The X-ray structure of the light, oxygen or voltage domain 2 of phototropin 1 from A. thaliana (AtLOV2) in its dark-adapted state has been determined. The N-terminal flanking A′α helix of AtLOV2 plays a role in its dimerization.
Keywords: LOV domains, N-terminal A′α helix, C-terminal Jα helix, coiled coil, dimerization, light-mediated signal transduction
Abstract
A key role in signal transduction and dimerization mediated by Per–Arnt–Sim (PAS) domains is played by α-helical linkers that flank the structurally similar α/β cores of these domains. However, crystal-packing forces and the different construct lengths and sequences of the PAS domains influence the final length and orientation of the linkers relative to the core and create uncertainty in the exact mechanism of the linker function. Thus, structural characterization and comparison of the linkers within isolated PAS-domain constructs and/or full-length PAS-containing proteins is important for clarification of the mechanism. The plant blue-light photoreceptors phototropins possess two N-terminal flavin mononucleotide-based light, oxygen or voltage (LOV) domains (LOV1 and LOV2) that comprise a subclass of the PAS family and one C-terminal serine/threonine kinase domain whose enzymatic activity is regulated by blue light. The dark-adapted state crystal structures of the Arabidopsis thaliana phototropin 1 and phototropin 2 LOV1-domain constructs flanked by an N-terminal A′α helix and the structure of the phototropin 2 core LOV2 domain are known. Here, the crystal structure of the A. thaliana phototropin 1 LOV2 domain has been determined in its dark-adapted state. The core is flanked by an N-terminal A′α helix and a C-terminal Jα helix similar to those in the previously reported structure of Avena sativa phototropin 1 LOV2. In contrast to the monomeric A. sativa LOV2, A. thaliana LOV2 is a dimer in which two A′α helices adopt a scissor-like orientation at the dimer interface and form a short α-helical coiled coil. The Jα helix predominantly interacts with the β-sheet and plays a role in coiled-coil formation and dimerization.
1. Introduction
Arabidopsis thaliana phototropin 1 (phot1) and phototropin 2 (phot2) are photosensory proteins that contain two N-terminal light, oxygen or voltage (LOV) domains (LOV1 and LOV2) and one C-terminal serine/threonine kinase domain (Sakai et al., 2001 ▶; Kagawa et al., 2001 ▶; Kinoshita et al., 2001 ▶; Sakamoto & Briggs, 2002 ▶; Takemiya et al., 2005 ▶; Folta & Spalding, 2001 ▶; Christie et al., 1998 ▶). Both LOV domains belong to the Per–Arnt–Sim (PAS) domain superfamily (Taylor & Zhulin, 1999 ▶; Henry & Crosson, 2011 ▶; Möglich et al., 2009b ▶), noncovalently bind flavin mononucleotide (FMN) in their dark state (Taylor & Zhulin, 1999 ▶) and possess a conserved α/β fold of their core flanked by α-helical linkers at their N-termini and C-termini (Harper et al., 2003 ▶; Halavaty & Moffat, 2007 ▶). Although LOV1 and LOV2 undergo identical reversible photochemical reactions (Kasahara et al., 2002 ▶; Salomon et al., 2000 ▶), they play different roles in signal transduction and oligomerization by phototropins (Christie et al., 2002 ▶; Matsuoka & Tokutomi, 2005 ▶; Nakasako et al., 2004 ▶, 2008 ▶; Salomon et al., 2004 ▶; Okajima et al., 2012 ▶; Nakasone et al., 2008 ▶; Christie, 2007 ▶).
The photocycle in plant phototropins begins with the light-driven formation of a covalent flavin-cysteinyl adduct between the C4a atom of FMN and the Sγ atom of a conserved cysteine residue (Cys512 in A. thaliana LOV2) in their LOV domains (Salomon et al., 2000 ▶; Swartz et al., 2001 ▶; Iwata et al., 2002 ▶; Halavaty & Moffat, 2007 ▶). Formation of this covalent bond triggers small structural changes in the FMN-binding pocket and its vicinity (Crosson & Moffat, 2002 ▶; Halavaty & Moffat, 2007 ▶; Harper et al., 2003 ▶) that ultimately activate autophosphorylation of phototropin by the kinase domain (Crosson et al., 2003 ▶). The photocycle is completed in the dark by slow spontaneous rupture of the covalent bond. Discovery of a C-terminal Jα helix flanking the core of the Avena sativa LOV2 domain (Harper et al., 2003 ▶) revealed that light-mediated displacement of the Jα helix from the core β-sheet and unfolding of Jα is an early step in autophosphorylation. Crystal-packing forces impeded these substantial structural changes in crystals of A. sativa LOV2(404–546) (denoted AsLOV2; Halavaty & Moffat, 2007 ▶). However, formation of the covalent adduct in the crystals resulted in the rearrangement of the hydrogen-bonding interactions in a linker region N-terminal to the core that contains a short α-helix denoted the A′α helix (Halavaty & Moffat, 2007 ▶). Upon light illumination, the A′α helix may unfold first and then displace the Jα helix (Zayner et al., 2012 ▶). In contrast, Takeda et al. (2013 ▶) have shown that the A′α helix unfolds after disruption of the Jα helix. Numerous crystal structures of homologous PAS domains show that the orientation and length of the flanking α-helices might depend on the specific PAS sequence and be affected by the length of the construct and/or by crystal packing [PDB entries 3sw1 (Circolone et al., 2012 ▶), 2gj3 (Key et al., 2007 ▶), 2v0u (Halavaty & Moffat, 2007 ▶), 3e4o (Zhou et al., 2008 ▶), 1d06 (Miyatake et al., 2000 ▶), 3cwf (Chang et al., 2010 ▶), 1xj3 (Key & Moffat, 2005 ▶), 4hj4 (Conrad et al., 2013 ▶), 4gcz (Diensthuber et al., 2013 ▶), 3c2w (Yang et al., 2008 ▶) and 3ewk (Ukaegbu & Rosenzweig, 2009 ▶)]. The flanking linkers form α-helical coiled coils and/or bundles through which signal transduction and dimerization is facilitated (Little et al., 2012 ▶; Slavny et al., 2010 ▶; Lee et al., 2008 ▶; Etzkorn et al., 2008 ▶; Nan et al., 2010 ▶; Key et al., 2007 ▶; Park et al., 2004 ▶; Miyatake et al., 2000 ▶; Diensthuber et al., 2013 ▶; Wang et al., 2013 ▶). Given the structural diversity of the flanking regions, a comparative sequence–structure analysis is increasingly important for understanding the exact mechanism(s) of their function, particularly since LOV domains are increasingly being used for optogenetic applications (Strickland et al., 2008 ▶, 2012 ▶; Wu et al., 2009 ▶; Christie, Gawthorne et al., 2012 ▶; Möglich et al., 2009a ▶; Möglich & Moffat, 2010 ▶; Drepper et al., 2011 ▶).
We have determined the crystal structure at 2.75 Å resolution of the dark-adapted state of the A. thaliana phot1 LOV2 domain construct spanning residues 452–615, denoted AtLOV2, that includes the N-terminal A′α helix and the C-terminal Jα helix. The A′α helix largely contributes to dimerization through an α-helical coiled coil. Despite its spatial proximity to the A′α helix, the Jα helix is not part of the coiled coil, but does appear to be involved in the coiling and dimerization of AtLOV2.
2. Materials and methods
2.1. Cloning, expression, purification and crystallization of AtLOV2
AtLOV2 was amplified from the genomic DNA and cloned into the pET-28c vector (EMD Serono, Rockland, Massachusetts, USA) via NdeI/HindIII restriction sites, expressed in Escherichia coli BL21 (DE3) cells (NEB, Ipswich, Massachusetts, USA) and purified as described previously (Halavaty & Moffat, 2007 ▶). In brief, the BL21 (DE3) cells were grown to an OD600 of 0.7 at 310 K at 200 rev min−1 followed by overnight induction at 289 K with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) in the dark. After induction, the cells were spun down and ruptured by sonication in 20 mM Tris–HCl buffer pH 7.4, 20 mM NaCl (buffer A) containing an EDTA-free protease-inhibitor cocktail tablet (Roche Diagnostic, Mannheim, Germany). The soluble fraction was loaded onto an Ni–NTA column and washed with 20 mM imidazole in buffer A. AtLOV2 was eluted with 250 mM imidazole in buffer A followed by overnight dialysis against buffer A. The N-terminal purification tag was cleaved by incubating the protein in the dark with thrombin (EMD Millipore, Merck, Darmstadt, Germany) according to the standard manufacturer’s protocol. The extent of cleavage was assayed by passing the reaction mixture over an Ni–NTA column and analysing the pooled fractions by SDS–PAGE. Cleaved AtLOV2 was further purified on a Superdex 200 gel-filtration column (GE Healthcare, Piscataway, New Jersey, USA) in buffer A plus 15% glycerol (buffer B).
AtLOV2 (21 mg ml−1) was crystallized in buffer B by mixing it with a candidate crystallization condition in a 1:1 volume ratio in the dark. Crystals suitable for data collection and structure determination were obtained by the sitting-drop vapor-diffusion technique using a condition from The JCSG+ Suite (Qiagen, Valencia, California, USA) consisting of 40 mM potassium phosphate, 16%(w/v) PEG 8000, 20%(v/v) glycerol at 287 K. Crystals of different sizes appeared in between one and two weeks. Prior to data collection, crystals were flash-cooled in liquid nitrogen in the dark.
2.2. Data collection and structure determination
Monochromatic oscillation X-ray data were collected at station 14-BM-C of BioCARS at the Advanced Photon Source (APS), Argonne National Laboratory (ANL) and were processed with HKL-3000 (Minor et al., 2006 ▶). The structure was determined by molecular replacement using Phaser (McCoy et al., 2007 ▶) from the CCP4 package (Winn et al., 2011 ▶) using AsLOV2 as a search model (PDB entry 2v0u; Halavaty & Moffat, 2007 ▶). The initial model was rebuilt with ARP/wARP (Morris et al., 2003 ▶), corrected in Coot (Emsley & Cowtan, 2004 ▶; Emsley et al., 2010 ▶), refined with REFMAC v.5.5 (Murshudov et al., 2011 ▶) and validated with the ADIT validation (http://deposit.pdb.org/validate/) and MolProbity (Chen et al., 2010 ▶; Davis et al., 2007 ▶) servers. Data-processing and structure-determination statistics are given in Table 1 ▶. The atomic coordinates were deposited in the PDB as entry 4hhd. Structure figures were prepared with PyMOL (DeLano, 2010 ▶) and LigPlot + (Laskowski & Swindells, 2011 ▶). Multiple sequence alignments were performed with ClustalW (Thompson et al., 1994 ▶) and figures were generated with ESPript2.2 (Gouet et al., 1999 ▶).
Table 1. Data-collection and refinement statistics.
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Wavelength (Å) | 0.9002 |
| Temperature (K) | 105 |
| Space group | P41212 |
| Unit-cell parameters (Å, °) | a = b = 63.4, c = 171.3, α = β = γ = 90.0 |
| Resolution range (Å) | 30.00–2.75 (2.80–2.75) |
| No. of unique reflections | 8574 (399) |
| R merge (%) | 7.0 (58.0) |
| Completeness (%) | 87.9 (86.6) |
| 〈I/σ(I)〉 | 17.5 (2.2) |
| Multiplicity | 3.3 (3.6) |
| Wilson B factor (Å2) | 66.3 |
| Refinement | |
| Resolution range (Å) | 29.72–2.75 (2.82–2.75) |
| No. of reflections | 8128 (545) |
| R work/R free (%) | 23.0/29.0 (29.0/47.0) |
| No. of protein molecules/non-H atoms | 2/2466 |
| No. of ligands/non-H atoms | 2/62 |
| No. of water O atoms | 29 |
| Mean temperature factor (Å2) | 48.4 |
| Coordinate deviation | |
| R.m.s.d., bonds (Å) | 0.01 |
| R.m.s.d., angles (°) | 1.31 |
| Ramachandran plot† | |
| Most favored (%) | 88.1 |
| Allowed (%) | 11.1 |
| Generously allowed (%) | 0.7 |
| Disallowed (%) | 0.0 |
Statistics are based on PROCHECK (Laskowski et al., 1993 ▶).
3. Results and discussion
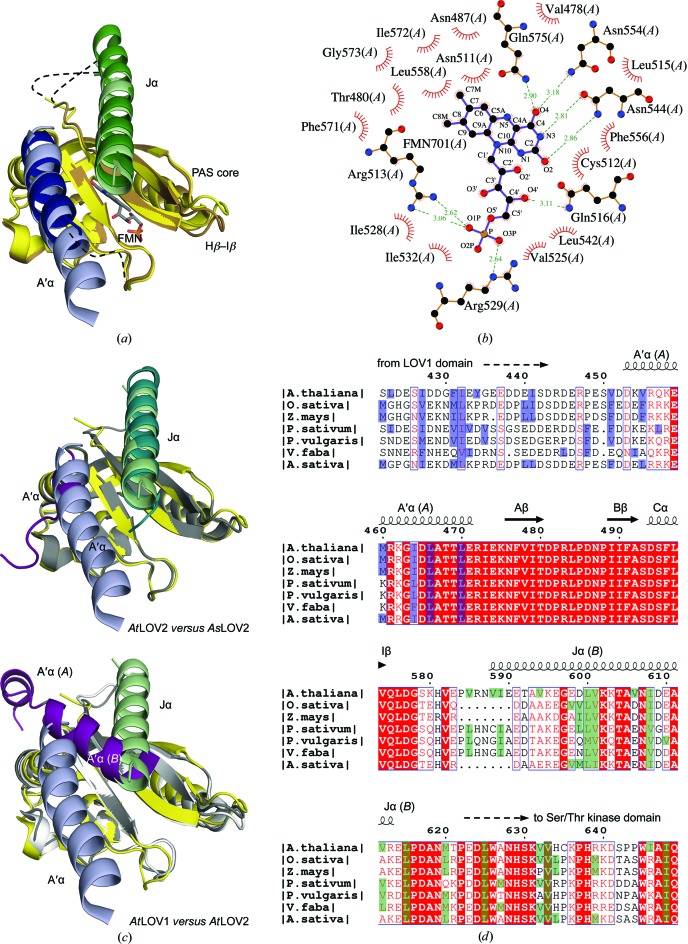
The asymmetric unit of the AtLOV2 crystal contains two monomers denoted chain A and chain B (r.m.s.d. of 0.9 Å over 144 Cα atoms; Fig. 1 ▶ a). The flanking A′α and Jα helices emerge on the same side of the β-sheet of each AtLOV2 monomer and vary in length. The FMN is embedded within the core of AtLOV2 and interacts with conserved residues (Fig. 1 ▶ b). The side chain of Cys512 poised to form the light-mediated covalent bond to the C4a atom of FMN is in the dark-state conformation (Halavaty & Moffat, 2007 ▶; Supplementary Fig. S11). The core of AtLOV2 resembles those of the phot1 AtLOV1 (r.m.s.d. of 1.2 Å over 105 Cα atoms; PDB entry 2z6c; Nakasako et al., 2008 ▶), the phot2 AtLOV1 (r.m.s.d. of 0.8 Å over 105 Cα atoms; PDB entry 2z6d; Nakasako et al., 2008 ▶), the phot2 LOV2 (r.m.s.d. of 0.9 Å over 108 Cα atoms; PDB entry 4eep; Christie, Hitomi et al., 2012 ▶) and the phot1 AsLOV2 (r.m.s.d. of 0.5 Å over 102 Cα atoms; PDB entry 2v0u; Halavaty & Moffat, 2007 ▶), thus confirming the high degree of structure conservation among LOV cores. However, the relative orientations and lengths of the flanking regions vary markedly among the crystal structures of LOV domains and evidently depend on the construct sequence/length and crystal packing (Figs. 1 ▶ a and 1 ▶ c).
Figure 1.
The AtLOV2 structure. (a) Superposition of the two AtLOV2 chains: in chain A the PAS core is shown in yellow, the A′α helix in light blue and the Jα helix in light green, while in chain B the PAS core is shown in brown, the A′α helix in dark blue and the Jα helix in dark green. (b) Coordination of FMN (chain A). (c) Top, superposition of AtLOV2 [chain A; colored as in (a)] and AsLOV2 (the PAS core is shown in gray, the A′α helix in magenta and the Jα helix in dark cyan). Bottom, superposition of AtLOV2 (chain A) and AtLOV1 (the PAS core is shown in gray in chain A and white in chain B; the A′α helix is in magenta for both chains). (d) Sequence alignment of plant phototropins showing the N- and C-terminal portions of the LOV2 domain only. Secondary-structure elements of AtLOV2 are displayed with the A′α helix from chain A and the Jα helix from chain B. The UniProtKB identifiers are as follows: Arabidopsis thaliana, O48963; Avena sativa, O49003; Oryzae sativa, O49003; Zea mays, O48547; Pisum sativum, P93489; Phaseolus vulgaris, Q5DW43; Vicia faba, Q8H935. The hydrophobic residues at positions a and d of the heptad repeat in the A′α helix and its preceding N-terminal regions are shown in blue and the Jα helix and its following C-terminal regions are shown in green.
The first six N-terminal residues in chain B were not resolved and its A′α helix is thus shorter than in chain A (Figs. 1 ▶ a and 1 ▶ c). The A′α helix in chain A curves slightly. Its sequence has an irregular heptad repeat of apolar residues characteristic of α-helical coiled coils (Fig. 1 ▶ d). However, the length of the hydrogen bonds in the proximity of these residues is not significantly shorter than elsewhere in the helix (Parry et al., 2008 ▶; Conway & Parry, 1990 ▶), yet the hydrogen-bond distances are set by the current 2.75 Å resolution, i.e. they are affected by the quality of the final model. The van der Waals interactions between the N-terminal residues of the A′α helix (chain A) and the residues of the Hβ–Iβ loop (chain B) may contribute to the bending of the helix. The interactions of the Jα helix with the PAS core are nearly identical in AtLOV2 and AsLOV2 (Halavaty & Moffat, 2007 ▶; Harper et al., 2003 ▶; Figs. 1 ▶ a and 1 ▶ c). A multiple full-length sequence alignment of the plant phototropins and structural comparison of AtLOV2 and AsLOV2 suggest that plant LOV2 sequences with a shorter Iβ–Jα junction (six residues, as in AsLOV2) may have a shorter Jα helix, whereas those with a longer junction (ten residues, as in AtLOV2) may adopt a longer helix (Fig. 1 ▶ d). In the AtLOV2 structure the disordered residues 581–592 of the junction in chain A and the partially ordered residues 580–586 in chain B presumably adopt a loop conformation, whereas residues 587–592 in chain B form part of the Jα helix that is slightly bent (Supplementary Fig. S1).
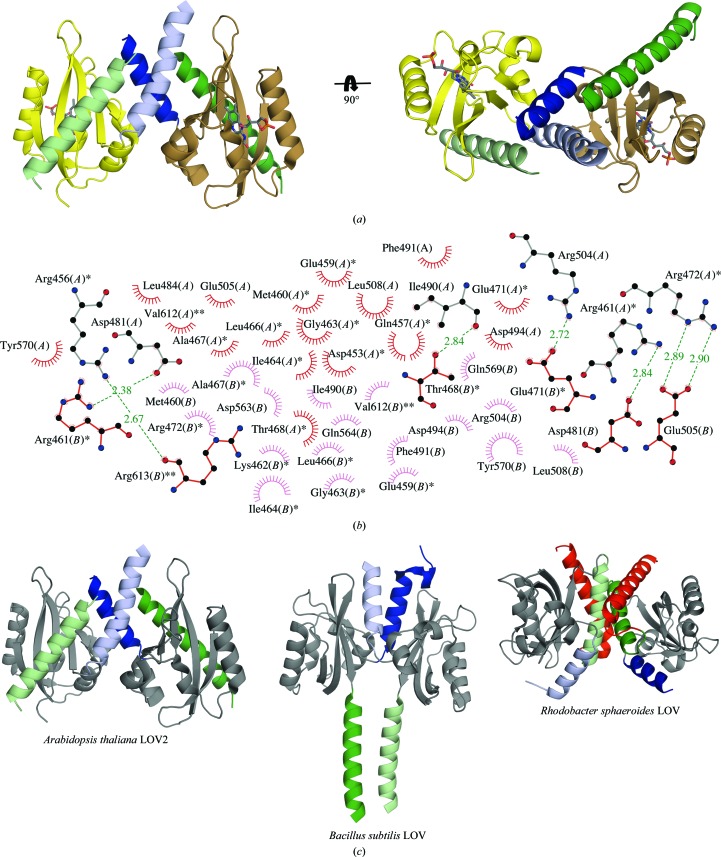
The dimer of AtLOV2 has a total buried surface area of 4500 Å2 contributed in each monomer by 23 residues from the A′α helix, 20 residues from the PAS core and three residues from the Jα helix (Figs. 2 ▶ a and 2 ▶ b). Similar scissor-like interactions between even shorter A′α helices have been reported in PAS/LOV structures both in the presence and the absence of the Jα helix [PDB entries 2gj3 (Key et al., 2007 ▶) and 3sw1 (Circolone et al., 2012 ▶)], in which dimerization is additionally stabilized by direct β-sheet-to-β-sheet contacts. Direct contacts are also found in short constructs which lack both the flanking A′α and Jα helices [PDB entries 1byw (Morais Cabral et al., 1998 ▶) and 1g28 (Crosson & Moffat, 2001 ▶); Supplementary Fig. S1], but direct contacts are absent in the AtLOV2 structure owing to the folding pattern of the Jα helix. The role of the A′α helix in dimerization of the current AtLOV2 construct is apparent, although the solution behavior of our AtLOV2(452–615) construct is unknown. Nakasone et al. (2006 ▶) showed that the AtLOV(449–586) construct that possesses the A′α helix and lacks the Jα helix dimerizes in its dark state in a concentration-dependent manner. In contrast, the AtLOV2(449–661) construct in which both flanking helices are present is a monomer even at high concentrations (Nakasone et al., 2008 ▶). Although the A′α helices in AtLOV2 and AsLOV2 are superimposable, the A′α helix in AsLOV2 spans only one turn, whereas the length of the structurally ordered N-terminus of AsLOV2 is half that of AtLOV2. In AsLOV2, Phe403 (remaining from the purification tag and replacing a conserved Asp403) lies in a hydrophobic pocket at the crystal contact (not shown). It thus may prevent extension of the A′α helix and bring about the monomeric form of AsLOV2. Some role of the Jα helix in the dimerization of AtLOV2 is possible, but details cannot be explicitly obtained from the current AtLOV2 structure. The sequences N-terminal to the A′α helix and C-terminal to the Jα helix show heptad periodicity and helical propensity based on the PSIPRED prediction (Jones, 1999 ▶; Buchan et al., 2010 ▶). They potentially form the extended or additional α-helical coiled coils and/or α-helical bundles found at the dimerization interface of certain PAS/LOV domains [Fig. 2 ▶ c and Supplementary Fig. S1; PDB entries 4gcz (Diensthuber et al., 2013 ▶), 3c2w (Yang et al., 2008 ▶), 4hj4 (Conrad et al., 2013 ▶) and 3e4o (Zhou et al., 2008 ▶)].
Figure 2.
Dimerization of AtLOV2. (a) AtLOV2 dimer in which the flanking linker helices form an α-helical coiled coil. (b) Dimerization interface of AtLOV2. Residues with one star belong to the A′α helix and those with two stars belong to the Jα helix; unlabeled residues are from the PAS core domain. (c) Varied dimerization modes of AtLOV2, Rhodobacter sphaeroides LOV (RsLOV; PDB entry 4hj4; Conrad et al., 2013 ▶) and the Bacillus subtilis LOV domain of FixL (PDB entry 4gcz; the histidine kinase domain of the chimera was omitted for clarity; Diensthuber et al., 2013 ▶). The A′α helix is colored light blue (chains A) and dark blue (chains B) and the Jα helix is depicted in light green (chains A) and dark green (chains B). The LOV core domains of the displayed structures are shown in gray. The Kα helix C-terminal to the Jα helix is shown in red for both chains in the RsLOV structure.
Overall, our structure supports numerous findings on the role of the flanking linker regions in the dimerization of PAS/LOV domains. Similar to the homologous PAS/LOV domains, these linkers exhibit amphipathic character and pack together in coiled-coil/α-helical bundle structures to facilitate the dimerization of AtLOV2. However, it still remains unclear exactly how AtLOV2 dimerizes, since we and others have explicitly shown that variations in sequence, construct design and crystal contacts undoubtedly affect the relative orientation and length of the flanking regions and ultimately the dimerization mode of these sensor domains.
Supplementary Material
PDB reference: phototropin 1 LOV2 domain, 4hhd
Supplementary material file. DOI: 10.1107/S1744309113029199/sw5066sup1.pdf
Acknowledgments
We thank Dr Vukica Srajer and the staff of sector 14-BM-C at APS, ANL for their assistance during data collection. This research was supported by National Institutes of Health (NIH) grant GM036452 to KM. BioCARS was formerly supported by NIH grant RR07707, now replaced by GM103543. Use of the Advanced Photon Source is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02-06CH11357.
Footnotes
Supplementary material has been deposited in the IUCr electronic archive (Reference: SW5066).
References
- Buchan, D. W., Ward, S. M., Lobley, A. E., Nugent, T. C., Bryson, K. & Jones, D. T. (2010). Nucleic Acids Res. 38, W563–W568. [DOI] [PMC free article] [PubMed]
- Chang, C., Tesar, C., Gu, M., Babnigg, G., Joachimiak, A., Pokkuluri, P. R., Szurmant, H. & Schiffer, M. (2010). J. Bacteriol. 192, 1156–1159. [DOI] [PMC free article] [PubMed]
- Chen, V. B., Arendall, W. B., Headd, J. J., Keedy, D. A., Immormino, R. M., Kapral, G. J., Murray, L. W., Richardson, J. S. & Richardson, D. C. (2010). Acta Cryst. D66, 12–21. [DOI] [PMC free article] [PubMed]
- Christie, J. M. (2007). Annu. Rev. Plant Biol. 58, 21–45. [DOI] [PubMed]
- Christie, J. M., Gawthorne, J., Young, G., Fraser, N. J. & Roe, A. J. (2012). Mol. Plant, 5, 533–544. [DOI] [PubMed]
- Christie, J. M., Hitomi, K., Arvai, A. S., Hartfield, K. A., Mettlen, M., Pratt, A. J., Tainer, J. A. & Getzoff, E. D. (2012). J. Biol. Chem. 287, 22295–22304. [DOI] [PMC free article] [PubMed]
- Christie, J. M., Reymond, P., Powell, G. K., Bernasconi, P., Raibekas, A. A., Liscum, E. & Briggs, W. R. (1998). Science, 282, 1698–1701. [DOI] [PubMed]
- Christie, J. M., Swartz, T. E., Bogomolni, R. A. & Briggs, W. R. (2002). Plant J. 32, 205–219. [DOI] [PubMed]
- Circolone, F., Granzin, J., Jentzsch, K., Drepper, T., Jaeger, K. E., Willbold, D., Krauss, U. & Batra-Safferling, R. (2012). J. Mol. Biol. 417, 362–374. [DOI] [PubMed]
- Conrad, K. S., Bilwes, A. M. & Crane, B. R. (2013). Biochemistry, 52, 378–391. [DOI] [PMC free article] [PubMed]
- Conway, J. F. & Parry, D. A. D. (1990). Int. J. Biol. Macromol. 12, 328–334. [DOI] [PubMed]
- Crosson, S. & Moffat, K. (2001). Proc. Natl Acad. Sci. USA, 98, 2995–3000. [DOI] [PMC free article] [PubMed]
- Crosson, S. & Moffat, K. (2002). Plant Cell, 14, 1067–1075. [DOI] [PMC free article] [PubMed]
- Crosson, S., Rajagopal, S. & Moffat, K. (2003). Biochemistry, 42, 2–10. [DOI] [PubMed]
- Davis, I. W., Leaver-Fay, A., Chen, V. B., Block, J. N., Kapral, G. J., Wang, X., Murray, L. W., Arendall, W. B., Snoeyink, J., Richardson, J. S. & Richardson, D. C. (2007). Nucleic Acids Res. 35, W375–W383. [DOI] [PMC free article] [PubMed]
- DeLano, W. L. (2010). PyMOL, v.1.5.0.4. http://www.pymol.org.
- Diensthuber, R. P., Bommer, M., Gleichmann, T. & Möglich, A. (2013). Structure, 21, 1127–1136. [DOI] [PubMed]
- Drepper, T., Krauss, U., Meyer zu Berstenhorst, S., Pietruszka, J. & Jaeger, K. E. (2011). Appl. Microbiol. Biotechnol. 90, 23–40. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. (2010). Acta Cryst. D66, 486–501. [DOI] [PMC free article] [PubMed]
- Etzkorn, M., Kneuper, H., Dünnwald, P., Vijayan, V., Krämer, J., Griesinger, C., Becker, S., Unden, G. & Baldus, M. (2008). Nature Struct. Mol. Biol. 15, 1031–1039. [DOI] [PubMed]
- Folta, K. M. & Spalding, E. P. (2001). Plant J. 26, 471–478. [DOI] [PubMed]
- Gouet, P., Courcelle, E., Stuart, D. I. & Métoz, F. (1999). Bioinformatics, 15, 305–308. [DOI] [PubMed]
- Halavaty, A. S. & Moffat, K. (2007). Biochemistry, 46, 14001–14009. [DOI] [PubMed]
- Harper, S. M., Neil, L. C. & Gardner, K. H. (2003). Science, 301, 1541–1544. [DOI] [PubMed]
- Henry, J. T. & Crosson, S. (2011). Annu. Rev. Microbiol. 65, 261–286. [DOI] [PMC free article] [PubMed]
- Iwata, T., Tokutomi, S. & Kandori, H. (2002). J. Am. Chem. Soc. 124, 11840–11841. [DOI] [PubMed]
- Jones, D. T. (1999). J. Mol. Biol. 292, 195–202. [DOI] [PubMed]
- Kagawa, T., Sakai, T., Suetsugu, N., Oikawa, K., Ishiguro, S., Kato, T., Tabata, S., Okada, K. & Wada, M. (2001). Science, 291, 2138–2141. [DOI] [PubMed]
- Kasahara, M., Swartz, T. E., Olney, M. A., Onodera, A., Mochizuki, N., Fukuzawa, H., Asamizu, E., Tabata, S., Kanegae, H., Takano, M., Christie, J. M., Nagatani, A. & Briggs, W. R. (2002). Plant Physiol. 129, 762–773. [DOI] [PMC free article] [PubMed]
- Key, J., Hefti, M., Purcell, E. B. & Moffat, K. (2007). Biochemistry, 46, 3614–3623. [DOI] [PubMed]
- Key, J. & Moffat, K. (2005). Biochemistry, 44, 4627–4635. [DOI] [PubMed]
- Kinoshita, T., Doi, M., Suetsugu, N., Kagawa, T., Wada, M. & Shimazaki, K. (2001). Nature (London), 414, 656–660. [DOI] [PubMed]
- Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. (1993). J. Appl. Cryst. 26, 283–291.
- Laskowski, R. A. & Swindells, M. B. (2011). J. Chem. Inf. Model. 51, 2778–2786. [DOI] [PubMed]
- Lee, J., Tomchick, D. R., Brautigam, C. A., Machius, M., Kort, R., Hellingwerf, K. J. & Gardner, K. H. (2008). Biochemistry, 47, 4051–4064. [DOI] [PubMed]
- Little, R., Slavny, P. & Dixon, R. (2012). PLoS One, 7, e46651. [DOI] [PMC free article] [PubMed]
- Matsuoka, D. & Tokutomi, S. (2005). Proc. Natl Acad. Sci. USA, 102, 13337–13342. [DOI] [PMC free article] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Adams, P. D., Winn, M. D., Storoni, L. C. & Read, R. J. (2007). J. Appl. Cryst. 40, 658–674. [DOI] [PMC free article] [PubMed]
- Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. (2006). Acta Cryst. D62, 859–866. [DOI] [PubMed]
- Miyatake, H., Mukai, M., Park, S.-Y., Adachi, S., Tamura, K., Nakamura, H., Nakamura, K., Tsuchiya, T., Iizuka, T. & Shiro, Y. (2000). J. Mol. Biol. 301, 415–431. [DOI] [PubMed]
- Möglich, A., Ayers, R. A. & Moffat, K. (2009a). J. Mol. Biol. 385, 1433–1444. [DOI] [PMC free article] [PubMed]
- Möglich, A., Ayers, R. A. & Moffat, K. (2009b). Structure, 17, 1282–1294. [DOI] [PMC free article] [PubMed]
- Möglich, A. & Moffat, K. (2010). Photochem. Photobiol. Sci. 9, 1286–1300. [DOI] [PubMed]
- Morais Cabral, J. H., Lee, A., Cohen, S. L., Chait, B. T., Li, M. & Mackinnon, R. (1998). Cell, 95, 649–655. [DOI] [PubMed]
- Morris, R. J., Perrakis, A. & Lamzin, V. S. (2003). Methods Enzymol. 374, 229–244. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Nakasako, M., Iwata, T., Matsuoka, D. & Tokutomi, S. (2004). Biochemistry, 43, 14881–14890. [DOI] [PubMed]
- Nakasako, M., Zikihara, K., Matsuoka, D., Katsura, H. & Tokutomi, S. (2008). J. Mol. Biol. 381, 718–733. [DOI] [PubMed]
- Nakasone, Y., Eitoku, T., Matsuoka, D., Tokutomi, S. & Terazima, M. (2006). Biophys. J. 91, 645–653. [DOI] [PMC free article] [PubMed]
- Nakasone, Y., Eitoku, T., Zikihara, K., Matsuoka, D., Tokutomi, S. & Terazima, M. (2008). J. Mol. Biol. 383, 904–913. [DOI] [PubMed]
- Nan, B., Liu, X., Zhou, Y., Liu, J., Zhang, L., Wen, J., Zhang, X., Su, X.-D. & Wang, Y.-P. (2010). Mol. Microbiol. 75, 1484–1494. [DOI] [PubMed]
- Okajima, K., Kashojiya, S. & Tokutomi, S. (2012). J. Biol. Chem. 287, 40972–40981. [DOI] [PMC free article] [PubMed]
- Park, H., Suquet, C., Satterlee, J. D. & Kang, C. (2004). Biochemistry, 43, 2738–2746. [DOI] [PubMed]
- Parry, D. A. D., Fraser, R. D. B. & Squire, J. M. (2008). J. Struct. Biol. 163, 258–269. [DOI] [PubMed]
- Sakai, T., Kagawa, T., Kasahara, M., Swartz, T. E., Christie, J. M., Briggs, W. R., Wada, M. & Okada, K. (2001). Proc. Natl Acad. Sci. USA, 98, 6969–6974. [DOI] [PMC free article] [PubMed]
- Sakamoto, K. & Briggs, W. R. (2002). Plant Cell, 14, 1723–1735. [DOI] [PMC free article] [PubMed]
- Salomon, M., Christie, J. M., Knieb, E., Lempert, U. & Briggs, W. R. (2000). Biochemistry, 39, 9401–9410. [DOI] [PubMed]
- Salomon, M., Lempert, U. & Rüdiger, W. (2004). FEBS Lett. 572, 8–10. [DOI] [PubMed]
- Slavny, P., Little, R., Salinas, P., Clarke, T. A. & Dixon, R. (2010). Mol. Microbiol. 75, 61–75. [DOI] [PubMed]
- Strickland, D., Lin, Y., Wagner, E., Hope, C. M., Zayner, J., Antoniou, C., Sosnick, T. R., Weiss, E. L. & Glotzer, M. (2012). Nature Methods, 9, 379–384. [DOI] [PMC free article] [PubMed]
- Strickland, D., Moffat, K. & Sosnick, T. R. (2008). Proc. Natl Acad. Sci. USA, 105, 10709–10714. [DOI] [PMC free article] [PubMed]
- Swartz, T. E., Corchnoy, S. B., Christie, J. M., Lewis, J. W., Szundi, I., Briggs, W. R. & Bogomolni, R. A. (2001). J. Biol. Chem. 276, 36493–36500. [DOI] [PubMed]
- Takeda, K., Nakasone, Y., Zikihara, K., Tokutomi, S. & Terazima, M. (2013). J. Phys. Chem. B, 10.1021/jp406109j. [DOI] [PubMed]
- Takemiya, A., Inoue, S., Doi, M., Kinoshita, T. & Shimazaki, K. (2005). Plant Cell, 17, 1120–1127. [DOI] [PMC free article] [PubMed]
- Taylor, B. L. & Zhulin, I. B. (1999). Mol. Biol. Rev. 63, 479–506. [DOI] [PMC free article] [PubMed]
- Thompson, J. D., Higgins, D. G. and Gibson, T. J. (1994). Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed]
- Ukaegbu, U. E. & Rosenzweig, A. C. (2009). Biochemistry, 48, 2207–2215. [DOI] [PMC free article] [PubMed]
- Wang, C., Sang, J., Wang, J., Su, M., Downey, J. S., Wu, Q., Wang, S., Cai, Y., Xu, X., Wu, J., Senadheera, D. B., Cvitkovitch, D. G., Chen, L., Goodman, S. D. & Han, A. (2013). PLoS Biol. 11, e1001493. [DOI] [PMC free article] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Wu, Y. I., Frey, D., Lungu, O. I., Jaehrig, A., Schlichting, I., Kuhlman, B. & Hahn, K. M. (2009). Nature (London), 461, 104–108. [DOI] [PMC free article] [PubMed]
- Yang, X., Kuk, J. & Moffat, K. (2008). Proc. Natl Acad. Sci. USA, 105, 14715–14720. [DOI] [PMC free article] [PubMed]
- Zayner, J. P., Antoniou, C. & Sosnick, T. R. (2012). J. Mol. Biol. 419, 61–74. [DOI] [PMC free article] [PubMed]
- Zhou, Y.-F., Nan, B., Nan, J., Ma, Q., Panjikar, S., Liang, Y.-H., Wang, Y. & Su, X.-D. (2008). J. Mol. Biol. 383, 49–61. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PDB reference: phototropin 1 LOV2 domain, 4hhd
Supplementary material file. DOI: 10.1107/S1744309113029199/sw5066sup1.pdf