Abstract
Dry eye is an inflammatory disease that results from activation of innate inflammatory pathways in resident ocular surface cells, as well as cytokines produced by recruited T helper (Th) cells. Cytokines produced by the infiltrating Th cells alter the normal cytokine balance on the ocular surface and cause ocular surface epithelial pathology. Changes in levels of Th cytokines on the ocular surface have been measured in dry eye and the biological effects of these cytokines have been documented in experimental culture and mouse model systems. The Th2 cytokine IL-13 has a homeostatic role in promoting goblet cell differentiation. In contrast, The Th1 cytokine IFN-γ antagonizes IL-13 and promotes apoptosis and squamous metaplasia of the ocular surface epithelia. The Th17 cytokine, IL-17 promotes corneal epithelial barrier disruption. The ocular surface epithelium expresses receptors to all of these Th cytokines. Therapies that maintain normal IL-13 signaling, or suppress IFN- γ and IL-17 have potential for treating the ocular surface disease of dry eye.
Keywords: Dry Eye, Tear Dysfunction, Inflammation, T cell, cytokine, interleukin 13, interferon gamma, interleukin 17
1.1. Introduction
It is now recognized that inflammation is a cause and consequence of dry eye disease. Decreased production of aqueous tears by the lacrimal glands or increased tear evaporation due to lipid deficiency or decreased blink rate can result in changes in tear composition that promote inflammation on the ocular surface and lid margins. Elevated tear osmolarity has been measured in all types of tear dysfunction (Tomlinson et al., 2006). Exposure of ocular surface epithelium to elevated osmolarity activates stress signaling pathways, including the JNK and NFκB pathways that promote production of inflammatory molecules, including cytokines, chemokines and matrix metalloproteinases (MMPs). Alternatively, ocular surface and lacrimal gland inflammation may develop in systemic autoimmune disease due to infiltration of these tissues with activated T lymphocytes (Stern et al., 2013).
The ocular surface has resident lymphoid cells, including dendritic cells, natural killer (NK) cells, B cells and conventional and γδ T cells that suppress (Zhang et al., 2012; Hattori et al., 2012; Khandelwal et al., 2013; Zhang et al., 2013) or promote (Zhang et al., 2012) immune responses. A major function of these cells is to defend against the variety of microbial agents that populate or infect the cornea and ocular surface (Knop and Knop, 2005; Ueta and Kinoshita, 2012). Conjunctival intraepithelial lymphocytes, such as NK or NKT cells, may also have a homeostatic function on the ocular surface by supporting differentiation of conjunctival goblet cells that secrete gel-forming mucin into the tears that has key stabilizing and protective functions (de Paiva et al., 2010b).
1.2. T helper Subsets
The immune response to foreign antigens requires a perfect coordination between sensor and effector cells. CD4+ T cells, also known as T helper (Th) cells, play a central role in immune protection. Naïve conventional CD4+ T cells have open to them at least 4 distinct fates that are determined by the pattern of signals they receive during antigen presentation. These 4 populations are Th1, Th2, Th17 and induced regulatory (iTreg) cells (Mosmann et al., 1986; Mosmann, 1992; Mosmann and Coffman, 1989; Mosmann and Sad, 1996; Zhu and Paul, 2008; Zhu and Paul, 2010). The cytokines secreted by these Th populations modulate various immune responses and are responsible for their functional roles.
Th1 cells secrete IFN-γ that activates macrophages that function in eradicating intracellular microorganisms, such as mycobacteria. Th1 cells also promote cytotoxic T cell development and delayed type hypersensitivity reactions (Mosmann et al., 1986; Mosmann, 1992; Mosmann and Coffman, 1989; Mosmann and Sad, 1996). Hence, Th1 cells are pro-inflammatory and may be involved in the pathogenesis and maintenance of some autoimmune diseases (Dardalhon et al., 2008).
Th2 cells produce IL-4, IL-5 and IL-13, cytokines that mediate immunity against parasitic infestations, and are pivotal in the development of atopic diseases, including seasonal allergy, asthma and atopic dermatitis/keratoconjunctivitis. IL-4 and IL-13 promote IgE switching by B cells (Mosmann et al., 1986; Mosmann, 1992; Mosmann and Coffman, 1989; Mosmann and Sad, 1996). IL-5 promotes activation and immunoglobulin secretion by B-cells and also eosinophil activation (Lee et al., 2013). IL-13 can also promote fibrosis (Doherty et al., 2007).
Th17 cells secrete IL-17, which induces production of pro-inflammatory molecules (cytokines, chemokines and MMPs) and recruits neutrophils (Bettelli et al., 2006; Bettelli et al., 2007). Th17 cells are involved in the early response to numerous extracellular pathogens, including bacteria and fungi, and have been found to be involved in autoimmunity and tissue inflammation (Chen and O'Shea, 2008).
It is thought that naïve CD4 cells, in the presence of TNF-α and IL-6, develop into Th22 cells that secrete IL-22 and TNF-α. These cells may be involved in epidermal immunity and remodeling in inflammatory skin diseases such as psoriasis, microbial infections, inflammatory and autoimmune diseases (Akdis et al., 2012; Eyerich et al., 2009; Kagami et al., 2010; Muhl et al., 2011; Qiao et al., 2011; Ryan-Payseur et al., 2011; Sanos and Diefenbach, 2013; Shao et al., 2012; Tian et al., 2013; Wolff et al., 2012; Zhang et al., 2011a).
Other Th subsets have a role in tolerance, rather than immunity. These include Tr1 cells (secreting IL-10), Th3 cells (secreting TGF-β), and Treg cells (secreting IL-10 and TGF-β) (Andolfi et al., 2012; Ankathatti et al., 2012; Battaglia et al., 2006; Carrier et al., 2007a; Carrier et al., 2007b; Zhu and Paul, 2008).
1.3. Th Cells in Dry Eye
There is a growing body of evidence documenting the presence and activity of CD4+ T cells in dry eye disease. Biopsies taken from patients with Sjögren Syndrome (SS) early in the course of the disease have shown lymphocytic infiltration in the lacrimal gland and activated T cells in the conjunctiva. (Calonge, 2001; Jones et al., 1994; Kunert et al., 2000; Pflugfelder et al., 1999; Solomon et al., 2001a; Stern et al., 2002). Mouse models of dry eye have provided the opportunity to investigate the role of specific T cell subsets in the pathogenesis of dry eye.
A great body of evidence supporting the pathogenicity of CD4+ T cells in dry eye comes from animal studies using either autoimmune (spontaneous) or induced animal models. Definitive studies using adoptively transferred CD4+ T cells from mice exposed to desiccating stress to naïve immunodeficient mice performed by our group established a direct pathogenic role for CD4+ T cells (Niederkorn et al., 2006). However, the transfer of CD4+ T cells into competent mice did not induce disease unless regulatory T cells were depleted (Niederkorn et al., 2006). Additional animal and human studies have shown that dry eye elicits mixed Th1 and −17 responses in the conjunctiva (Chauhan et al., 2009; de Paiva et al., 2009). Both Th subtypes have been found to have a pathogenic role (Chen et al., 2011; de Paiva et al., 2007a; de Paiva et al., 2009). Studies dissecting individual contributions of Th1 and Th17 have been performed [(Coursey et al., 2012) and manuscript submitted].
Dry eye syndrome has a myriad of clinical presentations, but it is frequently accompanied by corneal barrier disruption and loss of conjunctival goblet cells. Indeed, breakdown of corneal barrier is one of the hallmarks of dry eye disease and it is clinically characterized by increased uptake of fluorescent tracers, such as sodium fluorescein in human subjects. Using an environmentally induced dry eye model, our group has shown that matrix metalloproteinase 9 (MMP-9) is responsible for proteolytic degradation of tight junctions in the apical corneal epithelium, facilitating dye penetration into cornea (Pflugfelder et al., 2005). Corneal barrier disruption is accompanied by increased infiltration of CD4+ T cells in the conjunctiva; loss of PAS+ filled goblet cells and increased infiltration of CD11b+ cells into the cornea (Chauhan et al., 2009; de Paiva et al., 2006; de Paiva et al., 2007a).
Migration of CD4+ T cells into the conjunctival and cornea in dry eye disease may be modulated by chemokine ligands produced by the surface epithelium that increase in dryness. Pathogenic CD4 cells that infiltrate the ocular surface tissues express receptors to these ligands (de Paiva et al., 2009; Yoon et al., 2007; Yoon et al., 2010; Dohlman TH et al., 2013). It has also been shown that manipulation of afferent (migration of dendritic cells from the ocular surface to the regional lymph nodes) or efferent (migration of differentiated CD4+ T cells from the nodes to the cornea and conjunctiva) can ameliorate development of dry eye disease (Coursey et al., 2012; Goyal et al., 2009; Lee et al., 2011; Sadrai et al., 2012; Schaumburg et al., 2011) indicating that therapeutic strategies that interfere with various points in this immune circle may have clinical significance. As a matter of fact, cyclosporine A 0.01 % emulsion, the only FDA-approved drug to treat dry-eye disease has been shown to modulate several arms of the immune response by decreasing human leukocyte class II antigen (HLA-DR) expression in conjunctiva of dry eye patients (Baudouin et al., 2002; Brignole et al., 2000; Brignole et al., 2001) and decreasing expression of IL-17A and IFN-γ in conjunctiva of animals after desiccating stress (de Paiva et al., 2009).
Herein we review the association with and potential function of Th cytokines in dry eye disease.
2.1. Th2
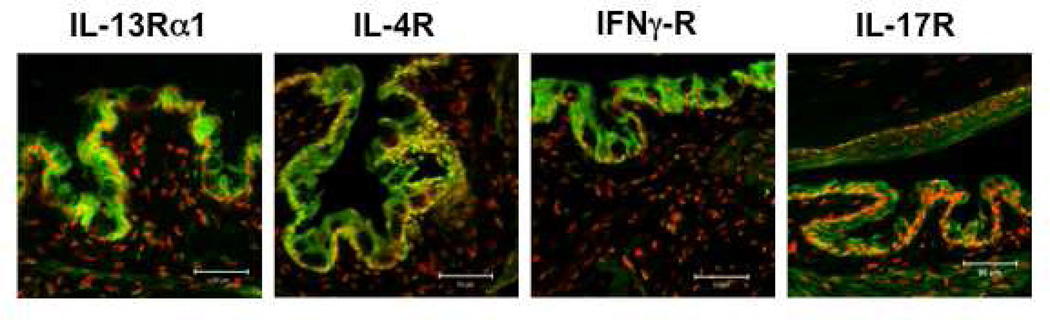
The signature Th2 cytokines, IL-4 and IL-13, bind to heterodimeric receptors that share the IL-4Rα1 subunit. IL-4 binds to the type I IL-4R complexes, composed of IL-4Rα and γc subunits (Hershey, 2003). Type I receptors are expressed by hematopoietic and mucosal epithelial cells. Type II IL-4R complexes are composed of IL-4Rα and IL-13Rα1. They are also present on hematopoietic and non-hematopoietic cells and bind to both IL-4 and IL-13. Both receptors are present in conjunctival epithelium (Figure 1). A second IL-13 receptor, IL-13Rα2, exclusively binds IL-13 and has been found to serve as a decoy receptor (Wilson et al., 2011). We have previously found expression of the IL-13Rα1 in the conjunctival epithelium, with the strongest expression in goblet cell rich areas Figure 1 (de Paiva et al., 2010b).
Figure 1.
Immunofluorescent staining (green) of receptors for T helper (Th) cytokines in the mouse ocular surface epithelium: Th2: IL-4Rα and IL-13Rα1; Th1: IFN-γR and Th17: IL-17RA. Nuclei are stained red with propidium iodide.
IL-4 and IL-13 both utilize the JAK–signal transducer and activator of transcription (STAT) pathway, specifically STAT6. Signaling is initiated by phosphorylation of IL-4Rα and JAK1, leading to the recruitment, phosphorylation and activation of STAT6. Activated STAT6 dimers translocate to the nucleus, bind specific canonic DNA elements and initiate transcription of downstream genes (Hershey, 2003).
Both IL-4 and IL-13 have been detected in human (Carreno et al., 2010; LaFrance et al., 2008; Lam et al., 2008; Weaver et al., 2007a) and mouse tears (Corrales et al., 2007) In one study, IL-13 was detected in all tear samples, while IL-4 was detectable in only 50% and the average concentration of IL-13 was twice as high as IL-4 (Carreno et al., 2010; Corrales et al., 2007). The effects of dry eye on tear concentration IL-4 has not been studied. In one study, there was no change in tear IL-13 concentration in patients with tear dysfunction with or without meibomian gland disease (MGD) (Lam et al., 2008). In experimental murine dry eye, IL-13 significantly decreased in tears after 5 and 10 days in Th-1 prone C57BL/6 mice, while it increased in BALB/C mice that have been found to develop less severe corneal and conjunctival disease in response to desiccating stress (Corrales et al., 2007). IL-5 was detected in normal tears (Carreno E et al., 2010), was found to be elevated in tears of dry eye patients and correlated with their symptom severity (Massingale et al., 2009)
We have reported that intraepithelial NK cells are the major source of IL-13 on the ocular surface. Similar to airway epithelia, IL-13 released from these cells appears to modulate goblet cell density in the conjunctival epithelium, because goblet cell density decreased more than 25% in NK cell depleted mice. Goblet cell density was found to decrease by approximately 30% in STAT6 deficient mice and subconjunctival IL-13 administration prevented loss of goblet cells in mice subjected to experimental desiccating stress (de Paiva et al., 2010b). One mechanism by which IL-13 promotes goblet cell differentiation in the airway epithelium is by increasing expression the SAM-pointed domain epithelial-specific transcription factor (SPDEF) (Chen et al., 2009). SPDEF has been found to be an essential for goblet cell differentiation in tracheobronchial and gastrointestinal epithelium of mice (Chen et al., 2009; Gregorieff et al., 2009). It also appears to be involved in conjunctival goblet cells differentiation, because SPDEF −/− mice fail to develop goblet cells (Marko et al., 2013). It remains to be determined if IL-13 regulates SPDEF in the conjunctival epithelium.
In non-ocular systems, the Th1 cytokine IFN-γ has been found to antagonize IL-13 signaling by multiple mechanisms (Hershey, 2003) that include up regulation of suppression of cytokine signaling (SOCS) genes and stimulated production of IL-13Rα2. As noted below, IFN-γ was found to decrease conjunctival goblet cell density in mice; however, it is not known to what extent this is attributed to suppression of IL-13 signaling. Preliminary studies performed in our lab have found that expression of the IFN-γR and IL-13Rα2 increase in patients with aqueous tear deficiency, both of which could suppress IL-13 signaling (Corrales and Pflugfelder, 2013). We have reported that dry eye decreases the IL-13/IFN-γ ratio (Lam et al., 2008). Treatment of experimental murine dry eye with topical cyclosporine (CsA) was found to increase the number of goblet cells (Pflugfelder et al., 2008; Strong et al., 2005), as well as the number of IL-13 producing NK cells (de Paiva et al., 2010b).
These studies suggest that IL-13 is the predominant Th2 cytokine on the normal ocular surface. It appears to have a homeostatic function in maintaining mucus secreting conjunctival goblet cells. IFN-γ can suppress IL-13 signaling by multiple mechanisms and this may be relevant to the conjunctival goblet cell loss that occurs in aqueous tear deficient dry eye where IFN-γ expression is increased.
In the non-obese diabetic (NOD) mouse model of Sjögren syndrome (SS), genetic deletion of IL-4 leads to decreased B-cell infiltration of the salivary and lacrimal glands, as well as preservation of glandular secretory function. These findings suggest that IL-4 promotes B-cell proliferation in this model (Nguyen and Peck, 2009). IL-4 has also been implicated in the pathogenesis of the hypergammaglobulinemia that develops in SS (Giron-Gonzalez et al., 2009).
3.1 Th1 pathway
IFN-γ is the signature cytokine from Th1 cells. It is produced by NK, NKT and activated, terminally differentiated CD4+ Th1+ cells although one study showed that salivary gland ductal epithelial cells can also produce IFN-γ (Ishimaru et al., 2008). Th1 cells can be identified by expression of CXCR3 and CCR5 surface receptors. IFN-γ receptor (Figure 1) has been detected in the conjunctival and corneal epithelium and CXCR3 and CCR5 ligands (CXCL-9, CXCL10 and CXCL11) have been found in tears and conjunctiva of humans and mice (Enriquez-de-Salamanca et al., 2010; Yoon et al., 2007; Yoon et al., 2010). IFN-g is a pleotropic cytokine involved in a variety of immune functions, including recruitment and polarization of naïve CD4 cells that once differentiated produce IFN-γ. IFN-γ production has an amplifying effect since its local production induces expression of IL-12 receptor (which facilitates Th-1 differentiation) and Th-1 chemokine ligands (CXCL-9, CXCL10 and CXCL11) that recruit and anchor differentiated Th-1 cells in tissues that subsequently produce IFN-γ and perpetuate the immune based inflammation in dry eye.
IFN-γ has been proposed as a biomarker for dry eye disease and Sjögren syndrome (SS) because elevated IFN-γ, either protein and/or RNA, has been detected in tears, saliva, conjunctiva, submandibular glands and blood [tears (Boehm et al., 2011; Corrales et al., 2007; de Paiva et al., 2007a; Enriquez-de-Salamanca et al., 2010; Lam et al., 2008; Massingale et al., 2009; Mrugacz et al., 2006; Riemens et al., 2012), conjunctiva (Chen et al., 2011; Chen et al., 2006; Corrales et al., 2007; de Paiva et al., 2007a; Zhang et al., 2011b) , saliva (Kang et al., 2011; Pertovaara et al., 2006), lacrimal (de Paiva et al., 2010a; Hayashi et al., 2012; Jie et al., 2010; Ogawa et al., 2002; Pelegrino et al., 2012; Rahimy et al., 2010b; Viau et al., 2011) submandibular glands (Brookes et al., 1995; Hayashi et al., 2012; Koarada et al., 2006; Kohashi et al., 2008; Mrugacz et al., 2006), and blood (Hagiwara et al., 1998; Szodoray et al., 2008)] Evidence from mouse models and human SS patients indicate that IFN-γ is a relevant therapeutic target (de Paiva et al., 2007; de Paiva et al., 2011; Ogawa et al., 2002).
Increased IFN-γ concentration in tears of dry eye patients measured by ELISA was reported more than a decade ago. More sensitive immunoassays, such as Luminex and antibody microarrays used in subsequent studies have confirmed the early findings (Boehm et al., 2011; Corrales et al., 2007; Enriquez-de-Salamanca et al., 2010; Massingale et al., 2009; Mrugacz et al., 2006; Riemens et al., 2012). In addition to dry eye, elevated tear IFN-γ concentration has also been found in patients with sicca symptoms after bone marrow transplantation (Riemens et al., 2012) and in tears of SS patients (Lam et al., 2008; Massingale et al., 2009). Among the various subsets of dysfunctional tear syndrome (DTS), those with meibomian gland disease (MGD) had lower IFN-γ concentration than those without MGD (Enriquez-de-Salamanca et al., 2010; Lam et al., 2008). Among all patients with DTS, tear IFN-γ concentration was found to correlate with corneal fluorescein staining score (Lam et al., 2008). It is possible that the inflamed lacrimal glands in patients with SS are one source for their increased tear IFN-γ.
Similar to tears (Riemens et al., 2012), increased IFN-γ has been found in saliva of SS patients and its presence correlated with severity of sicca symptoms (Kang et al., 2011). Interestingly, increased Th1/Th2 ratios was observed in more severe SS cases (Ajjan et al., 1998; Giron-Gonzalez et al., 2009; Konttinen et al., 1999), whereas increased Th-2 response correlated with milder SS (Kang et al., 2011; Mitsias et al., 2002; Pertovaara et al., 2006; van Woerkom et al., 2005). IFN-γ expression has been evaluated in minor salivary glands of human SS patients, as well as lacrimal and submandibular (SMG) gland biopsies in animal models. Virtually every mouse autoimmune model that mimics SS, or even environmentally-induced mouse dry eye models have shown increased expression of IFN-γ in submandibular and/or lacrimal glands (de Paiva et al., 2010a; Hayashi et al., 2012; Jie et al., 2010; Kohashi et al., 2008; Mitsias et al., 2002; Ogawa et al., 2002; Pelegrino et al., 2012; Rahimy et al., 2010b; Viau et al., 2011). In cultured labial salivary gland biopsies obtained from SS patients, the concentration of IFN-γ, but not IL-13, was associated with greater lymphocytic infiltration (Mitsias et al., 2002). Peck and colleagues identified a specific signature of IFN-γ inducible genes in LG and submandibular glands of C57BL/6.NOD-Aec1Aec2 (Peck and Nguyen, 2012). Increased expression of IFN-γ mRNA has also been observed in the conjunctiva, both in dry eye patients and mice with experimental dry eye (Chen et al., 2011; Chen et al., 2006; Corrales et al., 2007; de Paiva et al., 2007a; de Paiva et al., 2009; Zhang et al., 2011b).
IFN-γ is critical amplifying factor in immune reactions. Several studies have shown that treatment of glandular epithelial tissue with IFN-γ increases expression of HLA class I and II antigens in epithelial cells, CD80 and CD86 in dendritic cells and stimulates T cells to proliferate, thus, perpetuating the immune cascade (Brookes et al., 1995; Clark et al., 1994; De Saint et al., 1999; Manoussakis et al., 1999; Saito et al., 1993; Tsubota et al., 1999; Tsunawaki et al., 2002). Exposure of cultured glandular acini to IFN-γ led to breakdown of tight-junction proteins and increased epithelial apoptosis (Ewert et al., 2010; Katsiougiannis et al., 2010). It has also been shown that IFN-γ significantly decreases epithelial mucin expression (Albertsmeyer et al., 2010).
Recently, strategies to neutralize IFN-γ have been found to inhibit development of corneal and conjunctival epithelial disease in experimental dry eye. Neutralization of NK cells, early producers of IFN-γ (NK cells) following desiccating stress was found to decrease corneal fluorescein staining and inflammatory cytokine expression in the cornea and conjunctiva (Chen et al., 2011; Zhang et al., 2012) and also to decrease the Th-17 response (Zhang et al., 2012). IFN-γ KO mice are resistant to dry-eye induced goblet cell loss; reconstitution of these mice with exogenous IFN-γ induced the same changes observed in wild-type mice after desiccating stress, induced goblet cell loss and increased expression of cornified envelope precursor proteins by glandular the ocular surface epithelium (de Paiva et al., 2007a). Mice that received subconjunctival injections of anti- IFN-γ antibody showed decreased corneal and conjunctival apoptosis (Zhang et al., 2011b; Zhang et al., 2011c). Adoptive transfer of CD4+ T cells from anti-IFN-g treated donor mice exposed to desiccating stress were less pathogenic to immunodeficient recipient mice, yielding less corneal apoptosis and reduced loss of PAS+ filled goblet cells (Zhang et al., 2011c). Autoimmune-prone mouse strains with IFN-γ gene deletions have been shown to have less severe dacryoadenitis (Cha et al., 2004; Pelegrino et al., 2012).
Skurkovich and Skurkovich first proposed the concept of neutralizing cytokines to ameliorate autoimmune diseases in the mid-1970s (Skurkovich and Skurkovich, 2007; Skurkovich et al., 1987; Skurkovich et al., 2005b). Their team tested efficacy of anti-IFN-γ antibodies alone or in combination with anti-TNF-α in a variety of autoimmune diseases, such as rheumatoid arthritis (RA), multiple sclerosis, psoriasis and Type 1 diabetes, among others (Nasonova et al., 2008; Sigidin et al., 2001; Skurkovich et al., 2005a; Skurkovich et al., 2005b). A small series of RA patients showed significant improvement in their clinical signs 28 days post anti-IFN-γ treatment (Sigidin et al., 2001). In the same series, another group was treated with anti-TNF-α and showed similar improvement to those treated with anti-IFN-y (Sigidin et al., 2001). Interestingly, anti-TNF-α treatment is now an FDA approved treatment for RA, while anti-IFN-γ is still under investigation for treatment of Crohn’s disease (Hommes et al., 2006; Ishimaru et al., 2008).
Studies performed in mouse models of dry eye indicate that both IL-17 and IFN-γ may contribute to the development of ocular surface disease in dry eye. Consequently, neutralization of individual cytokines may only suppress disease manifestations attributable to that cytokine.
4.1. Th17
The Th17 subset produces IL-17A, IL-17F, IL-21 and IL-22 (Bettelli et al., 2006; Bettelli et al., 2007; Dardalhon et al., 2008; Stockinger et al., 2007).
TGF-β1 plus IL-6 and IL-21, the growth and stabilization factor (IL-23), and the transcription factors (STAT3, RORγt, and RORa) are involved in the differentiation of Th17 cells (Bettelli et al., 2006; Cooke, 2006; Ivanov et al., 2006; Iwakura and Ishigame, 2006; Kikly et al., 2006; Korn et al., 2007a; Korn et al., 2007b; Korn et al., 2008; Korn et al., 2009; Langrish et al., 2005; Lyakh et al., 2008; Mangan et al., 2006; Nurieva et al., 2008; Park et al., 2005; Weaver et al., 2007b).
IL-17A and IL-17F belong to the IL-17 family, which also includes IL-17B, IL-17C, IL-17D, and IL-E (IL-25). Cell type and tissue expression patterns differ greatly between the family members, but there is significant overlap in receptor binding patterns between IL-17 family members. IL-17A and IL-17F homodimers or IL-17A or IL-17F heterodimers are the principal drivers of inflammation and autoimmunity. The IL-17R family comprises five receptor subunits IL-17RA–IL-17RE. Despite considerable sequence divergence, many of the genes encoding the IL-17R family are linked, with clusters on human chromosome 3 (for IL-17RB, IL-17RC, IL-17RD and IL-17RE) and mouse chromosomes 6 (IL-17RA, IL-17RC and IL-17RE) and 14 (IL-17RB and IL-17RD) (Aggarwal and Gurney, 2002; Moseley et al., 2003). IL-17A and IL-17F both signal through IL-17RA and IL-17RC. IL-17 receptor (Figure 1) has been detected in the corneal and conjunctival epithelium (Chauhan et al., 2009).
Th17 cells can be identified by expression of CCR6 surface receptors (Coursey et al., 2012; Hirota et al., 2007; Wang et al., 2009). CCL20, the only known CCR6 ligand, is highly expressed after epithelial injury, including experimental desiccating stress (de Paiva et al., 2009; Li et al., 2011; Dohlman et al., 2013). Treatment of experimental dry eye with anti-CCL-20 antibody significantly decreased corneal fluorescein staining, infiltration of the conjunctiva with IL-17A+ cells, decrease infiltration of the cornea with CD11b+ cells and decrease expression of inflammatory cytokine and MMPs in the conjunctiva (Dohlman et al., 2013).
Dry eye has been demonstrated to cause inflammation on the ocular surface, evidenced by increased levels of inflammatory cytokines (IL-1, IL-6, TNF-α and IL-17) in the tear fluid and corneal and conjunctival epithelium, and an increased infiltration of DCs and T lymphocytes in the conjunctiva (Corrales et al., 2007; de Paiva et al., 2009; Niederkorn et al., 2006; Pflugfelder, 2004; Solomon et al., 2001a; Solomon et al., 2001b; Turner et al., 2000; Zhang et al., 2012; Zheng et al., 2009; Zheng et al., 2010). Recently, increased levels of IL-17, IL-23 and IL-6 were also found in saliva and salivary glands biopsies obtained from patients with the severe autoimmune dry eye condition, Sjögren syndrome (Katsifis et al., 2009; Nguyen et al., 2008; Sakai et al., 2008). Increased IL-17 mRNA and protein has been found in LG and SMG of mouse models of SS (de Paiva et al., 2010a; Nguyen et al., 2008; Pelegrino et al., 2012; Pitcher, III et al., 2011; Rahimy et al., 2010a; Turpie et al., 2009)
Evidence in mouse models of dry eye indicates that IL-17 stimulates production of MMP-3 and MMP-9 that contribute to disruption of corneal epithelial barrier function. Recent studies have shown that antibody neutralization of IL-17 ameliorated corneal barrier disruption in mice subjected to desiccating stress (Chauhan et al., 2009; de Paiva et al., 2009; Dohlman et al., 2013) and decreased expression of MMP-3 and −9 mRNA transcripts in the corneal epithelium (de Paiva et al., 2009) or conjunctiva (Dohlman et al., 2013), providing a definitive link between epithelial and immune cells in this process. Neutralization of IL-17 has also been found to inhibit corneal lymphangiogenesis (Chauhan et al., 2011). Memory Th cells, but not Th1 cells, were found to increase in a mouse model of chronic dry eye and these cells were capable of inducing severe corneal epithelial disease after adoptive transfer (Chen et al., 2013).
Novel therapeutic strategies aimed at inhibiting migration of Th17+CCR6+ cells to the ocular surface or production of IL-17 have been shown to decrease severity of dry eye disease in animal models of dry eye (Chauhan et al., 2009; Coursey et al., 2012; de Paiva et al., 2009; Sadrai et al., 2012; Zhang et al., 2012).
5. Conclusion
Levels of Th cytokines on the ocular surface have been found to change in dry eye and the biological effects of these cytokines have been investigated. The Th2 cytokine IL-13 has a homeostatic role in promoting goblet cell differentiation. In contrast, The Th1 cytokine IFN-γ antagonizes IL-13 and promotes apoptosis and squamous metaplasia of the ocular surface epithelia. The Th17 cytokine, IL-17 promotes corneal epithelial barrier disruption. Neutralization of IFN-γ or IL17 has been found to improve ocular surface epithelial disease.
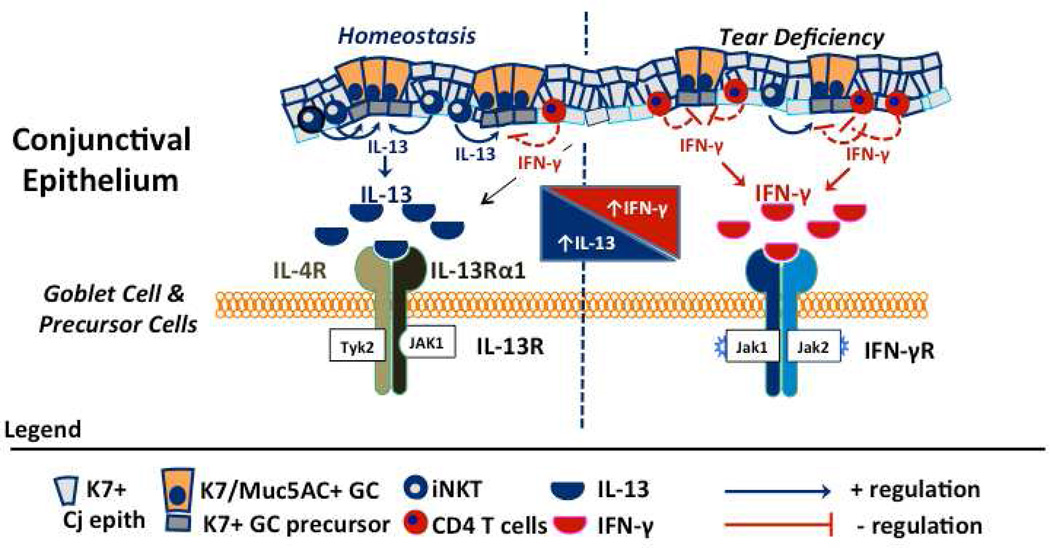
Figure 2.
Schematic summarizing balance of IL-13 and IFN-γ cytokines and receptors on the ocular surface in homeostasis and in tear deficiency. In the normal eye IL-13 produced by NKT and CD4+ cells residing in the conjunctival epithelium promotes goblet cell differentiation. Levels of IFN-γ are low. In contrast, IFN-γ expression increases in aqueous tear deficiency and IFN-γ suppresses IL-13 signaling by up regulation of suppression of cytokine signaling (SOCS) genes and stimulated production of IL-13Rα2 decoy receptor. Additionally, IFN-γ promotes cornification and apoptosis of the surface epithelium.
Table 1.
Th-related cytokines in tears and conjunctiva in dry eye disease
| Th Cytokine | Change in Dry Eye (tears and conjunctiva) | Associated Pathology in Dry Eye Disease |
|---|---|---|
| IL-4 | Human: Unknown Mouse: no change | unknown |
| IL-13 | Human: ↓ IL-13/IFN-γ ratio (Lam et al., 2008) Mouse: ↓tear concentration, ↓IL- 13/IFN-γ in C57/BL6 strain | Goblet cell loss |
| IFN-γ | Human: ↑ tear concentration (Lam et al., 2008; Massingale et al., 2009; (Boehm et al., 2011; Corrales et al., 2007; Enriquez-de-Salamanca et al., 2010; Massingale et al., 2009; Mrugacz et al., 2006; Riemens et al., 2012 Mouse: ↑ tear concentration (Corrales et al., 2007; de Paiva et al., 2007b) Mouse: ↑ Conjunctiva (Chen et al., 2011; Corrales et al., 2007; de Paiva et al., 2009; Zhang et al., 2011b) |
Goblet cell loss Epithelial apoptosis |
| IL-17 | Human: ↑ tear concentration (Katsifis et al., 2009) Mouse: ↑ tear concentration (de Paiva et al., 2009) Mouse: ↑ Conjunctiva (Chauhan et al., 2009; de Paiva et al., 2009) |
Corneal barrier disruption; Stimulation of MMP-3 and MMP-9 mRNA by corneal epithelium |
Research Highlights.
The Th2 cytokine IL-13 has a homeostatic role in conjunctival mucus production
Cytokines produced by T helper cells in dry eye alter the cytokine balance
Interleukin 17 stimulates MMP production and causes corneal epithelial disease
The Th1 cytokine IFN-γ causes apoptosis and conjunctival goblet cell loss
Acknowledgments
Financial Support: NIH Grant EY11915 (SCP), an unrestricted grant from Research to Prevent Blindness, New York, NY (SCP), the Oshman Foundation, Houston, TX (SCP), the William Stamps Farish Fund, Houston, TX (SCP), Hamill Foundation, Houston, TX (SCP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- Ajjan RA, McIntosh RS, Waterman EA, Watson PF, Franklin CD, Yeoman CM, Weetman AP. Analysis of the T-cell receptor Valpha repertoire and cytokine gene expression in Sjogren's syndrome. Br. J. Rheumatol. 1998;37:179–185. doi: 10.1093/rheumatology/37.2.179. [DOI] [PubMed] [Google Scholar]
- Akdis M, Palomares O, van dV, van SM, Akdis CA. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J. Allergy Clin. Immunol. 2012;129:1438–1449. doi: 10.1016/j.jaci.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Albertsmeyer AC, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp. Eye Res. 2010;90:444–451. doi: 10.1016/j.exer.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andolfi G, Fousteri G, Rossetti M, Magnani CF, Jofra T, Locafaro G, Bondanza A, Gregori S, Roncarolo MG. Enforced IL-10 expression confers type 1 regulatory T cell (Tr1) phenotype and function to human CD4(+) T cells. Mol. Ther. 2012;20:1778–1790. doi: 10.1038/mt.2012.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankathatti MM, Xu S, Freywald A, Xiang J. CD4+ Th2 cells function alike effector Tr1 and Th1 cells through the deletion of a single cytokine IL-6 and IL-10 gene. Mol. Immunol. 2012;51:143–149. doi: 10.1016/j.molimm.2012.02.120. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin. Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Baudouin C, Brignole F, Pisella PJ, De Jean MS, Goguel A. Flow cytometric analysis of the inflammatory marker HLA DR in dry eye syndrome: results from 12 months of randomized treatment with topical cyclosporin A. Adv. Exp. Med. Biol. 2002;506:761–769. doi: 10.1007/978-1-4615-0717-8_107. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Boehm N, Riechardt AI, Wiegand M, Pfeiffer N, Grus FH. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Invest Ophthalmol. Vis. Sci. 2011;52:7725–7730. doi: 10.1167/iovs.11-7266. [DOI] [PubMed] [Google Scholar]
- Brignole F, Pisella PJ, De Saint JM, Goldschild M, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in KCS: 6-month treatment with topical cyclosporin A. Invest Ophthalmol. Vis. Sci. 2001;42:90–95. [PubMed] [Google Scholar]
- Brignole F, Pisella PJ, Goldschild M, De Saint JM, Goguel A, Baudouin C. Flow cytometric analysis of inflammatory markers in conjunctival epithelial cells of patients with dry eyes. Invest Ophthalmol. Vis. Sci. 2000;41:1356–1363. [PubMed] [Google Scholar]
- Brookes SM, Price EJ, Venables PJ, Maini RN. Interferon-gamma and epithelial cell activation in Sjogren's syndrome. Br. J. Rheumatol. 1995;34:226–231. doi: 10.1093/rheumatology/34.3.226. [DOI] [PubMed] [Google Scholar]
- Calonge M. The treatment of dry eye. Surv. Ophthalmol. 2001;45(Suppl 2):S227–S239. doi: 10.1016/s0039-6257(00)00205-8. [DOI] [PubMed] [Google Scholar]
- Carreno E, Enriquez-de-Salamanca A, Teson M, Garcia-Vazquez C, Stern ME, Whitcup SM, Calonge M. Cytokine and chemokine levels in tears from healthy subjects. Acta Ophthalmol. 2010;88:e250–e258. doi: 10.1111/j.1755-3768.2010.01978.x. [DOI] [PubMed] [Google Scholar]
- Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J. Immunol. 2007a;178:179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. II. TGF-beta-transgenic Th3 cells rescue IL-2-deficient mice from autoimmunity. J. Immunol. 2007b;178:172–178. doi: 10.4049/jimmunol.178.1.172. [DOI] [PubMed] [Google Scholar]
- Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, Peck AB. A dual role for interferon-gamma in the pathogenesis of Sjogren's syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand. J. Immunol. 2004;60:552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- Chauhan SK, El AJ, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J. Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, Dana RA. novel pro-lymphangiogenic function for Th17/IL-17 Blood. 2011;118(17):4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Korfhagen TR, Xu Y, Kitzmiller J, Wert SE, Maeda Y, Gregorieff Clevers AH, Whitsett JA. SPDEF is required for mouse pulmonary goblet cell differentiation and regulates a network of genes associated with mucus production. J Clin Invest. 2009;119:2914–2924. doi: 10.1172/JCI39731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-{gamma}-secreting NK cells promote induction of dry eye disease. J Leukoc. Biol. 2011;89:965–972. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Chauhan SK, Soo Lee H, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2013 Apr 10; doi: 10.1038/mi.2013.20. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Mok H, Pflugfelder SC, Li DQ, Barry MA. Improved transduction of human corneal epithelial progenitor cells with cell-targeting adenoviral vectors. Exp. Eye Res. 2006;83:798–806. doi: 10.1016/j.exer.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, O'Shea JJ. Th17 cells: a new fate for differentiating helper T cells. Immunol. Res. 2008;41:87–102. doi: 10.1007/s12026-007-8014-9. [DOI] [PubMed] [Google Scholar]
- Clark DA, Lamey PJ, Jarrett RF, Onions DE. A model to study viral and cytokine involvement in Sjogren's syndrome. Autoimmunity. 1994;18:7–14. doi: 10.3109/08916939409014674. [DOI] [PubMed] [Google Scholar]
- Cooke A. Th17 cells in inflammatory conditions. Rev. Diabet. Stud. 2006;3:72–75. doi: 10.1900/RDS.2006.3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales RM, Pflugfelder SC. Regulators of MUC5AC gene expression in conjunctival epithelia of dry eye patients. Invest Ophthalmol Vis Sci. 2013;54 ARVO E- 913. [Google Scholar]
- Corrales RM, Villarreal A, Farley W, Stern ME, Li DQ, Pflugfelder SC. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26:579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. CCR6 KO Mice Are Resistant To Dry Eye Disease. ARVO Meeting Abstracts March. 2012;53:2324. [Google Scholar]
- Dardalhon V, Korn T, Kuchroo VK, Anderson AC. Role of Th1 and Th17 cells in organ-specific autoimmunity. J. Autoimmun. 2008;31:252–256. doi: 10.1016/j.jaut.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JI, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KS, Niederkorn JY, Stern ME, Li D-Q, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunology. 2009 May;2(3):243–253. doi: 10.1038/mi.2009.5. Epub 2009 Feb 25. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Corrales RM, Villarreal AL, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apical corneal barrier disruption in experimental murine dry eye is abrogated by methylprednisolone and doxycycline. Invest Ophthalmol Vis. Sci. 2006;47:2847–2856. doi: 10.1167/iovs.05-1281. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Hwang CS, Pitcher JD, III, Pangelinan SB, Rahimy E, Chen W, Yoon KC, Farley WJ, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. (Oxford) 2010a;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Raince JK, McClellan AJ, Shanmugam KP, Pangelinan SB, Volpe EA, Corrales RM, Farley WJ, Corry DB, Li DQ, Pflugfelder SC. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal. Immunol. 2010b;4(4):397–408. doi: 10.1038/mi.2010.82. Epub 2010 Dec 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma} Invest Ophthalmol. Vis. Sci. 2007a;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Villarreal AL, Corrales RM, Rahman HT, Chang VY, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Pflugfelder SC. Dry Eye-Induced Conjunctival Epithelial Squamous Metaplasia Is Modulated by Interferon-{gamma} Invest Ophthalmol. Vis. Sci. 2007b;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- de Paiva CS, Volpe EA, Gandhi NB, Zhang X, Zheng X, Pitcher JD, III, Farley WJ, Stern ME, Niederkorn JY, Li DQ, Flavell RA, Pflugfelder SC. Disruption of TGF-beta Signaling Improves Ocular Surface Epithelial Disease in Experimental Autoimmune Keratoconjunctivitis Sicca. PLoS. One. 2011;6:e29017. doi: 10.1371/journal.pone.0029017. Epub 2011 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saint JM, Brignole F, Feldmann G, Goguel A, Baudouin C. Interferon-gamma induces apoptosis and expression of inflammation-related proteins in Chang conjunctival cells. Invest Ophthalmol. Vis. Sci. 1999;40:2199–2212. [PubMed] [Google Scholar]
- Doherty T, Broide D. Cytokines and growth factors in airway remodeling in asthma. Curr Opin Immunol. 2007;19:676–680. doi: 10.1016/j.coi.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M, Sadrai Z, Dana R. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:4081–4091. doi: 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez-de-Salamanca A, Castellanos E, Stern ME, Fernandez I, Carreno E, Garcia-Vazquez C, Herreras JM, Calonge M. Tear cytokine and chemokine analysis and clinical correlations in evaporative-type dry eye disease. Mol. Vis. 2010;16:862–873. [PMC free article] [PubMed] [Google Scholar]
- Ewert P, Aguilera S, Alliende C, Kwon YJ, Albornoz A, Molina C, Urzua U, Quest AF, Olea N, Perez P, Castro I, Barrera MJ, Romo R, Hermoso M, Leyton C, Gonzalez MJ. Disruption of tight junction structure in salivary glands from Sjogren's syndrome patients is linked to proinflammatory cytokine exposure. Arthritis Rheum. 2010;62:1280–1289. doi: 10.1002/art.27362. [DOI] [PubMed] [Google Scholar]
- Eyerich S, Eyerich K, Pennino D, Carbone T, Nasorri F, Pallotta S, Cianfarani F, Odorisio T, Traidl-Hoffmann C, Behrendt H, Durham SR, Schmidt-Weber CB, Cavani A. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J. Clin. Invest. 2009;119:3573–3585. doi: 10.1172/JCI40202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Gonzalez JA, Baturone R, Soto MJ, Marquez M, Macias I, Montes de OM, Medina F, Chozas N, Garcia-Perez S. Implications of immunomodulatory interleukins for the hyperimmunoglobulinemia of Sjogren's syndrome. Cell Immunol. 2009;259:56–60. doi: 10.1016/j.cellimm.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Goyal S, Chauhan SK, Zhang Q, Dana R. Amelioration of murine dry eye disease by topical antagonist to chemokine receptor 2. Arch. Ophthalmol. 2009;127:882–887. doi: 10.1001/archophthalmol.2009.125. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium Gastroenterology. 2009;137:1333–1345. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Hagiwara E, Pando J, Ishigatsubo Y, Klinman DM. Altered frequency of type 1 cytokine secreting cells in the peripheral blood of patients with primary Sjogren's syndrome. J. Rheumatol. 1998;25:89–93. [PubMed] [Google Scholar]
- Hattori T, Saban DR, Emami-Naeini P, Chauhan SK, Funaki T, Ueno H, Dana R. Donor-derived, tolerogenic dendritic cells suppress immune rejection in the indirect allosensitization-dominant setting of corneal transplantation. J Leukoc Biol. 2012;91:621–627. doi: 10.1189/jlb.1011500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Shimoyama N, Mizuno T. Destruction of salivary and lacrimal glands by Th1-polarized reaction in a model of secondary Sjogren's syndrome in lupus-prone female NZB x NZWF(1) mice. Inflammation. 2012;35:638–646. doi: 10.1007/s10753-011-9356-y. [DOI] [PubMed] [Google Scholar]
- Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J. Allergy Clin. Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- Hirota K, Yoshitomi H, Hashimoto M, Maeda S, Teradaira S, Sugimoto N, Yamaguchi T, Nomura T, Ito H, Nakamura T, Sakaguchi N, Sakaguchi S. Preferential recruitment of CCR6-expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007;204:2803–2812. doi: 10.1084/jem.20071397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes DW, Mikhajlova TL, Stoinov S, Stimac D, Vucelic B, Lonovics J, Zakuciova M, D'Haens G, Van AG, Ba S, Lee S, Pearce T. Fontolizumab, a humanised anti-interferon gamma antibody, demonstrates safety and clinical activity in patients with moderate to severe Crohn's disease. Gut. 2006;55:1131–1137. doi: 10.1136/gut.2005.079392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru N, Arakaki R, Yoshida S, Yamada A, Noji S, Hayashi Y. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjogren's syndrome-like autoimmune exocrinopathy. J. Exp. Med. 2008;205:2915–2927. doi: 10.1084/jem.20080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Invest. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie G, Jiang Q, Rui Z, Yifei Y. Expression of interleukin-17 in autoimmune dacryoadenitis in MRL/lpr mice. Curr. Eye Res. 2010;35:865–871. doi: 10.3109/02713683.2010.497600. [DOI] [PubMed] [Google Scholar]
- Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjogren's syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium. Invest Ophthalmol. Vis. Sci. 1994;35:3493–3504. [PubMed] [Google Scholar]
- Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J. Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang EH, Lee YJ, Hyon JY, Yun PY, Song YW. Salivary cytokine profiles in primary Sjogren's syndrome differ from those in non-Sjogren sicca in terms of TNF-alpha levels and Th-1/Th-2 ratios. Clin. Exp. Rheumatol. 2011;29:970–976. [PubMed] [Google Scholar]
- Katsifis GE, Rekka S, Moutsopoulos NM, Pillemer S, Wahl SM. Systemic and local interleukin-17 and linked cytokines associated with Sjogren's syndrome immunopathogenesis. Am. J Pathol. 2009;175:1167–1177. doi: 10.2353/ajpath.2009.090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsiougiannis S, Tenta R, Skopouli FN. Activation of AMP-activated protein kinase by adiponectin rescues salivary gland epithelial cells from spontaneous and interferon-gamma-induced apoptosis. Arthritis Rheum. 2010;62:414–419. doi: 10.1002/art.27239. [DOI] [PubMed] [Google Scholar]
- Khandelwal P, Blanco-Mezquita T, Emami P, Lee HS, Reyes NJ, Mathew R, Huang R, Saban DR. Ocular mucosal CD11b+ and CD103+ mouse dendritic cells under normal conditions and in allergic immune responses. PLoS One. 2013;8(5):e64193. doi: 10.1371/journal.pone.0064193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikly K, Liu L, Na S, Sedgwick JD. The IL-23/Th(17) axis: therapeutic targets for autoimmune inflammation. Curr. Opin. Immunol. 2006;18:670–675. doi: 10.1016/j.coi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J. Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koarada S, Haruta Y, Mitamura M, Morito F, Tada Y, Ohta A, Nagasawa K. Ex vivo CD(+) T-cell cytokine expression from patients with Sjogren's syndrome following in vitro stimulation to induce proliferation. Rheumatology. 2006;45(Oxford):392–399. doi: 10.1093/rheumatology/kei182. [DOI] [PubMed] [Google Scholar]
- Kohashi M, Ishimaru N, Arakaki R, Hayashi Y. Effective treatment with oral administration of rebamipide in a mouse model of Sjogren's syndrome. Arthritis Rheum. 2008;58:389–400. doi: 10.1002/art.23163. [DOI] [PubMed] [Google Scholar]
- Konttinen YT, Kemppinen P, Koski H, Li TF, Jumppanen M, Hietanen J, Santavirta S, Salo T, Larsson A, Hakala M, Sorsa T. T(H)1 cytokines are produced in labial salivary glands in Sjogren's syndrome, but also in healthy individuals. Scand. J. Rheumatol. 1999;28:106–112. doi: 10.1080/030097499442577. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007a;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Korn T, Mitsdoerffer M, Croxford AL, Awasthi A, Dardalhon VA, Galileos G, Vollmar P, Stritesky GL, Kaplan MH, Waisman A, Kuchroo VK, Oukka M. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18460–18465. doi: 10.1073/pnas.0809850105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Semin. Immunol. 2007b;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert KS, Tisdale AS, Stern ME, Smith JA, Gipson IK. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch. Ophthalmol. 2000;118:1489–1496. doi: 10.1001/archopht.118.11.1489. [DOI] [PubMed] [Google Scholar]
- LaFrance MW, Kehinde LE, Fullard RJ. Multiple cytokine analysis in human tears: an optimized procedure for cytometric bead-based assay. Curr. Eye Res. 2008;33:525–544. doi: 10.1080/02713680802190085. [DOI] [PubMed] [Google Scholar]
- Lam H, Blieden L, de Paiva CS, Farley WJ, Stern ME, Pflugfelder SC. Tear Cytokine Profiles in Dysfunctional Tear Syndrome. Am. J. Ophthalmol. 2008 Nov 5; doi: 10.1016/j.ajo.2008.08.032. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Chauhan SK, Okanobo A, Nallasamy N, Dana R. Therapeutic Efficacy of Topical Epigallocatechin Gallate in Murine Dry Eye. Cornea. 2011 doi: 10.1097/ICO.0b013e31821c9b5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Choi JW, Jang WR, Kim JM, Kim JH. Activation of Eosinophils Is More Closely Linked with Interleukin-5 and Nitric Oxide Production than Tumor Necrosis Factor-α and Immunoglobulin E Levels. Acta Haematol. 2013;130(4):238–241. doi: 10.1159/000350474. [DOI] [PubMed] [Google Scholar]
- Li Z, Burns AR, Miller SB, Smith CW. CCL20, gammadelta T cells, and IL-22 in corneal epithelial healing. FASEB J. 2011;25:2659–2668. doi: 10.1096/fj.11-184804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyakh L, Trinchieri G, Provezza L, Carra G, Gerosa F. Regulation of interleukin-12/interleukin-23 production and the T-helper 17 response in humans. Immunol. Rev. 2008;226:112–131. doi: 10.1111/j.1600-065X.2008.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Manoussakis MN, Dimitriou ID, Kapsogeorgou EK, Xanthou G, Paikos S, Polihronis M, Moutsopoulos HM. Expression of B7 costimulatory molecules by salivary gland epithelial cells in patients with Sjogren's syndrome. Arthritis Rheum. 1999;42:229–239. doi: 10.1002/1529-0131(199902)42:2<229::AID-ANR4>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183:35–48. doi: 10.1016/j.ajpath.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin. Exp. Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley TA, Haudenschild DR, Rose L, Reddi AH. Interleukin-17 family and IL-17 receptors. Cytokine & Growth Factor Reviews. 2003;14:155–174. doi: 10.1016/s1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Mosmann TR. T lymphocyte subsets, cytokines, and effector functions. Ann. N. Y. Acad. Sci. 1992;664:89–92. doi: 10.1111/j.1749-6632.1992.tb39751.x. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- Mrugacz M, Kaczmarski M, Bakunowicz-Lazarczyk A, Zelazowska B, Wysocka J, Minarowska A. IL-8 and IFN-gamma in tear fluid of patients with cystic fibrosis. J. Interferon Cytokine Res. 2006;26:71–75. doi: 10.1089/jir.2006.26.71. [DOI] [PubMed] [Google Scholar]
- Muhl H, Bachmann M, Pfeilschifter J. Inducible NO synthase and antibacterial host defence in times of Th17/Th22/T22 immunity. Cell Microbiol. 2011;13:340–348. doi: 10.1111/j.1462-5822.2010.01559.x. [DOI] [PubMed] [Google Scholar]
- Nasonova VA, Lukina GV, Sigidin I. [Neutralisation of interferon gamma--a new trend in therapy of rheumatoid arthritis] Ter. Arkh. 2008;80:30–37. [PubMed] [Google Scholar]
- Nguyen CQ, Hu MH, Li Y, Stewart C, Peck AB. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederkorn JY, Stern ME, Pflugfelder SC, de Paiva CS, Corrales RM, Gao J, Siemasko K. Desiccating Stress Induces T Cell-Mediated Sjogren's Syndrome-Like Lacrimal Keratoconjunctivitis. J. Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CQ, Peck AB. Unraveling the pathophysiology of Sjogren syndrome-associated dry eye disease. Ocul Surf. 2009;7:11–27. doi: 10.1016/s1542-0124(12)70289-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren's syndrome. Arthritis Rheum. 2002;46:2730–2741. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck AB, Nguyen CQ. Transcriptome analysis of the interferon-signature defining the autoimmune process of Sjogren's syndrome. Scand. J. Immunol. 2012;76:237–245. doi: 10.1111/j.1365-3083.2012.02749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrino FS, Volpe EA, Gandhi NB, Li DQ, Pflugfelder SC, de Paiva CS. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res. Ther. 2012;14:R234. doi: 10.1186/ar4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertovaara M, Antonen J, Hurme M. Th2 cytokine genotypes are associated with a milder form of primary Sjogren's syndrome. Ann. Rheum. Dis. 2006;65:666–670. doi: 10.1136/ard.2005.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC. Anti-inflammatory therapy of dry eye. Am. J. Ophthalmol. 2004;137:337–342. doi: 10.1016/j.ajo.2003.10.036. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, de Paiva CS, Villarreal AL, Stern ME. Effects of sequential artificial tear and cyclosporine emulsion therapy on conjunctival goblet cell density and transforming growth factor-beta2 production. Cornea. 2008;27:64–69. doi: 10.1097/ICO.0b013e318158f6dc. [DOI] [PubMed] [Google Scholar]
- Pflugfelder SC, Farley W, Luo L, Chen LZ, de Paiva CS, Olmos LC, Li DQ, Fini ME. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am. J. Pathol. 2005;166:61–71. doi: 10.1016/S0002-9440(10)62232-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjogren's syndrome keratoconjunctivitis sicca. Curr. Eye Res. 1999;19:201–211. doi: 10.1076/ceyr.19.3.201.5309. [DOI] [PubMed] [Google Scholar]
- Pitcher J, III, de Paiva CS, Pelegrino F, McClellan A, Raince J, Pangelinan S, Rahimy E, Farley W, Stern M, Li D, Pflugfelder S. Pharmacological cholinergic blockade stimulates inflammatory cytokine production and lymphocytic infiltration in the mouse lacrimal gland. Invest Ophthalmol. Vis. Sci. 2011;52:3221–3227. doi: 10.1167/iovs.09-4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao D, Yang BY, Li L, Ma JJ, Zhang XL, Lao SH, Wu CY. ESAT-6- and CFP-10-specific Th1, Th22 and Th17 cells in tuberculous pleurisy may contribute to the local immune response against Mycobacterium tuberculosis infection. Scand. J. Immunol. 2011;73:330–337. doi: 10.1111/j.1365-3083.2011.02512.x. [DOI] [PubMed] [Google Scholar]
- Rahimy E, Pitcher JD, III, Pangelinan SB, Chen W, Farley JW, Niederkorn JY, Stern ME, Li D-Q, Pflugfelder SC, de Paiva CS. Spontaneous Autoimmune Dacryoadenitis in Aged CD25KO. Am J Pathol. 2010a Aug;177(2):744–753. doi: 10.2353/ajpath.2010.091116. Epub 2010 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimy E, Pitcher JD, III, Pangelinan SB, Chen W, Farley WJ, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC, de Paiva CS. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010b;177:744–753. doi: 10.2353/ajpath.2010.091116. Epub 2010 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol. Vis. 2012;18:797–802. [PMC free article] [PubMed] [Google Scholar]
- Ryan-Payseur B, Ali Z, Huang D, Chen CY, Yan L, Wang RC, Collins WE, Wang Y, Chen ZW. Virus infection stages and distinct Th1 or Th17/Th22 T-cell responses in malaria/SHIV coinfection correlate with different outcomes of disease. J. Infect. Dis. 2011;204:1450–1462. doi: 10.1093/infdis/jir549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadrai Z, Stevenson W, Okanobo A, Chen Y, Dohlman TH, Hua J, Amparo F, Chauhan SK, Dana R. PDE4 inhibition suppresses IL-17-associated immunity in dry eye disease. Invest Ophthalmol. Vis. Sci. 2012;53:3584–3591. doi: 10.1167/iovs.11-9110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I, Terauchi K, Shimuta M, Nishiimura S, Yoshino K, Takeuchi T, Tsubota K, Miyasaka N. Expression of cell adhesion molecules in the salivary and lacrimal glands of Sjogren's syndrome. J. Clin. Lab Anal. 1993;7:180–187. doi: 10.1002/jcla.1860070309. [DOI] [PubMed] [Google Scholar]
- Sakai A, Sugawara Y, Kuroishi T, Sasano T, Sugawara S. Identification of IL-18 and Th17 cells in salivary glands of patients with Sjogren's syndrome, and amplification of IL-17-mediated secretion of inflammatory cytokines from salivary gland cells by IL-18. J. Immunol. 2008;181:2898–2906. doi: 10.4049/jimmunol.181.4.2898. [DOI] [PubMed] [Google Scholar]
- Sanos SL, Diefenbach A. Innate lymphoid cells: from border protection to the initiation of inflammatory diseases. Immunol. Cell Biol. 2013 doi: 10.1038/icb.2013.3. [DOI] [PubMed] [Google Scholar]
- Schaumburg CS, Siemasko KF, de Paiva CS, Pflugfelder ME, Stern ME. Ocular Surface Antigen Presenting Cells are Necessary for Activation of Autoreactive T cells and Development of Autoimmune Lacrimal Keratoconjunctivtis. J Immunol. 2011 doi: 10.4049/jimmunol.1101442. in press. [DOI] [PubMed] [Google Scholar]
- Shao LL, Zhang L, Hou Y, Yu S, Liu XG, Huang XY, Sun YX, Tian T, He N, Ma DX, Peng J, Hou M. Th22 cells as well as Th17 cells expand differentially in patients with early-stage and late-stage myelodysplastic syndrome. PLoS. One. 2012;7:e51339. doi: 10.1371/journal.pone.0051339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigidin YA, Loukina GV, Skurkovich B, Skurkovich S. Randomized, double-blind trial of anti-interferon-gamma antibodies in rheumatoid arthritis. Scand. J. Rheumatol. 2001;30:203–207. doi: 10.1080/030097401316909530. [DOI] [PubMed] [Google Scholar]
- Skurkovich B, Skurkovich S. Autoimmune diseases are connected with disturbances in cytokine synthesis, and therapy with IFN-gamma blockers is their main pathogenetic treatment. Ann. N. Y. Acad. Sci. 2007;1109:167–177. doi: 10.1196/annals.1398.020. [DOI] [PubMed] [Google Scholar]
- Skurkovich S, Korotky NG, Sharova NM, Skurkovich B. Treatment of alopecia areata with anti-interferon-gamma antibodies. J. Investig. Dermatol. Symp. Proc. 2005a;10:283–284. doi: 10.1111/j.0022-202X.2005.10130_6.x. [DOI] [PubMed] [Google Scholar]
- Skurkovich S, Skurkovich B, Bellanti JA. A unifying model of the immunoregulatory role of the interferon system: can interferon produce disease in humans? Clin. Immunol. Immunopathol. 1987;43:362–373. doi: 10.1016/0090-1229(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Skurkovich S, Skurkovich B, Kelly J. Anticytokine therapy, particularly anti-IFN-gamma, in Th1-mediated autoimmune diseases. Expert. Rev. Clin. Immunol. 2005b;1:11–25. doi: 10.1586/1744666X.1.1.11. [DOI] [PubMed] [Google Scholar]
- Solomon A, Dursun D, Liu Z, Xie Y, Macri A, Pflugfelder SC. Pro-and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol. Vis. Sci. 2001a;42:2283–2292. [PubMed] [Google Scholar]
- Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br. J. Ophthalmol. 2001b;85:444–449. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ME, Gao J, Schwalb TA, Ngo M, Tieu DD, Chan CC, Reis BL, Whitcup SM, Thompson D, Smith JA. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002;43:2609–2614. [PubMed] [Google Scholar]
- Stern ME, Schaumburg CS, Pflugfelder SC. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013;32:19–41. doi: 10.3109/08830185.2012.748052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin. Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Strong B, Farley W, Stern ME, Pflugfelder SC. Topical Cyclosporine Inhibits Conjunctival Epithelial Apoptosis in Experimental Murine Keratoconjunctivitis Sicca. Cornea. 2005;24:80–85. doi: 10.1097/01.ico.0000133994.22392.47. [DOI] [PubMed] [Google Scholar]
- Szodoray P, Gal I, Barath S, Aleksza M, Horvath IF, Gergely P, Jr, Szegedi G, Nakken B, Zeher M. Immunological alterations in newly diagnosed primary Sjogren's syndrome characterized by skewed peripheral T-cell subsets and inflammatory cytokines. Scand. J. Rheumatol. 2008;37:205–212. doi: 10.1080/03009740801910361. [DOI] [PubMed] [Google Scholar]
- Tian T, Yu S, Ma D. Th22 and related cytokines in inflammatory and autoimmune diseases. Expert. Opin. Ther. Targets. 2013;17:113–125. doi: 10.1517/14728222.2013.736497. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Khanal S, Ramaesh K, Diaper C, McFadyen A. Tear film osmolarity: determination of a referent for dry eye diagnosis. Invest Ophthalmol. Vis. Sci. 2006;47:4309–4315. doi: 10.1167/iovs.05-1504. [DOI] [PubMed] [Google Scholar]
- Tsubota K, Fukagawa K, Fujihara T, Shimmura S, Saito I, Saito K, Takeuchi T. Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol. Vis. Sci. 1999;40:28–34. [PubMed] [Google Scholar]
- Tsunawaki S, Nakamura S, Ohyama Y, Sasaki M, Ikebe-Hiroki A, Hiraki A, Kadena T, Kawamura E, Kumamaru W, Shinohara M, Shirasuna K. Possible function of salivary gland epithelial cells as nonprofessional antigen-presenting cells in the development of Sjogren's syndrome. J. Rheumatol. 2002;29:1884–1896. [PubMed] [Google Scholar]
- Turner K, Pflugfelder SC, Ji Z, Feuer WJ, Stern M, Reis BL. Interleukin-6 levels in the conjunctival epithelium of patients with dry eye disease treated with cyclosporine ophthalmic emulsion. Cornea. 2000;19:492–496. doi: 10.1097/00003226-200007000-00018. [DOI] [PubMed] [Google Scholar]
- Turpie B, Yoshimura T, Gulati A, Rios JD, Dartt DA, Masli S. Sjogren's syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 2009;175:1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueta M, Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog. Retin. Eye Res. 2012;31:551–575. doi: 10.1016/j.preteyeres.2012.05.003. [DOI] [PubMed] [Google Scholar]
- van Woerkom JM, Kruize AA, Wenting-van Wijk MJ, Knol E, Bihari IC, Jacobs JW, Bijlsma JW, Lafeber FP, van Roon JA. Salivary gland and peripheral blood T helper 1 and 2 cell activity in Sjogren's syndrome compared with non-Sjogren's sicca syndrome. Ann. Rheum. Dis. 2005;64:1474–1479. doi: 10.1136/ard.2004.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau S, Pasquis B, Maire MA, Fourgeux C, Gregoire S, Acar N, Bretillon L, Creuzot-Garcher CP, Joffre C. No consequences of dietary n-3 polyunsaturated fatty acid deficiency on the severity of scopolamine-induced dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2011;249:547–557. doi: 10.1007/s00417-010-1576-6. [DOI] [PubMed] [Google Scholar]
- Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal. Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007a;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007b;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- Wilson MS, Ramalingam TR, Rivollier A, Shenderov K, Mentink-Kane MM, Madala SK, Cheever AW, Artis D, Kelsall BL, Wynn TA. Colitis and intestinal inflammation in IL10−/0− mice results from IL-13Rα2-mediated attenuation of IL-13 activity. Gastroenterology. 2011;140:254–64. doi: 10.1053/j.gastro.2010.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MJ, Leung JM, Davenport M, Poles MA, Cho I, Loke P. TH17, TH22 and Treg cells are enriched in the healthy human cecum. PLoS. One. 2012;7:e41373. doi: 10.1371/journal.pone.0041373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon KC, de Paiva CS, Qi H, Chen Z, Farley WJ, Li DQ, Pflugfelder SC. Expression of th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol. Vis. Sci. 2007;48:2561–2569. doi: 10.1167/iovs.07-0002. [DOI] [PubMed] [Google Scholar]
- Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, −10, −11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol. Vis. Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Pan HF, Ye DQ. Th22 in inflammatory and autoimmune disease: prospects for therapeutic intervention. Mol. Cell Biochem. 2011a;353:41–46. doi: 10.1007/s11010-011-0772-y. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen W, de Paiva CS, Corrales RM, Volpe EA, McClellan AJ, Farley WJ, Li DQ, Pflugfelder SC. Interferon-{gamma} Exacerbates Dry Eye Induced Apoptosis in Conjunctiva via Dual Apoptotic Pathways. Invest Ophthalmol. Vis. Sci. 2011b doi: 10.1167/iovs.10-7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Chen W, de Paiva CS, Volpe EA, Gandhi NB, Farley WJ, Li DQ, Niederkorn JY, Stern ME, Pflugfelder SC. Desiccating Stress Induces CD4(+) T-Cell-Mediated Sjogren's Syndrome-Like Corneal Epithelial Apoptosis via Activation of the Extrinsic Apoptotic Pathway by Interferon-gamma. Am J Pathol. 2011c;179:1807–1814. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Volpe EA, Gandhi NB, Schaumburg CS, Siemasko KF, Pangelinan SB, Kelly SD, Hayday AC, Li DQ, Stern ME, Niederkorn JY, Pflugfelder SC, de Paiva CS. NK cells promote Th-17 mediated corneal barrier disruption in dry eye. PLoS. One. 2012;7:e36822. doi: 10.1371/journal.pone.0036822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Schaumburg CS, Coursey TG, Siemasko KF, Volpe E, N.B. Gandhi NB, Li D-Q, Niederkorn JY, Stern ME, Pflugfelder SC, de Paiva CS. CD8+ Cells Regulate the T helper-17 Response in an Experimental Murine Model of Sjögren Syndrome. Mucosal Immuol. 2013 doi: 10.1038/mi.2013.61. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Bian F, Ma P, de Paiva CS, Stern M, Pflugfelder SC, Li DQ. Induction of Th17 differentiation by corneal epithelial-derived cytokines. J Cell Physiol. 2009;222(1):95–102. doi: 10.1002/jcp.21926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, de Paiva CS, Li DQ, Farley WJ, Pflugfelder SC. Desiccating stress promotion of Th17 differentiation by ocular surface tissues through a dendritic cell-mediated pathway. Invest Ophthalmol. Vis. Sci. 2010;51:3083–3091. doi: 10.1167/iovs.09-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunol. Rev. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]