Abstract
Background
Hypofractionated radiotherapy (hRT) is being explored for a number of malignancies. The potential benefit of giving concurrent chemotherapy with hRT is not known. We seek to predict the effects of combined modality treatment using mathematical models derived from laboratory data.
Methods
Data from 26 published clonogenic survival assays for cancer cell lines without and with the use of radiosensitizing chemotherapy were collected.
The first three data points of the radiotherapy (RT) arm of each assay were used to derive parameters for the Linear Quadratic (LQ) Model, the Multitarget (MT) Model, and the Generalized Linear Quadratic (gLQ) Model. For each assay and model, the difference between the predicted and observed surviving fraction at the highest tested RT dose was calculated.
The gLQ model was fit to all of the data from each RT cell survival assay, and the biologically equivalent doses in 2-Gy fractions (EQD2s) of clinically-relevant hRT regimens were calculated. The increase in cell kill conferred by the addition of chemotherapy was used to estimate the EQD2 of hRT along with a radiosensitizing agent. For comparison, this was repeated using conventionally-fractionated RT regimens.
Results
At a mean RT dose of 8.0 Gy, the average errors for the LQ, MT, and gLQ models were 1.63, 0.83, and 0.56 log units, respectively, favoring the gLQ model (p<0.05). Radiosensitizing chemotherapy increased the EQD2 of hRT schedules by an average of 28% to 82%, depending on disease site. This was similar to the gains predicted for the addition of chemotherapy to conventionally-fractionated RT.
Conclusions
Based on published in vitro assays, the gLQ equation is superior to the LQ and MT models in predicting cell kill at high doses of RT. Modeling exercises demonstrate that significant increases in biologically equivalent dose may be achieved with the addition of radiosensitizing agents to hRT. Clinical study of this approach is warranted.
Keywords: Radiosensitization, Hypofractionated, Radiosurgery, Generalized Linear Quadratic, Linear Quadratic
Introduction
The majority of patients receiving radiotherapy (RT) are treated with small, daily fractions over a number of weeks. In general, this approach maximizes tumor cell kill while limiting normal tissue toxicity. In recent years, technological advances in target definition, treatment planning, and setup verification have led to increased interest in the use hypofractionated radiotherapy (hRT) for a number of malignancies. For some diseases, RT delivered in one or a few fractions has already shown promise as being safe and effective.(1–4) In other situations where adequate clinical experience is lacking, clinicians often attempt to predict the likelihood of tumor control and normal tissue complications with a novel RT regimen by calculating its biologically effective dose (BED) or biologically equivalent dose in 2-Gy fractions (EQD2).
In practice, calculation of BED or EQD2 is most commonly performed using the linear quadratic (LQ) model.(5) This formula was initially derived to fit experimental observations of the effects of RT dose on cell survival in vitro. There is concern, however, that the LQ model is ill-suited to predict cell survival after high doses of RT.(6) The ability of the LQ model to predict clinical outcomes with hRT schedules has also been questioned.(7)
The addition of radiosensitizing chemotherapy to conventionally-fractionated RT has been shown to improve local control and overall survival for a number of disease sites.(8–14) In diseases where outcomes remain poor, further therapeutic gains may be possible through the combination of hRT and radiosensitizing agents. This will be an active area of study in coming years.
In this study we use published clonogenic assay data to compare the performance of several cell survival models at high doses of RT. We then utilize data from in vitro studies of radiosensitizing agents to quantify the potential gains in EQD2 that may be made through the combination of systemic therapy and hRT.
Methods
Evaluating Cell Survival Models
A literature search for publications containing clonogenic cell survival assay data for glioblastoma (GBM), head and neck cancer (HNC), pancreatic cancer, or non-small cell lung cancer (NSCLC) cell lines with and without the use of a clinically-used radiosensitizing agent was performed. Survival curves with four or more data points were included for this analysis.
Based on statistical considerations (see below), data from 26 experiments was collected. For papers in which numerical data were not provided, a customized script in MATLAB (Mathworks, Natick, MA) was used to extract data from digitized graphical survival curves.
The first portion of this study tested the accuracy of various cell survival models at high RT doses. The three models that were assessed were the LQ model(5), the multi-target (MT) model(6), and the generalized linear-quadratic (gLQ) model for constant dose-rate irradiation.(15) For this portion of the study, RT only data (no chemotherapy) was utilized.
Linear Quadratic Model:
Multi-target Model:
-
Generalized Linear Quadratic Model: , where
G = 2 [εT−1+e−εT]/ε2T2,
ε = μ + β2I0,
μ = ln(2)/Tr,
β2 = sqrt(β), and
I0 = d/T
S is surviving fraction; d is radiation dose in Gy. For model (3), values of 20 minutes for treatment time(T) and 24 minutes(15–21) for repair half-time(Tr) were used.
For each data set and model, least-squares optimization was performed on a log-linear plot of surviving fraction v. dose, using only the first three data points of each cell survival curve (RT only). Values for the parameters (α, β) for models (1) and (3) and (D0) for model (2) were restricted to be ≥ 0. In model (2), n was restricted to be ≥ 1.
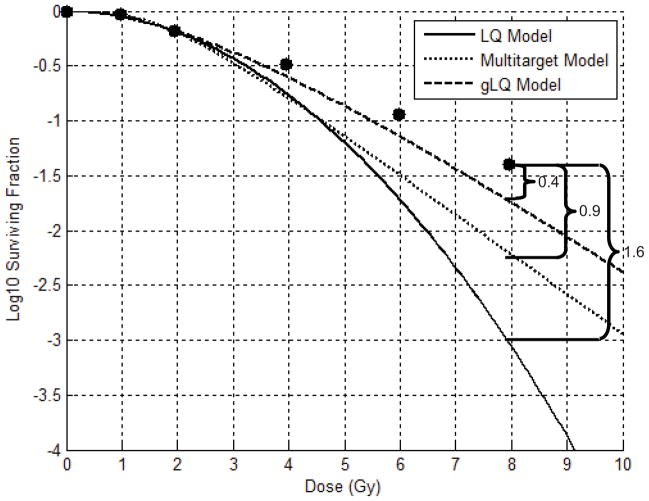
For each survival curve and model, the difference (in log units) between the predicted surviving fraction and the actual surviving fraction at the highest tested dose was recorded as the model error. (Figure 1) Assuming a standard deviation for model error of 0.75 log units, it was calculated that 26 survival assays should be utilized to provide a 90% probability of detecting a difference in mean error between models of 1 log unit.
Figure 1.
Example (data from van Bree, et al [46]) of Determining Model Errors: Each cell survival model is fit to only the first three data points from a clonogenic cell assay (radiotherapy only). At the highest tested dose (8 Gy), the Linear Quadratic (LQ), Multi-target, and Generalized Linear Quadratic (gLQ) models overestimate cell kill by 1.6, 0.9, and 0.4 log units, respectively.
To test for systematic error in each model, unpaired Student’s t-test was used to evaluate the hypothesis that the mean model error was equal to 0. To test the models against one another, paired Student’s t-test was utilized to compare the absolute value of the model errors.
Estimating EQD2 of hRT without and with drug
In the second portion of this study, we quantified the potential benefit of the addition of established radiosensitizing systemic agents to hRT. For each disease site, we evaluated a conventionally-fractionated radiotherapy regimen as well as one hRT regimen that has been utilized in published clinical reports. The selected RT schedules are listed in Table 3.
Table 3.
Biologically equivalent doses of conventional and hypofractionated radiotherapy regimens, with and without the addition of chemotherapy
| Disease | RT Schedule [reference] | RT: Mean EQD2 (95% CI) | RT+Chemo: Mean EQD2 (95% CI) | Mean EQD2 increase | p |
|---|---|---|---|---|---|
| GBM | 2.0 Gy x 30 fx = 60 Gy(8) | 60.0 Gy (NA) | 121.3 Gy (97.9 to 144.7 Gy) | 102% | 0.002 |
| GBM | 6.0 Gy x 5 fx = 30 Gy(51) | 51.6 Gy (40.6 to 62.6 Gy) | 77.8 Gy (72.3 to 83.3 Gy) | 39% | 0.015 |
| HNC | 2.0 Gy x 35 fx = 70 Gy(52) | 70.0 Gy (NA) | 105.2 Gy (81.3 to 129.1 Gy) | 50% | 0.034 |
| HNC | 5.0 Gy x 6 fx = 30 Gy(53, 54) | 38.4 Gy (32.3 to 44.6 Gy) | 49.7 Gy (39.5 to 59.9 Gy) | 28% | 0.045 |
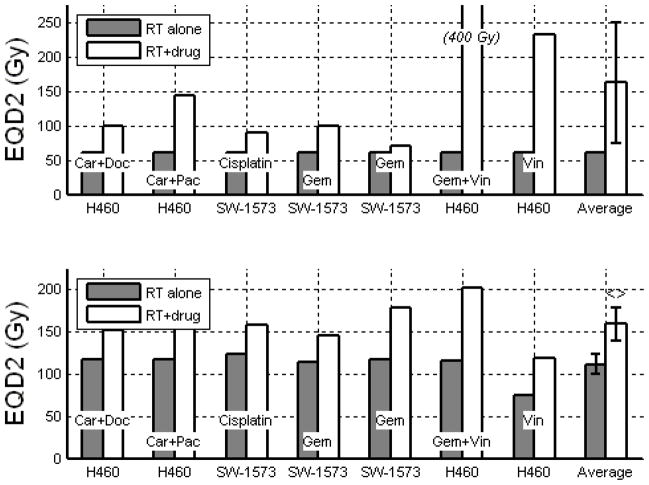
| NSCLC | 2.0 Gy x 30 fx = 60 Gy(10) | 60.0 Gy (NA) | 161.6 Gy (74.1 to 249.2 Gy) | 169% | 0.063 |
| NSCLC | 10.0 Gy x 5 fx = 50 Gy(55, 56) | 111.5 Gy (99.4 to 123.5 Gy) | 159.3 Gy (139.9 to 178.6 Gy) | 42% | 0.001 |
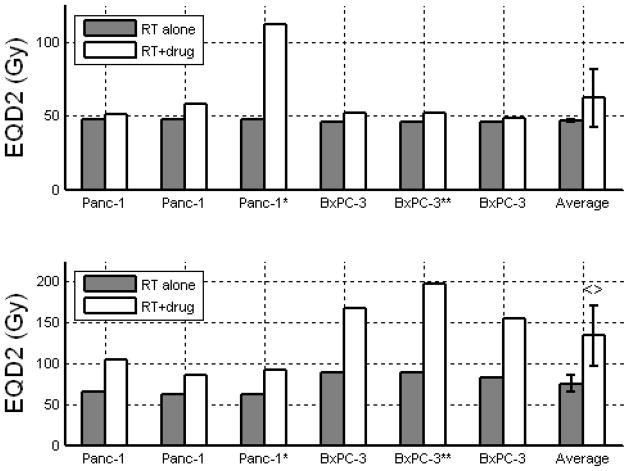
| Pancreas | 1.8 Gy x 28 fx = 50.4 Gy(57) | 46.8 Gy (46.2 to 47.4 Gy) | 62.4 Gy (42.8 Gy to 82.1 Gy) | 34% | 0.175 |
| Pancreas | 10.0 Gy x 3 fx = 30 Gy(58) | 75.2 Gy (64.6 to 85.7 Gy) | 133.8 Gy (97.1 to 170.5 Gy) | 82% | 0.007 |
Modeled radiotherapy (RT) fractionation schedules. (fx = fraction, EQD2 = biologically equivalent dose of RT given in 2 Gy fractions, NA = not applicable)
Based on the findings from the first portion of this study (see below), we utilized the gLQ model for the remainder of this analysis. For each survival curve (RT alone, all data points), least-squares optimization was performed on a log-linear plot of surviving fraction v. dose to determine values of α and β. Again, α and β were restricted to be ≥ 0.
For each data set and RT regimen, EQD2 was determined as follows:
S2 is surviving fraction after 2 Gy of irradiation; EQD2 is the biologically equivalent dose of RT given in 2 Gy fractions. Note that for RT schedules with a fraction size of 2 Gy, EQD2 will simply be equal to the prescription dose, D.
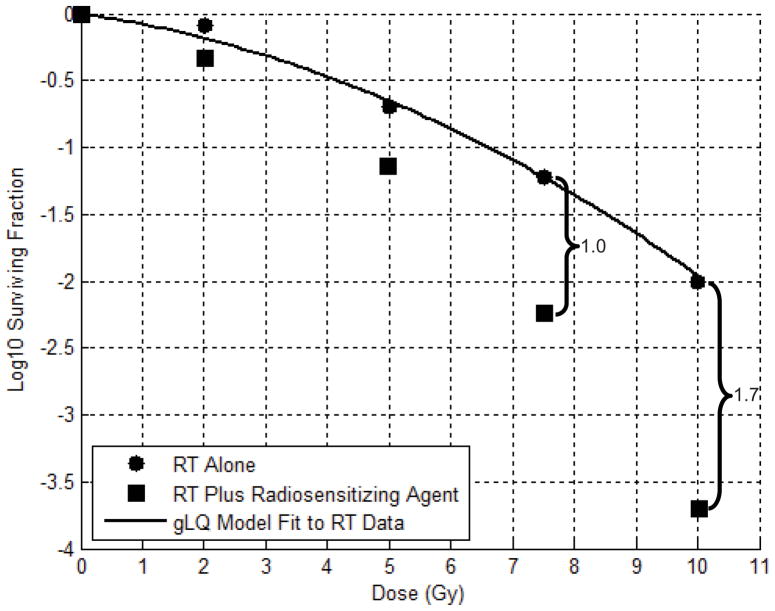
To determine the potential effects of adding radiosensitizing agents to RT, we first estimated the dose enhancement factor (DEF), defined as the increase in log cell kill, for each survival curve at each proposed fraction size. As DEF tends to increase with fraction size, a conservative estimate was made for each proposed fractionation scheme by using the DEF calculated using the surviving fraction values at the closest lower tested RT fraction size. (See Figure 2) Biologically equivalent dose for each data set and proposed chemoradiotherapy regimen was then calculated as follows:
Figure 2.
Example of determining biologically equivalent dose (EQD2) and dose enhancement factor (DEF): After fitting the Generalized Linear Quadratic (gLQ) model to survival assay data, estimates of log cell kill at 2 Gy and 10 Gy are 0.2 and 2.0, respectively. The EQD2 of a single fraction of 10 Gy is therefore 20 Gy. To determine EQD2 for chemoradiation, the DEF is defined as the increase in log cell kill at the closest lower tested radiotherapy (RT) dose. For example, a DEF of 1.0 would be used for fraction sizes between 7.5 and 10 Gy. For fraction sizes of 10 Gy or higher, a DEF of 1.7 is used. The EQD2 of a single fraction of 10 Gy with a radiosensitizing agent in this example is 37 Gy.
D is the total RT dose, d is the dose per fraction, Sd is the surviving fraction after chemoradiotherapy, and EQD2 is the biologically effective dose of chemoradiotherapy in 2 Gy fractions.
Results
Details regarding the 26 survival assays used in this analysis are provided in Table 1. The RT survival curves had a mean of 5.5 data points (range 5–7). The assays tested RT doses up to an average of 8.0 Gy (range 5.0–10.0 Gy). Least squares optimization was performed on all available data points from the RT alone (no radiosensitizing agent) survival curves to generate the model parameters listed in Table 1.
Table 1.
Cell survival assays utilized, survival model parameters using radiotherapy-only data, and average chemotherapy dose enhancement factors.
| Reference | Disease | Cell Line | Alpha (LQ) | Beta (LQ) | Alpha (gLQ) | Beta (gLQ) | D0 (MT) | n(MT) | Drug | Mean DEF |
|---|---|---|---|---|---|---|---|---|---|---|
| Charkravarti (38) | GBM | UN10 | 0.2 | 0.00 | 0.20 | 0.00 | 4.60 | 1.0 | TMZ | 0.757 |
| Charkravarti (38) | GBM | UN11 | 0.2 | 0.00 | 0.20 | 0.00 | 4.60 | 1.0 | TMZ | 0.748 |
| Kil (39) | GBM | U251 | 0.1 | 0.08 | 0.00 | 0.26 | 1.00 | 9.6 | TMZ | 0.333 |
| van Nifterik (40) | GBM | VU-122 | 0.08 | 0.07 | 0.00 | 0.18 | 1.40 | 4.2 | TMZ | 0.198 |
| van Nifterik (40) | GBM | VU-109 | 0.1 | 0.04 | 0.04 | 0.10 | 2.00 | 2.6 | TMZ | 0.077 |
| van Nifterik (40) | GBM | AMC 3046 | 0.03 | 0.06 | 0.00 | 0.13 | 1.60 | 4.4 | TMZ | 0.200 |
| van Rijn (41) | GBM | D384 | 0.1 | 0.06 | 0.01 | 0.16 | 1.60 | 3.2 | TMZ | 0.206 |
| Pekkola-Heino (42) | HNC | UM-SCC-1 | 0.45 | 0.00 | 0.45 | 0.00 | 2.20 | 1.0 | Cis | 0.331 |
| Pekkola-Heino (42) | HNC | UM-SCC-14A | 0.3 | 0.15 | 0.00 | 0.58 | 0.70 | 6.6 | Cis | 0.535 |
| Zhang (43) | HNC | UT-SCC-29 | 0.45 | 0.01 | 0.45 | 0.02 | 1.80 | 1.2 | Cis | 0.067 |
| Zhang (43) | HNC | UT-SCC-29 | 0.45 | 0.01 | 0.45 | 0.02 | 1.80 | 1.2 | Cis+Cet | 0.043 |
| Zhang (43) | HNC | UT-SCC-24A | 0.2 | 0.04 | 0.15 | 0.10 | 1.60 | 2.6 | Cis | 0.102 |
| Zhang (43) | HNC | UT-SCC-24A | 0.2 | 0.04 | 0.15 | 0.10 | 1.60 | 2.6 | Cis+Cet | 0.127 |
| Amorino (44) | NSCLC | H460 | 0 | 0.12 | 0.00 | 0.25 | 0.90 | 12.5 | Car+Doc | 0.454 |
| Amorino (44) | NSCLC | H460 | 0 | 0.12 | 0.00 | 0.25 | 0.90 | 12.0 | Car+Pac | 0.689 |
| Bergs (45) | NSCLC | SW-1573 | 0.1 | 0.06 | 0.00 | 0.19 | 1.20 | 7.6 | Cis | 0.315 |
| van Bree (46) | NSCLC | SW-1573 | 0.25 | 0.03 | 0.06 | 0.13 | 1.60 | 3.0 | Gem | 0.291 |
| van Bree (46) | NSCLC | SW-1573 | 0.1 | 0.04 | 0.05 | 0.10 | 2.00 | 2.4 | Gem | 0.420 |
| Zhang (47) | NSCLC | H460 | 0.35 | 0.04 | 0.00 | 0.26 | 1.00 | 9.2 | Gem+ Vin | 1.353 |
| Zhang (47) | NSCLC | H460 | 0.55 | 0.03 | 0.40 | 0.12 | 1.00 | 3.6 | Vin | 1.185 |
| Lawrence (48) | Pancreas | Panc-1 | 0.3 | 0.02 | 0.08 | 0.11 | 1.60 | 3.4 | Gem | 0.647 |
| Morgan (49) | Pancreas | Panc-1 | 0.4 | 0.04 | 0.08 | 0.23 | 1.00 | 7.4 | Gem | 0.353 |
| Morgan (49) | Pancreas | Panc-1 | 0.4 | 0.04 | 0.08 | 0.23 | 1.00 | 7.4 | Gem+Ox | 0.671 |
| Morgan (49) | Pancreas | BxPC-3 | 0.05 | 0.03 | 0.00 | 0.08 | 1.80 | 7.8 | Gem | 0.593 |
| Morgan (49) | Pancreas | BxPC-3 | 0.05 | 0.03 | 0.00 | 0.08 | 1.80 | 7.8 | Gem+Erl | 0.712 |
| Symon (50) | Pancreas | BxPC-3 | 0.15 | 0.03 | 0.00 | 0.12 | 1.60 | 5.6 | Gem | 0.668 |
LQ = linear quadratic model, gLQ = generalized linear quadratic model, MT = multi-target model, TMZ = Temozolomide, Cis = Cisplatin, Cet = Cetuxmiab, Car = Carboplatin, Doc = Docetaxel, Pac = Paclitaxel, Gem = Gemcitabine, Vin = Vinorelbine, Ox = Oxaliplatin, Erl = Erlotinib, DEF=dose enhancement factor
Determining Model Errors
Fitting the three cell survival models to the first three data points of each radiotherapy survival curve yielded mean errors at the last data point of −1.49, −0.44, and −0.18 log units for the LQ, MT, and gLQ models, respectively. (Table 2a) One sample Student’s t-test demonstrated that the mean error for the LQ model was significantly (p<0.05) lower than zero, indicating a systematic error (i.e. overestimation of cell kill by the LQ model). This was not the case for the gLQ or MT models.
Table 2a.
Mean differences between predicted (using models fit to low-dose RT data) and actual cell kill with high dose RT
| Model | Mean Error | 95% CI | p |
|---|---|---|---|
| LQ | −1.49 | −2.60 to −0.38 | 0.01 |
| MT | −0.44 | −1.00 to 0.12 | 0.12 |
| gLQ | −0.19 | −0.54 to 0.16 | 0.26 |
The mean absolute errors of the LQ, MT, and gLQ models were 1.63, 0.83, and 0.56 log units, respectively. Compared to the LQ model, both the gLQ and MT models demonstrated significantly lower absolute errors on paired Student’s t-test (p=0.01). Additionally, the absolute errors of the gLQ model were significantly lower than those of the MT model (p=0.02). The gLQ model was consequently chosen for the second portion of this study.
Estimating EQD2 of hRT without and with radiosensitizing chemotherapy
‘Mean DEF’, or the average gain in cell kill with the addition of drug to RT for each survival assay, ranged from 0.04 to 1.35 log units. Mean DEF for GBM, HNC, pancreatic cancer, and NSCLC cell lines averaged 0.36, 0.20, 0.61, and 0.67 log units, respectively. Mean DEF was significantly lower (p<0.05) for HNC cell lines than for pancreatic cancer or NSCLC on unpaired Student’s t test.
The average EQD2 values for each RT regimen and disease site, with and without radiosensitizing chemotherapy, are listed in Table 3. For conventionally-fractionated RT, the addition of chemotherapy yielded average EQD2 increases of 102%, 50%, 169%, and 34%, for GBM, HNC, NSCLC, and pancreatic cancer cell lines, respectively (mean: 89%). For the hRT regimens, corresponding EQD2 increases with the addition of chemotherapy were 39%, 28%, 42%, and 82% (mean: 48%). There was no statistically significant difference in the projected EQD2 increase using hRT compared to conventionally-fractionated RT (p = 0.345, using paired Students’ t-test). Of note, the predicted EQD2 gain when chemotherapy is added to hRT for pancreatic cancer was actually larger than that predicted for the combination of chemotherapy and conventionally-fractionated RT (mean 82% v. 34%, p<0.001 using paired Students’ t-test). This was not the case for other disease sites.
Discussion
We have demonstrated that, when fit to data from the low-dose range of clonogenic cell survival assays, the gLQ model outperforms both the LQ and MT models in predicting surviving fraction at higher radiotherapy doses. Our findings support the hypothesis, already established with a smaller sample size, (15) that the LQ model systematically overestimates cell kill at high fraction sizes. We have also shown that the effects of radiosensitizing chemotherapy seen in vitro, if reproduced in a clinical setting, would significantly increase the biologically equivalent dose delivered by commonly used hRT schema.
It has long been recognized that the LQ model is not well-suited to fit clonogenic cell survival assay data at high doses of RT.(22) In essence, the LQ equation predicts a cell survival curve that is continuously bending downward on a log-linear graph, while experimental data demonstrates a linear relationship between log cell kill and dose after an initial shoulder.(22, 23) This discrepancy has been addressed with the proposal of several novel cell survival models. These include the Lethal and Potentially Lethal (LPL) model, the Linear-Quadratic-Cubic (LQC) model, the Modified LQ (MLQ) model, the Universal Survival Curve (USC), and the gLQ model.(15, 24–28) For this exercise, we chose to compare the LQ model to the more traditional Multitarget (MT) model. The formulation of this equation, which assumes that a number of critical targets must be inactivated to kill each cell, generates a survival curve that is linear in the high dose range when plotted on a log-linear graph.
Radiotherapy works by damaging the DNA of tumor cells. One reason that the LQ model gained popularity over prior models, including the MT model, is that its parameters may be construed to reflect the formation of single- and double-strand breaks in DNA. We chose to test the gLQ model in this exercise because it maintains the form and mechanistic implications of the LQ model, with the addition of a factor to diminish the predicted cell kill at high fraction sizes. This attenuation of cell kill accounts for the fact that, at high RT fraction sizes, a large fraction of sublethal lesions are converted to lethal damage, and sublethal damage is essentially depleted.(15)
In the second portion of this study, we determined that, over a range of disease sites and hRT schedules, the addition of radiosensitizing chemotherapy would increase EQD2 by approximately 50%. This was similar to the increase in EQD2 predicted for the addition of chemotherapy to conventionally-fractionated RT schedules. As chemotherapy is commonly administered with conventionally-fractionated RT for HNC, NSCLC, pancreatic cancer, and GBM, this suggests that clinically-relevant gains may also be achieved with the addition of radiosensitizing agents to hRT.
In the case of HNC, data from randomized clinical trials has been used to estimate that the gain in local tumor control from the addition of chemotherapy to conventionally-fractionated RT equates to an increase in RT dose of 10–14%. (29, 30) This is significantly less than the 50% increase in EQD2 predicted by applying our methodology to in vitro data from HNC cell lines. Among the many potential explanations for the relatively modest gains seen in patients are the concepts that:
Tumor repopulation may accelerate during a lengthy treatment course and counteract gains in tumor control probability conferred by radiosensitizing agents.
Each fraction of a daily radiotherapy course may not be enhanced equally by intravenous chemotherapy that is administered every 3 weeks.
In vitro experiments cannot account for factors such as tumor oxygenation, therapeutic effects on stromal cells, and spatial variations of drug concentration within a tumor that likely affect tumor control.
Of note, limitations (1) and (2) may both be mitigated by a shift to a hypofractionated RT schedule. Modeling techniques to account for both accelerated repopulation and variations in radiosensitizing drug bioavailability have been described (29, 31, 32) but were beyond the scope of this analysis. Some investigators have also suggested that the effectiveness of hRT is enhanced by its effects on tumor microenvironment, particularly endothelial cells. (33–35) This is a potential mechanism by which tumor control rates with hRT may exceed those predicted based on clonogenic cell survival assays.
There are also reasons why a shift to hRT may not provide clinical benefit. Hypoxic cells are known to be relatively resistant to ionizing radiation.(6) Carlson et al have pointed out that the presence of transitory hypoxia will be more detrimental to the effectiveness of hRT than to that of conventionally-fractionated RT. (36) One solution may be to incorporate a hypoxic radiosensitizing agent, as suggested by the Stanford group. (37) Another approach might be to combine hRT with systemic anti-cancer agents that are equally active against hypoxic and normoxic cells.
An important limitation of our study is its dependence on in-vitro data and the consequent disregard of micro-environmental factors such as hypoxia. While it is important that we acknowledge these radiobiologic considerations, only careful clinical study can confirm if the combination of hRT and radiosensitizing chemotherapy will provide gains in tumor control.
The gLQ equation is superior to traditional cell survival models in predicting in vitro cell kill at high doses of RT. Mathematical modeling suggests that the addition of radiosensitizing agents to hRT may lead to clinically-significant gains in tumor control. These hypothesis-generating findings support the careful clinical investigation of hRT with concurrent systemic therapy.
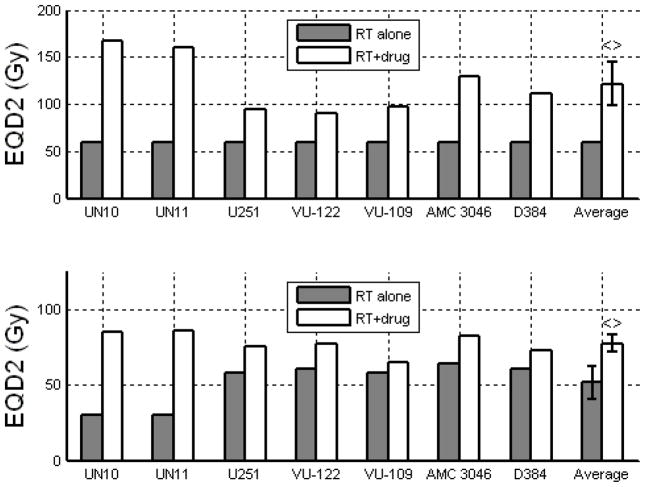
Figure 3.
EQD2 for GBM cell lines treated with 2.0 Gy x 30 (top) and 6 Gy x 5 (bottom), with and without concurrent temozolomide. Error bars indicate 95% confidence intervals. <> denotes a significant (p<0.05) increase in EQD2 with the addition of drug using paired Student’s t-test.
Figure 4.
EQD2 for HNC cell lines treated with 2.0 Gy x 35 (top) and 5.0 Gy x 6 (bottom), with and without concurrent cisplatin. * denotes addition of cetuximab. Error bars indicate 95% confidence intervals. <> denotes a significant (p<0.05) increase in EQD2 with the addition of drug using paired Student’s t-test.
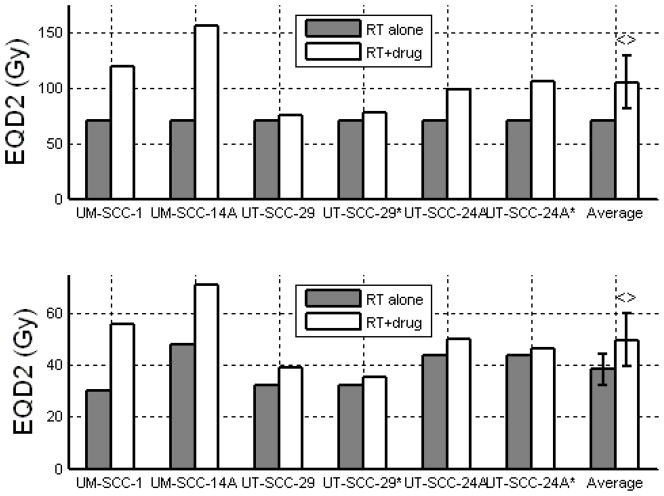
Figure 5.
EQD2 for NSCLC cell lines treated with 2.0 Gy x 30 (top) and 10.0 Gy x 5 (bottom). Car = Carboplatin, Doc = Docetaxel, Pac = Paclitaxel, Gem = Gemcitabine, Vin = Vinorelbine. Error bars indicate 95% confidence intervals. <> denotes a significant (p<0.05) increase in EQD2 with the addition of drug using paired Student’s t-test.
Figure 6.
EQD2 for Pancreatic cancer cell lines treated with 1.8 Gy x 28 (top) and 10 Gy x 3 (bottom), with and without concurrent gemcitabine. * denotes addition of oxaliplatin. ** denotes addition of erlotinib. Error bars indicate 95% confidence intervals. <> denotes a significant (p<0.05) increase in EQD2 with the addition of drug using paired Student’s t-test.
Table 2b.
Mean absolute differences between predicted (using models fit to low-dose RT data) and actual cell kill with high dose RT
| Model | Mean Absolute Error | 95% CI |
|---|---|---|
| LQ | 1.63 | 0.55 to 2.70 |
| MT* | 0.83 | 0.36 to 1.31 |
| gLQ*,** | 0.56 | 0.28 to 0.83 |
p<0.05 compared to LQ model;
p<0.05 compared to MT model
Footnotes
None of the authors has a financial disclosure or conflict of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nitin Ohri, Email: ohri.nitin@gmail.com, 111 South 11th Street, Room G-301, Bodine Center, Philadelphia, PA 19107, (215) 955-6702, (215) 955-0412 fax
Adam P. Dicker, Email: adamdicker@mac.com, 111 South 11th Street, Room G-301, Bodine Center, Philadelphia, PA 19107, (215) 955-6700, (215) 503-0013 fax
Yaacov Richard Lawrence, Email: yaacovla@gmail.com, 111 South 11th Street, Room G-301, Bodine Center, Philadelphia, PA 19107, (215) 955-6702, (215) 955-0412 fax
References
- 1.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.King CR, Brooks JD, Gill H, et al. Long-Term Outcomes from a Prospective Trial of Stereotactic Body Radiotherapy for Low-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2011 doi: 10.1016/j.ijrobp.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 4.Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976) 2007;32:193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 5.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679–694. doi: 10.1259/0007-1285-62-740-679. [DOI] [PubMed] [Google Scholar]
- 6.Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. Philadelphia, PA: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 7.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 9.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 10.Curran W, Scott C, Langer C. Longterm benefit is observed in a phase III comparison of sequential vs concurrent chemoradiation for patients with unresected stage III nsclc: RTOG 9410. Am Soc Clin Oncol. 2003;22:621. [Google Scholar]
- 11.Pignon JP, le Maitre A, Maillard E, et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14. doi: 10.1016/j.radonc.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 13.Gerard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 14.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 15.Wang JZ, Huang Z, Lo SS, et al. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Transl Med. 2010;2:39ra48. doi: 10.1126/scitranslmed.3000864. [DOI] [PubMed] [Google Scholar]
- 16.van Rongen E, Travis EL, Thames HD., Jr Repair rate in mouse lung after clinically relevant radiation doses per fraction. Radiat Res. 1995;141:74–78. [PubMed] [Google Scholar]
- 17.van Rongen E, Thames HD, Jr, Travis EL. Recovery from radiation damage in mouse lung: interpretation in terms of two rates of repair. Radiat Res. 1993;133:225–233. [PubMed] [Google Scholar]
- 18.Fowler JF, Whitsed CA, Joiner MC. Repair kinetics in mouse lung: a fast component at 1. 1 Gy per fraction. Int J Radiat Biol. 1989;56:335–353. doi: 10.1080/09553008914551501. [DOI] [PubMed] [Google Scholar]
- 19.Vegesna V, Withers HR, Taylor JM. Repair kinetics of mouse lung. Radiother Oncol. 1989;15:115–123. doi: 10.1016/0167-8140(89)90124-2. [DOI] [PubMed] [Google Scholar]
- 20.Parkins CS, Whitsed CA, Fowler JF. Repair kinetics in mouse lung after multiple X-ray fractions per day. Int J Radiat Biol. 1988;54:429–443. doi: 10.1080/09553008814551811. [DOI] [PubMed] [Google Scholar]
- 21.Travis EL, Thames HD, Watkins TL, et al. The kinetics of repair in mouse lung after fractionated irradiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1987;52:903–919. doi: 10.1080/09553008714552491. [DOI] [PubMed] [Google Scholar]
- 22.Puck TT, Marcus PI. Action of x-rays on mammalian cells. J Exp Med. 1956;103:653–666. doi: 10.1084/jem.103.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Astrahan M. Some implications of linear-quadratic-linear radiation dose-response with regard to hypofractionation. Med Phys. 2008;35:4161–4172. doi: 10.1118/1.2969065. [DOI] [PubMed] [Google Scholar]
- 24.Fowler JF. Differences in Survival Curve Shapes for Formal Multi-target and Multi-hit Models. Phys Med Biol. 1964;9:177. [Google Scholar]
- 25.Curtis SB. Lethal and potentially lethal lesions induced by radiation--a unified repair model. Radiat Res. 1986;106:252–270. [PubMed] [Google Scholar]
- 26.Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49:4825–4835. doi: 10.1088/0031-9155/49/20/012. [DOI] [PubMed] [Google Scholar]
- 27.Kehwar TS, Rathore RP, Supe SJ, et al. Many component model and its parameters to fractionated irradiation. Strahlenther Onkol. 1995;171:573–580. [PubMed] [Google Scholar]
- 28.Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose: useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 29.Fowler JF. Correction to Kasibhatla et al. How much radiation is the chemotherapy worth in advanced head and neck cancer? (Int j radiat oncol biol phys 2007;68:1491–1495) Int J Radiat Oncol Biol Phys. 2008;71:326–329. doi: 10.1016/j.ijrobp.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 30.Kasibhatla M, Kirkpatrick JP, Brizel DM. How much radiation is the chemotherapy worth in advanced head and neck cancer? Int J Radiat Oncol Biol Phys. 2007;68:1491–1495. doi: 10.1016/j.ijrobp.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Jones B, Dale RG. The potential for mathematical modelling in the assessment of the radiation dose equivalent of cytotoxic chemotherapy given concomitantly with radiotherapy. Br J Radiol. 2005;78:939–944. doi: 10.1259/bjr/40226390. [DOI] [PubMed] [Google Scholar]
- 32.Speke AK, Hill RP. Repopulation kinetics during fractionated irradiation and the relationship to the potential doubling time, Tpot. Int J Radiat Oncol Biol Phys. 1995;31:847–856. doi: 10.1016/0360-3016(94)00496-X. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 34.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8:89–91. doi: 10.1016/j.ccr.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology? Int J Radiat Oncol Biol Phys. 2008;71:324–325. doi: 10.1016/j.ijrobp.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Carlson DJ, Keall PJ, Loo BW, Jr, et al. Hypofractionation results in reduced tumor cell kill compared to conventional fractionation for tumors with regions of hypoxia. Int J Radiat Oncol Biol Phys. 2011;79:1188–1195. doi: 10.1016/j.ijrobp.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown JM, Diehn M, Loo BW., Jr Stereotactic ablative radiotherapy should be combined with a hypoxic cell radiosensitizer. Int J Radiat Oncol Biol Phys. 2010;78:323–327. doi: 10.1016/j.ijrobp.2010.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12:4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 39.Kil WJ, Cerna D, Burgan WE, et al. In vitro and in vivo radiosensitization induced by the DNA methylating agent temozolomide. Clin Cancer Res. 2008;14:931–938. doi: 10.1158/1078-0432.CCR-07-1856. [DOI] [PubMed] [Google Scholar]
- 40.van Nifterik KA, van den Berg J, Stalpers LJ, et al. Differential radiosensitizing potential of temozolomide in MGMT promoter methylated glioblastoma multiforme cell lines. Int J Radiat Oncol Biol Phys. 2007;69:1246–1253. doi: 10.1016/j.ijrobp.2007.07.2366. [DOI] [PubMed] [Google Scholar]
- 41.van Rijn J, Heimans JJ, van den Berg J, et al. Survival of human glioma cells treated with various combination of temozolomide and X-rays. Int J Radiat Oncol Biol Phys. 2000;47:779–784. doi: 10.1016/s0360-3016(99)00539-8. [DOI] [PubMed] [Google Scholar]
- 42.Pekkola-Heino K, Kulmala J, Grenman R. Carboplatin-radiation interaction in squamous cell carcinoma cell lines. Arch Otolaryngol Head Neck Surg. 1992;118:1312–1315. doi: 10.1001/archotol.1992.01880120038007. [DOI] [PubMed] [Google Scholar]
- 43.Zhang N, Erjala K, Kulmala J, et al. Concurrent cetuximab, cisplatin, and radiation for squamous cell carcinoma of the head and neck in vitro. Radiother Oncol. 2009;92:388–392. doi: 10.1016/j.radonc.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Amorino GP, Hamilton VM, Choy H. Enhancement of radiation effects by combined docetaxel and carboplatin treatment in vitro. Radiat Oncol Investig. 1999;7:343–352. doi: 10.1002/(SICI)1520-6823(1999)7:6<343::AID-ROI4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Bergs JW, Franken NA, ten Cate R, et al. Effects of cisplatin and gamma-irradiation on cell survival, the induction of chromosomal aberrations and apoptosis in SW-1573 cells. Mutat Res. 2006;594:148–154. doi: 10.1016/j.mrfmmm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 46.van Bree C, Castro Kreder N, Loves WJ, et al. Sensitivity to ionizing radiation and chemotherapeutic agents in gemcitabine-resistant human tumor cell lines. Int J Radiat Oncol Biol Phys. 2002;54:237–244. doi: 10.1016/s0360-3016(02)02891-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhang M, Boyer M, Rivory L, et al. Radiosensitization of vinorelbine and gemcitabine in NCI-H460 non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys. 2004;58:353–360. doi: 10.1016/j.ijrobp.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 48.Lawrence TS, Blackstock AW, McGinn C. The mechanism of action of radiosensitization of conventional chemotherapeutic agents. Semin Radiat Oncol. 2003;13:13–21. doi: 10.1053/srao.2003.50002. [DOI] [PubMed] [Google Scholar]
- 49.Morgan MA, Meirovitz A, Davis MA, et al. Radiotherapy combined with gemcitabine and oxaliplatin in pancreatic cancer cells. Transl Oncol. 2008;1:36–43. doi: 10.1593/tlo.07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Symon Z, Davis M, McGinn CJ, et al. Concurrent chemoradiotherapy with gemcitabine and cisplatin for pancreatic cancer: from the laboratory to the clinic. Int J Radiat Oncol Biol Phys. 2002;53:140–145. doi: 10.1016/s0360-3016(01)02790-0. [DOI] [PubMed] [Google Scholar]
- 51.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu KK, Pajak TF, Trotti A, et al. A Radiation Therapy Oncology Group (RTOG) phase III randomized study to compare hyperfractionation and two variants of accelerated fractionation to standard fractionation radiotherapy for head and neck squamous cell carcinomas: first report of RTOG 9003. Int J Radiat Oncol Biol Phys. 2000;48:7–16. doi: 10.1016/s0360-3016(00)00663-5. [DOI] [PubMed] [Google Scholar]
- 53.Roh KW, Jang JS, Kim MS, et al. Fractionated stereotactic radiotherapy as reirradiation for locally recurrent head and neck cancer. Int J Radiat Oncol Biol Phys. 2009;74:1348–1355. doi: 10.1016/j.ijrobp.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 54.Cengiz M, Ozyigit G, Yazici G, et al. Salvage Reirradiaton with Stereotactic Body Radiotherapy for Locally Recurrent Head-and-Neck Tumors. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 55.Takeda A, Sanuki N, Kunieda E, et al. Stereotactic body radiotherapy for primary lung cancer at a dose of 50 Gy total in five fractions to the periphery of the planning target volume calculated using a superposition algorithm. Int J Radiat Oncol Biol Phys. 2009;73:442–448. doi: 10.1016/j.ijrobp.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 56.Nagata Y, Hiraoka M, Mizowaki T, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys. 2009;75:343–347. doi: 10.1016/j.ijrobp.2009.02.087. [DOI] [PubMed] [Google Scholar]
- 57.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 58.Mahadevan A, Jain S, Goldstein M, et al. Stereotactic Body Radiotherapy and Gemcitabine for Locally Advanced Pancreatic Cancer. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]