Abstract
Objective
There are currently no targeted therapies against lung tumors with oncogenic K-ras mutations that are found in 25% to −40% of lung cancers and are characterized by their resistance to epidermal growth factor receptor inhibitors. The isozyme group IIa secretory phospholipase A2 (sPLA2IIa) is a potential biomarker and regulator of lung cancer cell invasion; however, the relationship between K-ras mutations and sPLA2IIa has yet to be investigated. We hypothesize that sPLA2IIa modulates lung cancer cell growth in K-ras mutant cells and that sPLA2IIa expression in human lung tumors is increased in K-ras mutant tumors.
Methods
Baseline sPLA2IIa expression in K-ras mutant lung cancer cell lines (A549, SW1573, H358, H2009) was assessed. Cells were treated with a specific sPLA2IIa inhibitor and evaluated for apoptosis and cell viability. Nuclear factor kappa-b (NF-κB) and extracellular signal-regulated kinase 1/2 activity were detected by Western blot. Human tumor samples were evaluated for sPLA2IIa mRNA expression by quantitative reverse-transcription polymerase chain reaction.
Results
Cytotoxicity of sPLA2IIa inhibition correlates with sPLA2IIa expression. Apoptosis in response to sPLA2 inhibition parallels attenuation in NF-κB activity. In addition, sPLA2IIa expression in human tumors correlates with squamous cell pathology and increasing stage of K-ras mutant lung tumors.
Conclusions
Baseline sPLA2IIa expression predicts response to sPLA2IIa inhibition in some K-ras mutant lung cancer cells. This finding is independent of p53 mutation status. Furthermore, squamous tumors and advanced-stage K-ras mutant tumors express more sPLA2IIa. These data support a role for sPLA2IIa as a potential global therapeutic target in the treatment of lung cancer.
Lung cancer remains the most lethal cancer in the world. Gain-of-function mutations in K-ras are found in 25% to 40% of lung cancers and are thought to be significant drivers of oncologic virulence. There are currently no targeted therapies that effectively treat K-ras mutant lung tumors. In fact, these mutations are characterized by their resistance to epidermal growth factor receptor (EGFR) inhibitors.1,2
Phospholipase A2 (PLA2) enzymes release arachidonic acid from cell membranes, initiating downstream production of tumor-promoting eicosanoids. Group IIa secretory phospholipase A2 (sPLA2IIa), a subgroup of PLA2s, has recently been identified as a potential lung cancer biomarker.3 In a cohort of patients with solitary pulmonary nodules, patients with lung cancer had higher plasma levels of sPLA2IIa than patients with a benign nodule. In addition, histologic analysis demonstrates overexpression of sPLA2IIa in lung tumors compared with normal lung tissue.3 From a functional perspective, sPLA2IIa has been shown to regulate lung cancer cell growth and invasion. RNA interference of sPLA2IIa decreases tumor growth and nuclear factor kappa-b (NF-κB) activity in a xenograft model of lung cancer.4 In addition, sPLA2IIa inhibition decreases intercellular adhesion molecule-1–associated invasion in vitro.5
Although cytosolic PLA2 activity is increased in K-ras mutant lung tumors,6–8 the relationship between sPLA2IIa and K-ras mutant lung cancer has not previously been explored. The aim of this study is to examine the potential of targeted sPLA2IIa inhibition in K-ras mutant lung cancer. Our purpose was twofold: (1) to investigate the role of sPLA2IIa in the growth of K-ras mutant lung cancer cells and (2) to examine sPLA2IIa expression in human tumor specimens and correlate the results with K-ras mutation status. We hypothesized that sPLA2IIa modulates lung cancer cell growth in K-ras mutant cells and that tumor expression of sPLA2IIa would correlate with K-ras status and cancer stage.
METHODS
Cells and Reagents
All cell lines tested were K-ras mutant derived from non–small cell lung cancers (Table 1). A549 cells were a gift from Dr Anirban Banerjee (Aurora, Colo). H358 cells were a gift from Dr Kim O’Neill (Provo, Utah). SW1573 and H2009 were a gift from Dr Mary Reyland (Aurora, Colo). Cells were maintained in a humidified atmosphere with 5% carbon dioxide using Ham’s F-12 (A549) or RPMI media (all other cell lines) with 10% fetal bovine serum (FBS). Tumor necrosis factor-alpha (TNF-α) (Sigma-Aldrich, St Louis, Mo) was used at a concentration of 20 ng/mL to mimic the inflammatory milieu of the tumor microenvironment. The sPLA2IIa inhibitor S3319 (Sigma Aldrich) was dissolved in dimethyl sulfoxide. Di-methyl sulfoxide served as a vehicle control for all experiments with a maximum experimental concentration of 0.08%. Rabbit anti-human antibodies for phosphorylated and total NF-κB p65, extracellular signal-regulated kinase (ERK) 1/2, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were obtained from Cell Signaling Technology, Inc (Beverly, Mass). For Western blot, the rabbit anti-human–specific sPLA2IIa antibody was from Abcam (Cambridge, Mass). For immunofluorescence, the rabbit anti-human sPLA2IIa antibody was obtained from BioVendor, LLC (Candler, NC).
TABLE 1.
Cell line characteristics
| Cell line | Tumor type | EGFR | K-ras | p53 | NF-kB with sPLA2 inhibition | ERK 1/2 with sPLA2 inhibition | sPLA2 |
|---|---|---|---|---|---|---|---|
| SW1573 | BAC | WT | Mut | WT | Strong decrease | Strong increase | More |
| H2009 | Adenoca | WT | Mut | Mut | Decrease | Strong increase | More |
| H358 | BAC | WT | Mut | Mut | Decrease | Strong increase | Less |
| A549 | Adenoca | WT | Mut | WT | Strong decrease | Decrease | Less |
Strong attenuation of NF-κB activity was correlated with WT p53. EGFR, Epidermal growth factor receptor; NF-κb, nuclear factor kappa-b; sPLA2, secretory phospholipase A2; ERK, extracellular signal-regulated kinase; BAC, bronchoalveolar carcinoma; WT, wild-type; Mut, mutant; Adenoca, adenocarcinoma.
Immunofluorescence Microscopy
We cultured 5000 cells per chamber on Lab-Tek II 8-well glass chamber slides (Thermo Scientific, Rochester, NY) in full media for 24 hours and then serum-starved them (0.5% FBS) for 24 hours. Cells were pretreated with incrementally increasing concentrations of the sPLA2 inhibitor in serum-starved media for 1 hour, then stimulated with 20 ng/mL TNF-α for 8 hours. Control cells were incubated with dimethyl sulfoxide in serum-starved media and stimulated with TNF-α for 8 hours. Immunofluorescence microscopy was performed to evaluate sPLA2IIa expression in A549 cells as previously described.9
Apoptosis Assay
We cultured 1 × 106 cells per well in 12-well plates in full media for 24 hours and then serum-starved them (0.5% FBS) for 24 hours before treatment. Cells were treated with sPLA2IIa inhibitor (20 or 40 μmol/L) or vehicle control in serum-starved media for 8 hours. A time-response experiment using 4- and 8-hour time points was used to determine the 8-hour time point chosen for this experiment (data not shown). Apoptosis was assessed by flow cytometric detection of annexin V and propidium iodide (BD Biosciences, San Jose, Calif).
Cell Viability Assay
We cultured 5 × 103 cells per well in 96-well plates in full media for 24 hours and then serum-starved them (0.5% FBS) for 24 hours before treatment. Cells were exposed to sPLA2IIa inhibitor (10–40 μmol/L) or vehicle control in serum-starved media for 24 hours. A time-response experiment using 12-, 24-, and 48-hour time points was used to determine the 24-hour time point chosen for this experiment (data not shown). Cell viability was measured with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to manufacturer instructions (Roche Applied Science, Indianapolis, Ind).
Western Blot
Cells were plated on 6-well plates at a density of 3 × 106 in full media for 24 hours, then serum-starved (0.5% FBS) for 24 hours, and then treated. Time trials with TNF-α were performed to determine the time of maximal initial increase in phosphorylation of NF-κB and ERK 1/2 in all cell lines. Cells were pretreated with sPLA2IIa inhibitor (10–40 μmol/L) for 1 hour, stimulated with TNF-α, then lysed with Laemmli buffer under reducing conditions. In separate experiments, untreated cells were lysed and analyzed for baseline sPLA2IIa expression. Proteins were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis techniques and transferred to a nitrocellulose membrane. Membranes were blocked in 5% nonfat milk in phosphate-buffered saline–Tween. Primary antibodies were dissolved in 5% bovine serum albumin in phosphate-buffered saline–Tween. Secondary antibodies were prepared in 5% nonfat milk in phosphate-buffered saline–Tween. The protein of interest was developed using Pierce ECL Chemiluminescent (Thermo Fisher Scientific, Inc, Rockford, Ill). Densitometric analysis was performed using ImageJ Software (National Institutes of Health, Bethesda, Md).
Quantitative Reverse-Transcription Polymerase Chain Reaction (RT-PCR)
Cells were plated on 12-well plates at a density of 1 × 106 in full media for 24 hours and serum-starved (0.5% FBS) for 24 hours before lysis and RNA isolation. RNA was prepared using the Qiagen RNeasy Plus Mini Kit according to the manufacturer’s protocol (Germantown, Md). Complementary DNA was synthesized using Bio-Rad iScript (Hercules, Calif). RT-PCR was performed using Applied Biosystems Sybr Green Master Mix (Carlsbad, Calif). The primer sequences used are as follows: sPLA2IIa (forward): CCC-TCC-TAC-TGT-TGG-CAG-TGA-T; sPLA2IIa (reverse): CCT-GTC-GTC-AAC-TTG-ATC-ATT-CTG; GAPDH (forward): CCT-GCA-CCA-CCA-ACT-GCT-TAG; GAPDH (reverse): TGA-GTC-CTT-CCA-CGA-TAC-CAA. Denaturation (95°C, 15 seconds), annealing (52°C, 30 seconds), and elongation (72°C, 30 seconds) steps were repeated for 40 cycles, flanked by a 10-minute denaturation and 5-minute elongation step. Data were analyzed by the ΔΔCt method.10
Lung Tumor Tissue Collection
Human lung tissue and corresponding clinical data were collected under a tissue banking protocol approved by the University of Colorado Institutional Review Board. Informed consent was obtained from each participant. The stage reported is the pathologic stage after surgical resection according to American Joint Committee on Cancer guidelines. Tumor samples and normal-appearing tissue from resected lobes were collected in RNALater (Qiagen). Complementary DNA and subsequent quantitative RT-PCR were prepared as described above.
Statistical Analysis
All data distributions were inspected before statistical analysis. The Student t test was performed for 2-group comparisons, and the analysis of variance test was performed when comparing 3 or more groups. No multiple comparison was conducted. StatView version 5.0 was used for all statistical analysis (SAS Institute, Inc, Cary, NC).
RESULTS
sPLA2IIa Expressed in K-ras Mutant Lung Cancer Cells
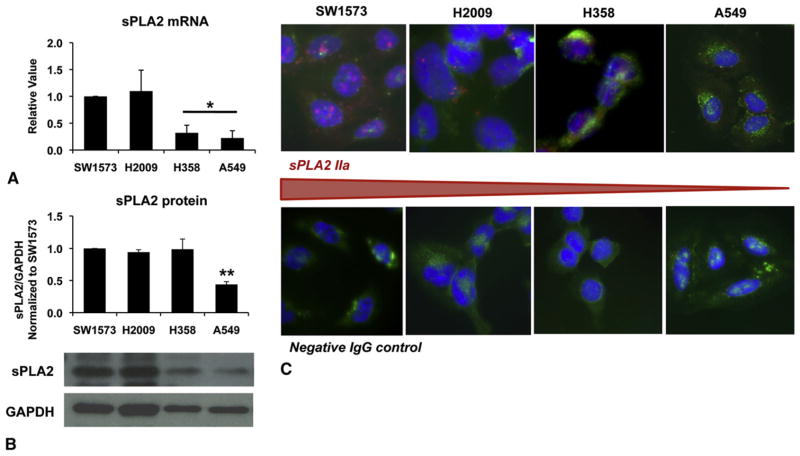
Group IIa sPLA2 expression varies in K-ras mutant lung cancer cells. SW1573 and H2009 express more sPLA2IIa messenger RNA (mRNA) compared with H358 and A549 cells (Figure 1, A). Protein analysis by Western blot and immunofluorescence corroborate this finding (Figure 1, B and C).
FIGURE 1.
K-ras mutant lung cancer cell lines analyzed for baseline group IIa secretory phospholipase A2 (sPLA2IIa) expression. Expression varies with SW1573 and H2009 cells expressing more sPLA2IIa compared with H358 and A549 cells. Messenger RNA (mRNA) was evaluated by quantitative reverse-transcription polymerase chain reaction (RT-PCR), mean ± SEM, *P<.05, n =5, run in triplicate (A). sPLA2IIa protein expression was evaluated by Western blot. Graph of densitometry, mean ± SEM, **P <.005, n =4 (B). Immunofluorescence for sPLA2IIa was used for additional protein, n =3 run in duplicate, sPLA2IIa =red, cell membrane =green, nuclei =blue (C). IgG, Immunoglobulin G; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Cell Survival in K-ras Mutant Lung Cancer Cells Depends on sPLA2IIa Expression
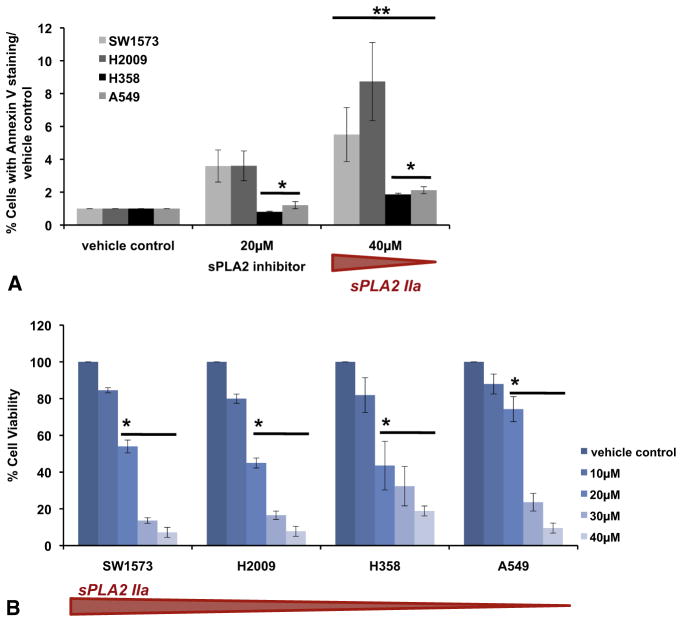
sPLA2IIa inhibition led to concentration-dependent apoptosis and cell death in all K-ras mutant lung cancer cells (Figure 2). Cells with higher baseline sPLA2IIa were more susceptible to cell death with sPLA2IIa inhibition. Early apoptosis detected by annexin V staining after 8 hours of sPLA2IIa inhibition was increased in SW1573 and H2009 cells compared with H358 and A549 cells (Figure 2, A). The MTT assay confirmed concentration- dependent cell death after 24 hours of sPLA2IIa inhibition, again with increased vulnerability in cells with higher sPLA2IIa expression (Figure 2, B).
FIGURE 2.
Group IIa secretory phospholipase A2 (sPLA2IIa) expression inhibition promotes concentration-dependent apoptosis and cell death in lung cancer cell lines, and cytotoxicity of sPLA2IIa inhibition parallels the level of sPLA2IIa expression. SW1573 and H2009 cells experience more apoptosis at 20 and 40 μmol/L compared with H358 and A549 cells. Percent of cells with annexin V staining were normalized to staining of the respective vehicle control, mean ± SEM, *P<.03 compared with SW1573, H2009. **P<.01 compared with vehicle control, n =3–4 (A). The 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide (MTT) assay was used to assay cell viability after sPLA2IIa inhibition. LD50 at 24 hours increases as sPLA2IIa expression decreases, mean ± SEM, *P<.003, n =3 run in triplicate (B).
sPLA2IIa Inhibition in K-ras Mutants Decreases NF-κB Phosphorylation
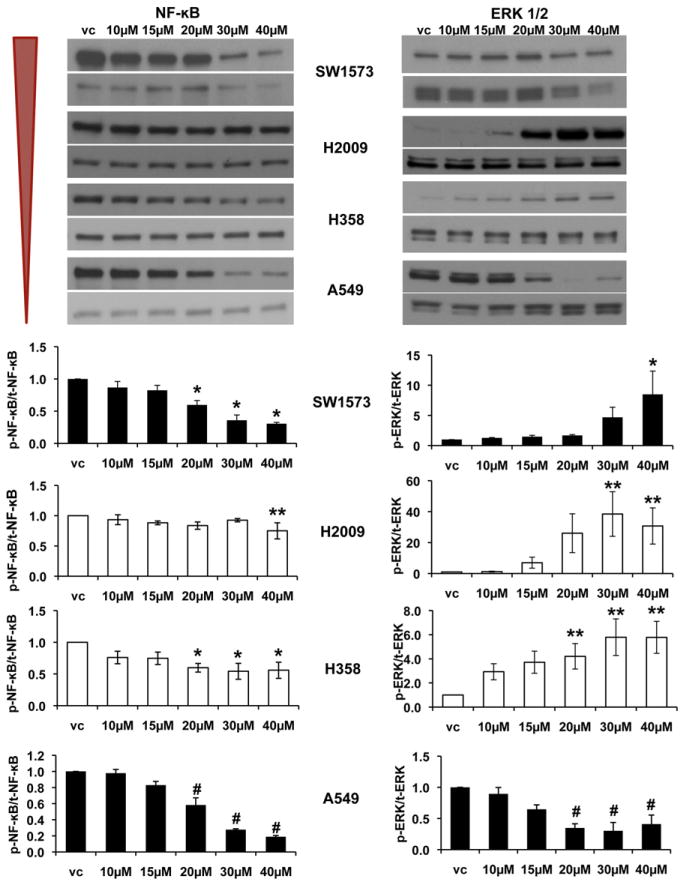
Previous work has demonstrated that sPLA2IIa inhibition–associated apoptosis is related predominantly to decreased NF-κB activity.4 We confirmed that NF-κB phosphorylation is decreased with sPLA2IIa inhibition in all K-ras mutant cells, whereas ERK 1/2 phosphorylation increases in 3 cell lines but decreases in A549 cells (Figure 3). H2009 and H358 cells have a more modest decrease in NF-κB compared with SW1573 and A549 cells. H2009 and H358 cells are both p53 mutant.
FIGURE 3.
Group IIa secretory phospholipase A2 (sPLA2IIa) inhibition attenuates nuclear factor kappa-b (NF-κB) activity in all cell lines, with a variable effect on extracellular signal-regulated kinase (ERK) 1/2 activity. NF-κB attenuation does not correlate with sPLA2IIa expression level. Both H2009 and H358 (white bar graphs) are mutant p53 and demonstrate a more modest decrease in NF-κB activity (vc, vehicle control). Representative Western blots with corresponding densitometry, mean ± SEM, *P<.05, **P<.01, #P<.001, n =3–5.
Human Lung Tumors Express sPLA2IIa
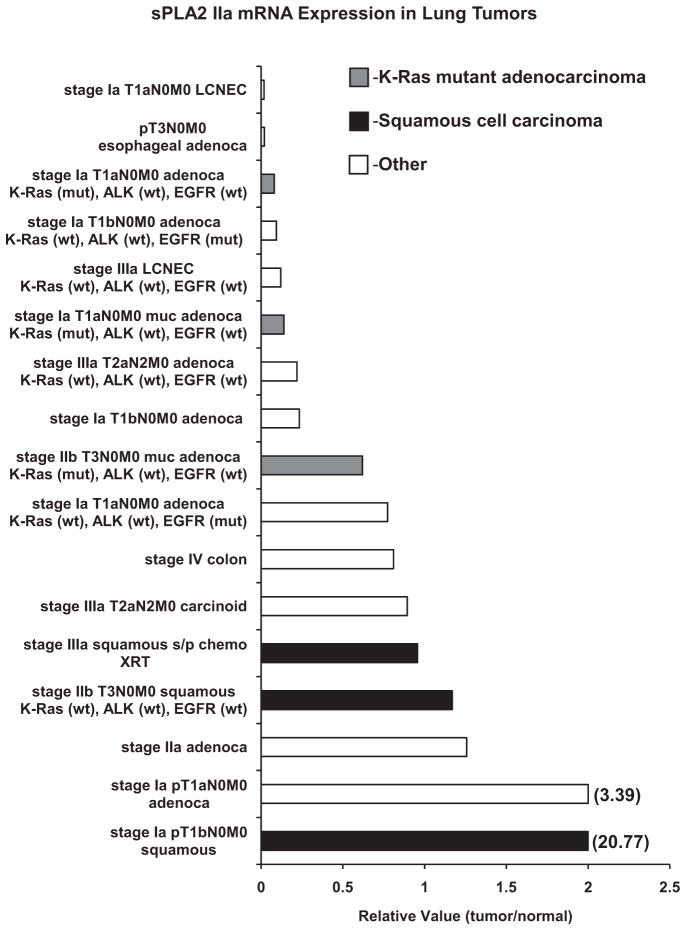
Fifteen lung tumors were examined for sPLA2IIa mRNA expression. One metastatic colon adenocarcinoma and 1 invasive esophageal adenocarcinoma were included in analysis for comparison. Lung tumors express detectable levels of sPLA2IIa mRNA (Figure 4). Although squamous and K-ras mutant tumors are less common in our cohort, there is a trend toward increased expression of sPLA2IIa in squamous tumors. In K-ras mutant tumors there is evidence of increased sPLA2IIa expression with increasing stage. Both large cell neuroendocrine tumors express low levels of sPLA2IIa.
FIGURE 4.
Human lung tumors express group IIa secretory phospholipase A2 (sPLA2IIa). Primary lung tumors (n =15), invasive esophageal adenocarcinoma (n =1), lung metastasis from colon adenocarcinoma primary (n =1). K-ras mutant adenocarcinomas (gray) express increasing levels of sPLA2IIa with increasing stage. Squamous cell carcinomas (black) express higher levels of sPLA2IIa. Samples that did not include K-ras, ALK, or epidermal growth factor receptor (EGFR) mutant status indicate patients that did not consent to mutation analysis. Messenger RNA evaluated by quantitative reverse-transcription polymerase chain reaction (RT-PCR), samples run in triplicate. LCNEC, Large cell neuroendocrine carcinoma; mut, mutant; wt, wild-type; s/p, status post; XRT, radiation therapy.
DISCUSSION
This is the first study to specifically explore the role of sPLA2IIa in K-ras mutant lung cancer. Consistent with our hypothesis, sPLA2IIa is a cytotoxic target in K-ras mutant lung cancer cells. Our finding that in vitro sensitivity to sPLA2IIa inhibition correlates with baseline sPLA2IIa expression, regardless of p53 mutation status, suggests that sPLA2IIa expression may predict response to sPLA2IIa inhibition. Analysis of sPLA2IIa expression in human lung tumors revealed some trends that we did not anticipate. Expression increases with cancer stage in K-ras mutant tumors, but it does not necessarily increase with stage of non–small cell lung cancers overall. In addition, we found a trend of relatively high expression in squamous cell tumors, another subset of lung tumors that presently lacks targeted therapy. These findings are intriguing. Not only do they suggest that sPLA2IIa may be a more sensitive biomarker for a subset of tumors, but small molecule inhibitors to sPLA2IIa have already been trialed for inflammatory disease,11 thus underscoring the possibility for rapid translational study in lung cancer.
Although sPLA2 inhibition significantly attenuated NF-κB activity in all lung cancer cell lines tested, cells with mutant p53 had a less robust decrease in NF-κB activity. Other investigators have demonstrated a relationship between mutant p53 and increased baseline NF-κB activity.12,13 Our data corroborate this observation and further advance our understanding of the relationship between sPLA2IIa and NF- κB by demonstrating that sPLA2IIa inhibition has a more modest effect on the activity of NF-κB in cancer cells where baseline activity is high. Additional studies are needed to determine whether this observation has implications for the effect of sPLA2IIa inhibition on downstream NF-κB targets such as metastasis-associated adhesion molecules and expression of inducible growth factors.5,14–17
An interesting and unexpected finding from our tumor analysis was that sPLA2IIa expression was increased in squamous tumors. Kupert and associates3 recently published strong sPLA2IIa protein staining in non–small cell lung tumor cores compared with normal lung tissue, including 100% of squamous cell tumor cores, although they did not quantify differences between histologic subtypes.3 This trend requires focused investigation of more squamous tumors inasmuch as there are currently no targeted therapies for this subset of non–small cell lung cancers. Furthermore, there is potential to examine the relationship between specific genetic alterations in squamous tumors and the tumor promoting activity of sPLA2IIa18.
The pathways most frequently implicated in phospholipase-related tumor growth are predominantly inflammatory, including NF-κB and the arachidonic acid–eicosanoid pathway.14,19,20 Mechanistic investigation of how sPLA2IIa effects production of tumor-promoting eicosanoids, including its interactions with other phospholipase enzymes, is ongoing. In addition, sPLA2IIa is a secreted molecule with potential autocrine and paracrine signaling capability.21 Ongoing studies aim to clarify the effect of sPLA2IIa inhibition on sPLA2IIa production and secretion with implications for understanding the role of sPLA2IIa in the tumor microenvironment to promote tumor progression and metastasis.
A limitation of this study is the use of a single small molecule inhibitor. We have previously presented comparable results with RNA interference of sPLA2IIa, resulting in decreased tumor growth in an animal model.4 In addition, our tumor tissue analysis was limited owing to small sample size. Furthermore, we were only able to analyze mRNA secondary to the limited amount of tissue available to us. The analysis of RNA and protein in the lung cancer cell lines mostly demonstrates a parallel relationship between sPLA2IIa mRNA transcript and sPLA2IIa protein expression. Future studies will need to include an analysis of protein expression of sPLA2IIa in tumors.
In summary, we have outlined the potential of sPLA2IIa as a global target for lung cancer therapy—sPLA2 inhibition is cytotoxic and potency correlates with baseline sPLA2 expression. Examination of human lung tumors suggests sPLA2IIa has particular relevance for K-ras mutant and squamous cell tumors, 2 lung cancer subsets that presently pose therapeutic challenges. These data contribute to growing evidence of a role for sPLA2IIa in lung cancer that deserves further translational investigation.
Abbreviations and Acronyms
- EGFR
epidermal growth factor receptor
- ERK
extracellular signal-regulated kinase
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- mRNA
messenger RNA
- MTT
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
- NF-κB
nuclear factor kappa-b
- PLA2
phospholipase A2
- RT-PCR
reverse-transcription polymerase chain reaction
- sPLA2IIa
group IIa secretory phospholipase A2
- TNF-α
tumor necrosis factor-alpha
Footnotes
Read at the 38th Annual Meeting of The Western Thoracic Surgical Association, Maui, Hawaii, June 27-30, 2012.
Disclosures: Authors have nothing to disclose with regard to commercial support.
References
- 1.Marks JL, Broderick S, Zhou Q, Chitale D, Li AR, Zakowski MF, et al. Prognostic and therapeutic implications of EGFR and KRAS mutations in resected lung adenocarcinoma. J Thorac Oncol. 2008;3:111–6. doi: 10.1097/JTO.0b013e318160c607. [DOI] [PubMed] [Google Scholar]
- 2.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6:201–5. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 3.Kupert E, Anderson M, Liu Y, Succop P, Levin L, Wang J, et al. Plasma secretory phospholipase A2-IIa as a potential biomarker for lung cancer patients with solitary pulmonary nodules. BMC Cancer. 2011;11:513–23. doi: 10.1186/1471-2407-11-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu JA, MD, Mauchley D, Li H, Meng X, Nemenoff RA, Fullerton DA, Weyant MJ. Knockdown of secretory phospholipase A2 IIa reduces lung cancer growth in vitro and in vivo. J Thorac Cardiovasc Surg. 2012;144:1185–92. doi: 10.1016/j.jtcvs.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu JA, Sadaria MR, Meng X, Mitra A, Ao L, Fullerton DA, et al. Lung cancer cell invasion and expression of intercellular adhesion molecule-1 (ICAM-1) are attenuated by secretory phospholipase A2 inhibition. J Thorac Cardiovasc Surg. 2012;143:405–11. doi: 10.1016/j.jtcvs.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Downward J. Targeting RAS signaling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 7.Heasley LE, Thaler S, Nicks M, Price B, Skorecki K, Nemenoff RA. Induction of cytosolic phospholipase A2 by oncogenic Ras in human non–small cell lung cancer. J Biol Chem. 1997;279:14501–4. doi: 10.1074/jbc.272.23.14501. [DOI] [PubMed] [Google Scholar]
- 8.Wang XQ, Li H, Van Putten V, Winn RA, Heasley LE, Nemenoff RA. Oncogenic K-Ras regulates proliferation and cell junctions in lung epithelial cells through induction of cyclooxygenase-2 and activation of metalloproteinase-9. Mol Biol Cell. 2009;20:791–800. doi: 10.1091/mbc.E08-07-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mauchley D, Meng X, Johnson T, Fullerton DA, Weyant M. Modulation of growth in human esophageal adenocarcinoma cells by group IIa secretory phospholipase A2. J Thorac Cardiovasc Surg. 2010;139:591–9. doi: 10.1016/j.jtcvs.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 10.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 11.Bradley JD, Dmitrienko AA, Kivitz AJ, Gluck OS, Weaver AL, Wisenhutter C, et al. A randomized, double-blinded, placebo-controlled clinical trial of LY333013, a selective inhibitor of group II secretory phospholipase A2, in the treatment of rheumatoid arthritis. J Rheumatol. 2005;32:417–23. [PubMed] [Google Scholar]
- 12.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–14. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement of NF-κB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–7. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadaria MR, Meng X, Fullerton DA, Reece TB, Shah RR, Grover FL, et al. Secretory phospholipase A2 inhibition attenuates intercellular adhesion molecule-1 expression in human esophageal adenocarcinoma cells. Ann Thorac Surg. 2011;91:1539–45. doi: 10.1016/j.athoracsur.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 15.Blum R, Jacob-Hirsch J, Amariglio N, Rechavi G, Kloog Y. Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1α, causing glycolysis shutdown and cell death. Cancer Res. 2005;65:999–1006. [PubMed] [Google Scholar]
- 16.Van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1αby NF-κB. Biochem J. 2008;412:477–84. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Granata F, Frattini A, Loffredo S, Staiano RI, Petraroli A, Ribatti D, et al. Production of vascular endothelial growth factors from human lung macrophages induced by group IIA and group X secreted phospholipases A2. J Immunol. 2010;184:5232–41. doi: 10.4049/jimmunol.0902501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discov. 2011;1:78–89. doi: 10.1158/2159-8274.CD-11-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Z, Liu Y, Scott KF, Levin L, Gaitonade K, Bracken RB, et al. Secretory phospholipase A2-IIa is involved in prostate cancer progression and may potentially serve as a biomarker for prostate cancer. Carcinogenesis. 2010;31:1948–55. doi: 10.1093/carcin/bgq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krysan K, Reckamp KL, Dalwadi H, Sharma S, Rozengurt E, Dohaldwala M, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non–small cell lung cancer cells in an epidermal growth factor receptor–independent manner. Cancer Res. 2005;65:6275–81. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 21.Hernández M, Martín R, García-Cubillas MD, Maeso-Hernández P, Nieto ML. Secreted PLA2 induces proliferation in astrocytoma through the EGF receptor: another inflammation-cancer link. Neuro Oncol. 2010;12:1014–23. doi: 10.1093/neuonc/noq078. [DOI] [PMC free article] [PubMed] [Google Scholar]