Abstract
Background
Increasing numbers of survivors of preterm birth are growing into adulthood today. Long-term health-effects of prematurity are still poorly understood, but include increased risk for diabetes, obesity and cardiovascular diseases in adult life. To test if reduced physical fitness may be a link in the causal chain of preterm birth and diseases in later life, the association of preterm birth and adult exercise capacity was investigated. The hypothesis was that preterm birth contributes independently of other risk factors to lower physical fitness in adulthood.
Methods and Findings
Population-based national cohort study of all males conscripting for military service in 1993–2001 and born in Sweden 1973–1983, n = 218,820. Data were retrieved from the Swedish Conscript Register, the Medical Birth Register and the Population and Housing Census 1990. Primary outcome was the results from maximal exercise test (Wmax in Watt) performed at conscription. Association to perinatal and socioeconomic risk factors, other co-variates and confounders were analysed. General linear modelling showed that preterm birth predicted low Wmax in a dose-response related pattern, with 25 Watt reduction in Wmax for the lowest gestational ages, those born ≤27 weeks. Low birth weight for gestational age also independently predicted low Wmax compared to normal and high birth weight (32 Watt reduction for those with a birth weight Standard Deviation Score <2). Low parental education was significantly associated with reduced Wmax (range 17 Watt), as well as both low and high current BMI, with severe obesity resulting in a 16 Watt deficit compared to Wmax top performance.
Conclusion
Being born preterm as well as being born small for gestational age predicts low exercise capacity in otherwise healthy young men. The effect size of being born preterm equal or exceed that of other known risk factors for unfitness in adults, such as low parental education and overweight.
Introduction
Advances in perinatal medicine have dramatically increased survival after preterm birth [1], [2]. Although this progress is very welcome for women delivering preterm and their families, there is an increasing concern that preterm birth may be an emerging risk factor for chronic lung problems [3]–[5], arterial hypertension [6]–[8] and type 2 diabetes [9]–[11], which predicts accelerated aging, cardiovascular disease and early death [12].
Physical Activity (PA) is important for well-being and has a well-documented favourable effect on many of the established risk factors for cardiovascular disease [13]–[15], such as diabetes [15], [16], hypertension [17] and overweight [15], [18]. Most studies report PA as health promoting independently of other factors [13], but the effect may also be mediated through changes in insulin sensitivity [15], physical fitness and body weight [15], [18].
Observational studies indicate that subjects born very or extremely preterm end up less physically active or less resilient to PA in later life [5], [19]–[22], however contemporary and large population-based follow-up studies on PA and exercise capacity in all adult survivors of preterm birth are lacking. Available reports are small [3]–[5], [19], [23], [24], some are old [24], and commonly focus on lung function in childhood [3], [4], [23]–[25] as bronchopulmonary dysplasia is a major morbidity of prematurity that may affect long-term respiratory health and influence exercise capacity. Results are conflicting, some showing decreased [3], [5], [25] and others normal exercise performance at follow-up [24], [26], [27]. Furthermore, several previous studies have used birth weight as a proxy for GA or included subjects according to birth weight [3], [5], [19], [23], resulting in a selection bias towards growth restricted infants. Any contribution to later PA and exercise capacity from fetal growth restriction - which is over-represented in pregnancies ending preterm - remains to be clarified.
We hypothesized that preterm birth is an independent risk factor for reduced exercise capacity in young adults. To test this hypothesis and control for potential confounding from fetal growth restriction, socioeconomic and familial factors, we studied the results of a graded cycling exercise test performed within the assessment for military service in a large, population-based cohort of 18-year-old Swedish men born 1973 through 1983.
Methods
Study Design
This cohort study was based on data from four population-based Swedish registers: the Medical Birth Register (MBR), the Conscript Register, the Population and Housing Census 1990 and the Multigeneration Register. The national registration number, assigned to each Swedish resident at birth, was used for individual record linkage. Linking of data from the different registers was performed by the Central Bureau of Statistics Sweden. The final dataset that was released to the authors and used for analysis was anonymous. Individual consent was not obtained from subjects included in the study and the approving ethical review board waived consent.
The MBR contains data on >99% of all births in Sweden. Starting at the first prenatal visit, information is prospectively collected on standardized forms and forwarded to the register. The MBR has been validated, and the quality was considered high [28].
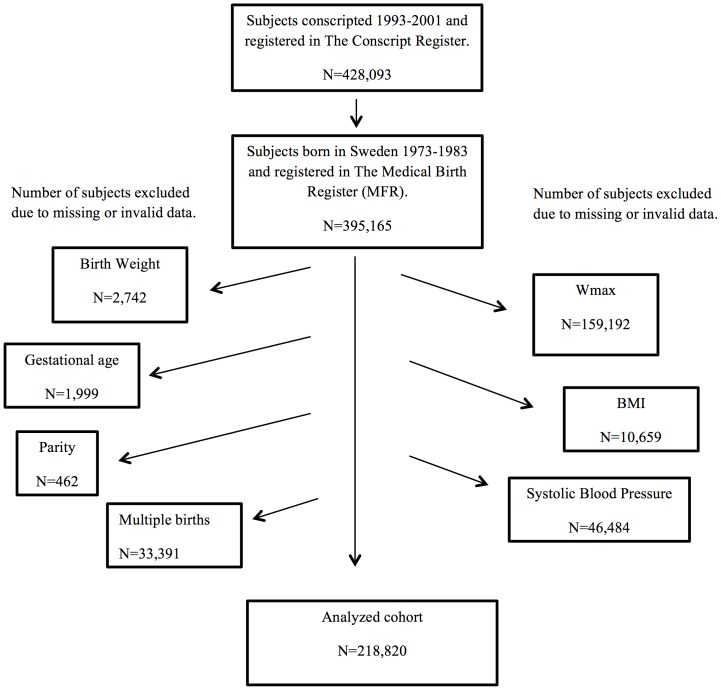
The Conscript Register contains information about young men assessed for military service. Conscription includes physical examination, health assessment and tests of exercise capacity. The results from maximal exercise test on cycle ergometer were used as outcome in this study. All men conscripted for military service in 1993–2001 and born in Sweden 1973–1983 were eligible for the study, but subjects without records of exercise capacity or perinatal risk factors were excluded (Figure 1). During that time period, conscription was mandatory and enforced by law, but men with severe handicaps or congenital malformations generally received an exemption. Other reasons for not being conscripted were moving out of the country, death or conscription during another time period. The study period was chosen because of changes in conscription rules and the availability of perinatal data in the MBR.
Figure 1. Formation of analyzed cohort.
One subject may have missing data in more than one category. Wmax = maximal exercise capacity, BMI = Body Mass Index.
The Population and Housing Census 1990 is a register to which adults in Sweden reported facts about education, profession, household, income and family structure in 1990. It was mandatory for all citizens 16 years and older. The response rate was 97.5% [29].
In The Multigeneration Register blood relationship of all Swedish citizens is registered. It was used to identify mothers and fathers of the conscripts.
Outcome
Exercise capacity was obtained from the Conscript Register and defined as the maximal load (Wmax, expressed in Watt, W) that the conscript could manage on cycle ergometer. Only conscripts with a normal electrocardiography and without diseases or injuries that could influence the results were allowed to perform the test. The test was performed according to the conscription protocol with initial workload determined by weight and estimated physical fitness (125 W for weight 70 kg, presumed average fitness, all levels described in File S1). After 5 min cycling on submaximal load with a pulse between 120 and 170, the load was increased by 25 W every minute as long as the conscript managed. The conscript was instructed to perform his maximum capacity.
Risk Factors, Confounders and Co-variates
Perinatal risk factors were obtained from the MBR. Gestational age (GA) in complete weeks was estimated from the date of the last menstrual period [30]. Subjects with records of GA <22 or >45 weeks were excluded. GA was categorized into 5 groups: extremely preterm (<28 weeks), very preterm (28–31 weeks), moderately preterm (32–36 weeks), term (37–41 weeks) and post term (≥42 weeks). Birth weight (BW) was measured in grams. Values <300 g and >7000 g were judged misclassified and excluded. Birth Weight Standard Deviation Score (BWSDS) was used as a measure for degree of large- and small-for-gestational age. BWSDS was calculated using a Swedish reference for normal fetal growth [31] and BWSDS was divided into five groups: <−2SD, −2SD to <−1SD, −1SD to <+1SD, +1SD to <+2 SD and ≥+2SD. For a baby born at 30 weeks GA a difference in BWSDS from −2SD to +2SD would mean a difference in birth weight from 1200 g to 2100 g. BWSDS exceeding ±6SD were excluded.
In addition, the following variables from the MBR were obtained: maternal age (<20, 21–25, 26–30, 31–35, 36–39, >40 years), maternal origin (born in Sweden, other Nordic countries, other European countries, Asia or other countries including North America, South America, Africa and Oceania), parity (primi- or multipara) and singleton or multiple birth.
Body Mass Index (BMI <18, 18–24.9, 25–29.9, 30–35, >35 kg/m2) and blood pressure (mmHg) - measured in the right arm after 5 to 10 minutes rest in the supine position - were collected from the conscript register.
A health assessment was performed by a physician at conscription. Based on physical examination, previous and current medical history, the conscripts were categorized in 12 health levels with A being the highest category. Level A represents fully healthy individuals without any minor health problem. Every lower level adds one or more health problem from mild allergies to asthma and severe diseases (described in detail in File S2).
Income of parents, including salary and income from finances, business and land or forest area, was retrieved from the Population and Housing Census 1990. Income was grouped in quartiles, the first quartile was considered as low, second and third as average and fourth quartile as high income. In this study, the highest income of mother or father was used.
The parental educational level of the conscript was reported in 8 levels and defined as the highest of mother or father. Socioeconomic index (SEI) categorizes people according to occupation. There are 15 index categories ranging from leading position, farmers and workers to non-occupied. Each index category has a dominance in relation to the others that is defined based on the expected impact on the child’s SEI [32]. In the study a subjects’ SEI was set as the most dominant SEI of the parents.
Statistical Methods
All data were presented using descriptive statistics, i.e. number of subjects, mean and standard deviation for continuous variables, and frequency and relative frequency (percentage) for categorical variables. Data were analysed using General Linear Model, Analysis of Variance, ANOVA, with gestational age, BWSDS, number of births, maternal country of birth, maternal age, parity, parental education, socioeconomic index, parental income, BMI, blood pressure, health status and age at conscription as fixed factors in univariate models and multivariate models. Least square means was used to calculate the point estimates in the multivariate model and thereby control for co-variates and evaluate the independent contribution by each factor to the outcome.
All tests were two-sided and p<0.05 was regarded as statistical significant. Analyses were performed using the STATISTICA software, version 9.0, Statsoft Inc., Tulsa, US.
Results
Population Characteristics
During the 1993 to 2001 period 428,092 men conscripted for military service, of who 395,164 (median age 18, range 18–26 years) were born in Sweden 1973–1983, comprising 71% of all male births these years. Most of the men born these years and not included in the study were conscripted during another period of time. After exclusion of subjects with missing or misclassified data, the analysed cohort consisted of 218,820 men (Figure 1). A comparison between the characteristics of conscripts in the study cohort, who performed the maximal exercise capacity test and non-eligible subjects, is presented in Table 1, 2 and 3. The most striking finding was the significantly lower proportion with full health (A-status) among men who did not perform the exercise test (Table 3).
Table 1. Perinatal characteristics of analyzed cohort vs. non-eligible subjects.
| Analyzed cohort | Non-eligible | |||
| N = 218,820 | N = 209,273 | |||
| N | % | N | % | |
| Gestational age (weeks) | ||||
| ≤27 | 56 | <0.1 | 109 | <0.1 |
| 28–31 | 726 | 0.3 | 684 | 0.3 |
| 32–36 | 9,930 | 4.5 | 8,738 | 4.2 |
| 37–41 | 182,490 | 83.4 | 146,487 | 70.0 |
| ≥42 | 25,618 | 11.7 | 18,327 | 8.8 |
| Missing | 0 | 0.0 | 34,928 | 16.7 |
| Birth Weight SDS | ||||
| <−2 | 7,052 | 3.2 | 5,418 | 2.6 |
| −2 to <−1 | 35,747 | 16.3 | 27,536 | 13.1 |
| −1 to <+1 | 147,819 | 67.6 | 117,797 | 56.3 |
| +1 to <+2 | 22,970 | 10.5 | 18,473 | 8.8 |
| ≥+2 | 5,232 | 2.4 | 4,378 | 2.1 |
| Missing | 0 | 0 | 35,671 | 17.1 |
| Multiple or single birth | ||||
| Singleton | 215,055 | 98.3 | 173,026 | 82.7 |
| Twins or N-tuplets | 3,765 | 1.7 | 2,856 | 1.4 |
| Missing | 0 | 0 | 33,391 | 15.9 |
| Maternal country of birth | ||||
| Sweden | 201,160 | 91.9 | 190,843 | 91,2 |
| Other Nordic countries | 9,723 | 4.4 | 8,944 | 4.3 |
| Europe | 5,430 | 2.5 | 5,250 | 2.5 |
| Asia | 1,252 | 0.6 | 2,492 | 1.2 |
| Other | 820 | 0.4 | 1,344 | 0.6 |
| Missing | 435 | 0.2 | 400 | 0.2 |
| Maternal age (years) | ||||
| ≤19 | 11,749 | 5.4 | 8,678 | 4.1 |
| 20–24 | 62,074 | 28.4 | 47,169 | 22.5 |
| 25–29 | 84,041 | 38.4 | 64,287 | 30.7 |
| 30–34 | 46,104 | 21.1 | 40,591 | 19.4 |
| 35–39 | 12,955 | 5.9 | 13,195 | 6.3 |
| ≥40 | 1,897 | 0.9 | 1,962 | 0.9 |
| Missing | 0 | 0 | 33,391 | 16.0 |
| Parity (number) | ||||
| 1 | 92,902 | 42.5 | 71,302 | 34.1 |
| ≥2 | 125,918 | 57.5 | 104,580 | 50.0 |
| Missing | 0 | 0 | 33,391 | 16.0 |
Table 2. Parental characteristics of analyzed cohort vs. non-eligible subjects.
| Analyzed cohort | Non-eligible | ||||
| N = 218,820 | N = 209,273 | ||||
| N | % | N | % | ||
| Parental education | |||||
| Post-graduate | 2,838 | 1.3 | 2,506 | 1.2 | |
| Post-secondary ≥3 years | 41,479 | 19.0 | 34,392 | 16.4 | |
| Post-secondary <3 years | 36,947 | 16.9 | 30,525 | 14.6 | |
| Upper secondary 3 years | 31,989 | 14.6 | 26,625 | 12.7 | |
| Upper secondary ≤2 years | 59,986 | 27.4 | 58,421 | 27.9 | |
| Primary/secondary 9–10 years | 24,511 | 11.2 | 24,475 | 11.7 | |
| Primary/secondary <9 years | 12,247 | 5.6 | 11,424 | 5.5 | |
| Unspecified | 8,823 | 4.0 | 20,900 | 10.0 | |
| Missing | 0 | 0 | 5 | <0.1 | |
| Socio Economic Index (SIE) | |||||
| Self-employed professionals | 10,111 | 4.6 | 7,146 | 3.4 | |
| Professionals/higher non-manual employees | 35,948 | 16.4 | 28,910 | 13.8 | |
| Entrepreneurs | 16,871 | 7.7 | 14,848 | 7.1 | |
| Intermediate non-manual employees | 52,830 | 24.1 | 40,977 | 19.6 | |
| Assistant non-manual employees, lower level | 19,788 | 9.0 | 16,103 | 7.7 | |
| Assistant non-manual employees, higher level | 6,438 | 2.9 | 5,646 | 2.7 | |
| Skilled employees in goods production | 27,978 | 12.8 | 26,750 | 12.8 | |
| Skilled employees in service production | 7,675 | 3.5 | 7,668 | 3.7 | |
| Unskilled employees in goods production | 12,032 | 5.5 | 13,557 | 6.5 | |
| Unskilled employees in service production | 15,020 | 6.9 | 16,939 | 8.1 | |
| Farmers | 5,638 | 2.6 | 4,062 | 1.9 | |
| Non-classified employees | 4,155 | 1.9 | 5,565 | 2.7 | |
| Non-active gainfully employed | 2,865 | 1.3 | 7,522 | 3.6 | |
| Missing | 1,471 | 0.7 | 13,580 | 6.5 | |
| Parental Income | |||||
| High | 96,400 | 44.1 | 72,258 | 34.5 | |
| Medium | 104,237 | 47.6 | 97,511 | 46.6 | |
| Low | 10,959 | 5.0 | 18,054 | 8.6 | |
| Missing | 7,224 | 3.3 | 21,450 | 10.2 | |
Table 3. Characteristics at conscription - analyzed cohort vs. non-eligible subjects.
| Analyzed cohort | Non-eligible | |||
| N = 218,820 | N = 209,273 | |||
| N | % | N | % | |
| Body Mass Index (BMI intervals) | ||||
| <18 | 6,400 | 2.9 | 7,275 | 3.5 |
| 18–24.9 | 180,691 | 82.6 | 125,242 | 59.9 |
| 25–29.9 | 26,824 | 12.3 | 24,461 | 11.7 |
| 30–34.9 | 4,225 | 1.9 | 6,472 | 3.1 |
| ≥35 | 680 | 0.3 | 2,236 | 1.0 |
| Missing | 0 | 0 | 43,587 | 20.8 |
| Systolic Blood Pressure (mmHg) | ||||
| –99 | 466 | 0.2 | 431 | 0.2 |
| 100–119 | 37,070 | 16.9 | 23,809 | 11.4 |
| 120–139 | 136,096 | 62.2 | 79,560 | 38.0 |
| 140–159 | 44,148 | 20.2 | 25,094 | 12.0 |
| 160–179 | 960 | 0.4 | 885 | 0.4 |
| 180– | 80 | <0.1 | 82 | <0.1 |
| Missing | 0 | 0 | 79,412 | 37.9 |
| Health Status | ||||
| A | 128,081 | 58.5 | 45,997 | 22.0 |
| B | 14,564 | 6.7 | 9,304 | 4.4 |
| D | 32,121 | 14.7 | 18,973 | 9.0 |
| E | 11,210 | 5.1 | 16,589 | 8.0 |
| J | 7,848 | 3.6 | 15,830 | 7.6 |
| JC | 98 | <0.1 | 5,498 | 2.6 |
| Y | 20,921 | 9.6 | 76,664 | 36.6 |
| Z | 750 | 0.3 | 3,139 | 1.5 |
| Missing | 3227 | 1.5 | 17,281 | 8.3 |
Determinants of Exercise Capacity
The overall mean exercise capacity in young Swedish men was 306 W +/−50 SD. In the univariate analysis (ANOVA) there was a linear increase in exercise capacity with increasing GA (from 278 W in men born extremely preterm to 307 W in men born at term, p<0.001, Table 4). Exercise capacity also increased with increasing BWSDS (from 287 W in men with BW <−2 SD to 322 W in men with BW ≥+2 SD, p<0.001, Table 2A). Other factors affecting the outcome of maximal exercise capacity in univariate analyses were maternal country of birth, maternal age, multiple births, parental education, socioeconomic index and income of the parents, as well as the conscripts current BMI and systolic blood pressure (Table 4, 5, 6 and 7). All results in the univariate analysis were statistically significant, p<0.001.
Table 4. Maximal exercise capacity by perinatal characteristics (univariate analysis).
| Wmax (Watt) | |||||
| N | Mean | SD | P | ||
| Multiple births | <0.001 | ||||
| Singleton | 215,055 | 306 | 50 | ||
| Twins or N-tuplets | 3,765 | 305 | 50 | ||
| Gestational age (weeks) | <0.001 | ||||
| ≤27 | 56 | 278 | 55 | ||
| 28–31 | 726 | 292 | 47 | ||
| 32–36 | 9,930 | 302 | 49 | ||
| 37–41 | 182,490 | 307 | 50 | ||
| ≥42 | 25,618 | 306 | 51 | ||
| Birth Weight SDS | <0,001 | ||||
| <−2 | 7,052 | 287 | 49 | ||
| −2 to −1 | 35,747 | 296 | 49 | ||
| −1 to 1 | 147,819 | 308 | 49 | ||
| 1 to 2 | 22,970 | 318 | 51 | ||
| >2 | 5,232 | 322 | 52 | ||
SDS = Standard Deviation Score.
Table 5. Maximal exercise capacity by maternal characteristics (univariate analysis).
| Wmax (Watt) | ||||
| N | Mean | SD | P | |
| Maternal country of birth | <0.001 | |||
| Sweden | 201,160 | 307 | 50 | |
| Other Nordic countries | 9,723 | 301 | 49 | |
| Europe | 5,430 | 301 | 51 | |
| Asia | 1,252 | 288 | 48 | |
| Unknown | 435 | 294 | 52 | |
| Other | 820 | 299 | 49 | |
| Maternal Age (years) | <0.001 | |||
| –19 | 11,749 | 296 | 51 | |
| 20–24 | 62,074 | 303 | 50 | |
| 25–29 | 84,041 | 309 | 50 | |
| 30–34 | 46,104 | 309 | 50 | |
| 35–39 | 12,955 | 307 | 50 | |
| 40– | 1,897 | 304 | 49 | |
| Parity (number) | <0.001 | |||
| 1 | 92,902 | 307 | 50 | |
| 2+ | 125,918 | 306 | 50 | |
Table 6. Maximal exercise capacity by parental characteristics (univariate analysis).
| Wmax (Watt) | ||||
| N | Mean | SD | P | |
| Parental education | <0.001 | |||
| Post-graduate | 2,838 | 316 | 48 | |
| Post-secondary ≥3 years | 41,479 | 316 | 48 | |
| Post-secondary <3 years | 36,947 | 314 | 49 | |
| Upper secondary 3 years | 31,989 | 307 | 49 | |
| Upper secondary ≤2 years | 59,986 | 302 | 50 | |
| Primary/secondary 9–10 years | 24,511 | 298 | 50 | |
| Primary/secondary <9 years | 12,247 | 292 | 52 | |
| Unspecified | 8,823 | 298 | 51 | |
| Socio Economic Index (SIE) | <0.001 | |||
| Self-employed professionals | 10,111 | 315 | 49 | |
| Professionals/higher non-manual employees | 35,948 | 315 | 48 | |
| Entrepreneurs | 16,871 | 304 | 50 | |
| Intermediate non-manual employees | 52,830 | 312 | 49 | |
| Assistant non-manual employees, lower level | 6,438 | 301 | 49 | |
| Assistant non-manual employees, higher level | 19,788 | 307 | 50 | |
| Skilled employees in goods production | 27,978 | 300 | 51 | |
| Skilled employees in service production | 7,675 | 303 | 49 | |
| Unskilled employees in goods production | 12,032 | 295 | 51 | |
| Unskilled employees in service production | 15,020 | 296 | 50 | |
| Farmers | 4,155 | 300 | 50 | |
| Non-classified employees | 5,638 | 304 | 49 | |
| Non-active gainfully employed | 2,865 | 289 | 51 | |
| Missing data | 1,471 | 299 | 47 | |
| Parental income | <0.001 | |||
| High | 96,400 | 313 | 49 | |
| Medium | 104,237 | 302 | 50 | |
| Low | 10,959 | 296 | 50 | |
| 7,224 | 298 | 51 | ||
Table 7. Maximal exercise capacity by conscript characteristics (univariate analysis).
| Wmax (Watt) | ||||
| N | Mean | SD | P | |
| Body Mass Index (BMI intervals) | <0.001 | |||
| −18 | 43,485 | 273 | 44 | |
| 18–25 | 143,606 | 313 | 47 | |
| 25–30 | 26,824 | 324 | 51 | |
| 30–35 | 4,225 | 314 | 51 | |
| 35– | 680 | 307 | 58 | |
| Systolic Blood Pressure (mmHg) | <0.001 | |||
| –99 | 466 | 293 | 51 | |
| 100–119 | 37,070 | 297 | 52 | |
| 120–139 | 136,096 | 307 | 50 | |
| 140–159 | 44,148 | 313 | 48 | |
| 160–179 | 960 | 317 | 50 | |
| 180– | 80 | 305 | 51 | |
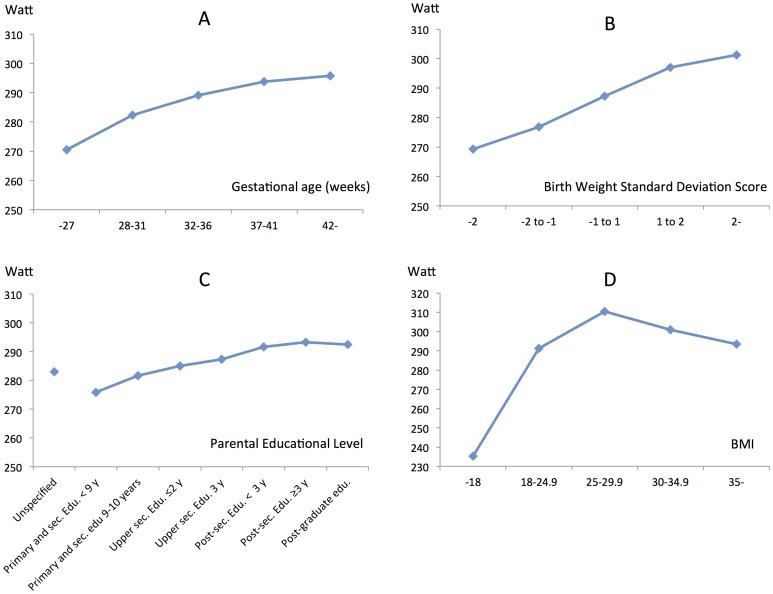
In the adjusted results from the multivariate analyses using general linear modelling, all levels of risk factors and categories of co-variates had significantly different effect on exercise capacity (p<0.001). The largest impact on Wmax was found for GA, BWSDS, educational level of parents and current BMI (Table 8). Least square means for exercise capacity increased with increasing GA (≤27∶271 W, 28–31∶282 W, 32–36∶289 W, 37–41∶294 W, ≥42∶296 W, Table 8, Figure 2). Exercise capacity increased with BWSDS as follows: <−2SD: 269 W, −2 to −1∶277 W, −1 to +1∶287 W, +1 to +2∶297 W, >+2∶301 W (Table 8, Figure 2). The interactive effect of low gestational age and low birth weight for gestational age was tested and found to be low. Maximal exercise capacity increased with higher parental educational level, p<0.001 (Table 8, Figure 2). There was an association between BMI at conscription and the results on cycle ergometer (Table 8). The highest exercise capacity was achieved by conscripts with a BMI 25–29.9 (LS mean 310 W) followed by the conscripts with a BMI 18–24.9 (LS mean 291 W). The lowest results were achieved by subjects being underweight, with a BMI≤18 (LS mean 235 W) (Table 8, Figure 2). The BMI, body weight and height at conscription according to gestational age categories are listed in File S3.
Table 8. Maximal exercise capacity and major risk factors (General Linear Modeling).
| LSMean | SE | 95%LL | 95%UL | N | P | |
| Gestational age (weeks) | <0.001 | |||||
| ≤27 | 271 | 6 | 258 | 283 | 56 | |
| 28–31 | 282 | 2 | 278 | 287 | 726 | |
| 32–36 | 289 | 1 | 287 | 292 | 9,93 | |
| 37–41 | 294 | 1 | 291 | 296 | 182,49 | |
| ≥42 | 296 | 1 | 293 | 298 | 25,618 | |
| Birth Weight SDS | <0.001 | |||||
| <−2 | 269 | 2 | 266 | 273 | 7,052 | |
| −2 to −1 | 277 | 2 | 273 | 280 | 35,747 | |
| −1 to 1 | 287 | 2 | 284 | 291 | 147,819 | |
| 1 to 2 | 297 | 2 | 293 | 301 | 22,970 | |
| >2 | 301 | 2 | 297 | 305 | 5,232 | |
| Parental education | <0.001 | |||||
| Post-graduate | 292 | 2 | 288 | 296 | 2,838 | |
| Post-secondary ≥3 years | 293 | 2 | 290 | 297 | 41,479 | |
| Post-secondary <3 years | 292 | 2 | 288 | 295 | 36,947 | |
| Upper secondary 3 years | 287 | 2 | 284 | 291 | 31,989 | |
| Upper secondary ≤2 years | 285 | 2 | 281 | 289 | 59,986 | |
| Primary/secondary 9–10 years | 282 | 2 | 278 | 285 | 24,511 | |
| Primary/secondary <9 years | 276 | 2 | 272 | 280 | 12,247 | |
| Unspecified | 283 | 2 | 279 | 287 | 8,823 | |
| Body Mass Index (BMI intervals) | <0.001 | |||||
| <18 | 235 | 2 | 232 | 239 | 6,400 | |
| 18–25 | 291 | 2 | 288 | 295 | 180,691 | |
| 25–30 | 310 | 2 | 307 | 314 | 26,824 | |
| 30–35 | 301 | 2 | 297 | 305 | 4,225 | |
| >35 | 294 | 3 | 289 | 298 | 680 |
Adjusted results expressed as Least Square Mean (LS Mean). SDS = Standard Deviation Score.
Figure 2. Maximal exercise capacity.
Adjusted results expressed as least squared means for maximal exercise capacity (Watt) in relation to the major risk factors. A - Gestational Age categories, B - Birth Weight Standard Deviation Score, C – Education level of the parents and D - BMI. Sec edu. = Secondary education, y = years, BMI = Body Mass Index.
Discussion
This is the first study to report that preterm birth predicts reduced exercise capacity in young healthy men, also after accounting for fetal growth. The association to preterm birth was found to be independent of other determinants of adult exercise capacity such as parental education, socioeconomic status and income, as well as of current BMI. The association was not limited to those men born very or extremely preterm, instead exercise capacity increased linearly with GA in a dose-response related manner. The effect size of being born preterm equalled or exceeded that of other known risk factors for unfitness in young adults, such as low parental education [33] and overweight [14].
The strengths of this study were the population-based design and the long-term follow-up, where prospectively collected data at birth could be linked to outcome 18–25 years later. The study was also large enough to assess inter-relationships between perinatal and other major determinants (parental education, socioeconomic status, income and current BMI) of exercise capacity in adulthood, while adjusting for maternal origin and multiple births. Importantly, we had sufficient power to separate the effects of preterm birth and fetal growth restriction on later exercise capacity. Low BWSDS - a proxy for poor fetal growth - was also found to predict low exercise capacity in young men. Accordingly, preterm birth is a perinatal risk factor for low exercise capacity in males later in life, even when taking fetal growth into account.
We have previously found that the conscription rate varied across the GA range, with the lowest conscription rate among men born extremely preterm [7]. Moreover, not all men who underwent conscription performed the exercise test. Comparing men who performed the exercise test with those who did not, the most striking finding was the significantly lower proportion with full health (A-status) among men who did not perform the exercise test (Table 3). This means that the conscripts who performed the maximal exercise test was a selection of the healthiest, which may in fact lead to an underestimation of the effect of preterm birth on adult exercise capacity in young men, particularly if applied to a more contemporary and unselected cohort of preterm survivors. Neonatal survival after preterm birth in 1973–1983 was lower than today and the neonatal period following prematurity was likely to be more traumatic. Hence, the effect of preterm birth on long-term fitness could have been expected to be greater than what we found in the present study. In view of the improvement of medical treatment in the neonatal period over time, the question has to be raised if the results of the present study are applicable on individuals born preterm in more recent years. Several circumstances indicate that the results can be expected to hold true. First, the survivors of prematurity in this study are likely to have been the healthiest, comparable to those with an uncomplicated neonatal course today. Second, exercise capacity is lower also in subjects born moderately preterm, a group less likely to require full intensive care. Third, there are several underlying physiological mechanisms irrespective of medical treatment that may explain a lower level of physical fitness in subjects born preterm [34]–[38]. As conscription for military service is no longer compulsory in Sweden after 2001, it is not possible to replicate this study in subjects born after 1983.
Yet another aspect that may affect the outcome on exercise capacity in subjects born preterm is psychological function. Subjects born preterm have been shown to have psychological difficulties to a greater extent than term subjects [39], but the effects of difficulties during up-bringing, neurodevelopmental and social aspects certainly warrants further studies. The gender aspect is the most obvious limitation of this study and by the design the conclusions can only with certainty be applied to males. We can only speculate about the result in females. Some studies have shown lower morbidity after preterm birth in females than in males [40], suggesting that if exercise capacity was tested in a population of both males and females the difference between those born term and preterm would be less pronounced. Other important limitations of this study include the lack of data on neonatal morbidity, infant nutrition, postnatal growth and smoking habits (of mothers and conscripts), which all could have had an impact on health outcomes after preterm birth. Given that smoking is more common in families with low education and socioeconomic index [41], which were included in our models, some of the effect of smoking on exercise capacity is corrected for in our model.
Our data demonstrate a linear relationship between preterm birth and exercise capacity, suggesting but not proving causality. Underlying mechanisms could be that preterm birth stops normal development and triggers adaptations in tissues and organs, ultimately resulting in lasting physiological alterations such as fewer alveoli [34], lower capillary density [35] and smaller vascular tree [36] - all impairing maximal oxygen uptake and transport - as well as lower leg power and coordination problems [19]. Cardiac function and performance could be involved, as indicated by remodeling of the heart in lambs and young adults born preterm [37]. Furthermore, a common genetic explanation for both preterm birth and low exercise capacity cannot be excluded. Finally, variations in behavior and motivation may clearly play a role for the result of the cycle ergometer test [20].
In this study the difference in maximal work load between male subjects born at term (306 W) and those born extremely preterm (278 W) is comparable to the difference in maximal aerobic capacity for an average man and an average woman [42]. The size of the difference would be clearly notable if two subjects were performing simultaneously. PA is often associated with some kind of competition. One could speculate that a child or adolescent not being able to perform at the same level as his peers may choose other activities, leading to a vicious circle of lower PA and lower exercise capacity. The level of fitness or PA has been shown to affect all-cause mortality [14], [43], risk of diabetes [16] and obesity [18], indicating that the lower level of fitness in young adults born preterm may be a link in the causal chain against morbidity in later life. Interestingly, higher systolic blood pressure in our cohort of young healthy males did not appear to be associated with reduced exercise capacity – a finding that may need to be explored further.
Preterm birth is a public health problem affecting between 6–12% [44] of all pregnant women. So far, the rates of this pregnancy complication have not declined, if anything, the opposite has been observed. Advances in perinatal medicine have, however, contributed to high survival rates after preterm birth today and have also significantly lowered the gestational age of viability [2]. The concern is now that increased survival may come at the cost of later morbidity. Young Swedish men and women born preterm have recently been shown to suffer from an increased mortality from cardiovascular and pulmonary causes [12]. The underlying mechanisms are poorly understood but in this context, our findings add to decrease this gap in knowledge. Preterm birth is associated both with low exercise capacity and high blood pressure [7], [8] and glucose intolerance [9], [10], risk factors for diabetes and cardiovascular diseases. The lower exercise capacity may be among the contributing factors for persons born preterm to develop these diseases.
Being born small was also associated with lower exercise capacity in later life. This finding was independent of gestational age and suggests that the effect of intrauterine growth restriction on future exercise capacity is similar after term as well as preterm birth. It also underlines the fact that to avoid significant health problems in coming generations [45], providing adequate care of the pregnant woman, including maternal nutrition during pregnancy, should be given highest priority in settings with on-going under- and/or malnutrition.
In conclusion, at an age where physical fitness and capacity peaks, healthy men born preterm have a lower exercise capacity compared to men born at term, irrespective of socioeconomic factors and current BMI. Reduced exercise capacity may add to future health problems faced by children growing up today following preterm birth and may play a role in the causal chain towards overweight, diabetes and hypertension. Early intervention programs to improve physical fitness in children and adults born preterm may be of value to reduce the risks for later disease and improve life-long health. Further studies on this issue, as well as on the underlying mechanisms, are warranted.
Ethical Approval
The study was approved by the Regional Ethical Review Board in Stockholm.
Supporting Information
Instructions for cycle ergometer exercise capacity test.
(DOCX)
Explanation of codes classifying health status at recruitment.
(DOCX)
BMI, body weight and height at conscription according to gestational age.
(DOCX)
Funding Statement
Funding to the authors during preparation of this work was received from Swedish Heart Lung Foundation, Stockholm County Council, Karolinska Institutet and Stiftelsen Samariten. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Finnström O, Olausson PO, Sedin G, Serenius F, Svenningsen N, et al. (1997) The Swedish national prospective study on extremely low birthweight (ELBW) infants. Incidence, mortality, morbidity and survival in relation to level of care. Acta Paediatr 86: 503–511. [DOI] [PubMed] [Google Scholar]
- 2. Fellman V, Hellström-Westas L, Norman M, Westgren M, Källén K, et al. (2009) One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 301: 2225–2233. [DOI] [PubMed] [Google Scholar]
- 3. Kilbride HW, Gelatt MC, Sabath RJ (2003) Pulmonary function and exercise capacity for ELBW survivors in preadolescence: effect of neonatal chronic lung disease. J Pediatr 143: 488–493. [DOI] [PubMed] [Google Scholar]
- 4. Welsh L, Kirkby J, Lum S, Odendaal D, Marlow N, et al. (2010) The EPICure study: maximal exercise and physical activity in school children born extremely preterm. Thorax 65: 165–172. [DOI] [PubMed] [Google Scholar]
- 5. Vrijlandt EJ, Gerritsen J, Boezen HM, Grevink RG, Duiverman EJ (2006) Lung function and exercise capacity in young adults born prematurely. Am J Respir Crit Care Med 173: 890–896. [DOI] [PubMed] [Google Scholar]
- 6. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB (2012) Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59: 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, et al. (2005) Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 112: 3430–3436. [DOI] [PubMed] [Google Scholar]
- 8. Bonamy AK, Bendito A, Martin H, Andolf E, Sedin G, et al. (2005) Preterm birth contributes to increased vascular resistance and higher blood pressure in adolescent girls. Pediatr Res 58: 845–849. [DOI] [PubMed] [Google Scholar]
- 9. Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, et al. (2004) Premature birth and later insulin resistance. N Engl J Med 351: 2179–2186. [DOI] [PubMed] [Google Scholar]
- 10. Hovi P, Andersson S, Eriksson JG, Järvenpää AL, Strang-Karlsson S, et al. (2007) Glucose regulation in young adults with very low birth weight. N Engl J Med 356: 2053–2063. [DOI] [PubMed] [Google Scholar]
- 11. Kaijser M, Bonamy AK, Akre O, Cnattingius S, Granath F, et al. (2009) Perinatal risk factors for diabetes in later life. Diabetes 58: 523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crump C, Sundquist K, Sundquist J, Winkleby MA (2011) Gestational age at birth and mortality in young adulthood. JAMA 306: 1233–1240. [DOI] [PubMed] [Google Scholar]
- 13. Thijssen DH, Maiorana AJ, O’Driscoll G, Cable NT, Hopman MT, et al. (2010) Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol 108: 845–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, et al. (2000) Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality - 6 y follow-up of middle-aged and elderly men and women. Int J Obes Relat Metab Disord 24: 1465–1474. [DOI] [PubMed] [Google Scholar]
- 15. Bassuk SS, Manson JE (2005) Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol 99: 1193–1204. [DOI] [PubMed] [Google Scholar]
- 16. LaMonte MJ, Blair SN, Church TS (2005) Physical activity and diabetes prevention. J Appl Physiol 99: 1205–1213. [DOI] [PubMed] [Google Scholar]
- 17. Hackam DG, Khan NA, Hemmelgarn BR, Rabkin SW, Touyz RM, et al. (2010) The 2010 Canadian Hypertension Education Program recommendations for the management of hypertension: part 2 - therapy. Can J Cardiol 26: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruiz JR, Rizzo NS, Hurtig-Wennlöf A, Ortega FB, Wärnberg J, et al. (2006) Relations of total physical activity and intensity to fitness and fatness in children: the European Youth Heart Study. Am J Clin Nutr 84: 299–303. [DOI] [PubMed] [Google Scholar]
- 19. Rogers M, Fay TB, Whitfield MF, Tomlinson J, Grunau RE (2005) Aerobic capacity, strength, flexibility, and activity level in unimpaired extremely low birth weight (<or = 800 g) survivors at 17 years of age compared with term-born control subjects. Pediatrics 116: e58–65. [DOI] [PubMed] [Google Scholar]
- 20. Saigal S, Stoskopf B, Boyle M, Paneth N, Pinelli J, et al. (2007) Comparison of current health, functional limitations, and health care use of young adults who were born with extremely low birth weight and normal birth weight. Pediatrics 119: e562–573. [DOI] [PubMed] [Google Scholar]
- 21. Kajantie E, Strang-Karlsson S, Hovi P, Räikkönen K, Pesonen AK, et al. (2010) Adults born at very low birth weight exercise less than their peers born at term. J Pediatr 157: 610–616 616: e611. [DOI] [PubMed] [Google Scholar]
- 22. Hack M, Cartar L, Schluchter M, Klein N, Forrest CB (2007) Self-perceived health, functioning and well-being of very low birth weight infants at age 20 years. J Pediatr 151: 635–641 641: e631–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kriemler S, Keller H, Saigal S, Bar-Or O (2005) Aerobic and lung performance in premature children with and without chronic lung disease of prematurity. Clin J Sport Med 15: 349–355. [DOI] [PubMed] [Google Scholar]
- 24. Baraldi E, Zanconato S, Zorzi C, Santuz P, Benini F, et al. (1991) Exercise performance in very low birth weight children at the age of 7–12 years. Eur J Pediatr 150: 713–716. [DOI] [PubMed] [Google Scholar]
- 25. Smith LJ, van Asperen PP, McKay KO, Selvadurai H, Fitzgerald DA (2008) Reduced exercise capacity in children born very preterm. Pediatrics 122: e287–293. [DOI] [PubMed] [Google Scholar]
- 26. Gross SJ, Iannuzzi DM, Kveselis DA, Anbar RD (1998) Effect of preterm birth on pulmonary function at school age: a prospective controlled study. J Pediatr 133: 188–192. [DOI] [PubMed] [Google Scholar]
- 27. Clemm H, Røksund O, Thorsen E, Eide GE, Markestad T, et al. (2012) Aerobic capacity and exercise performance in young people born extremely preterm. Pediatrics 129: e97–e105. [DOI] [PubMed] [Google Scholar]
- 28.The Swedish Medical Birth Register: a summary of content and quality. In: Stockholm SNBoHaW, (2003). Available at www.sos.se/fulltext/112/2003-112-3/2003-112-3.pdf. Accessed November 11, 2005.
- 29.Population and Housing Census 1990, Part 7: the Planning and Processing of the Population and Housing Census. Stockholm: Statistics Sweden; 1992.
- 30. Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M (1996) A United States national reference for fetal growth. Obstet Gynecol 87: 163–168. [DOI] [PubMed] [Google Scholar]
- 31. Marsál K, Persson PH, Larsen T, Lilja H, Selbing A, et al. (1996) Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 85: 843–848. [DOI] [PubMed] [Google Scholar]
- 32.Sweden SC (1982) MIS 1982:4 Socioekonomisk indelning SEI. Statistiska Centralbyrån. pp. 20–21.
- 33. Parizkova J (2008) Impact of education on food behaviour, body composition and physical fitness in children. Br J Nutr 99 Suppl 1S26–32. [DOI] [PubMed] [Google Scholar]
- 34. Moss TJ (2006) Respiratory consequences of preterm birth. Clin Exp Pharmacol Physiol 33: 280–284. [DOI] [PubMed] [Google Scholar]
- 35. Bonamy AK, Martin H, Jörneskog G, Norman M (2007) Lower skin capillary density, normal endothelial function and higher blood pressure in children born preterm. J Intern Med 262: 635–642. [DOI] [PubMed] [Google Scholar]
- 36. Schubert U, Müller M, Edstedt Bonamy A-K, Abdul-Khaliq H, Norman M (2011) Aortic growth arrest after preterm birth: a lasting structural change of the vascular tree. Journal of developmental origins of health and disease 2: 218–225. [DOI] [PubMed] [Google Scholar]
- 37. De Matteo R, Blasch N, Stokes V, Davis P, Harding R (2010) Induced preterm birth in sheep: a suitable model for studying the developmental effects of moderately preterm birth. Reprod Sci 17: 724–733. [DOI] [PubMed] [Google Scholar]
- 38. Lewandowski AJ, Augustine D, Lamata P, Davis EF, Lazdam M, et al. (2013) Preterm heart in adult life: cardiovascular magnetic resonance reveals distinct differences in left ventricular mass, geometry, and function. Circulation 127: 197–206. [DOI] [PubMed] [Google Scholar]
- 39. Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, et al. (2012) Preterm birth and psychiatric disorders in young adult life. Arch Gen Psychiatry 69: E1–8. [DOI] [PubMed] [Google Scholar]
- 40. Peacock JL, Marston L, Marlow N, Calvert SA, Greenough A (2012) Neonatal and infant outcome in boys and girls born very prematurely. Pediatr Res 71: 305–310. [DOI] [PubMed] [Google Scholar]
- 41. Hiscock R, Bauld L, Amos A, Platt S (2012) Smoking and socioeconomic status in England: the rise of the never smoker and the disadvantaged smoker. J Public Health (Oxf) 34: 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, et al. (2001) Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 104: 1694–1740. [DOI] [PubMed] [Google Scholar]
- 43. Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, et al. (1999) Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA 282: 1547–1553. [DOI] [PubMed] [Google Scholar]
- 44. Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, et al. (2010) The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ 88: 31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hult M, Tornhammar P, Ueda P, Chima C, Bonamy AK, et al. (2010) Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS One 5: e13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Instructions for cycle ergometer exercise capacity test.
(DOCX)
Explanation of codes classifying health status at recruitment.
(DOCX)
BMI, body weight and height at conscription according to gestational age.
(DOCX)