Abstract
Accumulating evidence implicates the growth hormone receptor (GHR) in carcinogenesis. While multiple studies show evidence for expression of growth hormone (GH) and GHR mRNA in human cancer tissue, there is a lack of quantification and only a few cancer types have been investigated. The National Cancer Institute’s NCI60 panel includes 60 cancer cell lines from nine types of human cancer: breast, CNS, colon, leukemia, melanoma, non-small cell lung, ovarian, prostate and renal. We utilized this panel to quantify expression of GHR, GH, prolactin receptor (PRLR) and prolactin (PRL) mRNA with real-time RT qPCR. Both GHR and PRLR show a broad range of expression within and among most cancer types. Strikingly, GHR expression is nearly 50-fold higher in melanoma than in the panel as a whole. Analysis of human metastatic melanoma biopsies confirmed GHR gene expression in melanoma tissue. In these human biopsies, the level of GHR mRNA is elevated in advanced stage IV tumor samples compared to stage III. Due to the novel finding of high GHR in melanoma, we examined the effect of GH treatment on three NCI60 melanoma lines (MDA-MB-435, UACC-62 and SK-MEL-5). GH increased proliferation in two out of three cell lines tested. Further analysis revealed GH-induced activation of STAT5 and mTOR in a cell line dependent manner. In conclusion, we have identified cell lines and cancer types that are ideal to study the role of GH and PRL in cancer, yet have been largely overlooked. Furthermore, we found that human metastatic melanoma tumors express GHR and cell lines possess active GHRs that can modulate multiple signaling pathways and alter cell proliferation. Based on this data, GH could be a new therapeutic target in melanoma.
Keywords: Growth hormone receptor, melanoma, growth hormone, prolactin receptor, cancer
Introduction
Growth hormone (GH) is secreted by the somatotrophs of the anterior pituitary. GH may also be expressed in other cell types, such as lymphocytes and neurons, where it could act in an autocrine and/or paracrine manner [1]. In addition to its effects through GHR, human GH can also initiate signaling via the prolactin receptor (PRLR) [2]. GH is capable of modulating many intracellular signaling pathways that are implicated in carcinogenesis, including JAK/STAT, MAPK/Erk and mTOR [3,4]. These pathways are known to play a role in mitogenic, anti-apoptotic and metabolic actions of GH; thus, the direct actions of GH could be involved in key processes of carcinogenesis [4,5]. Apart from the direct effects of GH, many of its biological actions are mediated via insulin-like growth factor (IGF)-1, a hormone known to have a role in cancer [4].
Several lines of evidence from human and animal studies support a role of GH in carcinogenesis. Patients with acromegaly, a condition of GH excess, have a higher incidence of colon polyps [6] and appear to have an increased risk of developing colon cancer and possibly other malignancies including thyroid cancer [7,8]. At the other physiological extreme of GH action, Laron Syndrome patients with inactive GHR rarely develop tumors and do not experience cancer mortality [9]. These data from human studies are supported by numerous animal studies, which show a decrease in cancer incidence and tumor burden in several rodent lines with reduced GH action [10–13]. For example, the GHR-null mouse has fewer cancer deaths than wild-type controls [10]. Collectively, these data strongly suggest a role for GH in cancer.
While several studies have shown both GH and GHR mRNA and protein expression in human cancer biopsies and explants, such as breast, prostate and colorectal cancer tissues [14–19], the studies are limited by a reliance on immunohistochemistry and non-quantitative RT-PCR. A lack of quantification and the limited number of cancer types investigated to date has left many questions unanswered as to the role of these proteins in the development or progression of cancer. In the present study, expression of GHR, PRLR, GH and PRL mRNA was quantitatively assessed with real time RT qPCR in the US National Cancer Institute’s NCI60 panel, which contains 60 human cell lines that represent nine types of cancer including breast, CNS, colon, leukemia, melanoma, non-small cell (NSC) lung, ovarian, prostate and renal [20]. Based on gene-expression results from the NCI60 panel, we also examined GHR expression in human melanoma tumor biopsies and determined the effect of GH treatment on cell proliferation and signaling pathway activation in several melanoma cell lines. The novel data presented here suggest that further studies are warranted to determine the role of GH in metastatic melanoma.
Materials and methods
NCI60 cancer cell line RNA samples
Total RNA from the 60 human cancer cell lines included in the NCI60 panel (Table 1) was provided by the National Cancer Institute’s Development Therapeutics Program. RNA quality and quantity was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies).
Table 1.
Cell lines included in the NCI60 panel
| Breast | CNS | Colon | Leukemia | Melanoma | NSC Lung | Ovarian | Prostate | Renal |
|---|---|---|---|---|---|---|---|---|
| MCF7 | SF-268 | COLO205 | CCRF-CEM | LOX IMVI | A549/ATCC | IGROV1 | PC-3 | 786-0 |
| MDA-MB-231 | SF-295 | HCC-2998 | HL-60(TB) | MALME-3M | EKVX | OVCAR-3 | DU-145 | A498 |
| MDA-MB-468 | SF-539 | HCT-116 | K-562 | M14 | HOP-62 | OVCAR-4 | ACHN | |
| HS 578T | SNB-19 | HCT-15 | MOLT-4 | MDA-MB-435 | HOP-92 | OVCAR-5 | CAKI-1 | |
| BT-549 | SNB-75 | HT29 | RPMI-8226 | SK-MEL-2 | NCI-H226 | OVCAR-8 | RXF393 | |
| T-47D | U251 | KM12 | SR | SK-MEL-28 | NCI-H23 | NCI/ADR-RES | SN12C | |
| SW-620 | SK-MEL-5 UACC-257 UACC-62 |
NCI-H322M NCI-H460 NCI-H522 |
SK-OV-3 | TK-10 UO-31 |
Real-time RT qPCR
mRNA expression was assessed with an iCycler iQ machine (Bio-Rad) using Maxima First Strand cDNA Synthesis Kit and a Maxima SYBR Green/Fluorescein qPCR Master Mix (Thermo Scientific). SDHA and HPRT were found to be stably expressed across the panel and were used as reference genes for normalization (data not shown). Further details can be found in supplementary materials.
Human melanoma biopsy cDNA array
cDNA arrays from human metastatic melanoma tumor biopsies (Origene MERT101) containing 40 tumor samples were analyzed for expression of GHR, IGF-1 and IGF-1R mRNA. These arrays contain cDNA from three non-tumor skin sample controls and 40 tumor biopsies that include both male and female stage III (regional) and stage IV (distant) metastatic melanoma tumor samples. HPRT was used as a reference gene.
Broad-Novartis Cancer Cell Line Encyclopedia
The Broad Institute and the Novartis Institutes for Biomedical Research have collaborated to generate gene expression data on 957 cancer cell lines in a project termed the Cancer Cell Line Encyclopedia (CCLE) [21]. Public access is provided to gene expression data from microarray studies (http://www.broadinstitute.org/ccle). Gene expression data was extracted from CCLE_Expression_Entrez_2012-10-18.res. Through the CCLE Terms of Access, we declare that, “those who carried out the original analysis and collection of the data bear no responsibility for the further analysis or interpretation of it.”
Melanoma cell culture
Human melanoma cell lines MDA-MB-435, UACC-62 and SK-MEL-5 were obtained from NCI’s Development Therapeutics Program and were used for all GH treatment studies. Cell lines were maintained at 37°C in 5% CO2 with complete media composed of RPMI 1640 media containing 2.5 mM L-glutamine (Thermo Scientific SH30027.FS), 5% fetal bovine serum (Thermo Scientific SH30071.03) and 1x antibiotic-antimycotic (Gibco 15240-062).
Cell proliferation
Recombinant human GH (hereafter referred to as GH) was produced using a method modified from Patra et al. [22]. Human melanoma cell lines in passage 3–8 were seeded on 96-well plates at 2,000 to 6,000 cells/well and cultured overnight in complete media. The second day, media was replaced with serum-free media. The following day, cells were treated with GH and cultured for 48 hours in serum-free media. Cell proliferation was determined using PrestoBlue Cell Viability Reagent (Invitrogen A-13261). Following preliminary GH dose-response studies spanning several orders of magnitude, 100 nM GH elicited the greatest proliferative response and was selected for further experiments.
STAT5 signaling assays
Cells were grown on 24-well plates (Seahorse Biosciences, 100777-004) until they reached 75–90% confluence. Cells were switched to serum-free media, starved for 16 hours and then treated with 100 nM GH for 10–30 minutes. Total and phosphorylated STAT5 (Y694) were measured by cell-based ELISA (Ray Biotech, CBEL-Stat-SK). Data is presented as a ratio of the ODs obtained from the phosphorylated STAT5 ELISA and the total STAT5 ELISA.
Erk1/2 and mTOR signaling assays
Cells were seeded on 12-well plates and grown to 75–90% confluency, then starved for 16 hours in RPMI without serum. Cells were then treated for 10–30 minutes with 100 nM GH. Total and phospho-Erk1/2 (T202/Y204 of Erk1 and Y185/Y187 of Erk2) ELISAs were purchased from Ray Biotech (PEL-Erk-T202-002). ELISAs for total and phosphorylated (S2448) mTOR were obtained from Cell Signaling (7974S and 7976S).
Data analysis
qPCR data was analyzed with qBasePLUS v2.3 (Biogazelle) as described in supplementary materials and subject to the Kruskal-Wallis rank sum test with Dunn’s multiple comparison post-hoc. Data spanned several orders of magnitude so geometric mean was used to describe expression levels. For proliferation data, untreated samples were set to one. Multiple independent proliferation experiments (n=3–7) were analyzed by randomized block design ANOVA. One-way ANOVA with Tukey’s post-hoc was used for analysis of ELISA data, where 10 and 30 minute time points were combined. Statistical analyses were conducted with GraphPad Prism 5 (GraphPad Software). A P-value <0.05 was considered significant.
Results
Gene expression in the NCI60 panel
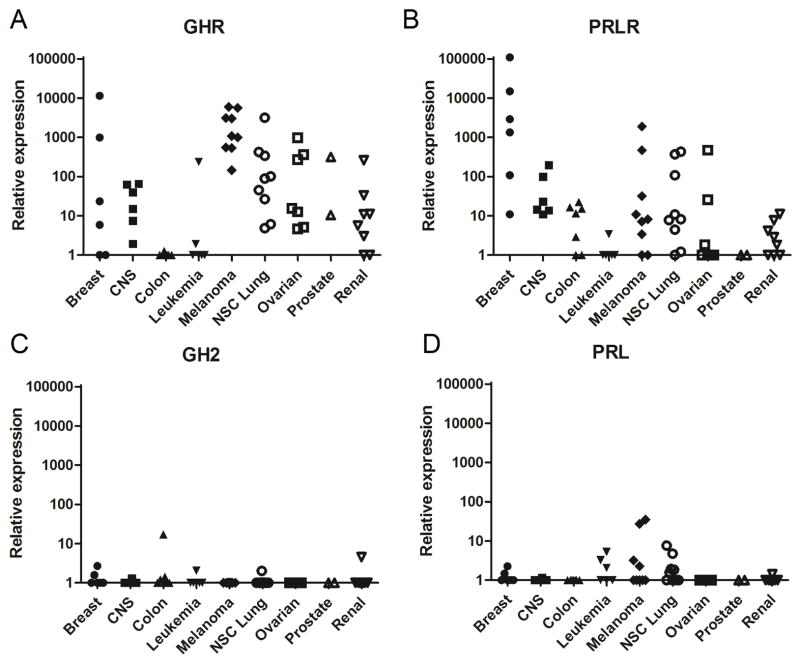
GHR, PRLR, GH and PRL mRNA levels were examined in the NCI60 cell line panel using real-time RT qPCR. GHR shows the highest mean mRNA level across the entire panel at 28.5 relative expression units, followed by PRLR at 11.2 (supplementary Table S2). We were unable to detect expression of the GH1 gene (“normal” GH). However, low levels of GH2 mRNA are detected in several cell lines. GH2 and PRL show the lowest overall levels at 1.1 and 1.4, respectively.
GHR mRNA is widely expressed in the NCI60 panel cell lines (Fig. 1A and supplementary Fig. S1). With the notable exception of colon and melanoma, all other cancer types contain both high and low GHR expressing cell lines (Fig. 1A). GHR is barely detectable in colon cancer, whereas all melanoma cell lines express high levels of GHR mRNA (Fig. 1A, supplementary Fig. S1). NSC lung and ovarian cancers both have several cell lines with high GHR expression. Yet, GHR levels in melanoma are 16-fold higher than in NSC lung and 29-fold higher than in ovarian.
Fig. 1.
Relative mRNA expression in the NCI60 panel. GHR (A), PRLR (B), GH (C) and PRL (D) expression was analyzed by real-time RT qPCR using the delta Cq method. Each symbol represents the expression of a single cell line, organized by cancer type.
PRLR mRNA expression is highest in breast cancer (Fig. 1B and supplementary Table S2), which has 44 times higher expression then the next highest type, CNS. Third and fourth in PRLR expression are melanoma and NSC lung cancer, which have nearly equal PRLR mRNA levels ~88-fold lower than breast cancer. As with GHR, leukemia and renal lines are among the three lowest in terms of PRLR expression. Prostate cancer has express the lowest amount of PRLR mRNA, but is only represented by two cell lines.
To evaluate the potential for paracrine and autocrine GH and PRL action in cancer cells, we assessed their mRNA expression in the NCI60 panel. GH1 mRNA was not detected. Both GH2 and PRL mRNAs were very low in all cancer types examined (Fig. 1C and D and supplementary Table S2).
mRNA expression in the CCLE dataset
The CCLE contains gene expression data on 957 cancer cell lines. In agreement with our studies, melanoma is one of the highest GHR expressing cancer types while those with the lowest levels in the CCLE include leukemia colon (supplementary Fig. S2; referred to as T-cell/B-cell acute lymphoblastic leukemia and colorectal in the CCLE). Our finding that PRLR mRNA expression is highest in breast cancer cell lines was also confirmed with CCLE data.
Expression of GHR in metastatic melanoma tumor biopsies
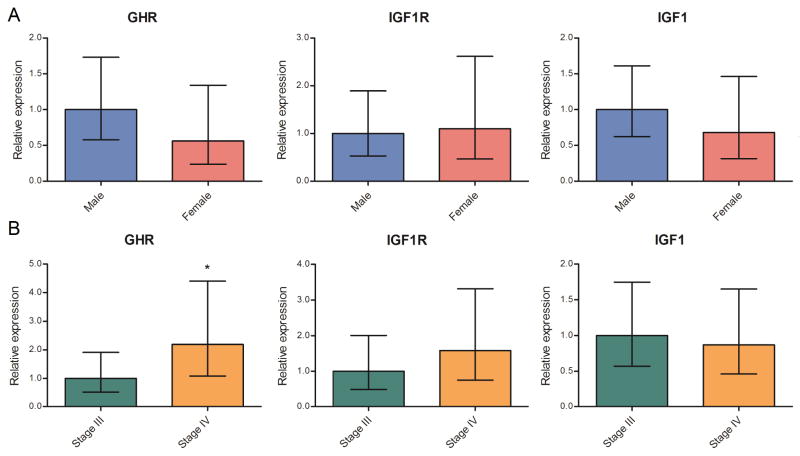
Due to the high levels of GHR detected in all NCI60 melanoma cell lines, the potential in vivo role of GHR in human melanoma biopsies was explored using tumor cDNA panels. Most tumor samples express GHR (Fig. 2), with more than a fourth showing levels greater than 100 relative expression units. GHR expression was undetectable in six samples. The three control skin samples express relatively high levels of GHR. When categorized by sex, both GHR and IGF1 mRNA levels are nearly double in tumors from male patients (Fig. 3A). GHR mRNA expression is more than twice as high in advanced stage IV compared to stage III tumors (one-tailed Mann-Whitney test P<0.05; Fig. 3B). Additionally, IGF1R mRNA expression appears to be markedly increased in the stage IV tumors, although this difference did not reach statistical significance (Fig. 3B).
Fig. 2.
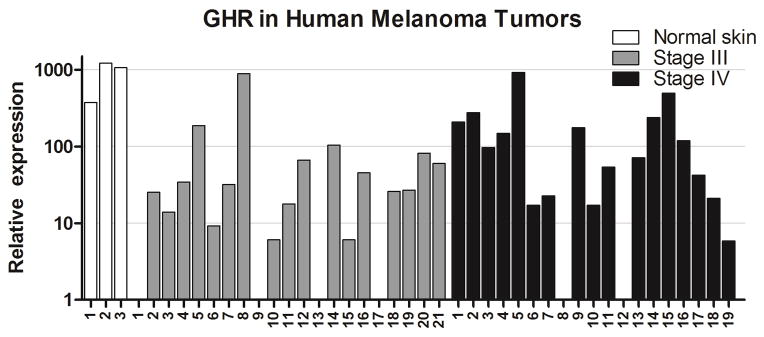
GHR mRNA expression in melanoma tumor biopsies. Human metastatic melanoma tumor cDNA was analyzed for GHR expression by real-time RT qPCR. Results are normalized and scaled to be comparable to the quantification of GHR in NCI60 cell lines as presented in Fig. 1. Three normal skin (white bars), 21 stage III melanoma (grey bars) and 19 stage IV melanoma (black bars) biopsies are shown.
Fig. 3.
GHR, IGF1 and IGF1R mRNA levels in metastatic melanoma samples analyzed by sex (A) and tumor grade (B). Geometric mean of gene expression with 95% CI is shown.
Effect of GH treatment on proliferation of metastatic melanoma cell lines
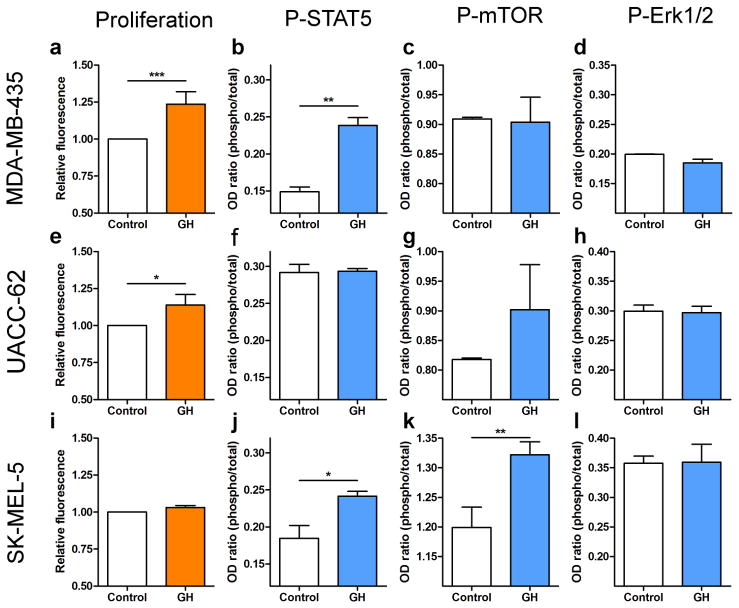
The greatest response to GH is seen in MDA-MB-435 cells, which show a 23.5% increase in growth with treatment (P<0.001; Fig. 4a). In UACC-62, GH increases proliferation by 13.8% (P<0.05; Fig. 4e). GH treatment did not significantly alter proliferation of SK-MEL-5 cells (Fig. 4i).
Fig. 4.
Effect of GH treatment of metastatic melanoma cell lines. The effect of 100 nM GH treatment on proliferation and the phosphorylation status of STAT5, mTOR and Erk1/2 is shown for MDA-MB-435 (a–d), UACC-62 (e–h) and SK-MEL-5 (i–l). For protein activation, the OD ratio of phosphorylated to total protein as determined by ELISA is reported. Error bars represent the SD.
Effect of GH on signaling pathways in metastatic melanoma cell lines
The same three cell lines used for proliferation were assessed for the effect of GH treatment on the activation of several signal transduction pathways. GH treatment increases STAT5 phosphorylation in MDA-MB-435 and SK-MEL-5 with GH treatment (Fig. 4b and 4j; P<0.05), although no change is observed in UACC-62 (Fig. 4f). mTOR activation is significantly induced in SK-MEL-5 (P<0.01; Fig. 4k). In UACC-62, mTOR phosphorylation shows a trend to be higher with GH treatment (Fig. 4g), but no change is observed for MDA-MB-435. There is no effect of GH treatment on MAPK activation, assessed by measuring total and phosphorylated Erk1/2 (Fig. 4d, 4h and 4l).
Discussion
We utilized the NCI60 cancer cell line panel to characterize nine types of human cancer for the potential ability to respond to GH through GHR and PRLR signaling. Seven of the cancer types examined show a broad range of GHR expression. Colon, on the other hand, had very low GHR expression while melanoma strikingly had the highest. Because melanoma clearly stood out with high GHR expression, this cancer type was examined further. Treatment of human metastatic melanoma cell lines with GH activated STAT5 and mTOR and stimulated cell growth in a cell line dependent manner. The elevation of GHR mRNA in advanced stage IV metastatic melanoma tumors is a novel finding with potential clinical relevance.
Several cancer types showed high levels of PRLR mRNA expression in a subset of cell lines, including NSC lung, CNS, melanoma and ovarian. PRLR has previously been observed in several ovarian cancer cell lines [23], whereas we could not find reports of PRLR mRNA expression in NSC lung, CNS or melanoma cancer cell lines. PRLR mRNA was highest in breast cancer cell lines, which is in agreement with a large body of literature showing high PRLR expression and activity in mammary carcinoma tissue [24]. Our novel findings of high PRLR mRNA levels in several other cancer types suggest that research should expand to include cancers of other tissues. GH1 expression was undetectable, and just a few lines exhibit low-level GH2 expression, a GH variant best known for its expression in placental tissue [25]. Similarly, PRL was only detectable in a few cell lines. Where GH2 and PRL are expressed, they are at low levels, suggesting that it is unlikely that GH or PRL act in an autocrine or paracrine manner in the NCI60 cell lines.
The most surprising result from this study is the universally high GHR expression in melanoma cell lines, a finding that we independently verified with data from the CCLE database. To follow up our in vitro findings, we examined human metastatic melanoma tumors for GHR expression. We observed GHR mRNA expression in a majority of human metastatic melanoma biopsies examined, in agreement with previous data from IHC studies [16,26,27]. The level of GHR mRNA was relatively high in the control skin samples. Previous research has detected, but not quantified, expression of GHR protein and mRNA in human skin tissue and a non-melanoma skin cancer [28–30]. Skin is composed of many different cell types, while the tumors presumably derive exclusively from melanocytes. The level of GHR produced by melanocytes in normal skin tissue is unknown. One possibility is that melanocytes are not a major contributor to the high GHR expression found in the control skin samples, yet a subset of melanoma tumors maintain a level of GHR that confers an invasive phenotype to the cancer cell or promotes tumor establishment upon metastasis to a distant site. Future work to examine the role of GHR in healthy skin and metastatic melanoma tumors is needed to test this hypothesis.
The potential for GH to play a role in metastasis and invasion led us to analyze expression of GHR by cancer stage. We found higher expression of GHR in more advanced stage IV samples compared to stage III, which suggests that inhibiting the GHR axis with GH-suppressive or GHR-inhibiting therapeutics may be worth pursuing in patients whose tumors show high GHR mRNA levels.
GH treatment of melanoma cell lines altered growth and/or cell signaling, showing that GH activity is biologically relevant. STAT5 was activated by GH treatment in two cell lines, and the third exhibited high basal activation of STAT5. Hassel et al. found that STAT5 has a role in anti-apoptotic signaling in melanoma cells [31], suggesting a mechanism whereby GH could promote cancer cell survival. STAT5 activation has also been correlated with advanced stages of melanoma in a fish model of the disease [32], which suggests that the GH could promote invasion and metastasis through STAT5 activation. All three lines have mutations in the proto-oncogene BRAF that activate the MAPK/Erk pathway [33] which likely explains the lack of an effect of GH on Erk1/2 activation in these cells. mTOR, a key signaling protein that can promote tumorigenesis in melanoma [34], was activated in one cell line by GH treatment. mTOR activity is often upregulated in cancer and two drugs that inhibit its action are approved for treatment of advanced metastatic renal cell cancer [35]. Further studies are needed to determine if GH activation of mTOR in melanoma is clinically relevant and whether mTOR inhibitors could also be effective against a subset of melanoma tumors.
Intriguingly, two recent case studies support the hypothesis that GHR could play a causative role in melanoma. In the first, a 26 year old was diagnosed with malignant melanoma following multiple hormone replacement therapy that included GH [36]. In the second, a husband and wife were both diagnosed with melanoma within two weeks of each other following three months of daily GH injections taken as part of an “anti-aging regimen” [37]. Although a cause and effect issue cannot be distilled here, the short length of time between the start of GH exposure and diagnosis of melanoma in these patients is remarkable.
In summary, we have examined the GH and PRL pathways in 60 cell lines from nine human cancer types. Most cancer types showed a wide range of GHR and PRLR mRNA expression, suggesting that different types of cancer may have specific sub-types that could potentially be sensitive to inhibition of GHR and/or PRLR signaling. The novel finding of high GHR mRNA expression in melanoma led us to examine the effect of GH treatment on signaling pathways and cell proliferation. These data show that metastatic melanoma cells possess active GH receptors that can modulate multiple signaling pathways and stimulate cell growth. Further research is warranted to explore the potential for treatment of metastatic melanoma with drugs that lower GH levels (somatostatin analogs) or inhibit GH action (pegvisomant). To this end, we currently have studies underway to further characterize melanoma cell lines for their response to GH and GHR antagonists.
Supplementary Material
Highlights.
Most cancer types of the NCI60 have sub-sets of cell lines with high GHR expression
GHR is highly expressed in melanoma cell lines
GHR is elevated in advanced stage IV metastatic tumors vs. stage III
GH treatment of metastatic melanoma cell lines alters growth and cell signaling
Acknowledgments
Funding
Supported by the State of Ohio’s Eminent Scholar Program, which includes a gift from Milton and Lawrence Goll, AMVETS, the Diabetes Institute at Ohio University and NIH (P01AG031736).
We thank Susan Holbeck and the NCI for providing RNA samples and cell lines.
Footnotes
Declaration of interest
JJK is an inventor of US patent 5350836 “Growth hormone antagonists”.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harvey S. Extrapituitary growth hormone. Endocrine. 2010;38:335–359. doi: 10.1007/s12020-010-9403-8. [DOI] [PubMed] [Google Scholar]
- 2.Bernichtein S, Touraine P, Goffin V. New concepts in prolactin biology. J Endocrinol. 2010;206:1–11. doi: 10.1677/JOE-10-0069. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi AA, Proud CG. The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab. 2007;292:E1647–55. doi: 10.1152/ajpendo.00674.2006. [DOI] [PubMed] [Google Scholar]
- 4.Chhabra Y, Waters MJ, Brooks AJ. Role of the growth hormone–IGF-1 axis in cancer. Expert Rev Endocrinol Metab. 2011;6:71–84. doi: 10.1586/eem.10.73. [DOI] [PubMed] [Google Scholar]
- 5.Clayton PE, Banerjee I, Murray PG, Renehan AG. Growth hormone, the insulin-like growth factor axis, insulin and cancer risk. Nat Rev Endocrinol. 2011;7:11–24. doi: 10.1038/nrendo.2010.171. [DOI] [PubMed] [Google Scholar]
- 6.Martino A, Cammarota G, Cianci R, Bianchi A, Sacco E, Tilaro L, Marzetti E, Certo M, Pirozzi G, Fedeli P, et al. High prevalence of hyperplastic colonic polyps in acromegalic subjects. Dig Dis Sci. 2004;49:662–666. doi: 10.1023/b:ddas.0000026315.91800.b2. [DOI] [PubMed] [Google Scholar]
- 7.Renehan AG, Brennan BM. Acromegaly, growth hormone and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22:639–657. doi: 10.1016/j.beem.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Dagdelen S, Cinar N, Erbas T. Increased thyroid cáncer risk in acromegaly. Pituitary. 2013 Jul 09; doi: 10.1007/s11102-013-0501-5. Epub. [DOI] [PubMed] [Google Scholar]
- 9.Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng CW, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. doi: 10.1126/scitranslmed.3001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64:522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollak M, Blouin MJ, Zhang JC, Kopchick JJ. Reduced mammary gland carcinogenesis in transgenic mice expressing a growth hormone antagonist. Br J Cancer. 2001;85:428–430. doi: 10.1054/bjoc.2001.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Luque RM, Kineman RD, Ray VH, Christov KT, Lantvit DD, Shirai T, Hedayat S, Unterman TG, Bosland MC, et al. Disruption of growth hormone signaling retards prostate carcinogenesis in the Probasin/TAg rat. Endocrinology. 2008;149:1366–1376. doi: 10.1210/en.2007-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanson SM, Unterman TG. The growth hormone-deficient spontaneous dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis. 2002;23:977–982. doi: 10.1093/carcin/23.6.977. [DOI] [PubMed] [Google Scholar]
- 14.Wu ZS, Yang K, Wan Y, Qian PX, Perry JK, Chiesa J, Mertani HC, Zhu T, Lobie PE. Tumor expression of human growth hormone and human prolactin predict a worse survival outcome in patients with mammary or endometrial carcinoma. J Clin Endocrinol Metab. 2011;96:E1619–29. doi: 10.1210/jc.2011-1245. [DOI] [PubMed] [Google Scholar]
- 15.Decouvelaere C, Peyrat JP, Bonneterre J, Djiane J, Jammes H. Presence of the two growth hormone receptor messenger RNA isoforms in human breast cancer. Cell Growth Differ. 1995;6:477–483. [PubMed] [Google Scholar]
- 16.Lincoln DT, Sinowatz F, Kolle S, Takahashi H, Parsons P, Waters M. Up-regulation of growth hormone receptor immunoreactivity in human melanoma. Anticancer Res. 1999;19:1919–1931. [PubMed] [Google Scholar]
- 17.Mertani HC, Garcia-Caballero T, Lambert A, Gerard F, Palayer C, Boutin JM, Vonderhaar BK, Waters MJ, Lobie PE, Morel G. Cellular expression of growth hormone and prolactin receptors in human breast disorders. Int J Cancer. 1998;79:202–211. doi: 10.1002/(sici)1097-0215(19980417)79:2<202::aid-ijc17>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Gebre-Medhin M, Kindblom LG, Wennbo H, Törnell J, Meis-Kindblom JM. Growth hormone receptor is expressed in human breast cancer. Am J Pathol. 2001;158:1217–1222. doi: 10.1016/S0002-9440(10)64071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiss-Messer E, Merom O, Adi A, Karry R, Bidosee M, Ber R, Kaploun A, Stein A, Barkey RJ. Growth hormone (GH) receptors in prostate cancer: Gene expression in human tissues and cell lines and characterization, GH signaling and androgen receptor regulation in LNCaP cells. Mol Cell Endocrinol. 2004;220:109–123. doi: 10.1016/j.mce.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein JN. Spotlight on molecular profiling: “integromic” analysis of the NCI-60 cancer cell lines. Mol Cancer Ther. 2006;5:2601–2605. doi: 10.1158/1535-7163.MCT-06-0640. [DOI] [PubMed] [Google Scholar]
- 21.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV, Sonkin D, et al. The cancer cell line encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:7391–307. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patra AK, Mukhopadhyay R, Mukhija R, Krishnan A, Garg LC, Panda AK. Optimization of inclusion body solubilization and renaturation of recombinant human growth hormone from Escherichia coli. Protein Expr Purif. 2000;18:182–192. doi: 10.1006/prep.1999.1179. [DOI] [PubMed] [Google Scholar]
- 23.Tan D, Chen KE, Khoo T, Walker AM. Prolactin increases survival and migration of ovarian cancer cells: Importance of prolactin receptor type and therapeutic potential of S179D and G129R receptor antagonists. Cancer Lett. 2011;310:101–108. doi: 10.1016/j.canlet.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 24.Swaminathan G, Varghese B, Fuchs SY. Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13:81–91. doi: 10.1007/s10911-008-9068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoo EJ, Cajiao I, Kim JS, Kimura AP, Zhang A, Cooke NE, Liebhaber SA. Tissue-specific chromatin modifications at a multigene locus generate asymmetric transcriptional interactions. Mol Cell Biol. 2006;26:5569–5579. doi: 10.1128/MCB.00405-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lincoln DT, Sinowatz F, Kolle S, Takahashi H, Parsons P, Waters M. Growth hormone receptor expression in the nucleus and cytoplasm of normal and neoplastic cells. Histochem Cell Biol. 1998;109:141–159. doi: 10.1007/s004180050212. [DOI] [PubMed] [Google Scholar]
- 27.Ginarte M, Garcia-Caballero T, Fernandez-Redondo V, Beiras A, Toribio J. Expression of growth hormone receptor in benign and malignant cutaneous proliferative entities. J Cutan Pathol. 2000;27:276–282. doi: 10.1034/j.1600-0560.2000.027006276.x. [DOI] [PubMed] [Google Scholar]
- 28.Stanimirovic A, Cupic H, Bosnjak B, Tomas D, Kruslin B, Belicza M. TP53, bcl-2 and growth hormone receptor expression in cutaneous squamous cell carcinoma. Acta Dermatovenerol Croat. 2005;13:201–205. [PubMed] [Google Scholar]
- 29.Lobie PE, Breipohl W, Lincoln DT, Garcia-Aragon J, Waters MJ. Localization of the growth hormone receptor/binding protein in skin. J Endocrinol. 1990;126:467–471. doi: 10.1677/joe.0.1260467. [DOI] [PubMed] [Google Scholar]
- 30.Tavakkol A, Elder JT, Griffiths CE, Cooper KD, Talwar H, Fisher GJ, Keane KM, Foltin SK, Voorhees JJ. Expression of growth hormone receptor, insulin-like growth factor 1 (IGF-1) and IGF-1 receptor mRNA and proteins in human skin. J Invest Dermatol. 1992;99:343–349. doi: 10.1111/1523-1747.ep12616668. [DOI] [PubMed] [Google Scholar]
- 31.Hassel JC, Winnemoller D, Schartl M, Wellbrock C. STAT5 contributes to antiapoptosis in melanoma. Melanoma Res. 2008;18:378–385. doi: 10.1097/CMR.0b013e32830ce7d7. [DOI] [PubMed] [Google Scholar]
- 32.Schartl M, Wilde B, Laisney JA, Taniguchi Y, Takeda S, Meierjohann S. A mutated EGFR is sufficient to induce malignant melanoma with genetic background-dependent histopathologies. J Invest Dermatol. 2010;130:249–258. doi: 10.1038/jid.2009.213. [DOI] [PubMed] [Google Scholar]
- 33.Ikediobi ON, Davies H, Bignell G, Edkins S, Stevens C, O’Meara S, Santarius T, Avis T, Barthorpe S, Brackenbury L, et al. Mutation analysis of 24 known cancer genes in the NCI-60 cell line set. Mol Cancer Ther. 2006;5:2606–2612. doi: 10.1158/1535-7163.MCT-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie X, White EP, Mehnert JM. Coordinate autophagy and mTOR pathway inhibition enhances cell death in melanoma. PloS One. 2013;8:e55096. doi: 10.1371/journal.pone.0055096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomez-Pinillos A, Ferrari AC. mTOR signaling pathway and mTOR inhibitors in cancer therapy. Hematol Oncol Clin North Am. 2012;26:483–505. doi: 10.1016/j.hoc.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Caldarola G, Battista C, Pellicano R. Melanoma onset after estrogen, thyroid, and growth hormone replacement therapy. Clin Ther. 2010;32:57–59. doi: 10.1016/j.clinthera.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 37.Handler MZ, Ross AL, Shiman MI, Elgart GW, Grichnik JM. Potential role of human growth hormone in melanoma growth promotion. Arch Dermatol. 2012;148:1179–1182. doi: 10.1001/archdermatol.2012.2149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.