Abstract
We explore how and to what extent prescription drug insurance expansions affects incentives for pharmaceutical advertising. When insurance expansions make markets more profitable, firms respond by boosting advertising. Theory suggests this effect will be magnified in the least competitive drug classes, where firms internalize a larger share of the benefits from advertising. Empirically, we find that the implementation of Part D coincides with a 14% to 19% increase in total advertising expenditures. This effect is indeed concentrated in the least competitive drug classes. The additional advertising raised utilization among non-elderly patients outside the Part D program by about 3.6%. This is roughly half of the direct utilization effect of Part D on elderly beneficiaries. The results suggest the presence of considerable spillover effects from publicly subsidized prescription drug insurance on the utilization and welfare of consumers outside the program.
Keywords: Medicare, prescription drugs, insurance, advertising
Introduction
The Medicare Modernization Act (MMA) implemented in 2006 represented one of the most far-reaching reforms to the Medicare program since its inception. A key component of the legislation was the implementation of Medicare Part D, which provided subsidies to millions of Medicare beneficiaries for the purchase of privately provided prescription drug insurance. A wealth of previous research demonstrated that Part D improved access to prescription drug insurance coverage, lowered out-of-pocket prices, reduced deadweight loss, and expanded the utilization of drugs among Medicare beneficiaries (Lichtenberg and Sun 2007; Duggan and Scott Morton 2008; Ketcham and Simon 2008; Yin, Basu et al. 2008; Lakdawalla and Sood 2009).
Yet, one of the unique and relatively under-studied aspects of Part D is its effect on the behavior of private firms. Part D significantly altered the landscape of the pharmaceutical market and may have dramatically altered the incentives faced by pharmaceutical companies. The behavioral responses of these large and powerful firms may have had a variety of unintended consequences that extended, multiplied, or mitigated the intended effects of the legislation. Moreover, the relationship between Part D and pharmaceutical firm behavior creates a channel through which the program might have significantly affected the well-being of patients outside the Medicare program entirely.
Pharmaceutical advertising provides an important and instructive example.1 Pharmaceutical firms spend roughly one-fifth of their revenue on marketing their products. Presumably, these large investments are made with an eye towards influencing consumer and physician choices. If Part D changed the incentives governing this process, the effects on the prescription drug market might have been quite significant. Moreover, since advertising – unlike insurance – cannot be precisely targeted to Medicare participants alone, these consequences might have spilled over onto prescription drug prices and utilization among non-Part D beneficiaries. The result may have been unintended benefits or costs for consumers that are entirely unconnected to the Medicare program.
In this study, we investigate whether and how prescription drug insurance theoretically affects firms’ incentives to advertise, and we quantify the empirical implications of these findings. There is a substantial theoretical literature on advertising in imperfectly competitive product markets. Our stylized model of oligopoly advertising by branded pharmaceutical firms summarizes three salient predictions from this long line of research:
More profitable markets draw more advertising. More profitable markets generate greater returns to capturing new consumers, and in turn stimulate more intense advertising effort.
Markets with more competitors draw less advertising. The presence of more competitors lowers an individual firm’s private gain from expanding the size of the entire market, and also may create stiffer resistance for individual firms trying to gain market share in a more crowded marketplace.
Greater competition mutes the effect of profitability on advertising. In more competitive markets, each individual firm captures a smaller slice of a given gain in profits. The effect of a fixed change in total profitability is thus dampened.
Applying these results to the problem of insurance expansion, we conclude that greater insurance provision boosts advertising if insured consumers are more profitable than uninsured consumers. Since earlier research suggests that Part D increased the profitability of prescription drug provision (Friedman 2009), one might then hypothesize that Part D intensified advertising effort. Moreover, the theoretical literature suggests this effect would have been largest in the least competitive drug classes.
Our empirical analysis confirms and quantifies these predicted relationships. We find that the implementation of Part D generates a 14% to 19% increase in total advertising expenditures. Consistent with economic theory, this effect is concentrated in the least competitive drug classes. This additional advertising boosted overall prescription drug utilization by 3.6% among non-elderly patients not covered by Part D. By way of comparison, the direct impact of the Part D program was to boost utilization by 6–12% among the elderly Medicare beneficiaries (Lichtenberg and Sun 2007; Yin, Basu et al. 2008; Duggan and Scott Morton 2010).
We use a unique database containing detailed advertising data for the top 1000 pharmaceutical products sold in the US in 2005. The identification strategy relies on the premise that Part D has the biggest impact on incentives to advertise drugs that display the most use by elderly patients. However, that we focus on how advertising investments affects utilization by non-Medicare consumers. These represent spillover effects of Part D outside the population of Part D-eligibles.
Several papers have studied the effects of advertising on the prescription drug market. Earlier research has shown that advertising primarily increases drug utilization rather than prices (Berndt, Bui et al. 1995; Hurwitz and Caves 2002; Rosenthal, Berndt et al. 2003; Donohue, Berndt et al. 2004; Iizuka and Jin 2005; Bradford, Kleit et al. 2006),2 although some have found that advertising lowers price elasticity of demand and product differentiation (Rizzo 1999; King 2002). Related studies have also focused on the differences between direct-to-consumer (DTC) advertising and direct-to-physician (DTP) advertising. As their names imply, DTC targets patients, and DTP targets physicians. DTC has been shown to increase total demand for a drug class, without substantially altering relative market shares of drugs within a class (Ling, Berndt et al. 2003; Rosenthal, Berndt et al. 2003; Donohue, Berndt et al. 2004; Iizuka and Jin 2005). Some have suggested, however, that this mechanism works entirely through increases in patient adherence, rather than the initiation of new prescriptions (Calfee, Winston et al. 2002). There is also evidence that the demand effects of DTC are magnified by the presence of generous insurance coverage (Wosinska 2002). On the other hand, advertising to physicians has a significant effect on drug choice within a class (Azoulay 2002; Iizuka and Jin 2005).
Our study brings together and complements the existing literatures on prescription drug advertising and on Medicare Part D. We focus on how prescription drug insurance affects the incentives of firms to advertise, and how this creates a mechanism for utilization spillovers outside public prescription drug insurance programs. We identify and quantify these spillover effects, which appear large enough to warrant consideration by policymakers.
Theoretical Framework
Advertising has a dual nature. “Cooperative” advertising grows the entire market for a firm and its competitors. “Predatory” advertising steals share from competitors, but keeps the total size of the market fixed. This insight goes back at least as far as Alfred Marshall (1923), and has been developed in a long and distinguished line of research over the subsequent decades (cf, Scherer 1970; Schmalensee 1976; Friedman 1983; Slade 1995; Piga 1998; Depken and Snow 2008).
It has also been noted previously that the private incentives for “cooperative” advertising become weaker as the number of firms in a marketplace grows – see, for example, Scherer’s (1970, p. 334) discussion in his influential textbook on industrial organization. However, as Scherer also notes, the empirical question of whether and how the number of firms affects advertising effort depends on the extent to which advertising is cooperative or predatory.
We develop a simple and stylized model to illustrate and summarize the implications of these two widely understood aspects of advertising. We base our approach on a sequence of models that trace back at least to Schmalensee (1976). A key feature of the Schmalensee model, mimicked by a number of later authors, is the focus on promotional competition only, and a deliberate decision to abstract from price competition. As Schmalensee writes:
It is a generally accepted ‘stylized fact’ of industrial organization that price competition is relatively rare in…markets [with few sellers and differentiated products]. Prices generally change infrequently, and sellers compete, if at all, mainly through product variation and promotional expenditures. (p. 493)
This assumption is maintained and developed further in a variety of later papers (cf, Slade 1995; Depken and Snow 2008). It is also consistent with the existing empirical literature cited above, namely that pharmaceutical promotion is associated with greater utilization but not greater prices.
Borrowing from Schmalensee and successors, our stylized model regards price as being set independently of advertising decisions. While this is a widely used assumption, it is worth noting that its failure has relatively little impact on the simple conclusions that have been drawn in the literature about market size, competition, and the returns to advertising.
To illustrate the findings of the earlier theoretical literature, we follow in particular the form of the model developed recently by Depken and Snow (2008), and include a very simple extension to allow for two, distinct and isolated market segments – uninsured and insured customers.
Consider the production and sale of goods in a pharmaceutical class with N on-patent products, produced and marketed by N oligopolistic firms that compete with each other. Let Ai and pi denote the advertising and price decisions of firm i. denotes the total advertising by all of firm i’s competitors, and is the vector of prices for all firm i’s competitors. The marginal cost of production is δ. The total quantity of drugs sold in the class is , the market share in terms of quantity of firm i is , and the absolute price mark-up of firm i (equal to price minus marginal cost) is . Firm i solves the following problem, taking as given optimal values for its competitors’ decisions, and :
Since we wish to summarize short-term effects, we hew closely to the static model developed by Depken and Snow. A dynamic model with a finite patent-horizon would be more realistic in its assumptions (cf, Bhattacharya and Vogt 2003), but would add notational complexity without significantly enriching the simple comparative static results we wish to describe and summarize.
To reflect the “cooperative” aspect of advertising, suppose that total demand has the constant elasticity form, , where γ > 1, and p̄ is the (quantity-weighted) average price in the marketplace.3 To reflect its “predatory” aspect, assume that spi is independent of advertising, and that s is homogeneous of degree zero in Ai and . In other words, a firm can steal market share when it increases advertising relative to competitors.
Both Schmalensee (1976) and Depken and Snow (2008) simply assume a fixed price and analyze advertising independent of pricing decisions. Our approach is slightly less rigid, but extremely similar in spirit. Here, the constant elasticity form of demand implies a convenient form for the price and the equilibrium oligopoly mark-up, both of which depend only on the elasticity γ and the marginal cost δ.4 Since price is “pinned down” by these two exogenous parameters, optimal advertising for firm i is accurately characterized by the solution to the simple, univariate maximization problem:
where represents firm i’s market share as a function of advertising levels. We assume the problem is strictly concave, in that . We also stabilize the strategic interaction among firms by assuming that it is harder to win new customers when competitors are advertising more heavily, or that . Failure of this assumption leads to multiple equilibria, because an individual firm’s return on advertising may theoretically be unaffected by large uniform movements in the advertising levels of all other firms.
Firm Behavior in an Unsegmented Market
The economics of oligopoly advertising in an unsegmented market have been developed by a variety of other authors, whose contributions we highlight as we present the theoretical implications.
In this case, the firm’s optimal advertising decision satisfies:
This first-order condition has a number of well-known implications.5
Advertising levels rise when unit revenues in the marketplace rise
Define “unit revenues” as U ≡ p̄1−γmi, which equals revenues per unit of quantity the firm sells.6 When unit revenues rise, firms will advertise more aggressively, both because each unit of market share is more valuable and because a unit change in market size is more valuable.
Advertising levels rise with total and marginal market quantity
Holding unit revenues fixed, exogenous increases in total market size, q, or in the marginal quantity effect of advertising, q′, also raise the incentive to advertise.
Holding prices fixed, increases in the number of competitors reduce advertising investments
Increases in competition: (1) Weakly lead to more free-riding off the advertising investments of other firms, and (2) Make it harder to gain market share through advertising.
Formally, result (1) on “free-riding” follows because, in a symmetric equilibrium, . Therefore, at a given level of Ai, more competitors weakly decrease the firm’s return on growing the total market, . Note that this effect is exactly zero if q′ = 0, in which case advertising is entirely predatory with no cooperative aspect. This reflects Scherer’s (1970) nuanced observation that the effects of competition depend on the nature of advertising within an industry. As long as advertising has some cooperative aspect, increases in the number of competitors strictly weaken incentives to advertise.
Result (2) on “market share” follows from our assumption of degree-zero homogeneity of s, provided we make one modest simplification. Homogeneity implies that for some increasing function g. In equilibrium, . A simple and intuitive way to satisfy these assumptions is to follow Schmalensee’s (1972) suggestion to equate relative advertising effectiveness and market share: in particular, one can satisfy the “adding up” and degree-zero homogeneity assumptions by stipulating that , for all possible Ai.7 As a result, . Therefore, for constant Ai, increases in N lower s1 and thus also lower . This comparative statics argument implies that greater competition lowers the return to advertising investments.
Holding market size fixed, increased competition mutes the effect of increased unit revenues on advertising
In other words, when baseline profits grow, the incentive to advertise rises, as shown above. However, this effect is weaker if the number of competitors rises as well. This result follows since the effect of profits on the return to advertising ( ) falls with the number of competitors, N. The comparative statics argument is similar to the one demonstrating that competition reduces advertising investments in general, and relies on the result that greater competition lowers s1q and sq′.
It is worth noting that, in this highly stylized model, the number of competitors (N) is intimately related to the market share held by competitors. Thus, one can interpret these results as applying equally to increases in the number of competitors, or increases in the market share of competitor firms.
Insurance Expansions
Now suppose that firms sell to two segmented markets of insured consumers and uninsured consumers. The total number of consumers in each group g is given by Mg, where g = I, U for the insured and uninsured groups, respectively. Scale the quantities to be per capita: Qg represents per capita quantity in group g, where . Note that the firm can perfectly price-discriminate across the two markets, but cannot perfectly target advertising expenditures. For example, television or radio ads may be viewed by consumers in both markets, or marketing to physicians may affect both uninsured and insured patients at that physician. In reality, firms have some ability to imperfectly target their advertising expenditures, but this simply limits the magnitudes of the spillovers across market segments rather than eliminates them.
The firm’s profit maximization problem now becomes:
The firm’s optimal advertising condition now satisfies:
To simplify the analysis, suppose that advertising has the same effect on market shares in both the insured and uninsured segments, but that all other parameters can vary across segments. Shifting the marginal patient from the uninsured to the insured group has the following impact on the return to advertising:
Insurance expansion raises advertising if: total revenues are higher in the insured marketplace ( ); and marginal revenues due to advertising are higher in the insured marketplace ( )
This result follows directly by inspection of the comparative static expression showing the impact of expansion on the return to advertising.
The assumptions given above – that insured consumers are more profitable in absolute terms and on the margin – appear consistent with the balance of the empirical evidence. First, prior empirical literature on Medicare Part D supports the assumption that total revenues are higher in the insured marketplace. For example, Zhang et al (2009) find that Medicare Part D was associated with a 74% increase in drug spending among elderly with no prescription drug insurance prior to Part D. Similarly, Duggan and Scott Morton (2010) also find evidence of increased spending on drugs after implementation of Medicare Part D. Finally, Friedman (2009) finds that the passage of Medicare Part D was associated with larger increases in stock prices of firms launching brand-name drugs with high exposure to the Medicare market. This is also consistent with the notion that the pharmaceutical industry and financial markets expected greater sales of branded drugs by consumers newly insured due to implementation of Medicare Part D.
Second, prior research supports the assumption that insurance coverage might make consumers more responsive to advertising on the margin. Intuitively, an undecided consumer might be more likely to try a new drug after being exposed to advertising (or after her physician is exposed to advertising) if out of pocket costs are lower. Consistent with this intuition, Wosinska (2002) finds that advertising influences demand more for drugs that have preferential status and lower out of pocket costs. If these two assumptions are indeed consistent with the empirical evidence on the pharmaceutical industry, it suggests that expanding insurance leads to more advertising.
Increases in competition mute the effect of insurance expansion on advertising
Increases in N lower s and s1, by the same logic as before, and this compresses the marginal impact of shifting patients into insured status.
The results with a segmented insurance market represent a fairly straightforward corollary from long-established analysis of advertising and oligopoly (cf, Scherer 1970). When unit revenues are higher among the insured, expanding insurance increases the size of the market, makes each firm’s share of the market more valuable, and increases the return to capturing an additional percentage point of share. In this case, insurance expansion strengthens the incentives for both cooperative and predatory advertising. Moreover, the effects of market-expansion are watered down in a highly competitive environment, in which more of the benefits of advertising “leak away” to other firms.
Implications for Welfare
Oligopoly production typically implies that there is under-provision of a good. Greater advertising expenditure by a firm with market power is welfare-improving, so long as output moves towards (but not past) its efficient competitive level. From this perspective, if Part D boosts advertising that expands utilization for the non-elderly, this is a positive spillover of the program – barring an “overshoot” of the efficient quantity -- and vice-versa.
Several caveats are in order for the pharmaceutical market. First, unlike in many other oligopoly markets, the presence of health insurance decouples the oligopoly price of drugs from the consumer’s out-of-pocket price. As a result, under-utilization and deadweight loss are mitigated, or even eliminated, in spite of manufacturer market power (Lakdawalla and Sood 2009). As such, increases in utilization might lead to over-use. Second, even when under-use is present, it is possible that advertising would increase by so much that output ends up exceeding its efficient level (Lakdawalla and Philipson Forthcoming). Notably, this is true even when a monopolist can perfectly set the level of advertising in the marketplace. Third, market demand may not reflect true willingness-to-pay: consumers may not be well-informed; physicians may be imperfect agents for their patients; or advertising may lead to extreme misperceptions of value. As a result, it is uncertain whether equilibrium demand is inefficiently high or low, even though oligopoly is present.
Despite these caveats, advertising is still predicted to create spillovers in utilization for the non-elderly. While estimating the social value (or cost) of additional drug utilization lies beyond the scope of this paper, our theoretical model suggests the importance of accounting for these spillovers when evaluating drug insurance expansions. Indeed, our empirical analysis documents and confirms the presence of these utilization spillovers.
Empirical Framework
We are interested in the causal effect of Medicare Part D on pharmaceutical advertising. Consistent with the implications of theory, the empirical model allows for heterogeneous effects by degree of competition in a market. We are also interested in quantifying the spillover effects of Part D on utilization by the non-elderly. We hypothesize that this effect will be magnified in drug classes with less competition.
Empirically, we think of a “market” as being defined by a drug class. We measure the degree of competitiveness in the drug class market in two different ways: (1) using the number of advertised drugs within the drug class prior to Part D implementation; and (2) comparing drugs that are “dominant,” in the sense that they accounted for the highest share of advertising before the implementation of Part D, to their “non-dominant” peers. Under the first measurement strategy, we stratify our results across drug classes with more or less competition. Under the second, we stratify them across dominant and non-dominant drugs, where we think of dominant drugs as facing less competition than non-dominant ones. The stylized model above illustrates how the number of competitors matters. The distinction of dominant versus non-dominant drugs is an alternative approach that permits drug-level, rather than class-level analysis, in a relatively tractable way.
For drug d, of type j ∈ {More Competition, Less Competition, Dominant, Non – Dominant}, in quarter q, we begin by estimating empirical models as in:
| (1) |
The unit of observation for this model is drug-quarter. The main outcome variables are total dollars spent on direct-to-consumer advertising and total dollars spent on direct-to-physician advertising. In alternate models, we also look at total advertising expenditures.
The effect of primary interest is the coefficient on the interaction between: a dummy variable indicating the implementation of Part D, and the share of the drug’s utilization as measured by number of prescriptions (in 2002–03) exposed to Part D. A positive value of the coefficient on the interaction, , would indicate that Medicare Part D increased advertising for drug type j relative to its peers.
The empirical model also includes drug-specific fixed-effects, θd, and time (quarter) fixed-effects, . The former absorbs time-invariant differences across drugs. The latter absorbs secular time trends present within each type of drug. We also control for other important determinants of advertising that might have changed over time, including: the number of drug safety warnings (so called “black box” warnings) issued by the FDA, the number of new brand name drugs, and the number of new generic entrants in a drug class. Finally, in alternate models, we relax the assumption of a common secular time trend for all drugs of type j and allow time trends to vary by the approval date of the drug to capture changes in advertising over a drug’s life cycle.
Identification rests on the assumption that Part D implementation did not coincide with other factors that discretely shifted advertising for drugs heavily exposed to Part D, relative to drugs that were less exposed. While there are no obvious candidates for such a shift, validity would still be compromised if secular trends in advertising differed across drugs that were heavily and less heavily exposed to Part D. To test for this scenario, we assessed whether trends in advertising prior to Part D implementation were similar for drugs more heavily exposed to Part D and drugs less heavily exposed to it.
Table 1 reports results from these tests for total advertising expenditures. To test differences in time trends prior to Part D, we evaluate the coefficient on the interactions between Medicare share and the quadratic time trend. If the interactions are jointly significant, this indicates that secular trends prior to Part D differ across drugs with different levels of exposure; this would pose a problem for our identification strategy if true. However, Table 1 provides no evidence of a significant interaction. As an additional test, we stratified the analysis by estimating separate models for direct to consumer and direct to physician advertising; the interaction terms still remained insignificant.
Table 1.
Test of Pre-Existing Difference in Advertising Trends by Medicare Share Prior to 2006
| Variables | Drug Types
|
|||
|---|---|---|---|---|
| More Competition | Less Competition | Dominant | Non-Dominant | |
|
| ||||
| Quarter* Medicare Market Share | 125 (927) | −1578 (1944) | −4338 (4318) | 258 (792) |
| Quarter Square* Medicare Market Share | −36 (77) | 179 (141) | 418 (320) | −17 (57) |
| Joint Significance of Pre-existing Difference in Time Trend (p-value of F-Test) | 0.42 | 0.13 | 0.16 | 0.95 |
| Number of Drugs | 180 | 173 | 57 | 294 |
| R-Squared | 0.87 | 0.94 | 0.94 | 0.90 |
| N | 2333 | 2235 | 741 | 3801 |
Note: Total advertising is in thousands of dollars. All models include drug fixed effects, quadratic time trend, number of black box warnings, number of generic drugs, and number of brand name drugs entering drug class as covariates. Clustered standard error by drugs are reported in the parentheses.
Significance:
p<0.10,
p<0.05,
p<0.01.
In addition to estimating the effect of Part D on advertising, we are also interested in the subsequent effect of increases in advertising on utilization. This requires a two-step estimation approach. The first step is, as above, estimating an equation that predicts advertising by drug and quarter. The second stage predicts utilization. Since advertising has spillover effects on the utilization of other drugs in the class, the second-stage equation models the effect of advertising on utilization at the drug class level.8
In addition, since our data on utilization (from the Medical Expenditure Panel Survey) are available only at an annual frequency, we model utilization by drug class and year. This leads to the second-stage model for utilization of drug class c in year t:
| (2) |
We also control in this equation for other important determinants of utilization including the number of drug safety warnings, the number of new brand name drugs and new generic drugs entering the drug class. In addition, note that we are modeling utilization by the non-elderly. Since Medicare implementation affected out-of-pocket costs for the elderly, we cannot disentangle price effects from advertising effects in this population. This is not a major limitation for our purposes, since our focus is on spillovers effects in Part D.
Since advertising is endogenous to drug utilization, we use a two-step instrumental variables approach to estimate this effect. The underlying instrument is Medicare Part D implementation interacted with Medicare market share. The estimation procedure begins by using equation (1) -- stratified across drug classes with more and less competition -- to predict advertising for each drug-quarter.9 Intuitively, this is the advertising predicted to occur with the implementation of Part D. Using these predictions, we then aggregate up to predict advertising at the drug class-year level, because the unit of observation in the second-stage equation is the drug class-year. This prediction of Part D-related drug class-year advertising serves as the instrument for observed drug class-year advertising in the second-stage equation.
Furthermore, note from the above equation that utilization is specified as a function of the existing stock of advertising. This specification recognizes that advertising in a given year can have long-lived effects–an idea that was first proposed by Nerlove and Arrow (1962) and has been subsequently been used in several empirical papers that followed this seminal work (Berndt, Bui et al. 1995; Berndt, Bui et al. 1997; Rizzo 1999; Ling, Berndt et al. 2003).
Unlike its flow, the stock of advertising cannot be observed directly. To construct it, we follow the prior literature and model advertising as a durable good where current stock of advertising equals the depreciated value of last years’ stock plus the current period flow of advertising.
| (3) |
There are two challenges to implementing this approach in practice. First, the depreciation rate (γ) is unknown. Prior studies either assume a particular value of the depreciation rate (cf, Donohue, Berndt et al. 2004), or use grid search to choose a depreciation rate that best fits the data (Berndt, Bui et al. 1997; Ling, Berndt et al. 2003). We follow the latter approach and find the value of the depreciation rate that minimizes the root mean square error of the model in equation (3). The estimated annual depreciation rate turns out to be 30% per year, which is quite similar to the depreciation rate used by others in the literature (Rizzo 1999), although there is a fair amount of dispersion across studies.
Second, the stock of advertising depends in principle on the flow of advertising for all years after product introduction. However, we do not have advertising data prior to 2003, even though some of our drugs will have launched before that. Thus, as an approximation, we construct the stock of advertising as a function of flows from the current year and preceding three years, assuming that advertising flows were constant for the years prior to 2003. This approach has been used in the prior literature and is aided by the relatively high rate of estimated depreciation for advertising flows (Rizzo 1999).
Implementation of Part D interacted with Medicare exposure is a valid instrument for advertising in the second-stage equation as long as: (1) It strongly predicts advertising, and (2) It does not directly affect class-level utilization through any channel other than class-level advertising itself. The empirical results from equation 1 will show that the instrument is a sufficiently strong predictor of advertising. As an indirect test of the second identifying assumption, we test whether implementation of Part D interacted with Medicare exposure predicts out of pocket expenses (an important determinant of utilization) in the non-elderly market. The results from this test are shown in Table 2 and support the validity of our identification assumption, because there is no significant relationship between the instrument and out of pocket expenses in the non-elderly market. However, we cannot rule out whether non-price factors such as drug utilization review or step therapy are correlated with our instrument.
Table 2.
Does Part D Implementation interacted with Medicare Share Predict Changes in Average Out-of-Pocket Expense In Non-Elderly Market
| Variables | Average Out-of-Pocket Expense
|
|
|---|---|---|
| Model 1 | Model 2 | |
|
| ||
| Post2006* Medicare Market Share | −1.195 (5.386) | −0.809 (5.607) |
| Year Fixed Effect | Yes | Yes |
| Drug Class Fixed Effect | Yes | Yes |
| Covariates | No | Yes |
| Average Out of Pocket Expense per Script | 24.74 | 24.74 |
| N | 290 | 290 |
Note: Data are from the Medical Expenditure Panel Surveys 2003 to 2007. Dependent variable is out of pocket cost per script. All models include drug fixed effects and time fixed effects. Model 2 includes number of black box warnings, number of generic drugs, and number of brand name drugs entering drug class as covariates. Clustered standard errors by drug class are reported in the parentheses.
Significance:
p<0.10,
p<0.05,
p<0.01.
Data
Pharmaceutical Marketing
We use data from the IMS Advertising Database for this analysis. The dataset contains individual drug level data on advertising for the top 1000 drugs (based on 2005 dollar sales). The advertising data is reported quarterly and spans six years from 2002-Q4 to 2008-Q3. For each drug, the data contain measures of direct-to- consumer advertising expenditures, along with direct-to-physician advertising including: medical journal advertisements, promotional visits to physicians, and samples dispensed. Table 3 describes the various measures of advertising available in the dataset.
Table 3.
Advertising Data Elements
| Advertising Measure | Definition/Measurement |
|---|---|
| Direct to Consumer Advertising: | |
| DTC Dollars | Total dollars spent on direct to consumer (DTC) advertising in television, radio, newspaper, magazine, and outdoor media. These data are obtained from TNS Media Intelligence the leading advertising intelligence firm that supplies data to advertising agencies, advertisers, broadcasters, and publishers. The company’s tracking technologies collect occurrence and expenditure data of more than 1 million brands. |
| Physician Advertising: | |
| Journal Advertising | This measure is an estimate of the cost of product advertising in medical journals. This figure is tabulated by relating observable advertising characteristics (for example, position, color, circulation) to standard rates and charges of advertising in different medical journals. |
| Detailing | This measure is an estimate of the cost, in dollars, for a particular detailing visit to a physician or other health care provider. The cost of contact is based on an estimate of the proportion of time spent on a particular product during an office visits and the total cost of an office visit. The cost of the office visits is based on an IMS survey of pharmaceutical firms to obtain information on salary, training, and fringe benefits such as bonus, car, and insurance of medical sales representatives. Cost of samples dispensed in not included in this measure |
| Samples | This is the retail value of the product sampling activities or pharmaceutical representatives that are directed to office-based physicians. A panel of front- office personnel reports the quantity of product samples provided to office-based physicians through in-person discussions, service visits, and the mail. |
Part D Market Share
Our identification strategy relies in part on differential exposure to Part D, across different drugs. Therefore, we need to estimate the share of each drug’s market that is exposed to the Part D legislation. Following Duggan and Scott Morton (2008), we will use the 2002–2003 Medical Expenditure Panel Survey (MEPS) to estimate Part D market share (i.e., the share of demand accounted for by the Medicare-eligible population) prior to the passage of Part D legislation10. The MEPS contains data on medical care use including prescription drug use and costs from a nationally representative sample of the civilian non-institutionalized population residing in the U.S. The MEPS data are organized in several files that report use either at the person level or medical event level. The most relevant file for our analysis is the prescribed medicine file, whose unit of observation is a prescription. For each prescription, the data report the name of the drug, therapeutic class of the drug, and total cost by payer. The prescribed medicine file from 2002 and 2003 contains data on more than 600,000 prescriptions received by MEPS respondents. The file can be linked to the person-level file, to obtain the age and insurance status of the person receiving the prescription. Therefore, for each prescription in the MEPS, we can determine whether the person receiving the prescription was covered by Medicare. We aggregate the prescriptions data by 3 digit Multum drug subclass to calculate the share of prescriptions accounted for by the population covered by Medicare. We also identify drugs that are exclusively covered by Medicare Part B using guidance documents issued by the Medicare program; these drugs should not be affected by Part D and were thus excluded from the analytic sample. For example, Part B covers vaccinations, nearly all physician injectibles and many specific types of patient injectibles, certain oral anti-cancer drugs as well as drugs taken in combination with chemotherapy (such as anti-nausea drugs), and immunosuppressive therapy for transplant patients. Finally, we use data from MEPS to model drug utilization and average out of pocket costs at the drug class-year level.
Black Box Warning, New Drug and Generic Drug Entrance
We obtained black box warning data from the monthly summaries of drug safety labeling changes at the FDA MedWatch website. We calculated the number of new brand drugs and generic drugs entrances by quarter and drug class using the FDA Orange Book.
Analysis Sample
We started with the top 1000 drugs listed in the IMS Advertising Database based on their 2005 sales. We first excluded 132 OTC drugs in the sample. All versions of the same drug representing different package size or strength were combined into a single group. There are 748 drugs in the database after this treatment. We then excluded the drugs in the IMS Advertising Database that were covered by Medicare Part B. The 2005 Average Sales Price (ASP) Drug Pricing Files from the Centers for Medicare and Medicaid Services provides a comprehensive list of drugs covered by Medicare Part B in year 2005 (Centers for Medicare and Medicaid Services 2005). We identified 222 drugs covered by Medicare Part B using the 2005 ASP Drug Pricing File. We merged the 526 remaining drugs in the IMS Advertising Data with the 2002–2003 MEPS data. Of these 526 drugs, 126 did not appear in either the 2002 or 2003 MEPS data. 71 of the 126 missing drugs were approved after 2003 and not included in the 2002/2003 MEPS data; the rest are likely missing in MEPS because their level of utilization is too small to be reflected in utilization by the MEPS sample patients.
Next, we removed two drugs from the sample -- Plavix and Meridia – that experienced unique utilization changes that coincided with Part D implementation. Plavix experienced significant changes in clinical guidelines for its use in 2006. Meridia’s average out-of-pocket expense more than doubled since 2006, and it also exhibited a very low Medicare market share.11 We then excluded 23 drugs that did not have information on approval dates in the Orange Book 12. To reduce measurement error in calculating Medicare share, we keep only those drugs belonging to drug classes with at least 100 MEPS respondents taking drugs in that drug class. This excludes 17 drugs from our analysis13. Finally, five drugs in the “Miscellaneous uncategorized agents” class were excluded14. The final analytic sample contained 353 branded drugs from about 57 drug classes and each drug has advertising information for 24 quarters15 from the fourth quarter of 2002 to the third quarter of 2008.
Results
Descriptive Statistics
Table 4 provides the descriptive statistics for our analysis sample of 353 drugs. The table shows that mean DTC and DTP advertising per drug per quarter was $1.8 million and $10.5 million respectively. The data show a secular decline in advertising after the implementation of Medicare Part D. However, this likely reflects changes in life-cycle advertising, as the cohort of drugs in our sample becomes older over time and faces both patent expiration and generic entry. The average Medicare market share for drugs in our sample was 37%, but there is significant variation in Medicare share for drugs in our sample ranging from 4% to 96%. In the Medicare share distribution, the interquartile range is 25% to 51%.
Table 4.
Mean of Variables in the Analytical Sample
| Variables | Full Sample | Before 2006 | After 2006 | P |
|---|---|---|---|---|
|
| ||||
| Quarterly Direct to Consumer (DTC) Dollars (in Thousands) | 1797.50 (6909.45) | 1906.76 (7133.97) | 1668.84 (6633.94) | 0.11 |
| Quarterly Journal Advertising Dollars (in Thousands) | 131.63 (470.85) | 170.25 (527.30) | 86.15 (389.33) | 0.00 |
| Quarterly Detailing Dollars (in Thousands) | 3018.65 (5802.71) | 3513.67 (6252.53) | 2435.71 (5163.87) | 0.00 |
| Quarterly Sampling Dollars (in Thousands) | 7344.44 (17786.59) | 8207.58 (19078.12) | 6327.98 (16076.76) | 0.00 |
| Quarterly Total Direct to Physician (DTP) Dollars (in Thousands) | 10494.72 (23202.63) | 11891.50 (24884.34) | 8849.85 (20934.65) | 0.00 |
| Quarterly Total Advertising Dollars (in Thousands) | 12292.23 (27807.38) | 13798.26 (29716.72) | 10518.68 (25264.29) | 0.00 |
|
|
||||
| Percent of Drugs Ever Have Positive DTC Dollars | 0.34 | |||
| Percent of Drugs Ever Have Positive DTP Dollars | 0.96 | |||
| Percent: Sample Total DTC/All Top 1000 Total DTC (2003) | 0.87 | |||
| Percent: Sample Total DTP/All Top 1000 Total DTP (2003) | 0.87 | |||
| Years Since FDA Approval as of 2006 | 12.37 | |||
| Average Medicare Market Share by Individual Drug | 0.34 | |||
| Average Medicare Market Share by Drug Class | 0.37 | |||
| Number of Drugs | 353 | |||
| Number of Drug Classes | 59 | |||
| Number of Quarters | 24 | |||
|
|
||||
| Number of Observations | 8447 | 4568 | 3879 | |
Note: Total physician advertising dollars include journal advertising dollars, detailing dollars and sampling dollars. Total advertising dollars include direct to consumer dollars and total physician advertising dollars. Standard errors are reported in parenthesis. P-values associated with the t-tests of variables before and after 2006 are reported in the last column. The approval year for drugs is left censored at 1982 (i.e. drugs approved before 1982 are assigned 1982 as the approval year).
Table 5 shows that the drugs in our analysis sample are largely representative of advertised drugs in the US pharmaceutical market. The 353 drugs in the sample account for 76% of all DTC advertising and 81% of all direct-to-physician advertising. These drugs also account for 46% of total prescriptions, 38% of total patients and 61% of total expenditure of the entire drug market. Note that the utilization shares are lower than the advertising shares, because generic drugs appear in utilization figures, but almost never advertise.
Table 5.
Sample Drugs in Context
| Variable | Sample Total | % of Total Market |
|---|---|---|
|
| ||
| DTC Advertising | $2.5B | 76% |
| DTP Advertising | $16.9B | 81% |
| Total Advertising | $19.4B | 80% |
|
| ||
| For Non-Elderly Population Only: | ||
|
| ||
| Total Prescription | 782M | 48% |
| Total Patients | 190M | 38% |
| Total Expenditure | $63.5B | 63% |
|
| ||
| For Entire Population: | ||
|
| ||
| Total Prescription | 1.29B | 46% |
| Total Patients | 285M | 38% |
| Total Expenditure | $108B | 61% |
Note: All calculations are based on year 2003. Data on advertising expenditures of the entire market were from GAO(2006). Total prescriptions, patients and expenditure of the entire market were estimated using all drugs in the MEPS data. B stands for billion. M stands for million.
Graphical Analysis
Figure 1 shows trends in total advertising by Medicare share. For these figures, we classify drugs into two groups -- above and below the median Medicare market share of 36%. The left panel of the figure shows trends in advertising for “dominant” drugs that accounted for the highest share of advertising in a drug class prior to implementation of Part D. The right panel shows trends in advertising for non-dominant drugs. The raw or unadjusted trends in advertising are consistent with the predictions from the theoretical model. The left panel shows that Medicare Part D was associated with an increase in total advertising for dominant drugs with above median Medicare share, relative to trends in advertising for dominant drugs with below median Medicare share. In contrast, there is little divergence in advertising trends across Medicare share for non-dominant drugs. Figure 2 shows similar patterns when competition is measured by the number of competitors in the drug class. Finally, patterns are similar for both direct-to-physician and direct-to-consumer advertising types.
Figure 1.
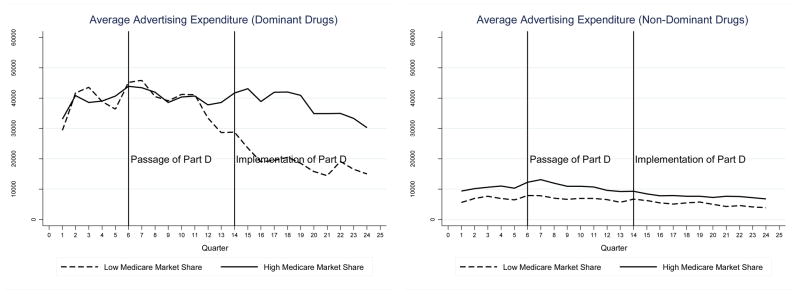
Trends in Total Advertising Expenditures by Medicare Share for Dominant vs. Non-Dominant Drugs
Figure 2.
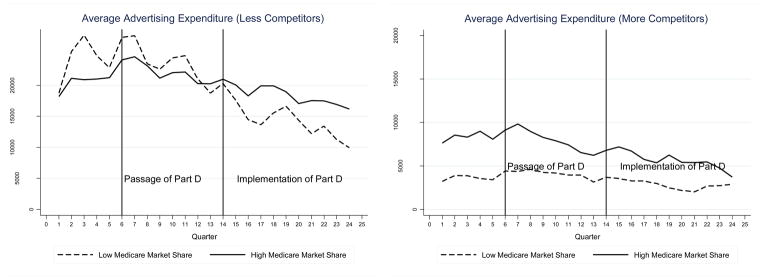
Trends in Total Advertising Expenditures by Medicare Share for Drugs with More or Less Competitors
Regression Results for Drug Advertising
Table 6 reports regression results for total advertising. The four columns in the table report results from the specification in equation (1) for each drug type. The results show that Medicare Part D led to a significant increase in total advertising for dominant drugs and for drugs with fewer competitors in their classes. In contrast, Medicare Part D was not associated with a statistically detectable change in total advertising for non-dominant drugs or drugs that faced more competition. These results imply that total advertising increased by $15.2 million per quarter, or by about 38% of average advertising expenditures for dominant drugs. Overall, these results suggest an 18% increase in advertising expenditures across all study drugs. Even if Part D had no impact on advertising for any drug outside our sample, this would still have increased aggregate pharmaceutical advertising by 14%. Similarly, the results for models stratified by the degree of competition in drug class imply that total advertising expenditures increased $6.8 million dollars per quarter, or by about 31% of average advertising expenditures for drugs with less competition prior to implementation of Part D. These models imply a 24% increase in advertising expenditures across all study drugs. Even if Part D had no impact on advertising for any drug outside our sample, this would still have increased aggregate pharmaceutical advertising by 19%.
Table 6.
Effects of Medicare Part D on Total Advertising by Drug Type
| Drug Types
|
||||
|---|---|---|---|---|
| More Competition | Less Competition | Dominant | Non-Dominant | |
| Part D* Medicare Share | −1,706.72 [3,946.67] | 16,294.07 [6,815.57]** | 36,011.67 [13,809.82]** | −1,172.73 [3,839.06] |
| Number of Black Box Warnings | −3,035.50 [1,940.45] | −2,176.59 [3,227.92] | −1,390.39 [6,109.50] | −3,087.45 [2,079.28] |
| Number of New Brand Drugs Entering Drug Class | −542.92 [314.57]* | 5.585 [665.39] | −469.87 [1,799.22] | −542.42 [284.67]* |
| Number of New Generic Drugs Entering Drug Class | −13.17 [211.84] | 702.69 [629.81] | 1,056.04 [1,600.43] | 83.13 [234.26] |
| Constant | 5,570.10 [796.82]*** | 18,672.41 [1,245.86]*** | 31,932.55 [2,545.51]*** | 8,309.25 [763.70]*** |
| Observations | 4312 | 4135 | 1368 | 7031 |
| R-squared | 0.78 | 0.86 | 0.88 | 0.8 |
All models include drug fixed effects and time fixed effects. Clustered standard errors by drug class are reported in the parentheses.
Significance:
p<0.10,
p<0.05,
p<0.01.
We also tested for an anticipatory change in advertising in the quarters following the passage of Part D but before the implementation of Part D. We found no statistically significant change in advertising during this period.
The above results might be confounded by changes in advertising over the life cycle of drugs. For example, we would overestimate the effects of Part D if newer drugs were more likely to have high Medicare share and advertising decreased over the life cycle of a drug. To account for this potential confounding, we estimated models that accounted for changes in advertising over the life cycle of a drug. In particular, we classified drugs into 4 groups based on FDA approval date (prior to 1982prior to 1983–1989–1990 – 1999 – 2000 or after) and allowed each group to have a different quadratic time trend in advertising. Our results are robust to the inclusion of these covariates and are available upon request.
We also tested robustness of results to alternate measures of dominant drugs. In particular, instead of estimating separate models by dominant versus non-dominant drugs we used a continuous measure of dominance which equaled the drug’s share of advertising within its drug class in the pre Part D period. The results from this model were consistent with the results from the model presented in table 6 and suggested that drugs that accounted for a higher share of advertising in the drug class experienced a larger change in advertising following the passage of Part D. For example, the results from this model suggest that total advertising expenditures across all drugs increased by 16% while the results from the model in table 6 for dominant vs. non-dominant drugs suggests that advertising expenditures increased by about 18%. Similarly, we tested robustness of results by estimating a model with a continuous measure of competition: the number of advertised drugs in the drug class. Again the results were consistent with previous model and suggested that Part D increased advertising more for drugs in less competitive drug classes. The results from this model suggested that total advertising expenditures increased by about 28% across all study drugs, compared to our baseline estimate of 24%.
Table 7 reports regression results for DTC and DTP advertising separately. Again the results are consistent with the predictions from theoretical model and confirm that both direct to consumer advertising and physician advertising increased for dominant drugs and for drugs with less competition. Overall these models imply a 69% to 73% increase in DTC advertising expenditures across all drugs. Similarly, the results from DTP advertising models imply a 10% to 17% increase in physician advertising expenditures across all drugs. The difference between the DTP and DTC percentage effects are striking. Of course, DTC is about one-fourth to one-fifth the size of DTP, so that the absolute changes in advertising are much closer in magnitude. In any event, the prior literature suggests a reason why the DTC effect might be larger: DTP typically involves more “predatory” advertising, while DTC is more “cooperative” (Rosenthal, Berndt et al. 2002; Ling, Berndt et al. 2003; Rosenthal, Berndt et al. 2003; Donohue, Berndt et al. 2004; Iizuka and Jin 2005). Given these findings, economic theory would predict larger effects on DTC instead of DTP.
Table 7.
Effects of Medicare Part D on Direct to Consumer and Physician advertising by Drug Type
| Drug Types
|
||||
|---|---|---|---|---|
| More Competition | Less Competition | Dominant | Non-Dominant | |
| DTC Advertising | ||||
| Part D* Medicare Share | 587.25 [1,379.80] | 6,711.25 [2,617.70]** | 19,148.95 [6701.49]*** | −211.37 [1,102.99] |
| Physician Advertising | ||||
| Part D* Medicare Share | −2,293.96 [3,430.94] | 9,582.80 [5,039.59]* | 16,862.72 [9,810.79]* | −961.36 [3,062.06] |
| Observations | 4312 | 4135 | 1368 | 7031 |
All models include drug fixed effects and time fixed effects. Models also include number of black box warnings, number of generic drugs, and number of brand name drugs entering drug class as covariates. Clustered standard errors by drug class are reported in the parentheses.
Significance:
p<0.10,
p<0.05,
p<0.01.
Drug Utilization
Table 8 provides the results from the instrumental variable regressions. The coefficient in the first stage is positive and significant at the 1% level, and the F-statistic is larger than the “rule of thumb” value of 10.0. The results in Table 8 show that a $1,000 increase in advertising stock for a drug class is associated with about 9 new prescriptions and 2 new patients in the drug class.
Table 8.
Effect of Advertising on Drug Utilization at the Drug Class Level
| Dependent Variable | Annual Scripts | Annual Patients |
|---|---|---|
| Advertising Stock ($000s) | 9.07*** (0.33) | 1.95*** (0.0.09) |
| Sample Size | 290 | 290 |
|
| ||
| First-Stage: Predicted Advertising | 1.06*** (0.329) | |
| First-Stage F-Statistic | 10. 32 | |
Data are from the Medical Expenditure Panel Surveys 2003 to 2007. All models include number of black box warnings, number of generic drugs, and number of brand name drugs entering drug class as covariates. Clustered standard errors by drug class are reported in the parentheses.
Significance:
p<0.10,
p<0.05,
p<0.01.
These results, along with our estimated rate of advertising depreciation, predict the effects of an increase in the flow of advertising on current and future drug utilization16. Specifically, a $1,000 increase in current advertising flow is associated with about 23 new prescriptions and 5 new patients in the long-run. The implied elasticity of drug utilization with respect to advertising expenditures is 0.22. Results presented earlier showed that the Part D program increased advertising by 31% for drugs facing less competition and by 18% across all study drugs. Combining the percentage change in advertising with the elasticity of utilization with respect to advertising implies that Part D increased non-elderly utilization in advertised drug classes by about 4%. Finally, since advertised drug classes account for about 90% of utilization (in the MEPS), Part D is estimated to have increased overall non-elderly utilization by about 3.6%.
There are several important points to note about these utilization effects. First, the increase is sizeable and suggests an important spillover effect of Part D to the non-elderly market. By contrast, previous estimates of the effects of Part D suggest that elderly utilization increased by about 6 to 12%. Second, the utilization effects are concentrated in drug classes that face less competition or where access problem are likely to be the greatest. However, the possible welfare effects of these increases in utilization should be viewed in light of the important caveats discussed earlier, namely that advertising may lead to misperceptions of value, or overconsumption relative to the efficient utilization point. Finally, it is worth noting that our estimate of the elasticity of class level drug utilization with respect to advertising are consistent with the previous literature which finds drug class level elasticity estimates ranging from 0.02 to 0.57 (Berndt, Bui et al. 1997; Rosenthal, Berndt et al. 2003).
Conclusions
We studied the theoretical and empirical effects of prescription drug insurance on pharmaceutical advertising behavior. Since insurance is likely to make the market more profitable, more advertising will tend to result, particularly in the least competitive segments of the pharmaceutical market. The implementation of Medicare Part D appeared to cause a 14% to 19% increase in total advertising spending, which resulted in a 4% increase in total utilization of drugs among the non-elderly. This represents a spillover effect onto a group not directly targeted by the legislation.
Our findings suggest the importance of accounting for spillover effects when assessing the utilization and welfare effects of prescription drug insurance expansion. The welfare effects of advertising in the pharmaceutical market are complex. Even though market power typically leads to under-provision, the presence of insurance that lowers consumer out-of-pocket prices makes it unclear whether there is under-use or over-use. The possibility of asymmetric information and agency problems between physicians and patients complicates matters further. It is thus unclear in general whether greater utilization is better or worse for the non-elderly consumers experiencing spillover effects. We leave to future research the question of under-use or over-use in the market.
Our findings also suggest the importance of additional research concerning the strategic interaction among firms competing through advertising. To focus on a few salient implications, we abstracted from several complexities, including the competition between large and small firms. Large firms may have “predatory” incentives to advertise against small firms with limited resources to respond. This could create negative spillovers in advertising in certain competitive settings. In addition, there are interactions between incentives for advertising and incentives for innovation. Profitability, for instance, boosts the returns to both advertising and innovation investments (Blume-Kohout and Sood Forthcoming). To the extent that innovation might foster future competition, this may suggest that the advertising effects of insurance expansions are self-limiting in the long-run, when competition drives advertising down. Finally, we have borrowed from the existing literature the finding that Part D makes the pharmaceutical market more profitable. Not all such insurance expansions will have this effect. For instance, more insurance creates larger private insurers, and potentially encourages the government itself to negotiate prices directly with manufacturers. Both of these would tend to drive down profitability – the increase in utilization enjoyed by manufacturers might be partially or even wholly offset by reductions in price.
The implementation of Part D reflects the growing interest among health policymakers in legislating through and in concert with the private-sector. The involvement of the private-sector might generate considerable efficiencies at a time when the public-sector can ill-afford large, centrally administered public programs. Yet, they also raise the possibility of various behavioral responses that can mitigate or reinforce the intended effects of the policies themselves. As a result, policymakers must be mindful of the economic forces unleashed by public-private partnership. Health economists should do their part to identify and predict these impacts.
Acknowledgments
For their many helpful comments and suggestions, we thank meeting participants at the Harvard-USC Medicare conference, the NBER Summer Institute, the ASHE annual meeting, and the Pharmaceutical Economics and Policy Council, along with seminar participants at USC, UCLA, UC-Berkeley, and Stanford. All errors and omissions are ours. We acknowledge research support from NIA grants RC4 AG039036 and P01 AG033559.
Footnotes
We use the term advertising to refer to all promotion and marketing activities, not just advertisements in popular media.
A recent exception to this literature is Dave and Saffer (2012), who find that direct-to-consumer advertising gives a significant boost to price.
The use of average prices here sacrifices little generality when studying symmetric equilibria.
Specifically, . The standard Lerner mark-up condition is a special case in which sp = 0.
This condition is related to that derived in the classic paper by Dorfman and Steiner (1954) proving that the marginal value product of advertising by a monopolist is equal to the ordinary elasticity of demand faced by the firm. Scherer and Ross (1990) derive an analogous condition for oligopoly.
Note that total revenue is given by , and the firm’s quantity is q * s. It thus follows that p̄1−γmi is equal to unit revenue.
Friedman (1957) is an early paper to explore this form for the market share function. Moreover, it is difficult to construct other market share functions that satisfy the adding-up constraint for all possible configurations of Ai.
Implementation of Part D interacted with Medicare exposure is a valid instrument in a second-stage model of drug-class utilization, but not in a model of drug-level utilization. At the drug-level, advertising can influence utilization of one drug by influencing the advertising of other drugs in a class; this would pose a validity problem in such a specification, because competitor advertising would be an omitted variable correlated with the instrument.
Note that the first-stage models rely on the equations stratified by more and less competitive classes, not on stratification across dominant and non-dominant drugs.
Prior work by the census bureau shows that MEPS undercounts the number of people on Medicaid and thus over estimates the rate of uninsurance. We do not know of any work that examines mis-reporting of Medicare status in MEPS, however it is possible that some mis-reporting exists.
Including these two drugs has little effect on our estimates.
Many of those 23 drugs were marketed in the U.S. without formal FDA approval. For example, prescription prenatal vitamins (e.g. Prenate Elite) and papain-containing drug products in topical form (e.g. Accuzyme) do not need FDA approval for marketing. For the rest of those drugs, the reason for not included in the Orange Book is unclear. However, the official Orange Book website states that drugs approved only on the basis of safety (e.g. Librax) or pre-1938 drugs are not included in the Orange Book.
As an alternate measure of exposure to Part D we also estimate the share of total prescriptions on a particular drug accounted for by the population covered by Medicare. However, this measure of exposure to Part D might suffer from significant measurement error due to the limited number of MEPS respondents taking certain drugs. For example, if we limit our sample to drugs with at least 100 MEPS respondents we would exclude 69% of the remaining drugs from our analysis.
The five drugs are: Accutane, Amnesteem, Nicotrol, Sotret and Xenical.
For drugs that were approved after year 2002, the advertising information before the approval date was missing.
The predicted long term effect of an increase in flow of advertising is simply: b(1 + [1 − γ] + [1 − γ]2 + [1 − γ]3). Where b is the estimated coefficient on stock of advertising and γ is the depreciation rate.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azoulay P. Do Pharmaceutical Sales Respond to Scientific Evidence? Journal of Economics and Management Strategy. 2002;11(4):551–594. [Google Scholar]
- Berndt ER, Bui L, et al. Information, Marketing, and Pricing in the U.S. Antiulcer Drug Market. The American Economic Review. 1995;85(2):100–105. [PubMed] [Google Scholar]
- Berndt ER, Bui LT, et al. The Roles of Marketing, Product Quality and Price Competition in the Growth and Composition of the U.S. Antiulcer Drug Industry. Economics of New Products, Studies in Income and Wealth. 1997;58:277–322. [Google Scholar]
- Bhattacharya J, Vogt WB. A Simple Model of Pharmaceutical Price Dynamics. Journal of Law and Economics. 2003;46(2):599–626. [Google Scholar]
- Blume-Kohout ME, Sood N. Market Size and Innovation: Effects of Medicare Part D on Pharmaceutical Research and Development. Journal of Public Economics. doi: 10.1016/j.jpubeco.2012.10.003. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford WD, Kleit AN, et al. Effects of Direct-to-Consumer Advertising of Hydroxymethylglutaryl Coenzyme A Reductase Inhibitors on Attainment of LDL-C Goals. Clinical Therapeutics. 2006;28(12):2105–2118. doi: 10.1016/j.clinthera.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Calfee JE, Winston C, et al. Direct-to-Consumer Advertising and the Demand for Cholesterol-Reducing Drugs. Journal of Law and Economics. 2002;45(2):673–690. [Google Scholar]
- Centers for Medicare and Medicaid Services. October ASP Pricing File and ASP NOC Pricing File. Centers for Medicare and Medicaid Services; 2005. [Google Scholar]
- Dave D, Saffer H. Impact of Direct-to-Consumer Advertising on Pharmaceutical Prices and Demand. Southern Economic Journal Vol. 2012;79(1):97–126. [Google Scholar]
- Depken CA, Snow A. The strategic nature of advertising in segmented markets. Applied Economics. 2008;40:2987–2994. [Google Scholar]
- Donohue JM, Berndt ER, et al. Effects of pharmaceutical promotion on adherence to the treatment guidelines for depression. Med Care. 2004;42(12):1176–1185. doi: 10.1097/00005650-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Donohue JM, Berndt ER, et al. Effects of pharmaceutical promotion on adherence to the treatment guidelines for depression. Medical Care. 2004;42(12):1176–1185. doi: 10.1097/00005650-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Dorfman R, Steiner PO. Optimal Advertising and Optimal Quality. The American Economic Review. 1954;44(5):826–836. [Google Scholar]
- Duggan M, Scott Morton F. The Effect of Medicare Part D on Pharmaceutical Prices and Utilization. Cambridge, MA: National Bureau of Economic Research; 2008. [DOI] [PubMed] [Google Scholar]
- Duggan M, Scott Morton F. The Effect of Medicare Part D on Pharmaceutical Prices and Utilization. American Economic Review. 2010;100(1):590–607. doi: 10.1257/aer.100.1.590. [DOI] [PubMed] [Google Scholar]
- Friedman J. The Incidence of the Medicare Prescription Drug Benefit: Using Asset Prices to Assess its Impact on Drug Makers. Cambridge, MA: Harvard University; 2009. [Google Scholar]
- Friedman JW. Advertising and Oligopolistic Equilibrium. Bell Journal of Economics. 1983;14(2):464–473. [Google Scholar]
- Friedman L. Game theory models in the allocation of advertising expenditures. Operations research. 1957;6:699–709. [Google Scholar]
- Hurwitz MA, Caves RE. The economics of intellectual property. Volume 3. Empirical evidence, trade secrets and trademarks. Vol. 145. International Library of Critical Writings in Economics; Cheltenham, U.K: Northampton, Vt: Elgar; 2002. Persuasion or Information? Promotion and the Shares of Brand Name and Generic Pharmaceuticals; pp. 439–60. distributed by American International Distribution Corporation, Williston, Vt. R. Towse and R. Holzhauer. [Google Scholar]
- Iizuka T, Jin GZ. Drug Advertising and Health Habits. National Bureau of Economic Research, Inc; 2005. NBER Working Papers: 11770. [Google Scholar]
- Iizuka T, Jin GZ. The Effect of Prescription Drug Advertising on Doctor Visits. Journal of Economics and Management Strategy. 2005;14(3):701–727. [Google Scholar]
- Ketcham JD, Simon K. Medicare Part D’s Effects on Elderly Drug Costs and Utilization. National Bureau of Economic Research, Inc; 2008. NBER Working Papers: 14326. [PubMed] [Google Scholar]
- King C. Product Differentiation and Competition in the Market for Antiulcer Drugs. Harvard Business School; 2002. [Google Scholar]
- Lakdawalla D, Sood N. Innovation and the Welfare Effects of Public Drug Insurance. Journal of Public Economics. 2009;93(3–4):541–548. doi: 10.1016/j.jpubeco.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakdawalla DN, Philipson TJ. Intellectual Property and Marketing in the Pharmaceutical Industry. Journal of Law and Economics Forthcoming. [Google Scholar]
- Lichtenberg FR, Sun SX. The impact of Medicare Part D on prescription drug use by the elderly. Health Affairs. 2007;26(6):1735–1744. doi: 10.1377/hlthaff.26.6.1735. [DOI] [PubMed] [Google Scholar]
- Ling DC, Berndt ER, et al. Deregulating Direct-to-Consumer Marketing of Prescription Drugs: Effects on Prescription and Over-the-Counter Product Sales. Journal of Law and Economics. 2003;45:691–723. [Google Scholar]
- Marshall A. Industry and Trade. London: MacMillan; 1923. [Google Scholar]
- Nerlove M, Arrow KJ. Optimal Advertising Policy under Dynamic Conditions. Economica. 1962;29(114):129–142. [Google Scholar]
- Piga C. A Dynamic Model of Advertising and Product Differentiation. Review of Industrial Organization. 1998;13(5):509–522. [Google Scholar]
- Rizzo JA. Advertising and Competition in the Ethical Pharmaceutical Industry: The Case of Antihypertensive Drugs. Journal of Law and Economics. 1999;42(1):89–116. [Google Scholar]
- Rosenthal MB, Berndt ER, et al. Demand effects of recent changes in prescription drug promotion. Frontiers in Health Policy Research. 2003;6:1–26. [Google Scholar]
- Rosenthal MB, Berndt ER, et al. Promotion of prescription drugs to consumers. N Engl J Med. 2002;346(7):498–505. doi: 10.1056/NEJMsa012075. [DOI] [PubMed] [Google Scholar]
- Scherer FM. Industrial Market Structure and Economic Performance. Chicago: Rand McNally; 1970. [Google Scholar]
- Scherer FM, Ross D. Industrial Market Structure and Economic Performance. Boston: Houghton Mifflin; 1990. [Google Scholar]
- Schmalensee R. The economics of advertising. Amsterdam: North-Holland Pub. Co; 1972. [Google Scholar]
- Schmalensee R. A Model of Promotional Competition in Oligopoly. Review of Economic Studies. 1976;43(3):493–507. [Google Scholar]
- Slade ME. Product Rivalry with Multiple Strategic Weapons: An Analysis of Price and Advertising Competition. Journal of Economics and Management Strategy. 1995;4(3):445–476. [Google Scholar]
- Wosinska M. Just What the Patient Ordered? Direct-to-Consumer Advertising and the Demand for Pharmaceutical Products. SSRN eLibrary; 2002. [Google Scholar]
- Yin W, Basu A, et al. The Effect of the Medicare Part D Prescription Benefit on Drug Utilization and Expenditures. Ann Intern Med. 2008;148(3):169–177. doi: 10.7326/0003-4819-148-3-200802050-00200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Donohue JM, et al. The Effect of Medicare Part D on Drug and Medical Spending. New England Journal of Medicine. 2009;361(1):52–61. doi: 10.1056/NEJMsa0807998. [DOI] [PMC free article] [PubMed] [Google Scholar]


