To determine the molecular basis underlying the ligand specificity of the ligand-binding regions of Pseudomonas chemotactic transducer A and B, complexes with amino acids have been purified and crystals have been produced.
Keywords: Pseudomonas aeruginosa, chemoreceptors PctA and PctB
Abstract
Pseudomonas aeruginosa is an opportunistic pathogen and one of the major model organisms for the study of chemotaxis. The bacterium harbours 26 genes encoding chemoreceptors, most of which have not been annotated with a function. The paralogous chemoreceptors PctA and PctB (Pseudomonas chemotactic transducer A and B) were found to mediate chemotaxis towards l-amino acids. However, the ligand spectrum of the receptors is quite different since the recombinant ligand-binding region (LBR) of PctA binds 17 different l-amino acids whereas that of PctB recognizes only five. To determine the molecular basis underlying this ligand specificity, PctA-LBR and PctB-LBR have been purified and crystals have been produced after pre-incubation with l-Ile and l-Arg, respectively. Initial crystallization conditions have been identified by the counter-diffusion method and X-ray data have been collected at 2.5 Å (PctA-LBR bound to l-Ile) and 3.14 Å (PctB-LBR bound to l-Arg) resolution. Crystals belonged to space groups P212121 and P3121, with unit-cell parameters a = 72.2, b = 78.5, c = 116.6 Å and a = b = 111.6, c = 117.4, respectively, for PctA-LBR and PctB-LBR. Molecular-replacement methods will be pursued for structural determination.
1. Introduction
The adaptation of bacteria to environmental changes is mediated by different signal transduction mechanisms. Genome analyses suggest that bacterial signal transduction is primarily achieved through the action of one-component systems, two-component systems and chemoreceptor-based signalling (Galperin, 2005 ▶). Typically, chemoreceptors are composed of a periplasmic ligand-binding region (LBR) and a cytosolic signalling domain. Ligand binding at the LBR creates a molecular stimulus that is transmitted across the membrane, where it modulates the autokinase activity of bound histidine kinase CheA and subsequently transphosphorylation activity of the CheY response regulator (Hazelbauer et al., 2008 ▶). Chemoreceptor-based signalling cascades mediate flagellum-mediated chemotaxis, type IV pili-mediated taxis or are involved in the regulation of alternative cellular processes (Hickman et al., 2005 ▶; Zusman et al., 2007 ▶; Wuichet & Zhulin, 2010 ▶).
The primary model in the study of chemosensory pathways is the chemotactic response of enterobacteria towards l-Ser and l-Asp, which is mediated by the Tsr and Tar chemoreceptors, respectively. Both receptors possess an LBR that forms a four-helix bundle structure (Milburn et al., 1991 ▶; Tajima et al., 2011 ▶). The full-length receptors as well as the recombinant LBRs recognize their cognate ligands l-Asp (Tar) and l-Ser (Tsr) with similar affinities in the lower micromolar range (Clarke & Koshland, 1979 ▶; Milligan & Koshland, 1993 ▶).
The chemotactic system in bacteria is characterized by a large degree of diversity (Krell et al., 2011 ▶), which is also reflected in the cellular abundance and molecular architecture of chemoreceptors (Lacal et al., 2010 ▶). An analysis of all available chemoreceptor sequences showed that one such difference is the size of the chemoreceptor LBR that can be classified into cluster I (around 150 amino acids) and cluster II regions (around 250 amino acids). The well studied cluster I receptors include the four-helix bundle-type LBRs (Milburn et al., 1991 ▶) of the enterobacterial receptors. Although an estimated 40% of chemoreceptors possess cluster II LBRs, there is very little information available on this protein family and the corresponding sequences are unannotated in the InterPro database. We have recently reported the structure of the cluster II LBR of the McpS chemoreceptor that is composed of two stacked four-helix bundles (Pineda-Molina, Reyes-Darias et al., 2012 ▶ ▶; Lacal et al., 2010 ▶).
The paralogous PctA, PctB and PctC chemoreceptors of the human pathogen Pseudomonas aeruginosa also possess a cluster II LBR. These receptors were found to mediate chemotaxis towards l-amino acids (Taguchi et al., 1997 ▶), intermediates of the amino-acid metabolism (putrescine, cadaverine and GABA; Taguchi et al., 1997 ▶) as well as chemorepulsion to different toxic chlorinated compounds like trichloroethylene and chloroform (Shitashiro et al., 2003 ▶).
We have recently reported the generation of recombinant PctA-LBR, PctB-LBR and PctC-LBR (Rico-Jiménez et al., 2013 ▶). Using isothermal titration calorimetry, we have shown that PctA-LBR and PctB-LBR recognize 17 and five l-amino acids, respectively (Rico-Jiménez et al., 2013 ▶). l-Gln is one of the three natural l-amino acids that did not bind to PctA-LBR, but is by far the ligand that bound with highest affinity to PctB-LBR. We have therefore proposed that the physiological relevance of PctA may lie in mediating chemotaxis to most amino acids, whereas PctB may have evolved to mediate taxis to low concentrations of l-Gln with elevated specificity (Rico-Jiménez et al., 2013 ▶). Most interestingly, these proteins recognize exclusively the l-isomers and have no affinity for d-amino acids. Both regions are highly paralogous and share approximately 70% sequence identity. To elucidate the molecular basis for ligand recognition and isomer specificity, structural information is indispensable. Here, we report the crystallization and preliminary crystallographic analysis of the LBRs of PctA and PctB in complex with different l-amino acids.
2. Materials and methods
2.1. Protein expression and purification
The cloning, expression and purification of histidine-tagged LBR of PctA (UniProt accession No. G3XD24) and PctB (UniProt accession No. Q9HW91) by affinity chromatography have been described recently (Rico-Jiménez et al., 2013 ▶). The DNA fragments encoding amino acids 30–278 and 30–277 of PctA and PctB, respectively, were amplified by PCR using genomic DNA of P. aeruginosa PAO1. The primers contained restriction sites for NdeI and BamHI. The resulting PCR products were digested with these enzymes and cloned into the expression plasmid pET28b(+). The resulting plasmids were termed pET28-PctA-LBR and pET28-PctB-LBR and the sequence was verified by DNA sequencing of the insert and flanking regions. In the case of PctA, the construct encoded a fusion protein of the LBR and an N-terminal extension of 20 amino acids harbouring the hexahistidine tag with a total molecular weight of 29.4 kDa (Fig. 1 ▶). In the reverse primer for the PctB construct the stop codon was deleted. As a consequence, the LBR of PctB was produced in fusion with extensions on its N- and C-termini each harbouring a hexahistidine tag, giving rise to a protein of 31.8 kDa (Fig. 1 ▶).
Figure 1.
Protein sequence of the two proteins crystallized. The fragments highlighted in yellow are extensions harbouring the hexahistidine tag.
Escherichia coli BL21 (DE3) was transformed with either pET28-PctA-LBR or pET28-PctB-LBR. Cultures were grown in 2 l Erlenmeyer flasks containing 500 ml LB medium supplemented with 50 µg ml−1 kanamycin at 303 K until an OD660 of 0.6 was reached, at which point protein production was induced by adding 0.1 mM IPTG. Growth was continued at 291 K overnight prior to cell harvesting by centrifugation at 10 000g for 30 min. Cell pellets were resuspended in buffer A [30 mM Tris–HCl, 300 mM NaCl, 5%(v/v) glycerol pH 7.0] containing 10 mM imidazole and broken by a French press at 6.9 MPa. After centrifugation at 20 000g for 1 h, the supernatant was loaded onto a 5 ml HisTrap column (Amersham Bioscience) previously equilibrated with buffer A containing 10 mM imidazole. In the case of PctA-LBR the column was washed with buffer A containing 40 mM imidazole and, because PctB-LBR contained two His tags, washing was carried out with buffer A containing 80 mM imidazole. Protein was eluted with a linear gradient to 1 M imidazole in buffer A.
To further increase the purity of the protein an additional size-exclusion chromatography step was carried out. To this end, fractions from the affinity-chromatography step were pooled, dialyzed against 50 mM Tris–HCl pH 7.0, 0.5 M NaCl and loaded onto a 150 ml HiPrep 26/60 Sephacryl S-200 HR gel-filtration column (GE Healthcare Life Sciences). The protein was eluted at a constant flow rate of 1 ml min−1 in the same buffer.
2.2. Identification of proteins by peptide fingerprinting
Protein bands were excised from SDS–PAGE gels and sent for analysis to the Proteomics Service of the Complutense University in Madrid. Proteins were digested with trypsin and the resulting peptides analyzed by MALDI–TOF to obtain the peptide fingerprint. Proteins were identified by matching experimental masses with those of theoretical digests of bacterial proteins.
2.3. Crystallization
PctA-LBR was subjected to buffer exchange with analysis buffer (5 mM Tris–HCl, 5 mM PIPES, 5 mM MES pH 7.0) using 0.5 ml concentrator units (Amicon). PctA-LBR at a concentration of 0.8 mM (25 mg ml−1) was then incubated with 3 mM l-Ile for 30 min on ice. The excess ligand was removed by buffer exchange with the same buffer. PctB-LBR was treated in the same way but was pre-incubated with 3 mM l-Arg.
Initial crystallization conditions for purified PctA-LBR and PctB-LBR were determined by the counter-diffusion technique (García-Ruiz, 2003 ▶) using capillaries of 0.1 mm inner diameter with the CSK-24 crystallization screening kit (Triana S&T, Granada, Spain). PctA-LBR crystals were grown at 277 K in 6 M sodium formate, presenting a rod-like shape. Crystals were extracted from the capillary and subsequently cryoprotected in mother liquor supplemented with 15%(v/v) glycerol.
Single PctB-LBR crystals were obtained in 1.25 M sodium citrate and 0.1 M Na HEPES pH 7.5 at 293 K in capillaries of 0.1 mm inner diameter. For cryoprotection, capillaries were equilibrated overnight in their mother liquor supplemented with 15%(v/v) glycerol. Crystals obtained showed a final size of approximately 100 µm.
2.4. Data collection and structure determination
Native data sets were collected on ESRF beamline ID23-1 for crystals of both PctA-LBR plus l-Ile and PctB-LBR pre-incubated with l-Arg using an ADSC Quantum 4 CCD detector. For PctA-LBR 220 frames were collected (oscillation range 0.92°, crystal-to-detector distance of 326.07, 100 K temperature) whereas for PctB-LBR the number of frames was 80 (oscillation range 2°, crystal-to-detector distance of 497.02 mm, 100 K temperature). The diffraction data were processed using XDS (Kabsch, 2010 ▶) and scaled using SCALA from the CCP4 suite (Winn et al., 2011 ▶) to resolutions of 2.5 and 3.1 Å, respectively.
3. Results and discussion
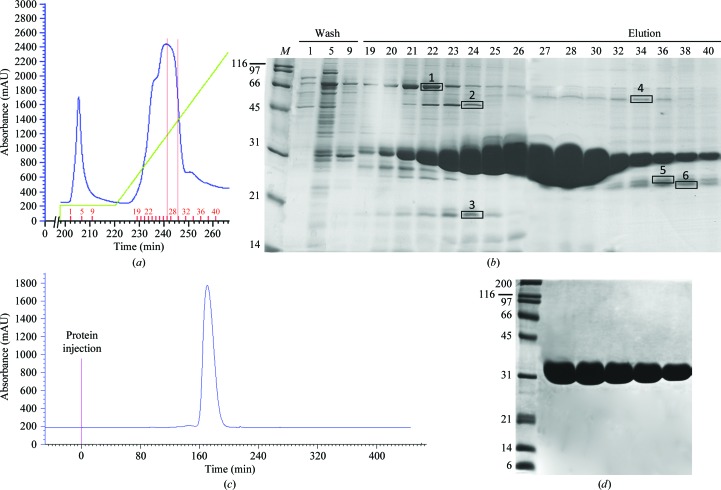
The cloning, expression and purification of PctA-LBR and PctB-LBR have been reported recently (Rico-Jiménez et al., 2013 ▶). However, in this publication protein expression was not discussed explicitly. PctA-LBR was produced as a fusion protein containing an N-terminal His tag, whereas PctB-LBR was cloned containing His tags at both termini (Fig. 1 ▶). Proteins were purified from the soluble fraction of the cellular lysate of a 0.5 l E. coli culture. After protein immobilization the column was washed with buffer containing 80 mM imidazole without loss of bound PctB-LBR as illustrated by fractions 1, 5 and 9 in Fig. 2 ▶(a). The corresponding washing step in the purification of the single His tag containing PctA-LBR could be carried out with a maximal imidazole concentration of 40 mM. PctB-LBR was eluted with a linear gradient of 1 M imidazole (Fig. 2 ▶ a). As shown in Fig. 2 ▶(b) the protein present in the final part of the elution peak was of remarkably high purity (fractions 27–30). Several proteins that partially co-eluted with purified PctB-LBR were identified by MALDI–TOF mass spectrometry (bands boxed in Fig. 2 ▶ b) and are listed in the legend to this figure. Bands 5 and 6 corresponded to proteolytic fragments of PctB-LBR, but the remaining proteins did not coincide with protein identified as major contaminants in immobilized metal-ion chromatography as reported previously (Bolanos-Garcia & Davies, 2006 ▶).
Figure 2.
Purification of PctB-LBR by affinity chromatography using immobilized metal-affinity chromatography (IMAC) and size-exclusion chromatography. (a) Part of the FPLC profile corresponding to the washing and elution step of IMAC. Shown in blue is the absorption at 280 nm. The green line indicates the imidazole gradient reaching 1 M. The fractions selected for SDS–PAGE analysis are marked by short red bars. The range of fractions used for subsequent size-exclusion chromatography are marked by the two red lines. (b) SDS–PAGE gel of selected fractions from IMAC chromatography (15 µl sample load). The boxed bands correspond to proteins that were identified by mass spectrometry as (1) chaperone protein DnaK (69 kDa, D8AJW5), (2) chaperone protein DnaJ (41 kDa, A7ZHA5), (3) 50S ribosomal protein L17 (14 kDa, A7ZSI3,), (4) d-lactate dehydrogenase (64 kDa P06149) and (5), (6) proteolytic fragments of PctB-LBR. (c) Chromatogram of size-exclusion chromatography. (d) SDS–PAGE gel of selected fractions from the size-exclusion chromatography step. Sample load varied between 32 and 17 µg per lane. All gels were stained with Coomassie Blue.
Fractions 27–30, representing 65 mg protein in 4 ml, were pooled, dialyzed and submitted to size-exclusion chromatography (Fig. 2 ▶ c). Representative fractions of this chromatographic step are shown in Fig. 2 ▶(d), where no further contaminating bands were visible on heavily loaded gels. In total, 40 mg pure protein was recovered for subsequent analyses. PctA-LBR was purified in the same way and comparable protein yields were obtained.
Using isothermal titration calorimetry we have shown that PctA-LBR and PctB-LBR recognize 17 and five l-amino acids, respectively (Rico-Jiménez et al., 2013 ▶). From these experiments we concluded that a good concentration to obtain an excess of ligand for cocrystallization experiments was 3 mM. We performed several cocrystallization experiments with different ligands (l-Ala, l-Arg, l-Thr and l-Trp for PctA-LBR and l-Gln and l-Asn for PctB-LBR) using 3 mM as a fixed ligand concentration. Here, we describe the crystallization and diffraction experiments for PctA-LBR bound to l-Ile and PctB-LBR bound to l-Arg which produced the best results.
As reported recently PctA-LBR and PctB-LBR bound l-Ile and l-Arg with affinities of 24 ± 5.6 and 64 ± 4 µM, respectively (Rico-Jiménez et al., 2013 ▶). A preliminary crystallization screen was performed with freshly purified PctA-LBR using the GCB-CSK-24 (Triana S&T) counter-diffusion screening kit with capillaries of 0.1 mm inner diameter (CP-01-50, Triana S&T) in the absence and presence of ligand. Initial optimization did not improve crystal quality so additional optimization experiments are ongoing in the laboratory. Vapour diffusion in a hanging-drop configuration was also attempted without success.
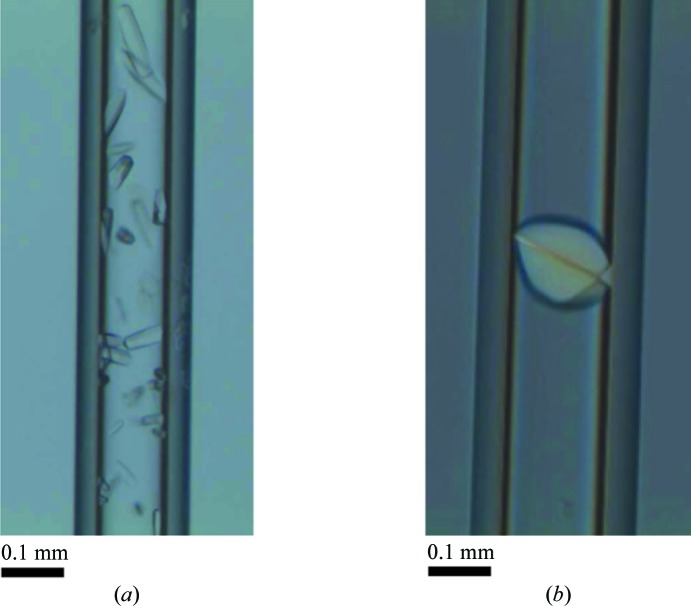
PctA-LBR crystals grown by counter-diffusion were observed only upon incubation with l-Ile (Fig. 3 ▶ a). They took 1 week to grow and achieved their final size (100 µm) after 2 weeks. These rod-like crystals appeared in high numbers inside the capillaries in 6 M sodium formate and were not sufficiently separated from each other to perform direct diffraction experiments. For this reason, crystals were extracted from the capillary and prepared independently in cryoloops. Cryoprotection was performed using mother liquor supplemented with 15%(v/v) glycerol. Crystals were finally flash-cooled in liquid nitrogen.
Figure 3.
Crystals used for data collection. (a) PctA-LBR crystals after pre-incubation with 3 mM l-Ile. (b) Single crystal of PctB-LBR grown after incubation with 3 mM l-Arg.
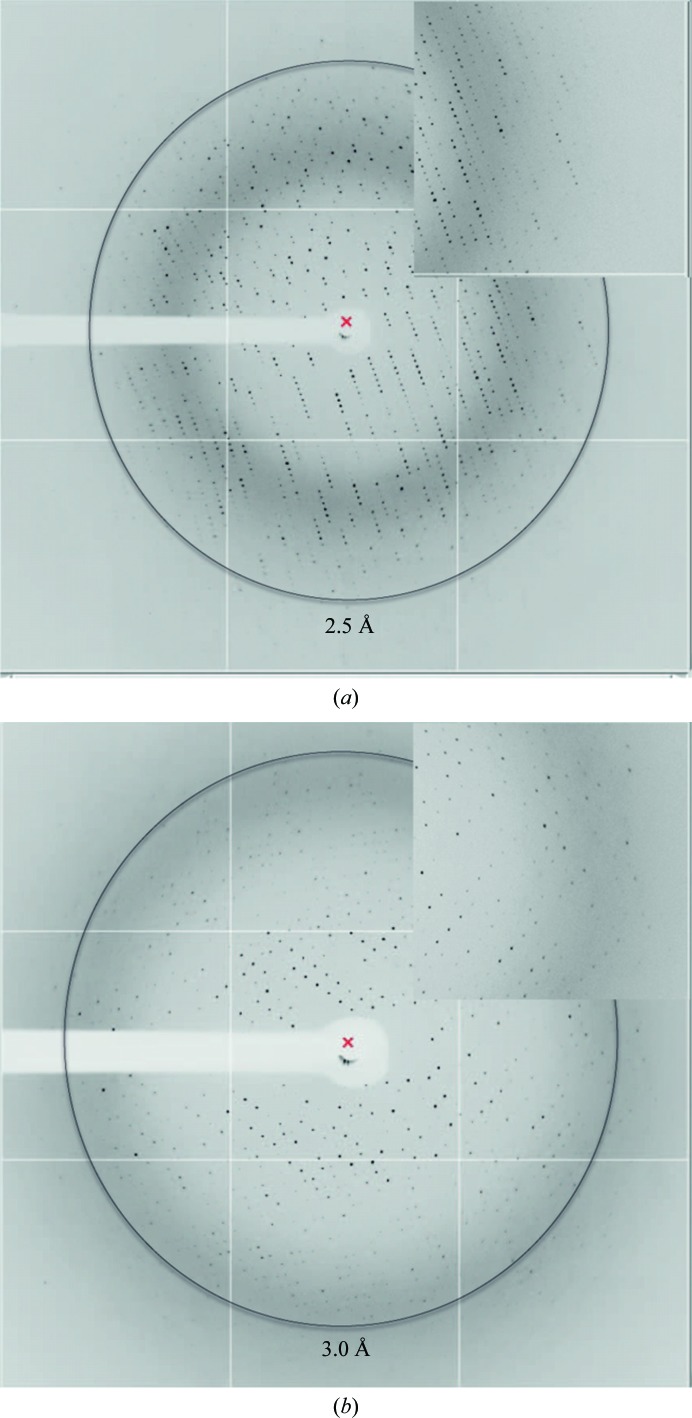
A single data set at 2.52 Å resolution was collected at ID23-1 (ESRF) and a representative diffraction pattern is shown in Fig. 4 ▶(a). Data indexing resulted in satisfactory statistics in space group P212121, with unit-cell parameters a = 72.2, b = 78.5, c = 116.6 Å. Complete data-collection statistics after processing with the XDS package (Kabsch, 2010 ▶) and crystal parameters are shown in Table 1 ▶. The asymmetric unit of this crystal form is likely to contain two molecules, corresponding to a solvent content of 53.8% and a Matthews coefficient of 2.66 Å3 Da−1 (Kantardjieff & Rupp, 2003 ▶; Matthews, 1968 ▶).
Figure 4.
Diffraction images. (a) X-ray diffraction pattern of a PctA-LBR crystal in complex with l-Ile after extraction and cryoprotection [15%(v/v) glycerol]. (b) Diffraction pattern of an un-extracted crystal of PctB-LBR pre-incubated with l-Arg obtained by counter-diffusion. High-resolution enlarged insets are shown in both cases.
Table 1. Parameters from the processing of X-ray data sets for PctA-LBR and PctB-LBR crystals.
Values in parentheses are for the outermost resolution shell.
| PctA–Ile | PctB–Arg | |
|---|---|---|
| Beamline | ID23-1, ESRF | ID23-1, ESRF |
| Space group | P212121 | P3121 |
| Unit-cell parameters (Å) | a = 72.2, b = 78.5, c = 116.6 | a = b = 111.6, c = 117.4 |
| Wavelength (Å) | 0.9786 | 0.9786 |
| Resolution (Å) | 48.0–2.52 (2.62–2.52) | 48.0–3.14 (3.36–3.14) |
| Total No. of reflections | 184734 | 142543 |
| Total unique reflections | 22972 | 15148 |
| R meas (%) | 11.3 (47.6) | 12.9 (60.5) |
| 〈I/σ(I)〉 | 12.5 (3.7) | 12.4 (3.6) |
| Completeness (%) | 99.5 (95.8) | 99.7 (98.3) |
| Multiplicity | 8.05 (7.6) | 9.4 (9.0) |
| Molecules per asymmetric unit | 2 | 3 |
| Matthews coefficient (Å3 Da−1) | 2.66 | 2.36 |
| Solvent content (%) | 53.8 | 47.84 |
The same crystallization procedure was applied to the crystallization of the PctB-LBR–l-Arg complex. Crystallization trials of PctB-LBR in complex with several ligands were carried out including its high-affinity target amino acid l-Gln. However, large crystals appeared for the protein in complex with l-Arg and were consequently selected for diffraction studies. For the complex with l-Gln only microcrystals were obtained and optimization experiments are ongoing in the laboratory to resolve this structure.
In analogy to PctA-LBR no crystals were obtained with the ligand-free protein. PctB-LBR–l-Arg crystals were grown in 1.25 M sodium citrate, 0.1 M Na HEPES pH 7.5. They appeared as single crystals showing a round morphology occupying the width of the capillary (Fig. 3 ▶ b). We have previously performed successful data collections using capillaries on ESRF automated sample changers (Pineda-Molina, Daddaoua et al., 2012 ▶ ▶). Owing to their optimal proportions and to avoid crystal extraction, capillary portions of approximately 1 cm, containing a single crystal, were equilibrated overnight with mother liquor supplemented with 15%(v/v) glycerol. Subsequently they were attached to the end of a SPINE standard cap, taking into account that they should not exceed the 22 mm fixed sample-holder length, and flash-cooled in liquid nitrogen.
A full data set was collected at 3.14 Å resolution on ID23-1 at the ESRF, of which a representative image is shown in Fig. 4 ▶(b). Data-collection details are summarized in Table 1 ▶. The unit cell (with parameters a = b = 111.6, c = 117.4 Å) is suspected to contain three protein molecules with 47% solvent content for the proposed space group P3121 .
We are currently attempting to solve the structures of PctA-LBR bound to l-Ile and PctB-LBR in complex with l-Arg using molecular replacement. Molecular-replacement searches are being carried out with MOLREP (Vagin & Teplyakov, 2010 ▶) using a search model. We have started with the higher-resolution data (PctA-Ile). We are performing molecular-replacement experiments together with iterative cycles of refinement to identify clearly the ligand in the structure.
Careful analysis of both structures will help us to understand the binding mode of PctA and PctB to their respective ligands.
Acknowledgments
This work was supported by the project ‘Factoría de Cristalización’ and by grants from the Junta de Andalucía (references P09-RNM-4509 and CVI-7335) and the Spanish Ministry of Economy and Competitiveness (reference Bio2010-16937). We are also grateful to the staff of beamline ID23-1 at the ESRF, Grenoble, France for their help during data collection and the ESRF for funding (MX-1398). EP-M is supported by a ‘Ramón y Cajal’ research contract (MICINN).
References
- Bolanos-Garcia, V. M. & Davies, O. R. (2006). Biochim. Biophys. Acta, 1760, 1304–1313. [DOI] [PubMed]
- Clarke, S. & Koshland, D. E. (1979). J. Biol. Chem. 254, 9695–9702. [PubMed]
- Galperin, M. Y. (2005). BMC Microbiol. 5, 35. [DOI] [PMC free article] [PubMed]
- García-Ruiz, J. M. (2003). Methods Enzymol. 368, 130–154. [DOI] [PubMed]
- Hazelbauer, G. L., Falke, J. J. & Parkinson, J. S. (2008). Trends Biochem. Sci. 33, 9–19. [DOI] [PMC free article] [PubMed]
- Hickman, J. W., Tifrea, D. F. & Harwood, C. S. (2005). Proc. Natl Acad. Sci. USA, 102, 14422–14427. [DOI] [PMC free article] [PubMed]
- Kabsch, W. (2010). Acta Cryst. D66, 125–132. [DOI] [PMC free article] [PubMed]
- Kantardjieff, K. A. & Rupp, B. (2003). Protein Sci. 12, 1865–1871. [DOI] [PMC free article] [PubMed]
- Krell, T., Lacal, J., Muñoz-Martínez, F., Reyes-Darias, J. A., Cadirci, B. H., García-Fontana, C. & Ramos, J. L. (2011). Environ. Microbiol. 13, 1115–1124. [DOI] [PubMed]
- Lacal, J., Alfonso, C., Liu, X., Parales, R. E., Morel, B., Conejero-Lara, F., Rivas, G., Duque, E., Ramos, J. L. & Krell, T. (2010). J. Biol. Chem. 285, 23126–23136. [DOI] [PMC free article] [PubMed]
- Lacal, J., García-Fontana, C., Muñoz-Martínez, F., Ramos, J. L. & Krell, T. (2010). Environ. Microbiol. 12, 2873–2884. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- Milburn, M. V., Privé, G. G., Milligan, D. L., Scott, W. G., Yeh, J., Jancarik, J., Koshland, D. E. & Kim, S.-H. (1991). Science, 254, 1342–1347. [DOI] [PubMed]
- Milligan, D. L. & Koshland, D. E. (1993). J. Biol. Chem. 268, 19991–19997. [PubMed]
- Pineda-Molina, E., Daddaoua, A., Krell, T., Ramos, J. L., García-Ruiz, J. M. & Gavira, J. A. (2012). Acta Cryst. F68, 1307–1310. [DOI] [PMC free article] [PubMed]
- Pineda-Molina, E., Reyes-Darias, J. A., Lacal, J., Ramos, J. L., Garc-a-Ruiz, J. M., Gavira, J. A. & Krell, T. (2012). Proc. Natl Acad. Sci. USA, 109, 18926–18931. [DOI] [PMC free article] [PubMed]
- Rico-Jiménez, M., Muñoz-Martínez, F., García-Fontana, C., Fernandez, M., Morel, B., Ortega, A., Ramos, J. L. & Krell, T. (2013). Mol. Microbiol. 88, 1230–1243. [DOI] [PubMed]
- Shitashiro, M., Kato, J., Fukumura, T., Kuroda, A., Ikeda, T., Takiguchi, N. & Ohtake, H. (2003). J. Biotechnol. 101, 11–18. [DOI] [PubMed]
- Taguchi, K., Fukutomi, H., Kuroda, A., Kato, J. & Ohtake, H. (1997). Microbiology, 143, 3223–3229. [DOI] [PubMed]
- Tajima, H., Imada, K., Sakuma, M., Hattori, F., Nara, T., Kamo, N., Homma, M. & Kawagishi, I. (2011). J. Biol. Chem. 286, 42200–42210. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Wuichet, K. & Zhulin, I. B. (2010). Sci. Signal. 3, ra50. [DOI] [PMC free article] [PubMed]
- Zusman, D. R., Scott, A. E., Yang, Z. & Kirby, J. R. (2007). Nature Rev. Microbiol. 5, 862–872. [DOI] [PubMed]