fractions from sera of chronic Chagasic patients with complex cardiac arrhythmias significantly decreased the heart rate from 131 to 98 bpm, whereas those from chronic Chagasic patients without complex cardiac arrhythmias or from patients with Wolff-Parkinson-White syndrome and control subjects had no effect 15. Similarly, a negative chronotropic effect on spontaneously beating cultured neonatal rat cardiomyocytes was significantly more prevalent using IgG from Chagasic and idiopathic dilated cardiomyopathic patients with sinus node dysfunction than from those who did not have sinus node dysfunction 15.
In both studies, the effects of sera/IgG were blocked by atropine, suggesting that the M2 muscarinic cholinergic receptor was involved. Furthermore, autoantibodies against the M2 muscarinic cholinergic receptor isolated from the sera of Chagasic patients produced significant bradycardia in isolated rat atria by reducing the production of cAMP 15 (Figure 3).
Characterization of the anti-M2 receptor antibodies showed that they dose-dependently shortened the action potential durations of isolated guinea pig ventricular cardiomyocytes, enhancing the outward K+ currents while inhibiting the L-type Ca2+ currents, effects similar to those of carbachol 16. More recently, a group of 14 patients with idiopathic dilated cardiomyopathy were all found to have high titers of antibodies against the M2 receptor and 43% of these sera induced a significant decrease in isoproterenol stimulated L-type Ca2+ currents in rabbit ventricular myocytes, whereas sera from healthy controls failed to do so 17. These results indicate that the anti-M2 receptor antibodies are biologically active with important electrophysiological consequences.
Conduction Abnormalities
Conduction disturbances such as bundle branch block and AV block are common with a 5 to 35% prevalence in patients with autoimmune diseases 11. Antibodies against the cardiac conduction system were found in 25% of patients with progressive systemic sclerosis and in 35% of patients with rheumatoid arthritis, and the presence of these antibodies was correlated with conduction abnormalities in rheumatoid arthritis patients with a p value of <0.001.
Autoantibodies are implicated in several cases of conduction abnormalities. Antibodies against β-adrenergic receptors were found in 35.7% of patients with conduction disturbances and no other cardiac abnormalities 9. Perfusion of a Langendorff mouse heart preparation with anti-β2 adrenergic receptor antisera produced bradycardia and conduction block that were reversible upon washout. 18. This is thought to be mediated via activation of Gi, which may activate G-protein regulated potassium channels, producing membrane hyperpolarization and inhibiting L-type calcium channels 18.
Sera and IgG from chronic Chagasic patients with complex cardiac arrhythmias produced AV block in Langendorff rabbit hearts. These effects were blocked by atropine, suggesting the presence of autoantibodies with muscarinic agonistic function 9. Similarly, IgG from sera of systemic lupus erythematosus patients with anti-Ro/SSA activity produced high grade AV block in Langendorff preparations of rabbit hearts, whereas IgG fractions with anti-RNP activity and from normal sera did not result in AV block 11, 15. Patch clamp studies in rabbit ventricular myocytes showed that the anti-Ro/SSA positive IgG inhibited the L-type Ca2+ current by 31.6%, whereas IgG from control serum had no effect.
Congenital Complete Heart Block
The vast majority of complete heart block diagnosed in utero or within the neonatal period is associated with mothers positive for anti-Ro/SSA, and the risk of congenital complete heart block in pregnant women positive for anti-Ro/SSA antibodies is about 2% 11, 15. In a large multi-center study, anti-Ro/SSA and anti-La/SSB antibodies were identified by ELISA in 100% and 76%, respectively, of mothers of infants with heart block.
Congenital complete heart block is produced by transplacental passage of maternal anti-Ro/SSA antibodies into the fetal circulation. Female mice actively immunized with human recombinant Ro/SSA antigens give birth to offspring with various conduction abnormalities including complete heart block 11. These autoantibodies are known to be arrhythmogenic. When perfused with anti-Ro/SSA antibodies, one third of Langendorff preparations of rabbit hearts exhibited heart block, whereas none exhibited heart block when perfused with control IgG. 11. Anti-Ro/SSA IgG also inhibited the slow inward L-type Ca2+ currents by 31.6% in isolated rabbit ventricular myocytes, suggesting that the antibodies directly modulate the activities of cardiac ion channels. A similar study demonstrated that this inhibition was produced by direct physical interaction between the antibodies and the pore-forming α subunit (α1C) of the L-type Ca2+ channel 13. These results indicate that anti-Ro/SSA antibodies are causally related to the development of complete heart block through inhibition of L-type Ca2+ currents (Figure 3). Chronic exposure to complete heart block-causing autoantibodies results in Ca2+ channel internalization and intracellular Ca2+ dysregulation, followed by progressive destruction of the AV node, leading to its fibrosis and calcification. These structural changes render the disturbance irreversible. The mechanisms and theories linking the cross-reactivity of autoantibodies with Ca2+ channels to the development of complete heart block have been extensively discussed in recent reviews 13, 19.
Bradyarrhythmias, associated autoantibodies, disease conditions, and postulated mechanisms are summarized in Table 1.
TABLE 1.
AUTOANTIBODIES AND BRADYARRHYTHMIAS
| Arrhythmias | Autoantibodies | Associated Diseases | Mechanisms |
|---|---|---|---|
| Sinus Bradycardia | Anti-sinus node | Sick sinus syndrome | |
| Anti-Ro/SSA, Anti-La/SSB | Neonatal lupus syndrome | Cross-reaction with α1D and α1C subunits of T- and L-type Ca2+ channels ↓ ICa,L, ↓ICa,T |
|
| Anti-M2 receptor | Dilated cardiomyopathy and Chagas” disease | Agonist-liked activity on M2 receptor ↓cAMP, ↑ IKACh, ↓ ICa,L |
|
| Conduction Abnormalities | Anti-β1 and anti-β2- adrenergic receptor | Idiopathic conduction disturbances (AV block and intraventricular conduction block) Dilated cardiomyopathy | Agonist-liked activity on β2-adrenergic receptor in conduction tissue ↑ Gi, ↑ GIRK, ↓ ICa,L |
| Anti-M2 receptor | Chagas’ disease | Agonist-liked activity on M2 receptor ↓cAMP, ↑ IKACh, ↓ ICa,L |
|
| Anti-Ro/SSA | Systemic lupus erythematosus | ↓ICa,L, ↓ICa,T | |
| Congenital Complete Heart Block | Anti-Ro/SSA | Neonatal lupus syndrome | Cross-reaction with α1C subunit of L-type Ca2+ channels → L-type Ca2+ channel internalization → [Ca2+]i dysregulation → cell death → inflammation → fibrosis ↓ ICa,L |
ICa,L, L-type calcium currents; ICa,T, T-type calcium currents; IKACh, acetylcholine-activated potassium currents; M2 receptor, muscarinic cholinergic type 2 receptor; Gi, inhibitory G protein; GIRK, G-protein activated inward rectifier K+ channel
TACHYARRHYTHMIAS – SUPRAVENTRICULAR TACHYCARDIAS
Supraventricular tachyarrhythmias are commonly observed in patients with autoimmune diseases, with concrete evidence linking autoantibodies to the development of these arrhythmias in several conditions as described below (Figure 2).
Inappropriate Sinus Tachycardia
Inappropriate sinus tachycardia is characterized by a non-paroxysmal persistent elevated resting heart rate and/or an exaggerated heart rate response to minimal physical activities 20. Understanding of the underlying mechanism of this challenging condition is incomplete, but sympathovagal imbalance and enhanced sinus nodal automaticity are suggested possibilities. Chiale et al. 21 found that anti-β adrenergic receptor antibodies were present in 52% of the patients with inappropriate sinus tachycardia, whereas no antiautonomic receptor antibodies were found in healthy controls. The IgG fractions from the patients induced a significant increase in cAMP with positive chronotropic effects.
Anti-β-adrenergic antibodies have been shown to exhibit agonist properties, apparently through interaction with the second extracellular loop where antigenicity resides 22. Binding of the antibody to the β-adrenergic receptor activates Gsα and adenylyl cyclase, promoting the accumulation of cAMP 21, 22 (Figure 3). This may constitute the basis of sympathetic hypersensitivity in some patients with inappropriate sinus tachycardia. A number of questions remain, but the subclassification of these patients according to the presence of anti-β-adrenergic receptor antibodies may allow targeted therapy based on disease mechanisms.
Atrial Fibrillation
Many factors contribute to the pathogenesis of atrial fibrillation, including electrical, structural, neurohumoral, and inflammatory processes 23. Autoimmune mechanisms are also implicated. Several types of antibodies are associated with atrial fibrillation (Figure 2). The first antibody detected in atrial fibrillation was that against myosin heavy chain 24.These antibodies were previously found in patients with myocarditis and dilated cardiomyopathy. Analysis of sera from 10 patients with paroxysmal atrial fibrillation and 10 age-matched controls showed the presence of immunoreactivity against cardiac myosin heavy chain in six patients versus one control subject, a statistically significant difference. Subsequently, antibodies against the Na/K ATPase 24, 25, the M2-muscarinic cholinergic receptors, the β1 adrenergic receptors, and the heat shock protein (HSP) 65 26 have also been implicated in promoting the development of atrial fibrillation in humans as reviewed below.
The sarcolemmal Na/K-ATPase regulates the intracellular Na+ and Ca2+ concentrations in cardiac myocytes and can affect the resting membrane potential, the inotropic properties, and the arrhythmogenic propensity of the cell 27 (Figure 3). In a cohort of 95 patients with congestive heart failure, antibodies against the α-catalytic subunit of Na/K-ATPase were detected in 16% of patients versus 0% of 48 age-matched controls 16, 26. Further analysis showed that the Na/K-ATPase antibodies were more prevalent in patients with dilated cardiomyopathy than in those with ischemic cardiomyopathy (28% vs. 4%, respectively), and this correlated with the incidence of paroxysmal atrial fibrillation (47% in dilated cardiomyopathy vs. 15% in ischemic cardiomyopathy, p<0.01) 26.
The M2 muscarinic cholinergic receptors mediate parasympathetic signaling in the heart and derangement of vagal tone promotes the development of atrial fibrillation 28. In a study examining the presence of autoantibodies in three age-matched groups of 104 subjects with dilated cardiomyopathy, chronic atrial fibrillation without ventricular dysfunction, and healthy controls, antibodies against the M2 muscarinic cholinergic receptors were present in 40%, 24%, and 8%, respectively 29. In patients with dilated cardiomyopathy, multivariate analysis showed that the presence of M2-receptor autoantibodies was an independent predictor for atrial fibrillation. When injected into chick embryos, the anti-M2 antibodies from patients with dilated cardiomyopathy and atrial fibrillation exerted agonist properties, resulting in negative chronotropic effects and the development of supraventricular arrhythmias 29. Furthermore, antibodies raised against the second extracellular loop of the human M2 muscarinic cholinergic receptors increased outward K+ currents in isolated guinea pig cardiac myocytes, similar to the effects of carbachol 9. These results suggest that the M2-autoantibodies may promote the development of atrial fibrillation through activation of IKACh (Figure 3).
The presence of antibodies against both the β1-adrenergic receptor and the M2 muscarinic cholinergic receptor was a very strong predictor of atrial fibrillation in 38 patients with Graves’ disease, with an odds ratio of 33.61 30. IgG from autoantibody positive patients induced hyperpolarization, decreased action potential duration, enhanced early afterdepolarization formation, and facilitated triggered firing in isolated canine pulmonary veins 30.
Heat shock proteins are stress response elements that function as intracellular chaperones for other proteins to help maintain proper protein folding and conformation. In a prospective study involving 329 patients undergoing elective primary coronary artery bypass grafting surgery, post-op atrial fibrillation developed in 62 patients (19%). Multivariate analysis showed that the presence of anti-HSP65 in the pre-op blood sample was significantly associated with atrial fibrillation, whereas C-reactive protein was not 25.
TACHYARRHYTHMIAS – VENTRICULAR TACHYARRHYTHMIAS
Ventricular Tachycardia
Autoantibodies are implicated in the development of ventricular tachyarrhythmias in several disease states. In patients with connective tissue disease who were positive for anti-Ro/SSA antibodies, 24 hour Holter studies revealed a high incidence of complex ventricular arrhythmias compared to patients who were anti-Ro/SSA negative (50% vs. 10%) in the absence of structural cardiac abnormalities 31. About half of the anti-Ro/SSA positive patients showed QTc interval prolongation, compared to only 0 to 5% of the anti-Ro/SSA negative patients 31. Interestingly, a patient with positive anti-Ro/SSA antibodies who presented with recurrent syncope, markedly prolonged QT and documented torsades de points was found to have abnormal IgGs that cross-react with KCNH2 channel, reducing channel protein expression and KCNH2 currents 32.
Likewise, anti-β-adrenergic antibodies appear to be eminently related to the pathogenesis of cardiomyopathy and the development of arrhythmias 16. Anti-β1-adrenergic autoantibodies were detectable in 35 to 80% of patients with Chagas’ disease 33, idiopathic dilated cardiomyopathy 22, and ischemic cardiomyopathy, but not in those with valvular or hypertensive heart disease. In Chagas’ heart disease, there is cross-reactivity between an antibody to the C-terminal region of the Trypanosoma cruzi ribosomal P2β protein and the second extracellular loop of the human β1 adrenergic receptor 33. In dilated and ischemic cardiomyopathy, autoantibodies interacted with different β1 receptor domains but only those directed against the second extracellular loop were capable of eliciting sympathomimetic effects 22. Inbred rats immunized against the second extracellular β1 receptor loop developed severe progressive left ventricular dilatation and dysfunction 22. In addition to activation of the adenylyl cyclase-cAMP-protein kinase A cascade, more recent studies have shown that in cardiac cells, anti-β1 adrenergic receptor antibodies can also activate the extracellular signal-regulated kinase (ERK)1/2 pathway, promoting cardiac hypertrophy and heart failure 34 (Figure 3).
There is a strong correlation between the presence of anti- β1-adrenergic autoantibodies and ventricular arrhythmias in patients with primary ventricular arrhythmias, idiopathic dilated cardiomyopathy, and Chagas’ disease; no such antibodies were detected in healthy controls 9. Studies have shown a high prevalence of anti-β-adrenergic antibodies in patients with primary cardiac electrical abnormalities as well as in patients with cardiomyopathy and viral hepatitis accompanied by ventricular arrhythmias 9. Application of β-adrenergic receptor antibodies obtained during immunoabsorption of patients with dilated cardiomyopathy to rabbit and human cardiac myocytes resulted in increased spontaneous beating frequency, increased action potential duration, enhanced L-type Ca2+ currents, and augmented contractility 9. Rabbits immunized against the second extracellular loop of the β1-adrenergic receptor showed prolonged action potential durations, possibly from down-regulation of Ito and IKS. These animals had an increased risk of sudden death and sustained ventricular tachycardia, and their cardiac tissues showed prolonged action potential with a higher propensity for induction of early afterdepolarizations 9. These findings strongly suggest that anti-β-adrenergic receptor antibodies promote the development of ventricular arrhythmias.
Sudden Cardiac Death
Using an enzyme-linked immunoabsorbent assay, autoantibodies recognizing the catalytic alpha subunit of the Na/K-ATPase and exerting antagonistic effects were detected in 26% of patients with dilated cardiomyopathy, versus only 2% of healthy controls 35. Ventricular ectopies and non-sustained ventricular tachycardias were significantly more frequent in antibody-positive (81%) versus antibody-negative (32%, p<0.001) patients, as was sudden cardiac death (26.9% vs. 5.4%, p =0.0006 with a hazard ratio of 22.5). Multivariate analysis showed that the presence of autoantibodies against Na/K-ATPase was an independent predictor of sudden cardiac death 35. The mechanism of electrical instability and sudden death by anti-Na/K-ATPase antibodies may resemble that of digitalis toxicity (Figure 3).
Similarly, autoantibodies against the second extracellular loop of β1-adrenergic receptors in patients with idiopathic dilated cardiomyopathy were shown to be associated with multiform ventricular ectopies, ventricular tachycardias, and increased all-cause cardiovascular mortality. Multivariate analysis further showed that the presence of anti-β1 adrenergic receptor antibodies was an independent predictor of sudden cardiac death 36. The underlying causes are many, but QT prolongation and development of triggered activities are implicated as important contributing factors 16.
Sudden cardiac death and serious ventricular arrhythmias are common in patients with Chagas’ disease, and dispersion of QTc intervals has been shown to be an independent predictor of sudden death in Chagas’ patients 37. QT interval dispersion was significantly greater in patients with anti-M2 muscarinic cholinergic receptor antibodies than in those without 37. Sera from anti-M2 antibody-positive patients produced a significant and reversible increase in QT interval in isolated rabbit hearts; this effect was abolished by atropine. Multivariate analysis showed that maximal QTc interval was an independent predictor of sudden cardiac death in these patients 37.
These findings suggest that in patients with cardiomyopathy, the presence of autoantibodies increases the propensity for sudden cardiac death. Cardiac repolarization abnormalities appear to be the common electrophysiological pathway leading to the development of lethal rhythm disturbances.
Tachyarrhythmias and associated autoantibodies and disease conditions as well as postulated mechanisms are summarized in Table 2.
TABLE 2.
AUTOANTIBODIES AND TACHYARRHYTHMIAS
| Arrhythmias | Autoantibodies | Associated Diseases | Mechanisms |
|---|---|---|---|
| Supraventricular Tachyarrhythmias | |||
| Sinus Tachycardia | Anti-β1 and anti-β2 adrenergic receptors | Inappropriate sinus tachycardia | Agonist-liked activity on β-adrenergic receptors, ↑ cAMP, ↑ chronotropic effect |
| Atrial Fibrillation | Anti-myosin heavy chain | Myocarditis, dilated cardiomyopathy, and idiopathic atrial fibrillation | Inflammation |
| Anti-Na/K-ATPase | Dilated cardiomyopathy | ↑ [Ca2+]i | |
| Anti-M2 receptor | Dilated cardiomyopathy, Grave’s hyperthyroidism | ↑ IKACh, ↓ APD, ↓ AERP, ↑ conduction velocity in atrial myocardium | |
| Anti-β1 receptors | Grave’s hyperthyroidism | ↑ hyperpolarization, ↓ APD, ↑ triggered activity | |
| Anti-HSP65 | Post-op CABG | Inflammation | |
| Ventricular Tachyarrhythmias | |||
| Ventricular Tachycardia | Anti-Ro/SSA | Connective tissue diseases | Cross-reaction with hERG K+ channel, ↓ IKr, ↑ QTc |
| Anti-β1-adrenergic receptor | Chagas’ disease, idiopathic dilated cardiomyopathy, ischemic cardiomyopathy, viral hepatitis myocarditis | Agonist-liked activity on β-adrenergic receptor, ↑ cAMP, ↑ chronotopic effects, ↑ APD, ↑ QT, ↑ ICa,L, ↓ Ito, ↓ IKs, ↑ EADs, ↑ ERK1/2 | |
| Sudden Cardiac Death | |||
| Anti-Na/K-ATPase | Dilated Cardiomyopathy | ↑ [Ca2+]i | |
| Anti-β1-adrenergic receptor | Dilated Cardiomyopathy | ↑ APD, ↑ QT, and triggered activity | |
| Anti-M2 receptor | Chagas’ disease | ↑ QT |
ICa,L, L-type calcium currents; IKACh, acetylcholine-activated potassium currents; IKr, rapid delayed rectifier potassium currents; IKS, slow delayed rectifier potassium currents; Ito, transient outward potassium currents; M2 receptor, muscarinic cholinergic type 2 receptor; Na/K-ATPase, sodium-potassium pump; APD, activation potential duration, AERP, atrial effective refractory period; EAD, early afterdepolarizations; [Ca2+]i, intracellular Ca2+.
CONCLUSION
The significance of autoantibodies in the pathogenesis of cardiovascular abnormalities and in the treatment of heart failure is receiving increasing attention. In particular, there is substantial evidence that autoantibodies play an important role in the development of cardiac arrhythmias in a wide range of disease conditions. Recent clinical studies have included autoantibodies against β1-adrenergic receptors as an independent variable in the evaluation of treatment efficacy 38, 39. It is anticipated that further studies will continue to illuminate our understanding of the role of autoantibodies in the development of cardiovascular diseases and cardiac arrhythmias. Autoantibodies are thus a promising therapeutic target in the treatment of autoimmune-mediated arrhythmias.
Acknowledgments
This study was supported by grants from the Mayo Clinic Foundation and from the National Institute of Health (HL74180 and HL080118)
Abbreviations
- AV
atrioventricular
- ICa,L
L-type calcium currents
- ICa,T
T-type calcium currents
- If
hyperpolarization-activated pacemaker currents
- IK
delayed rectifier potassium currents
- IKACh
acetylcholine-activated potassium currents
- IKS
slow delayed rectifier potassium currents
- Ito
transient outward potassium currents
- M2 receptor
muscarinic cholinergic type 2 receptor
- Na/K-ATPase
sodium-potassium pump
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Davidson A, Diamond B. Autoimmune diseases. N Engl J Med. 2001;345:340–350. doi: 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Amital H, Shoenfeld Y. Natural Autoantibodies, Heralding, Protecting and Inducing Autoimmunity. In: Shoenfeld Y, Meroni P-L, Gershwin ME, editors. Autoantibodies. 2. Amsterdam; Boston: Elsevier Science; 2007. pp. 7–12. [Google Scholar]
- 3.Toubi E, Shoenfeld Y. Protective autoimmunity in cancer (review) Oncology Reports. 2007;17:245–251. [PubMed] [Google Scholar]
- 4.Paul WE. Fundamental immunology. 6. Philadelphia: Wolters Kluwer/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 5.Nussinovitch U, Shoenfeld Y. Autoimmunity and heart diseases: pathogenesis and diagnostic criteria. Arch Immunol Ther Exp (Warsz) 2009;57:95–104. doi: 10.1007/s00005-009-0013-1. [DOI] [PubMed] [Google Scholar]
- 6.Nussinovitch U, Shoenfeld Y. Anti-troponin autoantibodies and the cardiovascular system. Heart. 2010;96:1518–1524. doi: 10.1136/hrt.2010.195255. [DOI] [PubMed] [Google Scholar]
- 7.Sitia S, Atzeni F, Sarzi-Puttini P, et al. Cardiovascular involvement in systemic autoimmune diseases. Autoimmun Rev. 2009;8:281–286. doi: 10.1016/j.autrev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Lazzerini PE, Capecchi PL, Guideri F, Acampa M, Galeazzi M, Laghi Pasini F. Connective tissue diseases and cardiac rhythm disorders: an overview. Autoimmun Rev. 2006;5:306–313. doi: 10.1016/j.autrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Lazzerini PE, Capecchi PL, Guideri F, et al. Autoantibody-mediated cardiac arrhythmias: mechanisms and clinical implications. Basic Res Cardiol. 2008;103:1–11. doi: 10.1007/s00395-007-0686-8. [DOI] [PubMed] [Google Scholar]
- 10.Costedoat-Chalumeau N, Amoura Z, Villain E, Cohen L, Piette JC. Anti-SSA/Ro antibodies and the heart: more than complete congenital heart block? A review of electrocardiographic and myocardial abnormalities and of treatment options. Arthritis Res Ther. 2005;7:69–73. doi: 10.1186/ar1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen A, Arnson Y, Dovrish Z, Hadary R, Amital H. Arrhythmias and Conduction Defects in Rheumatological Diseases-A Comprehensive Review. Semin Arthritis Rheum. 2008;39:145–156. doi: 10.1016/j.semarthrit.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Hu K, Qu Y, Yue Y, Boutjdir M. Functional basis of sinus bradycardia in congenital heart block. Circ Res. 2004;94:e32–38. doi: 10.1161/01.RES.0000121566.01778.06. [DOI] [PubMed] [Google Scholar]
- 13.Karnabi E, Boutjdir M. Role of calcium channels in congenital heart block. Scand J Immunol. 2010;72:226–234. doi: 10.1111/j.1365-3083.2010.02439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hernandez CC, Nascimento JH, Chaves EA, et al. Autoantibodies enhance agonist action and binding to cardiac muscarinic receptors in chronic Chagas’ disease. J Recept Signal Transduct Res. 2008;28:375–401. doi: 10.1080/10799890802262319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulze W, Kunze R, Wallukat G. Pathophysiological role of autoantibodies against G-protein-coupled receptors in the cardiovascular system. Exp Clin Cardiol. 2005;10:170–172. [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshikawa T, Baba A, Nagatomo Y. Autoimmune mechanisms underlying dilated cardiomyopathy. Circ J. 2009;73:602–607. doi: 10.1253/circj.cj-08-1151. [DOI] [PubMed] [Google Scholar]
- 17.Del Corsso C, de Carvalho AC, Martino HF, Varanda WA. Sera from patients with idiopathic dilated cardiomyopathy decrease ICa in cardiomyocytes isolated from rabbits. Am J Physiol Heart Circ Physiol. 2004;287:H1928–1936. doi: 10.1152/ajpheart.00044.2004. [DOI] [PubMed] [Google Scholar]
- 18.Escobar AL, Fernandez-Gomez R, Peter JC, Mobini R, Hoebeke J, Mijares A. IgGs and Mabs against the beta2-adrenoreceptor block A-V conduction in mouse hearts: A possible role in the pathogenesis of ventricular arrhythmias. J Mol Cell Cardiol. 2006;40:829–837. doi: 10.1016/j.yjmcc.2006.03.430. [DOI] [PubMed] [Google Scholar]
- 19.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Anti-Ro/SSA antibodies and cardiac arrhythmias in the adult: facts and hypotheses. Scand J Immunol. 2010;72:213–222. doi: 10.1111/j.1365-3083.2010.02428.x. [DOI] [PubMed] [Google Scholar]
- 20.Brady PA, Low PA, Shen WK. Inappropriate sinus tachycardia, postural orthostatic tachycardia syndrome, and overlapping syndromes. Pacing Clin Electrophysiol. 2005;28:1112–1121. doi: 10.1111/j.1540-8159.2005.00227.x. [DOI] [PubMed] [Google Scholar]
- 21.Chiale PA, Garro HA, Schmidberg J, et al. Inappropriate sinus tachycardia may be related to an immunologic disorder involving cardiac beta andrenergic receptors. Heart Rhythm. 2006;3:1182–1186. doi: 10.1016/j.hrthm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Jahns R, Boivin V, Lohse MJ. beta(1)-Adrenergic receptor function, autoimmunity, and pathogenesis of dilated cardiomyopathy. Trends Cardiovasc Med. 2006;16:20–24. doi: 10.1016/j.tcm.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Kourliouros A, Savelieva I, Kiotsekoglou A, Jahangiri M, Camm J. Current concepts in the pathogenesis of atrial fibrillation. Am Heart J. 2009;157:243–252. doi: 10.1016/j.ahj.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Fu M. Autoantibodies in atrial fibrillation: actors, biomarkers or bystanders? How far have we come? Cardiology. 2009;112:178–179. doi: 10.1159/000149151. [DOI] [PubMed] [Google Scholar]
- 25.Baba A, Fu M. Autoantibodies in atrial fibrillation: actor, biomaker or bystander? Autoimmunity. 2008;41:470–472. doi: 10.1080/08916930802031504. [DOI] [PubMed] [Google Scholar]
- 26.Baba A, Yoshikawa T, Chino M, et al. Autoantibodies: new upstream targets of paroxysmal atrial fibrillation in patients with congestive heart failure. J Cardiol. 2002;40:217–223. [PubMed] [Google Scholar]
- 27.Bers DM, Despa S. Na/K-ATPase--an integral player in the adrenergic fight-or-flight response. Trends Cardiovasc Med. 2009;19:111–118. doi: 10.1016/j.tcm.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen PS, Tan AY. Autonomic nerve activity and atrial fibrillation. Heart Rhythm. 2007;4:S61–64. doi: 10.1016/j.hrthm.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baba A, Yoshikawa T, Fukuda Y, et al. Autoantibodies against M2-muscarinic acetylcholine receptors: new upstream targets in atrial fibrillation in patients with dilated cardiomyopathy. Eur Heart J. 2004;25:1108–1115. doi: 10.1016/j.ehj.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Stavrakis S, Yu X, Patterson E, et al. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves’ hyperthyroidism. J Am Coll Cardiol. 2009;54:1309–1316. doi: 10.1016/j.jacc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lazzerini PE, Capecchi PL, Guideri F, et al. Comparison of frequency of complex ventricular arrhythmias in patients with positive versus negative anti-Ro/SSA and connective tissue disease. Am J Cardiol. 2007;100:1029–1034. doi: 10.1016/j.amjcard.2007.04.048. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Katayama Y, Kusano KF, et al. Anti-KCNH2 antibody-induced long QT syndrome: novel acquired form of long QT syndrome. J Am Coll Cardiol. 2007;50:1808–1809. doi: 10.1016/j.jacc.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 33.Labovsky V, Smulski CR, Gomez K, Levy G, Levin MJ. Anti-beta1-adrenergic receptor autoantibodies in patients with chronic Chagas heart disease. Clin Exp Immunol. 2007;148:440–449. doi: 10.1111/j.1365-2249.2007.03381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tutor AS, Penela P, Mayor F., Jr Anti-beta1-adrenergic receptor autoantibodies are potent stimulators of the ERK1/2 pathway in cardiac cells. Cardiovasc Res. 2007;76:51–60. doi: 10.1016/j.cardiores.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 35.Baba A, Yoshikawa T, Ogawa S. Autoantibodies produced against sarcolemmal Na-K-ATPase: possible upstream targets of arrhythmias and sudden death in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40:1153–1159. doi: 10.1016/s0735-1097(02)02075-2. [DOI] [PubMed] [Google Scholar]
- 36.Stork S, Boivin V, Horf R, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152:697–704. doi: 10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Medei E, Pedrosa RC, Benchimol Barbosa PR, et al. Human antibodies with muscarinic activity modulate ventricular repolarization: basis for electrical disturbance. Int J Cardiol. 2007;115:373–380. doi: 10.1016/j.ijcard.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 38.Miao GB, Liu JC, Liu MB, et al. Autoantibody against beta1-adrenergic receptor and left ventricular remodeling changes in response to metoprolol treatment. Eur J Clin Invest. 2006;36:614–620. doi: 10.1111/j.1365-2362.2006.01705.x. [DOI] [PubMed] [Google Scholar]
- 39.Nagatomo Y, Yoshikawa T, Kohno T, et al. A pilot study on the role of autoantibody targeting the beta1-adrenergic receptor in the response to beta-blocker therapy for congestive heart failure. J Card Fail. 2009;15:224–232. doi: 10.1016/j.cardfail.2008.10.027. [DOI] [PubMed] [Google Scholar]


