Abstract
Background
Adverse food reactions (AFR) have has recently attracted increased attention from the media and are now more commonly reported by patients. Its classification, diagnostic evaluation, and treatment are complex and present a considerable challenge in clinical practice. Non-immune-mediated types of food intolerance have a cumulative prevalence of 30% to 40%, while true (immune-mediated) food allergies affect only 2% to 5% of the German population.
Methods
We selectively searched the literature for pertinent publications on carbohydrate malabsorption, with special attention to published guidelines and position papers.
Results
Carbohydrate intolerance can be the result of a rare, systemic metabolic defect (e.g., fructose intolerance, with a prevalence of 1 in 25 000 persons) or of gastrointestinal carbohydrate malabsorption. The malabsorption of simple carbohydrates is the most common type of non-immune-mediated food intolerance, affecting 20% to 30% of the European population. This condition is caused either by deficient digestion of lactose or by malabsorption of fructose and/or sorbitol. Half of all cases of gastrointestinal carbohydrate intolerance have nonspecific manifestations, with a differential diagnosis including irritable bowel syndrome, intolerance reactions, chronic infections, bacterial overgrowth, drug side effects, and other diseases. The diagnostic evaluation includes a nutritional history, an H2 breath test, ultrasonography, endoscopy, and stool culture.
Conclusion
The goals of treatment for carbohydrate malabsorption are to eliminate the intake of the responsible carbohydrate substance or reduce it to a tolerable amount and to assure the physiological nutritional composition of the patient’s diet. In parallel with these goals, the patient should receive extensive information about the condition, and any underlying disease should be adequately treated.
Adverse food reactions (AFR) represent a growing problem in daily clinical practice. They are classified according to cause (1– 3). Nonspecific, immune-mediated (e.g., enzymatic, pharmacological, and toxic) AFR predominate, together accounting for 30% to 40% of all cases, while food allergies (FA)—antigen-specific immune reactions divided into types I to IV—are much less common, making up 2% to 5% of cases (1, 3, 4). Recently published data show that the number of studies has increased since 2000: from 54 to 77 publications per 2.5-year interval for AFR and from 454 to 991 for FA in the period up to June 2013. Patients with food-related symptoms seem to be taking the problem seriously. Around two thirds of patients with irritable bowel syndrome raise the subject of AFR with their physicians (2– 7, e1– e5).
Nonimmune-related AFR are frequently caused by carbohydrates and fats, less often by biogenic amines, and do not lead to specific FA (1– 5, e1– e3). Carbohydrate intolerance is playing a growing role due to the frequent industrial use of sugar substitutes (fructose, xylitol), e.g., in foodstuffs for diabetics. A healthy diet rich in fruit and vegetables will also often include fructose and sorbitol.
Our aim in this article is to describe the pathophysiology, clinical presentation, diagnosis, and treatment of carbohydrate malabsorption. A selective survey of the Medline database was carried out in June 2013 to identify recently published relevant original articles and review articles; the search terms were “carbohydrate malassimilation,” “carbohydrate maldigestion,” carbohydrate malabsorption,” “carbohydrate intolerance,” “lactose intolerance,” “lactase deficiency,” “fructose malabsorption,” and “sorbitol malabsorption.” The findings were compared with our experience of nonimmunological AFR. Data from guidelines and position papers on irritable bowel syndrome and on fructose malabsorption with reference to carbohydrate malabsorption were taken into consideration (8, 9). Although a large number of short-term studies on carbohydrate intolerance have been published, they have varying, sometimes discrepant endpoints. The lack of prospective, randomized long-term studies means that the evidence with regard to therapeutic interventions does not exceed level 2 or recommendation level B/C (8– 10, e6– e8).
Definition of carbohydrate intolerance
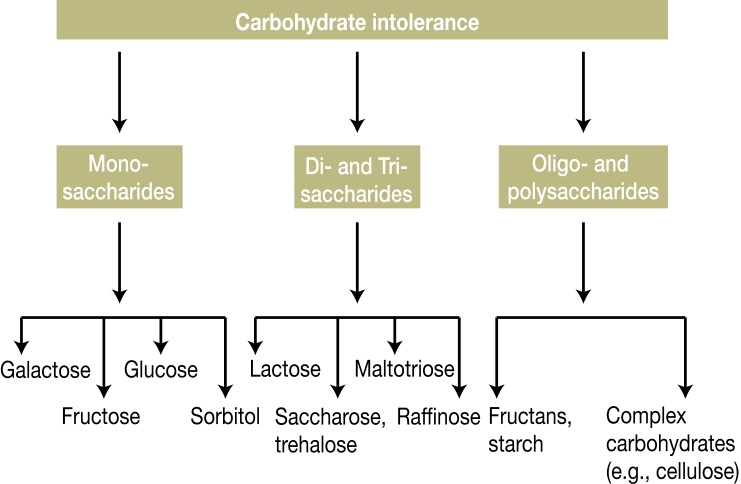
Intolerance to carbohydrates may result from gastrointestinal malabsorption of carbohydrates or, less frequently, from systemic metabolic defects (e.g., fructose intolerance) (Table 1). With regard to gastrointestinal intolerance, one distinguishes between simple and complex carbohydrates (Figure 1). The most frequent AFR occur with simple carbohydrates. The incidence of intolerance in the general population is estimated at 7% to 20% for lactose (dairy products), 15% to 25% for fructose, and 8 to 12% for sorbitol (Box) (5– 7, 11, e1, e3– e5, e8– e11). Clinically, carbohydrate intolerance induces malabsorption; this occurs in around half of all patients with nonspecific AFR and is not infrequently misinterpreted as an allergy (3, 6, 7, 11, 12, e12).
Table 1. Some important carbohydrate intolerances (5, 11, 13, 21, 33, e9, e16, e21).
| Enzyme structure | Target structure | Primary deficiency | Secondary deficiency or disorder |
|---|---|---|---|
| Combined disaccharide malabsorption syndrome | Lactase, saccharase and other disaccharides | Autosomal dominant | Severe intestinal inflammation (e.g., celiac disease) or extensive small intestinal resection |
| Isolated disaccharide intolerance | |||
| Lactase (ß-galactosidase) | Lactose | Physiological lactase reduction (>5–10 years of age); autosomal recessive familial lactase deficiency | Inflammatory intestinal diseases (e.g., Crohn’s disease, celiac disease); extremely rare, ca. 40 cases described |
| Saccharase (sucrase-isomaltase) | Saccharose | Autosomal recessive saccharase-isomaltase deficiency | Inflammatory intestinal diseases (e.g., Crohn’s disease, celiac disease) |
| Maltase (α -glucosidase) | Maltose | – | Medication with acarbose, miglitol |
| Trehalase | Trehalose | – | Chronic small intestinal diseases (e.g., celiac disease) |
| Differential diagnoses to carbohydrate malabsorption | |||
| Fructose intolerance (levulose intolerance) | Fructose (ketose)-1-phosphate aldolase (aldolase B) | Autosomal recessive 1: 25000 | – |
| Galactosemia | Galactokinase | Autosomal recessive 1: 80000 | – |
| Glucose-6-phosphate dehydrogenase*1 | Glucose-6-phosphate*2 | X-chromosomal inherited enzyme defect | Medication with sulfonamides |
*1Glucose-6-phosphate dehydrogenase deficiency is found particularly in inhabitants of Mediterranean countries with favism, in whom ingestion of fava beans may be followed by hemolytic crises, abdominal pain, fever, nausea, vomiting, and diarrhea
*2Despite a high rate of comorbidity with lactose maldigestion, favism can often be relatively well distinguished due to the presence of anemia, fever, recurrent hemolysis, and diarrhea
Figure 1.
Carbohydrate intolerance
Among the various forms of carbohydrate intolerance, those most commonly encountered in the European population are maldigestion of lactose and malresorption of fructose and sorbitol (sugar alcohol). Glucose malabsorption occurs with bacterial overgrowth in the small intestine (H2 breath test). Apart from the rarely occurring glucoamylase deficiency, malabsorption of complex and/or vegetable carbohydrates is predominantly secondary to other diseases in advanced stages (e.g., chronic pancreatitis, pancreatectomy) (e9, e11)
Box. Types of foods to be considered when documenting the nutritional history in commonly occurring adverse food reactions (AFR).
Questioning should first focus on identifying the group of nutrients (fats, carbohydrates, proteins) responsible for the AFR:
In the case of proteins, besides intolerance of biogenic amines the patient must be investigated for allergic reactions; this requires specific allergological tests (skin tests, specific IgE, provocation, etc. [1–4, e12]).
With regard to simple carbohydrates (mono- and disaccharides), it is diagnostically expedient to identify malabsorption of fructose or sorbitol by enquiring about meals with large amounts of fruit and/or vegetables, fruit juices, dried fruit, sugar-free chewing gum, low-energy products, candies, etc. (10– 12, 28, 31). Because of its high sweetness and low production costs, fructose is preferentially used in products for diabetics and as a substitute for saccharose (5, 6, 21, 31).
If maldigestion of lactose is suspected, the patient should be asked about consumption of dairy products, foods and drinks containing milk, and sources of concealed lactose (convenience foods, instant cappuccino, etc.) (5, 11, 13, 18).
| Biogenic amines | ||
| Putrescine, tyramine Serotonin Histamine |
Maggi seasoning, raw sausage meat Pineapple, banana, walnut Cheese, red wine, tuna, sauerkraut |
|
| Mono- and disaccharides | ||
| Fructose Lactose Sugar substitutes (sorbitol, xylitol) |
Fruit (apple), corn, raisins, honey, potato, fruit juices, soft drinks, sweeteners Dairy products, cappuccino, condensed milk, cheese, convenience foods, chocolate Fresh and dried fruit (grapes, pears, peaches, plums, dates), sugar substitutes, diabetic foods, chewing gum, candies |
|
| Oligo- and polysaccharides | ||
| Fructans (inulin, levans)Raffinose, stachyose | Wheat products, full-fat milk products, onion, artichokePulses, beans, lentils | |
Primary and secondary forms
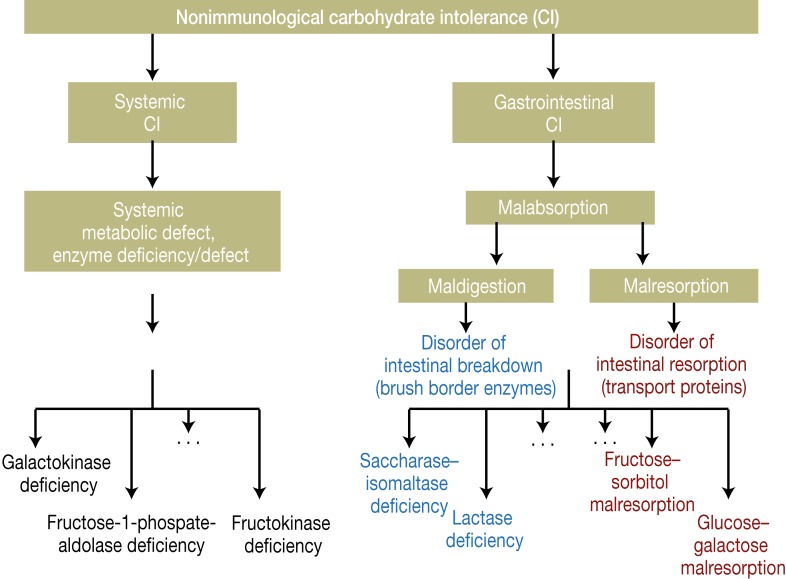
Primary disorders of carbohydrate tolerance are either caused by rare congenital defects of the enzymes and transport mechanisms involved in normal digestion or occur as a result of physiologically determined cessation of enzyme activity, as may be the case with lactase (so-called physiological lactase deficiency), provided no other associated underlying diseases are present (Table 1, Figure 2) (5, 6, 8, 9, 11). Persons with primary malabsorption generally show a specific intolerance to the corresponding carbohydrate, e.g., to lactose in lactase deficiency or to saccharose in saccharase–isomaltase deficiency.
Figure 2.
Mechanisms of the nonimmunologically mediated forms of carbohydrate intolerance (CI)
Secondary forms of carbohydrate maldigestion or malresorption are due to organ pathology or to loss of the brush border of the small intestinal mucosa. These disorders affect multiple carbohydrates in one patient. They may be polyetiologic (e.g., intestinal lymphangiectasia, mastocytosis), may result from inflammatory diseases (e.g., infections, celiac disease, chronic inflammatory bowel disease), or may represent reactions to toxic substances or treatments (e.g., alcohol consumption, chemotherapy, irradiation) (5, 6, 8– 11, e9, e10).
Clinical symptoms
The symptoms experienced by patients with carbohydrate malabsorption are caused by lack of breakdown or resorption of carbohydrates in the intestinal lumen. These carbohydrates are osmotically active, retain fluid in the lumen, are metabolized by bacteria, and lead to gassy, acid-forming stools (9– 12). This results in the typical cardinal symptoms of postprandial flatulence, nausea, meteorism, diarrhea, and nonspecific abdominal pain. Occasionally constipation, weight loss or extraintestinal symptoms (e.g., headache in fructose malabsorption) may be found (6, 9– 11). Because of the rapid passage of the carbohydrates through the gastrointestinal tract, the symptoms often begin as early as 30 min after ingestion. They can persist for 6 to 9 h after food intake. The patients usually have no symptoms at night or when they abstain from food. Serologically, there are usually no signs of increased inflammatory activity (erythrocyte sedimentation rate [ESR], C-reactive protein, protein electrophoresis) (5, 6, 10).
Nutritional history
Interviews with the patient will reveal information about nutrition, choice of foods, factors accompanying the symptoms, and presence of warning signs (dysphagia, fever, weight or blood loss). The patient should be asked what types of foods cause the symptoms (proteins, fats, carbohydrates, biogenic amines, dietary fiber) to help identify the group of foodstuffs responsible (Box). Next, the interviewer should enquire about intolerance of particular foods within that category as a prelude to specific diagnostic testing. In the case of fructose and sorbitol malabsorption, in which the patient typically mentions various kinds of fruit and vegetables, vegetable allergens and pollen-associated FA also have to be tested (1– 4, 9, 12).
Hydrogen exhalation test
Nowadays carbohydrate malabsorption is primarily established by means of the hydrogen exhalation test (H2 breath test), in which the patient is given a drink containing 50 g lactose or 25 g fructose, alternatively 5 to 10 g sorbitol or any other sugar that needs to be tested. Exhalation of H2 is then measured every 30 min for a period of 150 to 180 min (normal value <20 ppm). Since the human body itself does not produce hydrogen gas, H2 can occur only if bacteria come into contact with carbohydrates and H2, among other substances, is formed (pathological value >20 ppm) (8– 10). This happens only when carbohydrates encounter bacteria (bacterial overgrowth) in the small intestine or the normal bacterial flora in the colon. Therefore, an orally administered carbohydrate that is completely resorbed in a small intestine without bacterial overgrowth will not result in an increased amount of H2 in the exhaled air. Lactulose, a disaccharide that is not broken down in the human small intestine, normally always reaches the colon and leads to exhaled H2 of >20 ppm in persons who produce H2. In Europe this is around 85% of the population; the remaining 10% to 15% are so-called H2 nonproducers, whose bacterial flora does not produce hydrogen gas, so that no exhalation of H2 can be measured (9– 11). In these cases the H2 breath test always yields values of <20 ppm after administration of the above-mentioned carbohydrates and lactulose, so that the diagnosis has to be established on the basis of the clinical symptoms or the results of other tests.
Lactose intolerance
In around 70% of people worldwide, the activity of the lactose (glucose–galactose disaccharide)-cleaving lactase (beta-galactosidase) in the microvilli of the small intestinal brush border sinks below a critical threshold between the ages of 2 and 5 years; this is the most frequent cause of enzyme deficiency (9, 11, 13, e1, e9). The rate of primary lactase deficiency varies among ethnic groups (e.g., Asia 80% to 100%, Africa 70% to 95%, USA 15% to 80%, Europe as a whole 15% to 70%, Germany 15% to 20%) and is based on the nonpersistence of lactase after childhood (5, 10, 12– 14, e1, e8, e13, e14). The persistence of lactase after childhood in around 30% of the world population is due to alterations in the long arm of chromosome 2 of the lactase gene (9, 12– 14).
The absolute or relative deficiency of lactase means that orally consumed lactose reaches lower regions of the small intestine and the colon in nonhydrolyzed, osmotically active form. Breakdown by bacteria results in production of short-chain fatty acids, methane, carbon dioxide, hydrogen, etc., inducing meteorism, flatulence, abdominal pain, and diarrhea (11, 12).
Diagnostic confirmation of the gene mutation shows the primary lactase deficiency (11, 13), but cannot predict whether and from what dosage upwards a person will suffer increased symptoms after lactose intake. The severity of the symptoms is influenced not only by maldigestion but also by other variables such as gastric emptying time, small intestinal transit time, intestinal flora, and individual symptom threshold. Persons with primary lactose intolerance still possess residual lactase activity, so small amounts of lactose (<1–5 g daily) cause no discomfort. This is why patients with lactose intolerance do not react unfavorably to the minimal amounts of lactose (measured in milligrams) contained in tablets (11, 13, 17, e8).
The secondary forms of lactose intolerance that occur with certain diseases cannot be identified by genetic testing. In such cases the intolerance disappears, both in children and in those adults in whom lactase persists, as soon as the patient has recovered from the disease concerned (e.g., remission of Crohn’s disease) (13– 18).
Intolerance is diagnosed by means of the H2 breath test with 50 mg lactose (for children, 2 mg/kg body weight BW). The sensitivity and specificity of the test are 90% to 95% and 95% to 100%, respectively. The H2 breath test yields false-negative results in patients with no H2-producing intestinal flora (5, 6, 9, 13, 14). Alternatives to the H2 breath test are determination of disaccharidase activity in the small intestinal mucosa, either directly by biopsy or by blood test (determination of blood sugar) after administration of 50 g lactose (blood glucose increase >20 mg/dL). The blood test is thought to be somewhat less sensitive and specific (14– 17).
After diagnosis of lactose intolerance, the patient should avoid intake of lactose completely for 4 to 6 weeks. Thereafter, and following consultation with a dietician, the tolerated minimal dosage of lactose can be consumed (5, 6, 11, 13, e8, e9). It is important to train the patient to recognize which foods contain lactose (e.g., ice cream, sausages, bakery produce, and convenience foods) (11, 13– 16, e9, e14). Other treatment options are:
Intake of lactase tablets when dairy products are consumed (not covered by health insurance in Germany; median reduction of symptoms up to 88% [71% to 90%])
Consumption of special lactose-free dairy products (<0.1 g lactose/100 g)
Selection of fermented dairy products (yoghurt, curds), which have a lower lactose content than nonfermented foods due to bacterial–enzymatic breakdown of lactose (17, e8, e9, e13, e14). While 100 g of full-fat (in Germany, typically 3.5%) pasteurized milk contains ca. 5 g lactose, 100 g of a fermented product (yoghurt, curds, hard cheese, slicing cheese, soft cheese, cream cheese) will contain only 1 to 3 g (12– 16, e9).
Finally, intake of a diet regularly comprising the same proportions of carbohydrates (50%), protein (15% to 20%), and fat (25% to 30%) and without low-fat products can achieve prolongation of gastric emptying time and thus slower intestinal filling.
Altogether, these measures achieve remission in 40% to 100% of patients. The variance can be explained by differences among the study groups in terms of compliance, underlying diseases, and ethnic composition (5, 6, 11, 13– 17).
Fructose intolerance
Gastrointestinal malabsorption of fructose should not be confused with hereditary fructose intolerance (a metabolic disease affecting around 1 in 25 000 persons), in which the enzyme fructose-1-phosphate aldolase is missing from the patient’s tissues and fructose-1-phosphate accumulates, causing, among other symptoms, postprandial hypoglycemia in infants (Fgure 2) (5, 9, 12, e3, e9, e15, e16).
In humans the transport system SGLT-1 (sodium–glucose cotransporter) is responsible for the active resorption of glucose from the small intestine, while the glucose transport systems GLUT-5 (apical brush border membrane) and GLUT-2 (basolateral transporter) provide for the passive uptake of fructose (9– 12, e16). GLUT-5 has a low, saturable uptake capacity. If fructose consumption exceeds around 30 to 50 g/h, osmotically active fructose remains in the intestine. The uptake capacity can be raised by glucose or amino acids (Figure 3) (10, 19– 22). If symptoms of fructose malabsorption occur after consumption of less than 25 to 30 g fructose, the patient has symptomatic primary fructose malabsorption. This functional disorder usually results from dose-dependent overloading of the principal transport system GLUT-5. The time of occurrence of individual symptoms depends on the composition of the diet, fructose diffusion kinetics, dose (fruit juices, syrup), intestinal permeability, intestinal flora, and the underlying disease (6, 7, 20– 23). Acquired transport disorders have been described following intensive physical training (22), with a low-glucose diet, and with interaction of the fructose transporter with other osmotically active substances (mannitol, xylitol) (6, 21– 23). Moreover, sorbitol can be transformed into fructose within the intestine, blocking GLUT-5 (6, 19– 21, e4, e5). This leads to aggravation of the fructose uptake disorder.
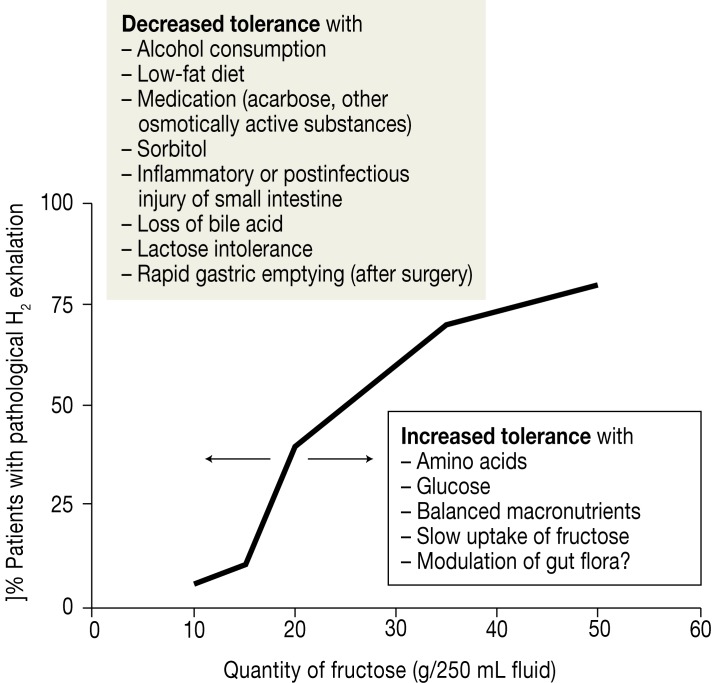
Figure 3.
Dependence of fructose resorption on the amount of fructose consumed, expressed as proportion of pathological H2 breath test results (>20 ppm increase)
This fructose resorption curve, calculated from the results of several studies, shows that doses of fructose from around 40 g upwards result in pathological H2 breath test results in more than 60% of persons, although not all of them develop symptoms (10, 19– 23). The resorption capacity for fructose is limited in humans (9– 11, 26, e16). Various factors can act to modulate fructose resorption (Figure 3): displacement of the fructose resorption curve to the left leads to manifestations of malabsorption on intake of lower amounts of fructose (decrease in tolerance), while displacement to the right results in tolerance of higher quantities of fructose. The extent to which congenital or acquired mechanisms influence the affinity and function of the transporter has not yet been conclusively established (9, 10, 19, 33)
In secondary fructose malabsorption the functional transport disorder of fructose uptake is accompanied by morphological injury of the intestinal epithelium or reduction of the area available for resorption (celiac disease, short bowel syndrome).
Fructose malabsorption is often characterized by:
Excessively rapid arrival of large quantities of fructose in the distal small intestine and colon
Changes in the anaerobic gut flora
Dose-dependent osmotic effects
Increased formation of short-chain fatty acids
Fructose and sorbitol malabsorption are frequently combined with AFR, FA, and irritable bowel syndrome (1, 3, 7, 10, e9, e12).
The diagnosis of fructose intolerance is confirmed by a positive H2 breath test (>20 ppm) and abdominal discomfort following oral administration of 25 g fructose (sensitivity and specificity both 80% to 90%) (19– 23, e4). If a positive H2 breath test is not accompanied by subjective symptoms, the patient has an asymptomatic fructose resorption disorder; symptoms may occur at higher test doses or with simultaneous administration of sorbitol (6, 19, 21). In H2 nonproducers (negative lactulose test), the diagnosis must be made on the basis of the clinical findings alone (6, 8, 9).
The treatment of fructose intolerance comprises reduction of fructose intake to <10 g/day together with complete abstinence from sugar alcohols and alcoholic beverages; any accompanying disease should be treated appropriately (9– 12, 20, e4). It is crucial to draw the patient’s attention to the importance of balanced consumption of glucose and fructose, because glucose can stimulate the GLUT-5 and GLUT-2 transporters (19– 24) and thus increase fructose uptake (Figure 3). This explains why patients with fructose malabsorption often tolerate cane or beet sugar (saccharose: glucose–fructose ratio 1 : 1) or bananas (glucose–fructose ratio 1.5 : 1) better than foods with a higher proportion of fructose (e.g., apples: glucose–fructose ratio 1 : 3) (12, 19, 20, 23– 25).
The conversion of fructose to glucose in the gut can be supported by the use of xylose isomerase as a dietary supplement; a recent study showed that intake of this enzyme ameliorated the symptoms of fructose intolerance (19). Increased efficiency of fructose absorption has also been described with amino acids and heavy meals, so patients with carbohydrate malabsorption should be informed of the importance of eating normal amounts of macronutrients (see section “Lactose intolerance” above) (9, 12, 20). These dietetic measures lead to remission in 60% to 90% of cases (6, 9, 19, 22– 26). Following initial symptom reduction by abstinence, the individual fructose tolerance threshold should be established. The patient can then consume safe amounts of fructose in the long term (22– 25).
Sorbitol malabsorption
Sorbitol, a hexavalent sugar alcohol, undergoes only slight intestinal resorption by passive diffusion. Sorbitol (E420) is used as a sugar substitute, as a vehicle for other substances, and as a humectant (hygroscopic properties) and is present in many different kinds of fruit (Box) (9, 10, 24– 27). It directly inhibits the GLUT-5 transporter, is osmotically active, and displaces the saturable fructose resorption curve to the left (Figure 3), so the symptoms are the same as those of fructose malabsorption. This is why some studies have shown associated sorbitol intolerance in persons with fructose malabsorption (10– 24, 27– 29, e4, e14, e16). In a few cases sorbitol has also been identified as the cause of “chewing gum diarrhea,” with flatulence, weight loss, meteorism, and abdominal pain (30, e15).
Sorbitol showed a higher malabsorption rate than fructose and xylitol on the H2 breath test. After intake of 25 g of each substance, a pathological increase in H2 exhalation was found in 84% of the sorbitol group, compared with 36% for fructose and 12% for xylitol (29). It can be concluded that sorbitol possesses a very high potential for induction or amplification of carbohydrate malabsorption (27, 29, 30, e4, e15).
The diagnosis of sorbitol malabsorption is established either clinically or by means of the H2 breath test after administration of 5 to 10 g sorbitol (8– 10, 25). Patients are treated by reduction of sorbitol intake as advised by a dietician, and if applicable glucose can be given to activate the GLUT-5 transporter and thus improve resorption of the fructose formed from sorbitol. For this reason attention should always also be paid to the fructose content of foodstuffs (27– 30).
Principal differential diagnoses
The main differential diagnoses and diagnostic strategies are listed in Table 2. The above-mentioned clinical symptoms of carbohydrate malabsorption are also found in patients with functional dyspepsia, irritable bowel, AFR, and bacterial overgrowth in the small intestine. Carbohydrate malabsorption may be associated with increased histological inflammatory activity. The patients often complain of AFR or have had sensitization to allergens identified (9, 30– 37, e9, e12). Interestingly, lactose, fructose, or sorbitol provocation induces typical symptoms in some irritable bowel patients; however, the incidence of carbohydrate malabsorption is no higher in this group than in the general population (10– 13, 28, 30– 37). Nevertheless, in patients with irritable bowel the symptoms of coexisting carbohydrate intolerance are more severe. Removal of the corresponding foods from the diet is less effective (40% to 50% remission) than in persons without irritable bowel syndrome (70% to 90% remission); apparently other pathophysiological mechanisms (histamine intolerance, salicylate intolerance, low-grade inflammation) play a role (5, 8, 28– 38, e12, e18– 20).
Table 2. Important differential diagnoses to carbohydrate malabsorption and tests to narrow down the diagnosis in persons with irritable bowel symptoms in whom adverse food reactions have been described.
| Disease / pathophysiology | Test |
|---|---|
Carbohydrate intolerance (malabsorption)
|
H 2 -BT 50g Lactose H 2 -BT 25g Fructose H 2 -BT 10g Sorbitol |
| Bacterial overgrowth | H2-BT 50g Glucose (e17) |
|
Chronic inflammatory bowel diseases Chronic pancreatitis |
Inflammatory activity, endoscopy and histology Elastase in stool, lipase, abdominal sonography |
| Infections | Stool tests |
Adverse food reactions (intolerance)
|
Test meal, abdominal sonography Provocation 75mg histamine Provocation Provocation 10–100–250mg ASA Functional blood test (39) |
Food allergies
|
Prick test Specific IgE serum or bowel (1, 4, 40, e9, e12) Urinary methylhistamine, provocation |
| Polyposis coli and neoplasms | Abdominal sonography and other imaging procedures; Endoscopy and histology |
| Dyspepsia and irritable bowel syndrome | For differential diagnosis see above; if indicated, interdisciplinary diagnosis involving allergology/dermatology, gynecology, if necessary endocrinology, psychosomatics |
| Celiac disease | Serum IgA antibodies to transglutaminase, endomysium, or to DGP-lgA (e9) |
ASA, acetylsalicylic acid; DGP-IgA, deamidated gliadine peptide; H2-BT, H2 breath test;
NSAID, nonsteroidal anti-inflammatory drugs
Key Messages.
Intolerance of simple carbohydrates results either from congenital metabolic defects or gastrointestinal malabsorption (maldigestion or malresorption).
Carbohydrate malabsorption is classed as a nonimmunologically mediated adverse food reaction (AFR), and the simple carbohydrates most frequently involved are the disaccharide lactose (maldigestion) and the monosaccharides fructose and sorbitol (malresorption).
Lactose intolerance results from deficiency of the enzyme lactase in the small intestinal brush border after childhood. Fructose and sorbitol malabsorption are caused either by a dose-dependent functional disorder or overloading (high quantities of fructose) of the transporters GLUT-5 and GLUT-2, essential for fructose uptake, or by inhibition of GLUT-5 by sorbitol.
The nonresorbed mono- and disaccharides are osmotically active in more distal segments of the gut, are metabolized by bacteria, lead to production of H2 and methane gas, and thus induce flatulence, meteorism, pain, diarrhea, etc., necessitating differentiation from other possible diagnoses (irritable bowel, bacterial overgrowth in the small intestine, etc.).
Carbohydrate malabsorption is best treated dietetically by reducing or completely abolishing intake of the corresponding mono- or disaccharide. The patient must be instructed accordingly and advised of the importance of eating normal amounts of macronutrients
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Zopf Y, Baenkler HW, Silbermann A, Hahn EG, Raithel M. The differential diagnosis of food intolerance. Dtsch Arztebl Int. 2009;106(21):359–369. doi: 10.3238/arztebl.2009.0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skypala I. Adverse food reactions - an emerging issue for adults. J Am Diet Assoc. 2011;111:1877–1891. doi: 10.1016/j.jada.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Osterballe M, Mortz CG, Hansen TK, Andersen KE, Bindslev-Jensen C. The prevalence of food hypersensitivity in young adults. Pediatr Allergy Immunol. 2009;20:686–692. doi: 10.1111/j.1399-3038.2008.00842.x. [DOI] [PubMed] [Google Scholar]
- 4.Möhrenschlager M, Ring J. Food allergy: An increasing problem for the elderly. Gerontology. 2011;57:33–36. doi: 10.1159/000316576. [DOI] [PubMed] [Google Scholar]
- 5.Wilder-Smith CH, Materna A, Wermelinger C, Schuler J. Fructose and lactose intolerance and malabsorption testing: the relationship with symptoms in functional gastrointestinal disorders. Aliment Pharmacol Ther. 2013;37:1074–1083. doi: 10.1111/apt.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hammer HF, Hammer J. Diarrhea caused by carbohydrate malabsorption. Gastroenterol Clin North Am. 2012;41:611–627. doi: 10.1016/j.gtc.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Niec AM, Frankum B, Talley NJ. Are adverse food reactions linked to irritable bowel syndrome? Am J Gastroenterol. 1998;93:2184–2190. doi: 10.1111/j.1572-0241.1998.00531.x. [DOI] [PubMed] [Google Scholar]
- 8.Layer P, Andresen V, Pehl C, et al. S3-Leitlinie der Deutschen Gesellschaft für Verdauungs- und Stoffwechselstörungen und der Deutschen Gesellschaft für Neurogastroenterologie und Motilität zu Definition, Pathophysiologie, Diagnostik und Therapie des Reizdarmsyndroms. Z Gastroenterol. 2011;49:237–292. doi: 10.1055/s-0029-1245976. [DOI] [PubMed] [Google Scholar]
- 9.Schäfer C, Reese I, Ballmer-Weber BK, et al. Fruktosemalabsorption: Stellungnahme der AG Nahrungsmittelallergie in der Deutschen Gesellschaft für Allergologie und klinische Immunologie (DGAKI) Allergo J. 2010;19:66–69. [Google Scholar]
- 10.Latulippe ME, Skoog SM. Fructose malabsorption and intolerance: Effects of fructose with and without simultaneous glucose ingestion. Clin Rev Food Sci Nutr. 2011;51:583–592. doi: 10.1080/10408398.2011.566646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Born P, Sekatcheva M, Rösch Th, Classen M. Carbohydrate malabsorption in clinical routine: a prospective observational study. Hepatogastroenterology. 2006;71:673–677. [PubMed] [Google Scholar]
- 12.Fernandez-Banares F, Rosinach M, Esteve M, Forne M, Espinos JC, Viver JM. Sugar malabsorption in functional abdominal bloating: A pilot study on the long-term effect of dietary treatment. Clinical Nutrition. 2006;25:824–831. doi: 10.1016/j.clnu.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Lomer MCE, Parkes GC, Sanderson JD. Review article: lactose intolerance in clinical practice - myths and realities. Aliment Pharmacol. 2008;27:93–103. doi: 10.1111/j.1365-2036.2007.03557.x. [DOI] [PubMed] [Google Scholar]
- 14.Kuokkanen M, Myllyniemi M, Vauhkonen M, et al. Biopsy-based quick test for duodenal hypolactasia in upper gastrointestinal endoscopy. Endoscopy. 2006;38:708–712. doi: 10.1055/s-2006-925354. [DOI] [PubMed] [Google Scholar]
- 15.Sibley E. Genetic variation and lactose intolerance. Am J Pharmacogenetics. 2004;4:239–245. doi: 10.2165/00129785-200404040-00003. [DOI] [PubMed] [Google Scholar]
- 16.Ridefelt P, Hakansson LD. Lactose intolerance: Lactose intolerance test versus genotyping. Scand J Gastroenterol. 2005;40:822–826. doi: 10.1080/00365520510015764. [DOI] [PubMed] [Google Scholar]
- 17.Montalto M, Curigliano V, Santoro L, et al. Management and treatment of lactose malabsorption. World J Gastroenterol. 2006;12:187–191. doi: 10.3748/wjg.v12.i2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casellas F, Aparici A, Casaus M, Rodriguez P, Malagelada JR. Subjective perception of lactose intolerance does not always indicate lactose malabsorption. Clin Gastroenterol Hepatol. 2010;8:581–586. doi: 10.1016/j.cgh.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 19.Komericki P, Akkilic-Materna M, Strimitzer T, Weyermair K, Hammer HF, Aberer W. Oral xylose isomerase decreases breath hydrogen excretion and improves gastrointestinal symptoms in fructose malabsorption-a double blind, placebo-controlled study. Aliment Pharmacol Ther. 2012;36:980–987. doi: 10.1111/apt.12057. [DOI] [PubMed] [Google Scholar]
- 20.Wächtershäuser A, Stein J. Kohlenhydrat-Intoleranzen. In: Stein J, Raithel M, Kist M, editors. Erkrankungen durch Lebensmittel.1. Aufl. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2011. pp. 18–31. [Google Scholar]
- 21.Kyaw MH, Mayberry JF. Fructose malabsorption: true condition or a variance from normality. J Clin Gastroenterol. 2011;45:16–21. doi: 10.1097/MCG.0b013e3181eed6bf. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa T, Mulligan K, Wada L, Schumacher L, Riby J, Kretschmer N. The effect of excercise on fructose absorption. Am J Clin Nutr. 1993;58:75–79. doi: 10.1093/ajcn/58.1.75. [DOI] [PubMed] [Google Scholar]
- 23.Born P, Zech J, Lehn H, Classen M, Lorenz R. Colonic bacterial activity determines the symptoms in people with fructose malabsorption. Hepatogastroenterology. 1995;42:778–785. [PubMed] [Google Scholar]
- 24.Hoekstra JH, van den Acker JHL. Facilitating effects of amino acids on fructose and sorbitol absorption in children. J Pediatric Gastroenterol & Nutrition. 1996;23:118–124. doi: 10.1097/00005176-199608000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Keller J, Francke A, Storr M, Wiedbruck F, Schirra J. Klinisch relevante Atemtests in der gastroenterologischen Diagnostik, Empfehlungen der Deutschen Gesellschaft für Neurogastroenterologie und Motilität sowie der Deutschen Gesellschaft für Verdauungs- und Stoffwechselerkrankungen. Z Gastroenterol. 2005;43:1071–1090. doi: 10.1055/s-2005-858479. [DOI] [PubMed] [Google Scholar]
- 26.Douard V, Ferraris RP. The role of fructose transporters in disease linked to excessive fructose intake. J Physiol. 2013;591:401–414. doi: 10.1113/jphysiol.2011.215731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidenhiler S, Rieger M. Glukose-, Fruktose- und Sorbitgehalt in Lebensmitteln. Der Allgemeinarzt. 2003;14:1113–1118. [Google Scholar]
- 28.Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary managment. J Am Diet Assoc. 2006;106:1631–1639. doi: 10.1016/j.jada.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 29.Born P, Zeck J, Stark M, Classen M, Lorenz R. Zuckeraustauschstoffe: Vergleichende Untersuchung zur intestinalen Resorption von Fructose, Sorbit und Xylit. Med Klinik. 1994;89:575–578. [PubMed] [Google Scholar]
- 30.Bauditz J, Norman K, Biering H, Lochs H, Pirlich M. Severe weight loss caused by chewing gum. Br Med J. 2008;336:96–97. doi: 10.1136/bmj.39280.657350.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with IBS: Randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765–771. doi: 10.1016/j.cgh.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 32.Vivinus-Nébot M, Dainese R, Anty R, et al. Combination of allergic factors can worsen diarrheic irritable bowel syndrome: role of barrier defects and mast cells. Am J Gastroenterol. 2012;107:75–81. doi: 10.1038/ajg.2011.315. [DOI] [PubMed] [Google Scholar]
- 33.Corlew-Roath M, Di Palma JA. Clinical impact of identifying lactose maldigestion or fructose malabsorption in irritable bowel syndrome or other conditions. South Med J. 2009;102:1010–1012. doi: 10.1097/SMJ.0b013e3181b64c7f. [DOI] [PubMed] [Google Scholar]
- 34.Posserud I, Stotzer PO, Björnsson ES, Abrahamsson H, Simrén M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–808. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riepe SP, Goldstein J, Alpers DH. Effect of secreted bacteroides proteases on human intestinal bruch border hydrolases. J Clin Invest. 1980;66:314–322. doi: 10.1172/JCI109859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yakoob J, Abbas Z, Khan R, Hamid S, Awan S, Jafri W. Small intestinal bacterial overgrowth and lactose intolerance contribute to irritable bowel syndrome symptomatology in Pakistan. Saudi J Gastroenterol. 2011;17:371–375. doi: 10.4103/1319-3767.87176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nucera G, Gabrielli M, Lupascu A, et al. Abnormal breath test to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005;21:1391–1395. doi: 10.1111/j.1365-2036.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- 38.Schmulson M, Chey WD. Abnormal immuneragulation and low-grade inflammation in IBS: does one size fit all? Am J Gastroenterol. 2012;107:273–275. doi: 10.1038/ajg.2011.427. [DOI] [PubMed] [Google Scholar]
- 39.Schafer D, Schmid M, Gode UC, Baenkler HW. Dynamics of eicosanoids in peripheral blood cells during bronchial provocation in aspirin-intolerant asthmatics. Eur Respir J. 1999;13:638–646. doi: 10.1183/09031936.99.13363899. [DOI] [PubMed] [Google Scholar]
- 40.Schwab D, Raithel M, Klein P, et al. Immunoglobulin E and eosinophilic cationic protein in segmental lavage fluid of the small and large bowel identifies patients with food allergy. Am J Gastroenterol. 2001;96:508–514. doi: 10.1111/j.1572-0241.2001.03467.x. [DOI] [PubMed] [Google Scholar]
- e1.Simren M, Mannsson A, Langkilde AM, et al. Food related gastrointestinal symptoms in irritable bowel syndrome. Digestion. 2001;63:108–115. doi: 10.1159/000051878. [DOI] [PubMed] [Google Scholar]
- e2.Zar S, Kumar D, Benson MJ. Review article: Food hypersensitivity and irritable bowel syndrome. Aliment Pharmacol Ther. 2001;15:439–449. doi: 10.1046/j.1365-2036.2001.00951.x. [DOI] [PubMed] [Google Scholar]
- e3.Jones HF, Butler RN, Moore DJ, Brooks DA. Developmental changes and fructose absorption in children: effect on malabsorption testing and dietary management. Nutrition Reviews. 2013;71:300–309. doi: 10.1111/nure.12020. [DOI] [PubMed] [Google Scholar]
- e4.Jain NK, Patel VP, Pitchumoni CS. Sorbitol intolerance in adults. Prevalence and pathogenesis on two continents. J Clin Gastroenterol. 1987;9:317–319. doi: 10.1097/00004836-198706000-00015. [DOI] [PubMed] [Google Scholar]
- e5.Szilagyi A, Malolpszy P, Yesovitch S, et al. Fructose malabsorption may be gender dependent and fails to show compensation by colonic adaptation. Dig Dis Sci. 2007;52:2999–3004. doi: 10.1007/s10620-006-9652-9. [DOI] [PubMed] [Google Scholar]
- e6.AWMF-Leitlinienreport zur AWMF-Leitlinie Nr. 027/018 „Diagnostik, Therapie und Management der Glutarazidurie Typ I (Synonym Glutaryl-CoA-Dehydrogenase-Defizienz)“. Erstellungsdatum 03/2011: S1-12. www.awmf.org/uploads/tx_szleitlinien/027-08I_S3_Glutarazidurie_Typ_I_2011-05_01.pdf (last accessed on 16 October 2013)
- e7.Atkins D, Best D, Briss PA, et al. GRADE Working group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328 doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e8.Shaukat A, Levitt MD, Taylor BC, et al. Systematic review: Effective management strategies for lactose intolerance. Ann Int Med. 2010;152:797–803. doi: 10.7326/0003-4819-152-12-201006150-00241. [DOI] [PubMed] [Google Scholar]
- e9.Stein J, Kist M, Raithel M. Stuttgart: Wissenschaftliche Verlagsgesellschaft; 2011. Erkrankungen durch Nahrungsmittel. [Google Scholar]
- e10.Keim V, Klar E, Poll M, Schoenberg MH, Michael H. Postoperative care following pancreatic surgery-surveillance and treatment. Dtsch Arztebl Int. 2009;106(48):789–794. doi: 10.3238/arztebl.2009.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Ladas SD, Giorgiotis K, Raptis S. Complex carbohydrate malabsorption in exocrine pancreatic insufficiency. Gut. 1993;34:984–987. doi: 10.1136/gut.34.7.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Vatn MH, Grimstad IA, Thorsen L, et al. Adverse reaction to food: Assessment by double-blind placebo-controlled food challenge and clinical, psychosomatic and immunologic analysis. Digestion. 1995;56:421–428. doi: 10.1159/000201270. [DOI] [PubMed] [Google Scholar]
- e13.Portincasa P, Di Ciaula A, Vacca M, Montelli R, Wang DQH, Palasciano G. Beneficial effects of oral tilactase on patients with hypolactasia. Eur J Clin Invest. 2008;38:835–844. doi: 10.1111/j.1365-2362.2008.02035.x. [DOI] [PubMed] [Google Scholar]
- e14.Ladas SD, Grammenos I, Tassios PS, Raptis SA. Coincidental malabsorption of lactose, fructose, and sorbitol ingested at low doses is not common in normal adults. Dig Dis Sci. 2000;45:2357–2362. doi: 10.1023/a:1005634824020. [DOI] [PubMed] [Google Scholar]
- e15.Goldberg LD, Ditchek NT. Chewing gum diarrhoea. Am J Dig Dis. 1978;23 doi: 10.1007/BF01072704. [DOI] [PubMed] [Google Scholar]
- e16.Naim HY, Zimmer KP. Congenital disease of dysfunction and absorption. In: Walker WA, Goulet OJ, Kleinmann RE, Sanderson IR, Sherman PM, Schneider BL, editors. Pediatric gastrointestinal disease. 4th edition. Amsterdam: Elsevier Science; pp. 880–906. [Google Scholar]
- e17.Lauritano EC, Gabriel M, Scarpellini E, et al. Antibiotic therapy in small intestinal bacterial overgrowth: rifaximin versus metronidazole. Eur Rev Med Pharmacol Sci. 2009;13:111–116. [PubMed] [Google Scholar]
- e18.Pimentel M. Review of rifaximin as treatment for SIBO and IBS. Expert Opin Invest Drugs. 2009;18:349–358. doi: 10.1517/13543780902780175. [DOI] [PubMed] [Google Scholar]
- e19.Raithel M, Baenkler HW, Naegel A, et al. Significance of salicylate intolerance in diseases of the lower gastrointestinal tract. J Physiol Pharmacol. 2005;56:89–102. [PubMed] [Google Scholar]
- e20.Weidenhiller M, Layritz Ch, Kuefner MA, Zopf Y, Hagel D, Raithel M. Histaminintoleranz-Syndrom (HIS): Vielfalt der Mechanismen von physiologischer, pathophysiologischer und toxischer Wirkung und deren Unterscheidung. Z Gastroenterol. 2012;50:1302–1309. doi: 10.1055/s-0032-1325487. doi: 10.1055/s-0032-1325487. [DOI] [PubMed] [Google Scholar]
- e21.Donner MG, Erhardt A, Häussinger D. Metabolic disorders of the liver. Part 2: glycogen storage diseases, hereditary fructose intolerance, galactosemia and hepatic porphyrias. Dtsch Med Wochenschr. 2010;135:2540–2547. doi: 10.1055/s-0030-1269424. [DOI] [PubMed] [Google Scholar]