Abstract
Maternally inherited Wolbachia (α-Proteobacteria) are widespread parasitic reproductive manipulators. A growing number of studies have described the presence of different Wolbachia strains within a same host. To date, no naturally occurring multiple infections have been recorded in terrestrial isopods. This is true for Armadillidium vulgare which is known to harbor non simultaneously three Wolbachia strains. Traditionally, such Wolbachia are detected by PCR amplification of the wsp gene and strains are characterized by sequencing. The presence of nucleotide deletions or insertions within the wsp gene, among these three different strains, provides the opportunity to test a novel genotyping method. Herein, we designed a new primer pair able to amplify products whose lengths are specific to each Wolbachia strain so as to detect the presence of multi-infections in A. vulgare. Experimental injections of Wolbachia strains in Wolbachia-free females were used to validate the methodology. We re-investigated, using this novel method, the infection status of 40 females sampled in 2003 and previously described as mono-infected based on the classical sequencing method. Among these females, 29 were identified as bi-infected. It is the first time that naturally occuring multiple infections of Wolbachia are detected within an individual A. vulgare host. Additionally, we resampled 6 of these populations in 2010 to check the infection status of females.
Introduction
Wolbachia are endosymbiotic α-Proteobacteria, closely related to the Rickettsia. Wolbachia are highly diversified and are currently divided into 11 supergroups (A to F and H to L, and supergroup G which is considered to be a recombination between A and B) [1-4]. They are mainly maternally inherited and infect a wide range of nematodes and arthropods [5-7]. Depending on both the bacterial lineage and the host, they may induce very diverse effects on host reproduction such as cytoplasmic incompatibility [8], male killing [9], thelytokous parthenogenesis [10], or feminization of genetic males [11]. All these manipulations enable the spread of Wolbachia by decreasing the expected productivity of uninfected females, or by distorting the sex-ratio in favour of infected females [12]. They can induce reproductive isolation, or even an alteration in host reproductive ecology [13-15]. As a result, many Wolbachia are considered to be parasites of reproduction and thus play a determining role in the infected hosts' evolution.
In 2008, Duron et al. [16] proposed that at least a third of arthropod species were infected by a diverse assemblage of maternally inherited bacteria and an important number of studies seems to indicate that, on both a population and individual scale, many of these cases of multiple infections involve different Wolbachia strains [17-19]. For instance, in the ant Formica exsecta, there can be up to five strains of Wolbachia within an individual host [12]. Thus, within a host, various interactions are expected to occur between coexisting symbionts and these will influence both the life history traits of the host and the dynamics of symbiont spread [20]. Theoretical predictions of either coexistence or exclusion of different strains suggest that if there are two Wolbachia strains inducing cytoplasmic incompatibility in a population with no co-infected individuals, the strain with the higher relative fitness will drive the other out of the population. However, in populations where co-infection in individual hosts is observed, uninfected, singly infected and co-infected hosts can co-occur. Within these populations, long-term persistence of co-infections may be possible, during which time both the parasites and the hosts are probably selected and evolve together to survive [21,22]. Moreover, Ironside et al. [23] proposed that the presence of two co-occuring feminising parasites in natural populations of Gammarus duebenii could be possible following either a recent invasion of a new parasite, a horizontal transmission of one or both parasites, or the spread of alleles for resistance to the most dominant parasite in host populations.
In terrestrial isopods (Crustacea, Oniscidea), Wolbachia induce cytoplasmic incompatibility in three species, Porcellio dilatatus petiti [24], Porcellio dilatatus dilatatus [25] and Cylisticus convexus [26] and feminization in many others, including members of the genus Armadillidium, such as Armadillidium vulgare [27] and Armadillidium nasatum [28]. In A. vulgare, two distinct feminizing Wolbachia strains (wVulC and wVulM) have been identified in various populations [29]. More recently, Verne et al. [30] showed that several natural populations of A. vulgare presented a third Wolbachia strain named wVulP. This latter strain showed evidence of recombination events between wVulC and wVulM that have occurred on the wsp gene [30]. Although multiple infections within a given individual host have never been observed in situ, the presence of different Wolbachia strains in the same terrestrial isopod host populations and the existence of recombination between feminizing strains suggest that co-infections are possible and expected. To date, few studies [31,32] have investigated the prevalence of Wolbachia in field populations of A. vulgare. Based on the classical sequencing method (amplification and sequencing of the wsp gene), these studies have failed to detect the presence of multiple infections. Indeed, in this case, only the main PCR product is generally detected. Thus, this classical methodology seems not suitable to detect multiple infections. Herein, we designed a novel method to detect and discriminate the three different Wolbachia strains known to infect A. vulgare. From the study of Verne et al. [30], we inferred that several insertion or deletion events have occurred within the wsp gene fragment. Thus, we designed a new primer set and, after amplification, different product sizes are expected with specific lengths for each Wolbachia strain.
In this paper, we tested the methodology by performing experimental mono-, bi-, and tri-injections of different Wolbachia strains in Wolbachia-free A. vulgare hosts. Using this new method, we also re-investigated the work of Verne et al. [32] on the prevalence of Wolbachia strains in several natural populations sampled in 2003. Additionally, we resampled 6 of these populations in 2010 to follow the dynamics of Wolbachia strains' prevalence over time. With this new genotyping method, we reveal for the first time the occurrence of multiple infections of Wolbachia within individual A. vulgare hosts originating from natural woodlice populations.
Materials and Methods
Ethic Statement
All experimental procedures and animal manipulations did not require an ethics statement.
Authorizations for field sampling
No specific permissions were required for the 7 sampled locations which are public sites. No specific permissions were required for our activities. We confirm that the field studies did not involve endangered or protected species.
A novel method to detect and genotype Wolbachia strains in Armadillidium vulgare
In order to discriminate the three Wolbachia strains known to infect A. vulgare, we designed a new primer pair able to amplify products whose lengths are specific to each Wolbachia strain. To this end, we aligned wsp sequences of each Wolbachia strain (about 600 bp) found in A. vulgare (wVulC, GenBank accession number: DQ778095; wVulM, GenBank accession number: DQ778097; wVulP, GenBank accession number: DQ778096). Primer 3® software [33] was used to design forward (5’TGGTGCAGCATATGTAAGCAA3’) and reverse (5’AAAACTTTGTGTGCGCCTTT3’) primers able to amplify a shorter PCR product (about 250 bp) which includes the variable region. PCRs were performed using a Trio-Thermoblock (BiometraGmBH) in a final volume of 12 μL [0.05 μL Taq polymerase (5 U/μL) (Promega), 2.5 μL of Taq buffer (5X), 0.5 μL of dNTP (8.3 mM), 0.5 μL of each primer (10 µM) and 1 μL of DNA template]. PCR cycling profile included an initial denaturing step of 5 min at 95°C, followed by 35 cycles of 30 s at 95°C, 30 s at 55°C, 1 min at 72°C, and a final step of 5 min at 72°C. The forward primer 5’TGGTGCAGCATATGTAAGCAA3’ was end-labelled with fluorescent phosphoramidite (6-FAM). The PCR products were run with the internal size standard GeneScan™- 500 ROX™ on an ABI PRISM 3130xl® automated sequencer. Allele sizes were scored using Genemapper® (Applied Biosystems).
Validation of the method by experimental mono- and multi-injections of Wolbachia strains in A. vulgare
In order to validate the methodology, Wolbachia-free female A. vulgare were injected with one, two or three strains of Wolbachia. The Wolbachia inoculates were obtained from host lineages, originating from 3 natural French populations that have been maintained in our laboratory for many years (the Méry-sur-Cher population harbours the wVulM Wolbachia strain; the St Cyr population harbours the wVulC strain; and the Poitiers population harbours the wVulP strain). One woodlouse lineage (from Nice, France) is Wolbachia-free and was used as a recipient for the experimental injections. Inoculates were obtained from the ovaries of 5 individuals from each woodlice line. The ovaries were crushed in 1 mL of Ringer buffer. The resulting suspensions were filtered through a 1.2 µm pore membrane to obtain inoculates. Using Quantitative-PCR, we estimated the Wolbachia concentrations for each inoculate to be 1.43 x 107, 1.15 x 107 and 3.24 x 107 wsp copy numbers/µL for wVulC, wVulM and wVulP, respectively. Wolbachia-free females of A. vulgare (Nice line) were injected with 1 µL of inoculate containing either no Wolbachia (negative control), one of the three Wolbachia strains (wVulM, wVulC or wVulP), or an equal mix of either two different strains (wVulM/wVulC, wVulM/wVulP, wVulC/wVulP) or three Wolbachia strains (wVulM/wVulP/wVulC), using a 10 µL Hamilton needle adapted with a 1 mm glass capillary. Five females were injected for each treatment. They were placed at 20°C, at a light-to-dark photoperiod of 18:6, and dissected 28 days later in order to isolate their ovaries from which we extracted DNA using the protocol described in Kocher et al. [34]. Moreover, DNA from each inoculate was also extracted. We amplified all of these DNA samples with the newly designed primer pair in order to compare and verify, on an ABI PRISM 3130xl® automated sequencer, the sizes of the amplified fragments. This PCR reaction was carried out in the same conditions as above and was qualified as the 'novel genotyping method' for Wolbachia strain detection in A. vulgare.
Field study
In 2003, Verne et al. [32] sampled 7 populations in the West of France. In these populations, the classical sequencing method revealed that, among 124 analyzed females, 40 were mono infected by Wolbachia (i.e. 7 females were infected by wVulM, 5 females by wVulP and 28 by wVulC) (Table 1). We used our novel genotyping method in order to re-investigate the infection status of these females [32]. Moreover, in order to estimate the evolution dynamics of the different Wolbachia strains in natural populations of A. vulgare, we resampled, in 2010, 6 of the 7 populations previously analyzed in Verne et al. [32]. We collected 85 females, extracted the DNA from ovaries following the protocol described above and then characterized the infection status using the novel genotyping method (Table 1).
Table 1. Prevalence of Wolbachia strain infection in natural populations of Armadillidium vulgare sampled in 2003 and 2010.
| Location | Sampling year | Sex ratio (♂/♀) | Number of analyzed females | Number of infected females (%) |
Number of females infected by different Wolbachia strains
|
||||
|---|---|---|---|---|---|---|---|---|---|
| wVulM | wVulC | wVulP | wVulM / C | wVulP / C | |||||
| Ensoulesse 46°38'6.00262’’N 00°23'30.63477’’E | 2003 | 1.11 | 9 | 2 (22.2) | 0 | 0 | 2 (22) | 0 | 0 |
| 2010 | 1.13 | 20 | 11 (55) | 0 | 0 | 11 (55) | 0 | 0 | |
| Poitiers 46°35'3.77006’’N 00°22'16.07919’’E | 2003 | 1.38 | 8 | 8 (100) | 1 (12.5) | 1 (12.5) | 4 (50) | 2 (25) | 0 |
| 2010 | 0.40 | 17 | 17 (100) | 0 | 1 (6) | 11 (65) | 4 (23) | 1 (6) | |
| Coulombiers 46°29'18.92092’’N 00°11'31.56164’’E | 2003 | 0.36 | 22 | 3 (14) | 0 | 0 | 0 | 3 (14) | 0 |
| 2010 | 0.90 | 12 | 5 (42) | 0 | 2 (17) | 0 | 1 (8) | 2 (17) | |
| Saint Maixent l'Ecole 46°24'58.01243’’N 00°11'56.61584’’W | 2003 | 0.17 | 35 | 18 (51) | 0 | 0 | 0 | 18 (51) | 0 |
| 2010 | 0.79 | 12 | 4 (33) | 0 | 3 (25) | 0 | 1 (8) | 0 | |
| La Crèche 46°21'40.08011’’N 00°18'21.95247’’W | 2003 | 0.77 | 22 | 3 (14) | 0 | 0 | 0 | 3 (14) | 0 |
| 2010 | 0.93 | 12 | 7 (58) | 0 | 4 (33) | 0 | 2 (17) | 1 (8) | |
| Beauvoir-Sur-Niort 46°10'35.91493’’N 00°28'30.45661’’W | 2003 | 0.89 | 18 | 3 (17) | 2 (11) | 0 | 0 | 1 (6) | 0 |
| 2010 | 0.47 | 12 | 7 (58) | 0 | 2 (17) | 1 (8) | 4 (33) | 0 | |
| Granzay-Gript 46°12'52.08761’’N 00°28'6.57765’’W | 2003 | 0.42 | 10 | 3 (30) | 1 (10) | 0 | 0 | 2 (20) | 0 |
| - | NA | NA | NA | NA | NA | NA | NA | NA | |
Results are obtained using the novel genotyping method. Sampled locations, their GPS coordinates (longitude and latitude in the World Geodetic System 1984 (WGS 84)), sampling year, sex ratio (♂/♀), number of analyzed females , number of infected females (percentage) are indicated in the table. Granzay-Gript was not sampled in 2010 (NA=not available).
Results
Validation of the methodology
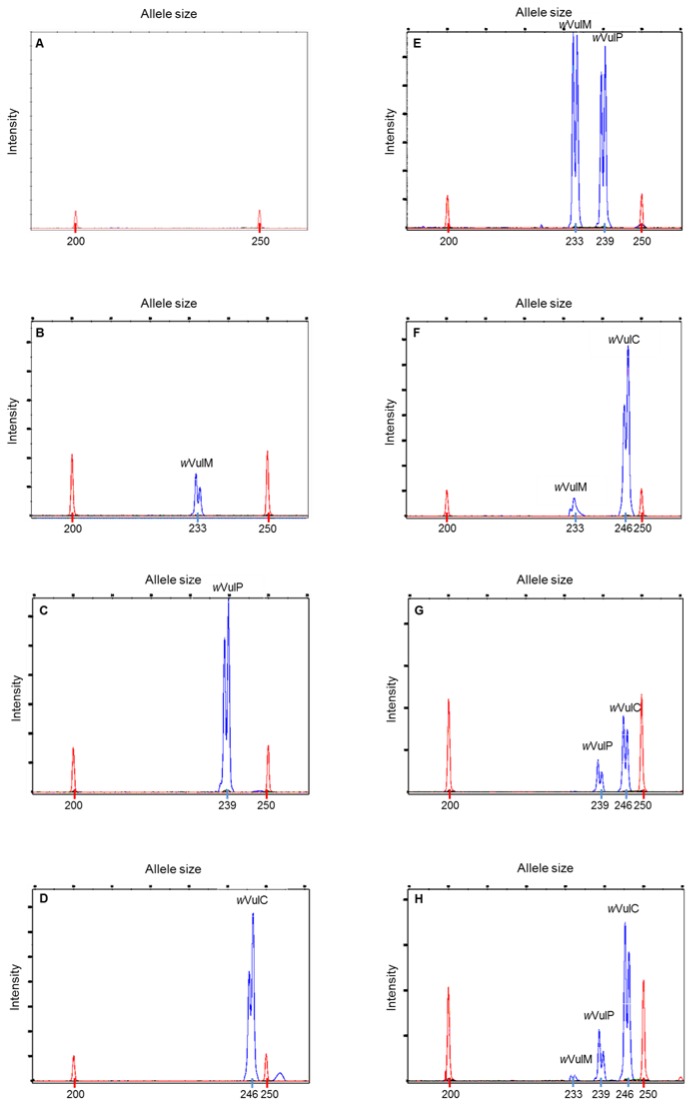
Both primers designed from the alignment of Wolbachia strain wsp sequences gave specific amplified fragments for each strain. Thus, we obtained amplification products of 233, 239 and 246 base pairs for wVulM, wVulP and wVulC, respectively. PCR amplification of the inoculate obtained from the Wolbachia-free females (Nice line) gave no amplification product. Results from the injection experiments showed patterns in accordance with the number and the size of injected strains. Whatever strain, one peak was observed on Genemapper® when inoculate was made up of only one strain. Two peaks were observed for doubly injected individuals, and three peaks were observed for the individuals injected by inoculate containing the three strains (Figure 1). No peak was observed when the individuals were injected by Wolbachia-free inoculate.
Figure 1. Chromatograms obtained from experimental injections of the different Wolbachia strains.
Chromatograms are obtained respectively when inoculate was made up of: A) no Wolbachia strain; B) wVulM strain; C) wVulP strain; D) wVulC strain; E) wVulM and wVulP strains; F) wVulM and wVulC strains; G) wVulP and wVulC strains; H) wVulM, wVulP and wVulC strains. Size markers appear in red. Wolbachia appear in blue. The fragment sizes for wVulM, wVulP and wVulC are 233, 239 and 246 bp respectively.
Wolbachia prevalence and dynamics of infection in natural populations
The results obtained using the novel genotyping method showed a very high prevalence of bi-infected individuals. Indeed, in 2003, classical sequencing analysis revealed 40 mono-infected females [32] whereas, from the same females, the novel genotyping method identified only 11 females as being mono-infected (i.e. 27.5%) and 29 females as being infected by both the wVulC and wVulM strains (i.e. 72.5%). Among the 11 mono-infected females, 4 harboured wVulM (observed in Poitiers, Beauvoir-sur-Niort and Granzay-Gript), 1 wVulC (observed in Poitiers) and 6 wVulP (observed in Ensoulesse and Poitiers).
In the comparative sampling carried out in 2010, 51 females on the 85 analyzed were infected by Wolbachia (Table 1). Among these, our method reveals that 35 individuals were mono-infected (12 wVulC and 23 wVulP) and 16 individuals were bi-infected (12 wVulC/wVulM and 4 wVulP/wVulC). No females harboured the wVulM strain alone (Table 1). We detected no bi-infections involving wVulP and wVulM, regardless of both the population and the sampling year.
Discussion
Multi-infections in A. vulgare
The experimental injections of different Wolbachia strains performed in the present study revealed that genotyping allows an evident discrimination of the three strains in A. vulgare which are characterized by specific amplified fragment sizes. This methodology is also very efficient to reveal multi-infections in A. vulgare from both experimental strain injections and individuals sampled in the field. Verne et al. [32] reported only mono-infected females based on sequencing analyses. Here, using the novel genotyping method to reanalyze the same samples, it would appear that multiple infections in A. vulgare are rather common with high proportions of bi-infected females (72.5%). Although co-infection of different Wolbachia strains in a single individual is commonly found in arthropods [18,35-38], this is the first time that doubly infected individuals have been observed in natural population of terrestrial isopods. This result is not really surprising as several recent studies have suggested that horizontal transfers of Wolbachia in A. vulgare may explain both the discordance between A. vulgare and Wolbachia phylogenies [32] and the presence of the recombinant strain wVulP [30]. Indeed, for recombination to occur, two strains need to be in close contact. Such proximity is possible if an individual host is infected by several strains. Previous studies have reported that haemolymph contact, predation and parasitism are possible routes for horizontal transfers of Wolbachia in A. vulgare [39-41]. Haemolymph contacts may be more frequent than previously thought, due to the fact that woodlice populations are often densely populated, and because of the abundance of injured individuals as a result of predations [42,43] or incidents during molting [39]. Thus, a given Wolbachia strain could spread through a population through such horizontal transfers and infect individuals already infected by another Wolbachia strain.
In 2003, all of the bi-infected females contained both the wVulC and wVulM strains but no bi-infections involving the wVulP strain was observed. This result is consistent with the prevalence of these strains in natural populations. Indeed, the Wolbachia strains wVulC and wVulM were more frequently observed in situ than the wVulP strain [32,40].
The Co-infection: a transition phase?
In theory, the co-existence of several feminizers within the same individual is unstable at equilibrium [44]. When two feminizing Wolbachia strains are in competition within the same host, the strain with the higher fitness is fixed [45]. Based on our results, it is difficult to give any firm conclusions concerning the evolution of Wolbachia strain prevalence between 2003 and 2010 , but we can expect that the wVulC strain will progressively replace the wVulM in the near future. Indeed, according to Cordaux et al. [29], wVulM is considered as a resident strain, with a transmission rate to the offspring lower than that of wVulC, this last strain being considered as an invasive strain. Recent experimental studies from challenged woodlice reveal that wVulC has a higher development rate than wVulM within the host tissue, suggesting that wVulC strain could be the most virulent and dominant strain (Johnson, unpublished data).
The wVulP Wolbachia strain is considered to be a recent strain resulting from the recombination of wVulC and wVulM [30]. According to evolutionary theory, it is expected that this strain would have a higher fitness than the others, leading to an increase in its prevalence in natural populations.. A follow up of the A. vulgare populations and their Wolbachia infection status could allow us to verify this hypothesis.
Conclusion
One of the main problems in the detection and characterization of different Wolbachia strains in A. vulgare was the absence of a rapid, inexpensive screening tool. Herein, we describe a novel PCR-based approach allowing the discrimination between wVulC, wVulM and wVulP on the basis of different amplification sizes by genotyping. For the first time, our study reports the presence of multiple Wolbachia strain infections in natural populations of A. vulgare, suggesting that such multiple infections are much more frequent than previously thought. Whether the presence of two different Wolbachia strains in a single individual is the result of horizontal transfer, hybrid introgression or co-divergence, as has recently been shown in other species complexes, awaits investigation although elements here support the idea of horizontal transfers. Additionally, three species closely related to A. vulgare, A. tunisiense, A. pelagicium, A. nasatum have showed amplification pattern corresponding to mono infected individuals suggesting that this technique could be efficient to check the infected status in several isopods species.
The method presented here offers new perspectives in the detection of multiple infections in natural populations of A. vulgare and related species and will also be invaluable in studies of infection dynamics after micro-injections of several strains in Wolbachia-free female hosts.
Acknowledgments
We are grateful to EBI laboratory and EES team and we thank more particularly Sophie Beltran for helpful comments on the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C (2002) How many Wolbachia supergroups exist? Mol Biol Evol 19: 341-346. doi: 10.1093/oxfordjournals.molbev.a004087. PubMed: 11861893. [DOI] [PubMed] [Google Scholar]
- 2. Bordenstein S, Rosengaus RB (2005) Discovery of a novel Wolbachia super group in Isoptera. Curr Microbiol 51: 393-398. doi: 10.1007/s00284-005-0084-0. PubMed: 16252129. [DOI] [PubMed] [Google Scholar]
- 3. Lo N, Evans TA (2007) Phylogenetic diversity of the intracellular symbiont Wolbachia in termites. Mol Phylogenet Evol 44: 461-466. doi: 10.1016/j.ympev.2006.10.028. PubMed: 17174110. [DOI] [PubMed] [Google Scholar]
- 4. Ros VI, Fleming VM, Feil EJ, Breeuwer JA (2009) How diverse is the genus Wolbachia? Multiple-gene sequencing reveals a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl Environ Microbiol 75: 1036-1043. doi: 10.1128/AEM.01109-08. PubMed: 19098217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Werren JH, Zhang W, Guo LR (1995) Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc Biol Sci 261: 55-63. doi: 10.1098/rspb.1995.0117. PubMed: 7644549. [DOI] [PubMed] [Google Scholar]
- 6. Werren JH (1997) Biology of Wolbachia . Annu Rev Entomol 42: 587-609. doi: 10.1146/annurev.ento.42.1.587. PubMed: 15012323. [DOI] [PubMed] [Google Scholar]
- 7. Bandi C, Anderson TJ, Genchi C, Blaxter ML (1998) Phylogeny of Wolbachia in filarial nematodes. Proc Biol Sci 265: 2407-2413. doi: 10.1098/rspb.1998.0591. PubMed: 9921679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Serbus LR, Casper-Lindley C, Landmann F, Sullivan W (2008) The genetics and cell biology of Wolbachia-host interactions. Annu Rev Genet 42: 683-707. doi: 10.1146/annurev.genet.41.110306.130354. PubMed: 18713031. [DOI] [PubMed] [Google Scholar]
- 9. Hurst GDD, Jiggins FM, Hinrich Graf von der Schulenburg J, Bertrand D, West SA et al. (1999) Male-killing Wolbachia in two species of insect. Proceedings of the Royal Society B: Biological Sciences 266: 735-740. [Google Scholar]
- 10. Weeks AR, Breeuwer JA (2001) Wolbachia-induced parthenogenesis in a genus of phytophagous mites. Proc Biol Sci 268: 2245-2251. doi: 10.1098/rspb.2001.1797. PubMed: 11674872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rigaud T, Moreau J, Juchault P (1999) Wolbachia infection in the terrestrial isopod Oniscus asellus: sex ratio distortion and effect on fecundity. Heredity (Edinb) 83: 469-475. doi: 10.1038/sj.hdy.6885990. PubMed: 10583549. [DOI] [PubMed] [Google Scholar]
- 12. Reuter M, Keller L (2003) High levels of multiple Wolbachia infection and recombination in the ant Formica exsecta . Mol Biol Evol 20: 748-753. doi: 10.1093/molbev/msg082. PubMed: 12679529. [DOI] [PubMed] [Google Scholar]
- 13. Bandi C, Dunn AM, Hurst GD, Rigaud T (2001) Inherited microorganisms, sex-specific virulence and reproductive parasitism. Trends Parasitol 17: 88-94. doi: 10.1016/S1471-4922(00)01812-2. PubMed: 11228015. [DOI] [PubMed] [Google Scholar]
- 14. Hurst GD, Werren JH (2001) The role of selfish genetic elements in eukaryotic evolution. Nat Rev Genet 2: 597-606. doi: 10.1038/89739. PubMed: 11483984. [DOI] [PubMed] [Google Scholar]
- 15. Charlat S, Hurst GDD, Merçot H (2003) Evolutionary consequences of Wolbachia infections. Trends in Genetics 19: 217-223. doi: 10.1016/S0168-9525(03)00024-6. PubMed: 12683975. [DOI] [PubMed] [Google Scholar]
- 16. Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L et al. (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6: 27. doi: 10.1186/1741-7007-6-27. PubMed: 18577218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rousset F, Solignac M (1995) Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proc Natl Acad Sci U S A 92: 6389-6393. doi: 10.1073/pnas.92.14.6389. PubMed: 7604001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kondo N, Ijichi N, Shimada M, Fukatsu T (2002) Prevailing triple infection with Wolbachia in Callosobruchus chinensis (Coleoptera: Bruchidae). Mol Ecol 11: 167-180. doi: 10.1046/j.0962-1083.2001.01432.x. PubMed: 11856419. [DOI] [PubMed] [Google Scholar]
- 19. Hiroki M, Tagami Y, Miura K, Kato Y (2004) Multiple infection with Wolbachia inducing different reproductive manipulations in the butterfly Eurema hecabe . Proc Biol Sci 271: 1751-1755. doi: 10.1098/rspb.2004.2769. PubMed: 15306297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mouton L, Dedeine F, Henri H, Boulétreau M, Profizi N et al. (2004) Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168: 181-189. doi: 10.1534/genetics.104.026716. PubMed: 15454536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frank SA (1998) Dynamics of Cytoplasmic Incompatability with Multiple Wolbachia Infections. J Theor Biol 192: 213-218. doi: 10.1006/jtbi.1998.0652. PubMed: 9735249. [DOI] [PubMed] [Google Scholar]
- 22. Engelstädter J, Telschow A, Hammerstein P (2004) Infection dynamics of different Wolbachia-types within one host population. J Theor Biol 231: 345-355. doi: 10.1016/j.jtbi.2004.06.029. PubMed: 15501467. [DOI] [PubMed] [Google Scholar]
- 23. Ironside JE, Smith JE, Hatcher MJ, Sharpe RG, Rollinson D et al. (2003) Two species of feminizing microsporidian parasite coexist in populations of Gammarus duebeni. J Evol Biol 16: 467-473. doi: 10.1046/j.1420-9101.2003.00539.x. PubMed: 14635846. [DOI] [PubMed] [Google Scholar]
- 24. Rousset F, Bouchon D, Pintureau B, Juchault P, Solignac M (1992) Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proc Biol Sci 250: 91-98. doi: 10.1098/rspb.1992.0135. PubMed: 1361987. [DOI] [PubMed] [Google Scholar]
- 25. Le Clec'h W, Raimond M, Guillot S, Bouchon D, Sicard M (2013) Horizontal transfers of feminizing versus non feminizing Wolbachia strains: from harmless passengers to pathogens. Environmental Microbiology. [DOI] [PubMed] [Google Scholar]
- 26. Moret Y, Juchault P, Rigaud T (2001) Wolbachia endosymbiont responsible for cytoplasmic incompatibility in a terrestrial crustacean: effects in natural and foreign hosts. Heredity (Edinb) 86: 325-332. doi: 10.1046/j.1365-2540.2001.00831.x. [DOI] [PubMed] [Google Scholar]
- 27. Juchault P, Rigaud T, Mocquard J-P (1992) Evolution of sex-determining mechanisms in a wild population of Armadillidium vulgare Latr.(Crustacea, Isopoda): competition between two feminizing parasitic sex factors. Heredity (Edinb) 69: 382-390. doi: 10.1038/hdy.1992.138. [DOI] [Google Scholar]
- 28. Juchault P, Legrand J (1989) Sex determination and monogeny in terrestrial isopods Armadillidium vulgare (Latreille, 1804) and Armadillidium nasatum Budde-Lund, 1885. Monografia. Monitore Zoologico Italiano 4: 359-375. [Google Scholar]
- 29. Cordaux R, Michel-Salzat A, Frelon-Raimond M, Rigaud T, Bouchon D (2004) Evidence for a new feminizing Wolbachia strain in the isopod Armadillidium vulgare: evolutionary implications. Heredity (Edinb) 93: 78-84. doi: 10.1038/sj.hdy.6800482. PubMed: 15138452. [DOI] [PubMed] [Google Scholar]
- 30. Verne S, Johnson M, Bouchon D, Grandjean F (2007) Evidence for recombination between feminizing Wolbachia in the isopod genus Armadillidium . Gene 397: 58-66. doi: 10.1016/j.gene.2007.04.006. PubMed: 17537593. [DOI] [PubMed] [Google Scholar]
- 31. Grandjean F, Rigaud T, Raimond R, Juchault P, Souty-Grosset C (1993) Mitochondrial DNA polymorphism and feminizing sex factors dynamics in a natural population of Armadillidium vulgare (Crustacea, Isopoda). Genetica 92: 55-60. doi: 10.1007/BF00057507. PubMed: 8163156. [DOI] [PubMed] [Google Scholar]
- 32. Verne S, Johnson M, Bouchon D, Grandjean F (2012) Effects of parasitic sex-ratio distorters on host genetic structure in the Armadillidium vulgare–Wolbachia association. J Evol Biol 25: 264-276. doi: 10.1111/j.1420-9101.2011.02413.x. PubMed: 22188300. [DOI] [PubMed] [Google Scholar]
- 33. Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132: 365-386. PubMed: 10547847. [DOI] [PubMed] [Google Scholar]
- 34. Kocher TD, Thomas WK, Meyer A, Edwards SV, Pääbo S et al. (1989) Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci U S A 86: 6196-6200. doi: 10.1073/pnas.86.16.6196. PubMed: 2762322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Breeuwer JA, Stouthamer R, Barns SM, Pelletier DA, Weisburg WG et al. (1992) Phylogeny of cytoplasmic incompatibility micro-organisms in the parasitoid wasp genus Nasonia (Hymenoptera: Pteromalidae) based on 16S ribosomal DNA sequences. Insect Mol Biol 1: 25-36. doi: 10.1111/j.1365-2583.1993.tb00074.x. PubMed: 1343772. [DOI] [PubMed] [Google Scholar]
- 36. Merçot H, Llorente B, Jacques M, Atlan A, Montchamp-Moreau C (1995) Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans . Genetics 141: 1015–1023. PubMed: 8582608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sinkins SP, Braig HR, O'neill SL (1995) Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proceedings of the Royal Society of London Series B. Biological Sciences 261: 325-330. doi: 10.1098/rspb.1995.0154. PubMed: 8587875. [DOI] [PubMed] [Google Scholar]
- 38. Kikuchi Y, Fukatsu T (2003) Diversity of Wolbachia endosymbionts in heteropteran bugs. Appl Environ Microbiol 69: 6082-6090. doi: 10.1128/AEM.69.10.6082-6090.2003. PubMed: 14532065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rigaud T, Juchault P (1995) Success and failure of horizontal transfers of feminizing Wolbachia endosymbionts in woodlice. J Evol Biol 8: 249-255. doi: 10.1046/j.1420-9101.1995.8020249.x. [DOI] [Google Scholar]
- 40. Cordaux R, Michel-Salzat A, Bouchon D (2001) Wolbachia infection in crustaceans: novel hosts and potential routes for horizontal transmission. J Evol Biol 14: 237-243. doi: 10.1046/j.1420-9101.2001.00279.x. [DOI] [Google Scholar]
- 41. Le Clec’h W, Chevalier FD, Genty L, Bertaux J, Bouchon D et al. (2013) Cannibalism and Predation as Paths for Horizontal Passage of Wolbachia between Terrestrial Isopods. PLOS ONE 8: e60232. doi: 10.1371/journal.pone.0060232. PubMed: 23593179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vandel A (1960) Faune de France: Isopodes terrestres. Paris: Paul Chevalier.
- 43. Warburg MR, Lisenmair KE, Bercovitz K (1984) The effect of climate on the distribution and abundance of isopods.Symposia of the Zoological Society of London 53: 339-367. [Google Scholar]
- 44. Taylor DR (1990) Evolutionary consequences of cytoplasmic sex ratio distorters. Evolutionary Ecology 4: 235-248. doi: 10.1007/BF02214332. [DOI] [Google Scholar]
- 45. Lachat M (2005) Impact de deux souches de Wolbachia sur les traits d’histoire de vie de leurs hôtes Armadillidiumvulgare. Thèse, Université de Poitiers. 169 pp. [Google Scholar]