Summary
Melanoma is an “immunogenic tumor”, often highly infiltrated with lymphocytes, which are capable of inducing regression of the primary tumor. The commonly observed phenomenon of regression suggests substantial cross-talk between immune cells and transformed melanocytes. An immune response to melanocyte differentiation antigens common to transformed and normal melanocytes manifests clinically at distant sites as melanoma associated vitiligo or halo nevi. Despite similar antigenic targets, the pathogenesis and prognosis differs between the different melanoma associated leukodermas. Understanding immunologic cross-talk between melanocytes and the immune system will aid the development of approaches to combat melanoma.
Keywords: melanoma, vitiligo, immunity, leukoderma, melanocyte, nevus
Introduction
Malignant melanoma is a highly aggressive tumor derived from transformed melanocytes, steadily rising in incidence. In the United States alone, there will be approximately 76,000 new cases of melanoma, and over 9,000 deaths in 2013 ( 2013). The incidence rose at 6% annually in the 1970s and continues to increase at 3% annually. While the overall survival is >90%, once the disease has spread, the 5 year survival is 62% and with distant metastases, drops to 15%. Clearly new therapies are needed, and there are currently two new drugs which are making an impact. Ipilimumab is an antibody capable of promoting an anti-tumor immune response by reducing normal down-regulation of immunity by the molecule CTLA-4 (Hodi et al., 2010). Vemurafenib is a small molecule inhibitor which leads to dramatic tumor regression in patients with a mutated BRAF signaling molecule (Sosman et al., 2012). Unfortunately, the frequency of patients responding to ipilimumab is only up to 10% (Mellman et al., 2011), and the duration of response to Vemurafenib is short, with only 5.3 months of progression free survival (Chapman et al., 2011). Most recently, blockade of another negative immune regulatory interaction, that of PD-1 (expressed by activated T cells) and its ligand, PD-L1 (expressed by tumor and stromal cells) has shown significant clinical activity in melanoma (Topalian et al., 2012, Brahmer et al., 2012), and is capable of inducing durable responses. Much work is still ahead to more fully understand the pathogenesis of melanoma, drug resistance mechanisms that evolve, and for the development of new drugs and combinations with superior clinical benefit. Better understanding of immunity to melanocytes in melanoma, which clinically manifest as leukodermas, could further advance these goals, by identifying potential targets for immunotherapy and novel small molecule inhibitors and by combining approaches.
Several types of leukoderma have been described in melanoma patients:
Primary melanoma regression – A progressive process replacing the tumor with fibrous stroma within the superficial dermis. Complete regression of a tumor is rare.
Halo nevus (Sutton’s nevus) – A rim of depigmentation surrounding a melanocytic nevus.
Melanoma-associated depigmentation: appearance of white patches in sites distant from the primary tumor, arising either spontaneously or following immunologic based treatments.
Here, we will review the current literature on melanoma-associated leukodermas and discuss their immunologic pathogeneses and clinical significance.
The melanocyte
Melanocytes are the pigment producing cells of the skin, providing color to the skin, hair and eyes as well as protection from ultraviolet light and free radicals (Bustamante et al., 1993). During embryogenesis, melanocytes migrate from the neural crest to the epidermis, hair follicle, leptomeninges, uveal tract and the inner ear. In the skin, the melanocyte resides within the basal layer of the epidermis, with its cell body sitting at the basal lamina and its dendrites in contact with approximately 30–40 surrounding keratinocytes, to which it transfers melanosomes. The synthesis of melanin takes place in the melanosomes, intracytoplasmatic organelles that produce eumelanin (brown or black melanin, more abundant in dark skin) and pheomelanin (yellow-red melanin, found in red hair, light and dark skin). Melanin is synthesized from its precursor, tyrosine, through an enzymatic process involving Tyrosinase, Tyrosinase-related protein 1 (TRP-1) and Tyrosinase-related protein 2 (TRP-2).
Melanosomes which produce eumelanin progress through four stages of maturation, with the first two stages generating a matrix favorable for eumelanin deposition and polymerization. It is thought that the melanin intermediates can be concentrated on this matrix, and perhaps stabilized or detoxified there. Gp100 (Pmel17) is a major component of the fibrillar matrix of early stage melanosomes, is maintained in most epidermal and uveal melanomas and thus makes an excellent target for immunotherapy (Theos et al., 2005). Hoashi and colleagues recently demonstrated that a secreted form of gp100 (sPmel17) is released by regulated proteolytic ectodomain shedding, so it may serve as a melanoma-specific serum biomarker for early detection (Hoashi et al., 2010). MART-1 (Melanoma Antigen Recognized by T cells, also known as Melan-A) forms a complex with gp100 and affects its expression, stability, trafficking and the processing which is required for melanosome structure and maturation. MART-1 is indispensable for gp100 function and plays an important role in pigmentation (Hoashi et al., 2005).
Melanosome antigens, being intracellular proteins, are expressed in peptide form on the surface of melanocytes and keratinocytes in the context of major histocompatibility complex (MHC) class I molecules. This is a process that allows for surveillance by the immune system. Negative selection of self-reactive T cells during ontogeny of the immune system reduces the risk of autoimmunity by reducing the high avidity self-reactive T cells. However, these melanosome containing cells may be recognized by remaining CD8+ T cells under inflammatory conditions that can increase antigen presentation to the immune system through maturation of antigen presenting cells (APC) to more optimal T cell stimulatory activity (which can support activation of naïve and lower avidity T cells), and increasing migration of APC, like dendritic cells (DC), from skin to lymph nodes, where they encounter a large number of T cells.
The subcellular location of these melanocytic proteins affects their processing and presentation. MART-1 has a transmembrane region which is composed of hydrophobic residues and contains the immunodominant HLA-A2 MHC class I epitope amino acids (MART-127-35). This location has been demonstrated to adversely affects its’ cell surface presentation and immunogenicity (Rimoldi et al., 2001). This may be balanced by its presence in multiple vesicles, including not only melanosomes but also the trans-golgi network, which may positively impact its’ processing (De Maziere et al., 2002). Much is known about the immunogenicity of MART-1 and the behavior of T cells specific to it because these T cells are present in the circulation at an unusually high frequency (approximately 100 times more than most self antigen-specific T cells). This was shown to be due to an unusually high thymic output of naïve CD8+ T cells specific to the MART-1 epitope restricted by the common HLA-A2 molecule (Zippelius et al., 2002).
Overwijk and Restifo proposed that because melanosomes are known to be related biochemically and developmentally to endosome and lysosome lineages that this allows loading of melanocyte differentiation antigens onto MHC class II through intersection of protein transport vesicles. This may result in more efficient activation of antigen specific CD4+ ‘helper’ T cells that may subsequently aid in the activation of more effective or “helped” CD8+ T cells (Overwijk and Restifo, 2000). The help provided by CD4+ T cells during the priming of CD8+ T cells is essential for development of immune memory and for clonal secondary expansion of CD8+ T cells. Without this help, these CD8+ T cells will undergo death mediated by TNF-related apoptosis-inducing ligand (TRAIL) (Janssen et al., 2005).
This melanosomal biology may confer some of the unique immunogenicity of melanoma. CD4+ T cell responses may be of particular importance in the setting of melanoma, as these tumors often express MHC class II on their surface, like APC, and unlike normal melanocytes, melanocytic nevi and most other tumor types. Expression of MHC class II by melanoma is associated with poor prognosis and higher metastatic dissemination. This indicates that the MHC class II does not serve to promote the type of CD4+ T cell help that supports protective anti-tumor immunity. It had recently been demonstrated that lymphocyte activation gene-3 (LAG-3), which is a ligand for MHC class II expressed on tumor infiltrating lymphocytes, plays a role in melanoma survival through protection of the tumor from FAS-mediated and drug induced apoptosis (Hemon et al., 2011).
Melanoma commonly over expresses several melanocyte lineage antigens, including Tyrosinase, TRP-1, TRP-2, gp100 and MART-1. They are highly expressed in most melanomas regardless of tumor stage (Barrow et al., 2006). Both MART-1 and gp100 are regulated by the transcription factor MITF (microphthalmia associated transcription factor), hence they are commonly over-expressed together (Du et al., 2003). MITF appears to be critical for the development of the melanocyte cell lineage (Hodgkinson et al., 1993). Several other genes have been identified as important in melanocyte development such as c-kit, snail/slug, sox10 and endothelins (Bandarchi et al., 2013). The role of MITF, c-kit, MART-1, HMB-45 and bcl-2 as biomarkers of melanocytic tumor progression was investigated through tissue microarray (Nazarian et al., 2010).
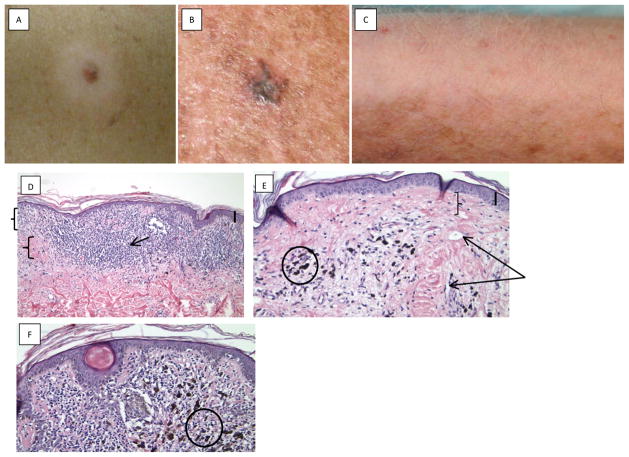
Melanoma has traditionally been described as an “immunogenic tumor”, one that the immune system is able to recognize. These tumors are often infiltrated by high levels of T lymphocytes unlike tumors derived from other tissues (Goff et al., 2010). A cytotoxic T cell response against “self” antigens like MART-1 and gp100, could cause destruction of normal melanocytes expressing normal levels of these proteins, as well (Irvine et al., 2002). Such spontaneous immunity is clinically evident as depigmentation either within a melanocytic lesion (regression), around melanocytic nevi (halo nevi) or in a distant site (melanoma-associated depigmentation) (Fig. 1).
Figure 1.
Photographic examples of melanoma and vitiligo. A: Halo nevus clinical photograph, showing a central nevus and surrounding depigmentation. Photograph courtesy of Dr. Oleg Akilov. B: Primary melanoma regression. Photograph courtesy of Dr. Larisa Geskin. C: Melanoma associated vitiligo, in a melanoma patient post treatment with ipilimumab (anti-CTLA4). Vitiligo appeared on the lateral aspects of arms, with poliosis. Photograph courtesy of Dr. John M. Kirkwood. D, E: Photomicrographs of primary melanoma regression; D. Dense band like lymphocytic infiltrate (arrow) and fibroplasia (lower bracket) of papillary dermis (upper bracket) seen focally (thick bar upper right denotes 0.5 mm); E. Late stage of regression reveals dermal fibrosis (bracket), vascular ectasia (two arrows) and sprinkling of lymphocytes and melanophages (circle). F: Photomicrograph of regressed nevus demonstrates residual junctional melanocytes with slight distortion of architecture, dense lymphocytic infiltrates and few melanophages (circle) (thick bar upper right denotes 0.6 mm).
Melanoma regression
Melanoma regression is a common manifestation, with variable histologic regression found in 10–35% of primary melanomas (Blessing and McLaren, 1992). Complete regression is rare, with approximately 40 total cases reported in the literature (High et al., 2005), all but one in metastatic patients. There is a single report of biopsy proven superficial spreading melanoma (Breslow thickness, 0.7mm) in which the patient refused treatment. Clinical follow up documented the gradual regression of the primary tumor until complete regression was observed approximately 4 years after initial diagnosis, with no evidence of systemic disease (Menzies and McCarthy, 1997). The prevalence of complete regression without metastases is unknown as it would not come to medical attention. Melanoma of unknown origin may represent fully regressed primary tumors. Bories et al examined 7 patients with lymph node metastatic melanoma with unknown primary lesion, in all patients a regressed melanoma was found through dermoscopy followed by biopsy (Bories et al., 2008).
In the process of regression, fibrous stroma progressively replaces the dermal portion of the tumor. Clinical signs of regression include areas of tumor depigmentation with a white, red, blue or gray color (Requena et al., 2009). Completely regressed tumors may have variable clinical presentation: depigmented, scar like to pink-white, blue, brown or black macules and papules. In darker regressed lesions, melanophages (macrophages which have taken up melanin) and pigmentary incontinence was found, but no melanoma cells (High et al., 2005).
Histologically, primary melanoma regression is identified as a delicate area of fibrosis of papillary dermis. There is an increased number of dilated endothelium lined vascular spaces in the region. Within this area, there are no viable melanoma cells present, instead there are variable numbers of lymphocytes and melanophages (Figure 1D, E). Melanoma may be present in adjacent epidermis or one or both sides of the fibrosed papillary dermis in tumors with partial regression. Complete regression can be identified histologically by presence of large aggregates of melanophages within the papillary and reticular dermis and associated fibrosis. Usually there is a flattening of the overlying epidermis with loss of rete pegs. In the vertical growth, phase one may see evidence of partial regression such as abundance of melanophages mingled with viable tumor cells.
The prognostic significance of melanoma regression is a subject of debate (Requena et al., 2009). It is considered by most to be associated with a poor prognosis, as the melanoma may have had a prior deeper invasive component (Olah et al., 2003), and the immunologic partial elimination of the tumor may have selected for more aggressive melanoma clones. Several studies (Fontaine et al., 2003, Morris et al., 2008, Kaur et al., 2008) examined sentinel lymph node involvement in patients with melanoma regression and did not find it to be related to a higher rate of lymph node metastases; others (Olah et al., 2003, Guitart et al., 2002) found the opposite. These differences may be attributed to the inconsistent histopathologic definition of regression and varying thickness of the melanomas examined. Nodal metastases in thin melanomas may imply that metastases might have occurred prior to regression (Rao et al., 2010).
Regression of melanoma has been attributed to immunologic mechanisms, with observation of an inflammatory reaction at the initial stages of regression involving a lymphocytic infiltrate composed predominantly of CD4+ T cells. Several pro-fibrotic cytokines were found in primary melanoma and regressed melanoma (IL-6, PDGF, TFG-Beta, bFGF) which partially explain the fibrosis observed in the late stage of regression (Moretti et al., 2007).
Halo Nevus
Halo nevus (also known as Sutton’s nevus) is a benign melanocytic nevus surrounded by a symmetrical, well demarcated, rim (halo) of hypopigmentation or depigmentation, usually affecting individuals under the age of 20 years with no predilection to gender or race (Bolognia et al., 2009). Halo nevi are located mostly on the trunk, particularly the upper back, and may persist for over a decade. The central nevus may partially or completely regress and associated halo may persist or repigment (Aouthmany et al., 2012).
Histologically, the nevus may be junctional, compound or dermal, with small lymphocytes surrounding or permeating the dermal component and sometimes even the junctional melanocytic cells. The nevus cells may become vacuolated, have pyknotic nuclei or their morphology may be obscured by the dense lymphocytic infiltrate (Figure 1F). In typical cases of halo nevi there is a denser lymphocytic infiltrate compared to regressing melanoma.
The lymphocytic infiltrate of halo nevus is composed predominantly of CD8+ T cells (Zeff et al., 1997, Moretti et al., 2007). CD4+ T cells also play a part as they support both cytotoxic T lymphocytes and B cells (Zeff et al., 1997). The role of a humoral response was suggested by Lewis and Copeman, when antibodies thought to be specific against malignant melanoma were found in patients with halo nevi. After surgical removal of the halo nevus or spontaneous resolution with repigmentation of halo, these antibodies could not be found (Lewis and Copeman, 1972). No correlation was found between the appearance of circulating antibodies and the regression of the central nevus, suggesting that antibody production was secondary to nevus cell destruction by cytotoxic T lymphocytes.
The peripheral white halo has little or no lymphocytic infiltrate. Two suggested mechanisms for the development of the halo are: 1. Inflammatory cytokines released, spreading to adjacent melanocytes, or 2. T-cell immune response directed not only to the antigens of the nevus melanocytes but also to adjacent epidermal melanocytes that express the same antigens. The diameter of the halo is correlated to the size of the central nevus, the larger the nevus, the larger the halo (Rongioletti et al., 2011).
The appearance of a halo may also be seen around melanoma, congenital nevi, spitz nevi and other dermatologic conditions such as dermatofibroma (Rubegni et al., 2009, Kolm et al., 2006). On the other hand, regression of nevi may occur without a surrounding halo (Speeckaert et al., 2011).
Regression of the central nevus is not associated with fibrosis, in contrast to the regression of melanoma. Morreti and colleagues found that the antifibrotic tumor necrosis factor (TNF)-α was expressed in higher levels in halo nevi compared to melanoma. They did however also find the profibrotic growth factor basic fibroblast growth factor (bFGF) in halo nevi, thus the cytokine environment does not completely explain this difference (Moretti et al., 2007).
Clinically, there is an association between halo nevi and melanoma, as multiple halo nevi in older adults may be a sign for melanoma (Bolognia et al., 2009). Halo nevi are also associated with vitiligo and a vitiligo-like phenomenon. Approximately 20% of individuals with halo nevi have vitiligo. Patients with multiple halo-nevi may have vitiligo-like lesions that were classified by van Geel and colleagues as halo nevi associated leukoderma. These lesions are distinct from generalized vitiligo as the patients are younger, with less associated autoimmune diseases. The lesions are asymmetric, without clear demarcation, limited in size and do not progress as vitiligo lesions do (van Geel et al., 2012). Musette et al found local expansion of limited subsets of oligoclonal T cells infiltrating halo nevi, but absent in the circulation (Musette et al., 1999). Melanoma with a halo around it has been reported. The halo of melanoma is more irregular than that seen in halo nevus and the patients are usually older.
Melanoma-associated Depigmentation
The appearance of white patches in melanoma patients had received much attention in past years. This phenomenon was most commonly referred to as vitiligo or melanoma-associated vitiligo (Ram and Shoenfeld, 2007, Hartmann et al., 2008, Nordlund et al., 1983). Vitiligo is a depigmenting skin disorder with varying clinical presentations classified to non segmental vitiligo (acrofacial, mucosal, generalized, universal or mixed) and segmental vitiligo. The term ‘vitiligo’ is used as an umbrella term for all non-segmental forms of vitiligo, yet melanoma-associated depigmentation is not considered a subtype of vitligo by recent international consensus group (Ezzedine et al., 2012). This vitiligo-like phenomenon in melanoma patients will be referred to in this review as melanoma-associated depigmentation.
Hartmann and colleagues compared vitiligo and melanoma-associated depigmentaion patients and found that vitiligo occurs at a younger age (27.6±16.5 years and 56.4±10.8 respectively), with more women affected in the vitiligo group (77%) compared to melanoma-associated depigmentation (~50%). Similar data regarding age and gender of melanoma-associated depigmentation patients was reported by Quaglino and colleagues (Quaglino et al., 2009). Vitiligo patients had a family history of vitiligo more often, associated autoimmune diseases and anti thyroid antibodies (Hartmann et al., 2008). Melanoma-associated depigmentation patients have more immune-mediated diseases (Quaglino et al., 2009). Most melanoma-associated depigmentation patients examined had a bilateral symmetric pattern corresponding to vitiligo vulgaris while a few had unilateral asymmetric or focal hypopigmentation with none having acrofacial pattern of vitiligo. No correlation was found between the distribution of leukoderma and the location of the primary tumor. Acquired leukodermas (hypopigmented scars, halo nevi) were found more commonly in melanoma-associated depigmentation patients. No histologic or immunohistologic differences were found between the two groups, in the clinically hypopigmented areas melanin and MART-1 positive melanocytes were markedly reduced or absent, with no differences in the numbers of intra-epidermal CD1-positive DC. At the transition to normally pigmented skin in many patients there was a sparse lymphohistiocytic infiltrate with predominantly CD4 positive T-cells (Hartmann et al., 2008).
Melanoma-associated depigmentation occurs in 2–16% of melanoma patients (Hartmann et al., 2008) and more commonly following treatment (Rosenberg and White, 1996). Most patients will have melanoma-associated depigmentation within a few years of diagnosis. In some, depigmented vitiligo-like lesions may appear many years prior to the diagnosis of melanoma, in which case the patients are younger (median age 38) with a more generalized distribution of lesions (Hartmann et al., 2008, Schallreuter et al., 1991, Quaglino et al., 2009). No survival differences were found in association with the onset time of melanoma-associated depigmentation (before diagnosis of melanoma or after) (Quaglino et al., 2009). The lesions may appear on the trunk or near the primary tumor or metastases and then spread centrifugally (Nordlund et al., 1983). Other reports did not find an association between the location of lesions and the primary tumor, and found the leukoderma to spread centripetally (Hartmann et al., 2008).
Melanoma-associated depigmentation portends a favorable prognostic factor in melanoma patients with significantly enhanced 5-year survival (Nordlund et al., 1983, Bystryn et al., 1987, Quaglino et al., 2010). Development of melanoma-associated depigmentation after immunotherapy has also been correlated with improved survival, as did appearance of other autoimmune manifestations (Rosenberg and White, 1996, Gogas et al., 2006, Boasberg et al., 2006).
Treatment of metastatic melanoma patients and metastatic renal cell cancer with high-dose IL-2 resulted in melanoma-associated depigmentation in approximately 20% of responding melanoma patients (Rosenberg and White, 1996). The relation between melanoma-associated depigmentation and melanoma regression was highly significant (p=0.0002). All patients with melanoma-associated depigmentation experienced at least partial tumor regression. None of the renal cell cancer patients treated developed any depigmented vitiligo-like lesions, likely due to the lack of cross-reactive antigens. Specific induction of immunity to melanosomal antigens via vaccination with DC transduced by Adenovirus encoding gp100 and/or MART-1 or with DC pulsed with MART-127-35 peptide (Butterfield et al., 2008, Butterfield et al.,2003, Haluska et al., 2000, Ribas et al., 2004, Tsao et al., 2002) had also resulted in leukoderma and melanoma-associated depigmentation.
Other immune based therapies reported to induce melanoma-associated depigmentation are interferon alpha (Yang et al., 2010) anti PD-1, ipilimumab, IL-2+GM-CSF (Boasberg et al., 2006, Quaglino et al., 2009) and radiotherapy (Teulings et al., 2013) (Figure 2). Adoptive lymphocyte transfer of highly selected tumor-reactive differentiation antigens resulted in regression of tumor in metastatic patients as well as appearance of melanoma-associated depigmentation in 3 of 13 patients (Dudley et al., 2002). In one melanoma patient, tumor-infiltrating lymphocytes had the same T cell receptor as depigmented peritumoral skin (Becker et al., 1999). Both overexpression of melanocyte antigens in tumor cells or the therapy-induced inflammatory reaction are possible mechanisms for breaking immunologic tolerance to these self antigens (Rosenberg and White, 1996) (Figure 2).
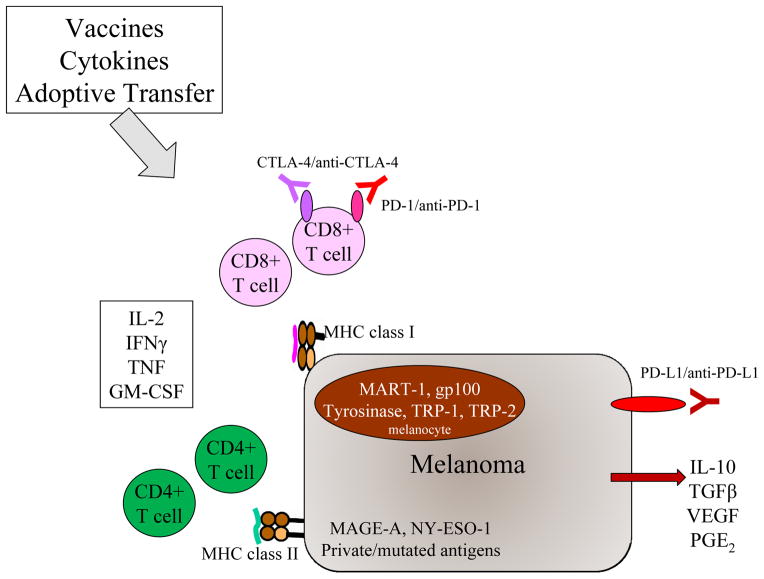
Figure 2.
Overview of melanoma immunotherapy strategies and targets. Spontaneous immune surveillance and therapeutic interventions such as vaccines, adoptive transfer and cytokines can result in activation of CD8+ and CD4+ T cells. These T cells can recognize peptides derived from melanocyte differentiation antigens like MART-1, cancer/testes antigens like MAGE-A family members, or mutated antigens, presented in MHC class I and II. Activated T cells can produce “type 1” cytokines like IL-2 and IFNγ, which support antitumor effects: Melanoma tumors produce cytokines like IL-10 and TGFβ which inhibit immunity, and also upregulate PD-L1. Previously activated T cells upregulate CTLA-4 and PD-1, which can be blocked by specific antibodies to further promote antitumor effects.
Discussion
The immune response elicited against melanocyte differentiation antigens links melanoma and its associated leukodermas. The field continues to decipher what makes one immune response to melanoma clinically effective and others not. A locally effective immune response against melanoma resulting in partial or complete regression of the tumor, as well as the improved survival in those with melanoma-associated depigmentation supports the role of immunotherapy in melanoma treatment. New immunologic therapies, targeting “immune checkpoints”, such as anti CTLA-4 and anti-PD-L1/PD-1 are coming into play, enhancing anti-tumor immunity and overcoming tumor induced immune suppression (Topalian et al., 2012, Brahmer et al., 2012, Hodi et al., 2010). Recent attempts to combine anti-CTLA4 and anti-PD-1 in patients with advanced melanoma showed rapid tumor regression in a substantial proportion of patients (Wolchok et al., 2013).
Primary melanoma regression, halo nevi and melanoma-associated depigmentation are distinct in pathogenesis, cytokine profile and prognostic outcome. The difference in prognosis likely results from different mechanisms as different types of pigment cells are destroyed (normal or malignant melanocytes and normal or atypical nevus cells) (Hann and Nordlund, 2000). The clinical association between melanoma and associated leukodermas is a two way street, on the one hand it may provide prognostic information or indicate response to treatment of a known melanoma patient, or may serve as a flag to the general dermatology patient appearing with several halo nevi (and being older than 40 years old), or the newly diagnosed vitiligo patient who may have an atypical distribution of lesions or other associated leukodermas (hypopigmented scars, halo nevi) and without family history of vitiligo. This may serve as a marker that would prompt a thorough complete body examination in search of suspected melanocytic lesions. Since melanoma-associated depigmentation may occur years before diagnosis of melanoma, regular total body examination follow-up is recommended.
Patients presenting with melanoma metastases with an unknown primary lesion should also be carefully evaluated for depigmented lesions or scars that may account for the regression of the primary lesion. Wood’s lamp examination may assist in identifying subtle hypopigmentation. Careful, detailed skin examination with the aid of a dermatoscope, with emphasis of the metastatic lymph node draining areas may yield further diagnoses of regressed melanoma.
It is important to understand why only a small fraction of the tumors undergo complete regression and what are the initiating events to this process. It is unknown how many cases of melanoma have true complete regression without any residual disease. It is possible that the immune system is activated once the tumor cells invade the lymph nodes, explaining the higher lymph node involvement in regressed tumors as well as the systemic involvement despite immunologic recognition. Observations of immunity spreading from one antigen to another in a tissue, seen in autoimmunity, is also seen in a series of clinical responding patients in cancer immunotherapy clinical trials, suggested that this process may be a critical parameter (Disis, 2011). Understanding the immunology behind tumor regression as well as the causes for the immune system’s failure to completely reject the tumor may be an important step in the development of new therapeutic approaches.
Insight into the mechanisms leading to spontaneous immune response against common melanocytic antigens is important in the ongoing efforts to develop effective immunotherapies for malignant melanoma as well as the understanding and treatment of pigmentation disorders.
Acknowledgments
We would like to thank Ravit Bickson for the graphic assistance; Drs. Larisa Geskin, John M. Kirkwood and Oleg Akilov for clinical photographs. This work was also supported by the University of Pittsburgh Skin SPORE Grant, P50 CA121973 (PI: J. M. Kirkwood; Project 2 PI: L. H. Butterfield).
References
- American Cancer Society. Cancer Facts & Figures. 2013. [Google Scholar]
- AOUTHMANY M, WEINSTEIN M, ZIRWAS MJ, BRODELL RT. The natural history of halo nevi: a retrospective case series. J Am Acad Dermatol. 2012;67:582–6. doi: 10.1016/j.jaad.2011.11.937. [DOI] [PubMed] [Google Scholar]
- BANDARCHI B, JABBARI CA, VEDADI A, NAVAB R. Molecular biology of normal melanocytes and melanoma cells. J Clin Pathol. 2013;66:644–8. doi: 10.1136/jclinpath-2013-201471. [DOI] [PubMed] [Google Scholar]
- BARROW C, BROWNING J, MACGREGOR D, DAVIS ID, STURROCK S, JUNGBLUTH AA, CEBON J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin Cancer Res. 2006;12:764–71. doi: 10.1158/1078-0432.CCR-05-1544. [DOI] [PubMed] [Google Scholar]
- BECKER JC, GULDBERG P, ZEUTHEN J, BROCKER EB, STRATEN PT. Accumulation of identical T cells in melanoma and vitiligo-like leukoderma. J Invest Dermatol. 1999;113:1033–8. doi: 10.1046/j.1523-1747.1999.00805.x. [DOI] [PubMed] [Google Scholar]
- BLESSING K, MCLAREN KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315–22. doi: 10.1111/j.1365-2559.1992.tb00988.x. [DOI] [PubMed] [Google Scholar]
- BOASBERG PD, HOON DS, PIRO LD, MARTIN MA, FUJIMOTO A, KRISTEDJA TS, BHACHU S, YE X, DECK RR, O’DAY SJ. Enhanced survival associated with vitiligo expression during maintenance biotherapy for metastatic melanoma. J Invest Dermatol. 2006;126:2658–63. doi: 10.1038/sj.jid.5700545. [DOI] [PubMed] [Google Scholar]
- BOLOGNIA JL, JORIZZO JL, RAPINI RP. Dermatologia (Bolognia, Dermatology) Mosby; 2009. [Google Scholar]
- BORIES N, DALLE S, DEBARBIEUX S, BALME B, RONGER-SAVLE S, THOMAS L. Dermoscopy of fully regressive cutaneous melanoma. Br J Dermatol. 2008;158:1224–9. doi: 10.1111/j.1365-2133.2008.08501.x. [DOI] [PubMed] [Google Scholar]
- BRAHMER JR, TYKODI SS, CHOW LQ, HWU WJ, TOPALIAN SL, HWU P, DRAKE CG, CAMACHO LH, KAUH J, ODUNSI K, PITOT HC, HAMID O, BHATIA S, MARTINS R, EATON K, CHEN S, SALAY TM, ALAPARTHY S, GROSSO JF, KORMAN AJ, PARKER SM, AGRAWAL S, GOLDBERG SM, PARDOLL DM, GUPTA A, WIGGINTON JM. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSTAMANTE J, BREDESTON L, MALANGA G, MORDOH J. Role of melanin as a scavenger of active oxygen species. Pigment Cell Res. 1993;6:348–53. doi: 10.1111/j.1600-0749.1993.tb00612.x. [DOI] [PubMed] [Google Scholar]
- BUTTERFIELD LH, COMIN-ANDUIX B, VUJANOVIC L, LEE Y, DISSETTE VB, YANG JQ, VU HT, SEJA E, OSEGUERA DK, POTTER DM, GLASPY JA, ECONOMOU JS, RIBAS A. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008;31:294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTERFIELD LH, RIBAS A, DISSETTE VB, AMARNANI SN, VU HT, OSEGUERA D, WANG HJ, ELASHOFF RM, MCBRIDE WH, MUKHERJI B, COCHRAN AJ, GLASPY JA, ECONOMOU JS. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- BYRNE KT, TURK MJ. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget. 2011;2:684–94. doi: 10.18632/oncotarget.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BYSTRYN JC, RIGEL D, FRIEDMAN RJ, KOPF A. Prognostic significance of hypopigmentation in malignant melanoma. Arch Dermatol. 1987;123:1053–5. [PubMed] [Google Scholar]
- CHAPMAN PB, HAUSCHILD A, ROBERT C, HAANEN JB, ASCIERTO P, LARKIN J, DUMMER R, GARBE C, TESTORI A, MAIO M, HOGG D, LORIGAN P, LEBBE C, JOUARY T, SCHADENDORF D, RIBAS A, O’DAY SJ, SOSMAN JA, KIRKWOOD JM, EGGERMONT AM, DRENO B, NOLOP K, LI J, NELSON B, HOU J, LEE RJ, FLAHERTY KT, MCARTHUR GA, GROUP BS. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–16. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE MAZIERE AM, MUEHLETHALER K, VAN DONSELAAR E, SALVI S, DAVOUST J, CEROTTINI JC, LEVY F, SLOT JW, RIMOLDI D. The melanocytic protein Melan-A/MART-1 has a subcellular localization distinct from typical melanosomal proteins. Traffic. 2002;3:678–93. doi: 10.1034/j.1600-0854.2002.30909.x. [DOI] [PubMed] [Google Scholar]
- DISIS ML. Immunologic biomarkers as correlates of clinical response to cancer immunotherapy. Cancer Immunol Immunother. 2011;60:433–42. doi: 10.1007/s00262-010-0960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU J, MILLER AJ, WIDLUND HR, HORSTMANN MA, RAMASWAMY S, FISHER DE. MLANA/MART1 and SILV/PMEL17/GP100 are transcriptionally regulated by MITF in melanocytes and melanoma. Am J Pathol. 2003;163:333–43. doi: 10.1016/S0002-9440(10)63657-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUDLEY ME, WUNDERLICH JR, ROBBINS PF, YANG JC, HWU P, SCHWARTZENTRUBER DJ, TOPALIAN SL, SHERRY R, RESTIFO NP, HUBICKI AM, ROBINSON MR, RAFFELD M, DURAY P, SEIPP CA, ROGERS-FREEZER L, MORTON KE, MAVROUKAKIS SA, WHITE DE, ROSENBERG SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EZZEDINE K, LIM HW, SUZUKI T, KATAYAMA I, HAMZAVI I, LAN CC, GOH BK, ANBAR T, SILVA DE CASTRO C, LEE AY, PARSAD D, VAN GEEL N, LE POOLE IC, OISO N, BENZEKRI L, SPRITZ R, GAUTHIER Y, HANN SK, PICARDO M, TAIEB A VITILIGO GLOBAL ISSUE CONSENSUS CONFERENCE P. Revised classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25:E1–13. doi: 10.1111/j.1755-148X.2012.00997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FONTAINE D, PARKHILL W, GREER W, WALSH N. Partial regression of primary cutaneous melanoma: is there an association with sub-clinical sentinel lymph node metastasis? Am J Dermatopathol. 2003;25:371–6. doi: 10.1097/00000372-200310000-00002. [DOI] [PubMed] [Google Scholar]
- GOFF SL, SMITH FO, KLAPPER JA, SHERRY R, WUNDERLICH JR, STEINBERG SM, WHITE D, ROSENBERG SA, DUDLEY ME, YANG JC. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother. 2010;33:840–7. doi: 10.1097/CJI.0b013e3181f05b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOGAS H, IOANNOVICH J, DAFNI U, STAVROPOULOU-GIOKAS C, FRANGIA K, TSOUTSOS D, PANAGIOTOU P, POLYZOS A, PAPADOPOULOS O, STRATIGOS A, MARKOPOULOS C, BAFALOUKOS D, PECTASIDES D, FOUNTZILAS G, KIRKWOOD JM. Prognostic significance of autoimmunity during treatment of melanoma with interferon. N Engl J Med. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
- GUITART J, LOWE L, PIEPKORN M, PRIETO VG, RABKIN MS, RONAN SG, SHEA CR, TRON VA, WHITE W, BARNHILL RL. Histological characteristics of metastasizing thin melanomas: a case-control study of 43 cases. Arch Dermatol. 2002;138:603–8. doi: 10.1001/archderm.138.5.603. [DOI] [PubMed] [Google Scholar]
- HALUSKA F, LINETTE G, JONASH S, HODI S, LONGERICH S, YANG S, WEBB I, STOWELL C, KAPLAN J, ROBERTS B, GOLDBERG M. Immunologic Gene Therapy of melanoma: Phase I Study of Therapy with Dendritic Cells Transduced with Recombinant Adenoviruses Encoding Melanoma Antigens (Abstract 1777) Proc Am Soc Clin Oncol (Abstract) 2000;19:453a. [Google Scholar]
- HANN SK, NORDLUND JJ. Vitiligo: A Monograph on the Basic and Clinical Science. Malden, MA: Wiley, John & Sons, Inc; 2000. [Google Scholar]
- HARTMANN A, BEDENK C, KEIKAVOUSSI P, BECKER JC, HAMM H, BROCKER EB. Vitiligo and melanoma-associated hypopigmentation (MAH): shared and discriminative features. J Dtsch Dermatol Ges. 2008;6:1053–9. doi: 10.1111/j.1610-0387.2008.06755.x. [DOI] [PubMed] [Google Scholar]
- HEMON P, JEAN-LOUIS F, RAMGOLAM K, BRIGNONE C, VIGUIER M, BACHELEZ H, TRIEBEL F, CHARRON D, AOUDJIT F, AL-DACCAK R, MICHEL L. MHC class II engagement by its ligand LAG-3 (CD223) contributes to melanoma resistance to apoptosis. J Immunol. 2011;186:5173–83. doi: 10.4049/jimmunol.1002050. [DOI] [PubMed] [Google Scholar]
- HIGH WA, STEWART D, WILBERS CR, COCKERELL CJ, HOANG MP, FITZPATRICK JE. Completely regressed primary cutaneous malignant melanoma with nodal and/or visceral metastases: a report of 5 cases and assessment of the literature and diagnostic criteria. J Am Acad Dermatol. 2005;53:89–100. doi: 10.1016/j.jaad.2005.03.006. [DOI] [PubMed] [Google Scholar]
- HOASHI T, TAMAKI K, HEARING VJ. The secreted form of a melanocyte membrane-bound glycoprotein (Pmel17/gp100) is released by ectodomain shedding. FASEB J. 2010;24:916–30. doi: 10.1096/fj.09-140921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOASHI T, WATABE H, MULLER J, YAMAGUCHI Y, VIEIRA WD, HEARING VJ. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J Biol Chem. 2005;280:14006–16. doi: 10.1074/jbc.M413692200. [DOI] [PubMed] [Google Scholar]
- HODGKINSON CA, MOORE KJ, NAKAYAMA A, STEINGRIMSSON E, COPELAND NG, JENKINS NA, ARNHEITER H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- HODI FS, O’DAY SJ, MCDERMOTT DF, WEBER RW, SOSMAN JA, HAANEN JB, GONZALEZ R, ROBERT C, SCHADENDORF D, HASSEL JC, AKERLEY W, VAN DEN EERTWEGH AJ, LUTZKY J, LORIGAN P, VAUBEL JM, LINETTE GP, HOGG D, OTTENSMEIER CH, LEBBE C, PESCHEL C, QUIRT I, CLARK JI, WOLCHOK JD, WEBER JS, TIAN J, YELLIN MJ, NICHOL GM, HOOS A, URBA WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IRVINE DJ, PURBHOO MA, KROGSGAARD M, DAVIS MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- JANSSEN EM, DROIN NM, LEMMENS EE, PINKOSKI MJ, BENSINGER SJ, EHST BD, GRIFFITH TS, GREEN DR, SCHOENBERGER SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- KAUR C, THOMAS RJ, DESAI N, GREEN MA, LOVELL D, POWELL BW, COOK MG. The correlation of regression in primary melanoma with sentinel lymph node status. J Clin Pathol. 2008;61:297–300. doi: 10.1136/jcp.2007.049411. [DOI] [PubMed] [Google Scholar]
- KOLM I, DI STEFANI A, HOFMANN-WELLENHOF R, FINK-PUCHES R, WOLF IH, RICHTIG E, SMOLLE J, KERL H, SOYER HP, ZALAUDEK I. Dermoscopy patterns of halo nevi. Arch Dermatol. 2006;142:1627–32. doi: 10.1001/archderm.142.12.1627. [DOI] [PubMed] [Google Scholar]
- LEWIS MG, COPEMAN PW. Halo naevus--a frustrated malignant maelanoma. Br Med J. 1972;2:47–8. doi: 10.1136/bmj.2.5804.47-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELLMAN I, COUKOS G, DRANOFF G. Cancer immunotherapy comes of age. Nature. 2011;480:480–9. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MENZIES SW, MCCARTHY WH. Complete regression of primary cutaneous malignant melanoma. Arch Surg. 1997;132:553–6. doi: 10.1001/archsurg.1997.01430290099020. [DOI] [PubMed] [Google Scholar]
- MORETTI S, SPALLANZANI A, PINZI C, PRIGNANO F, FABBRI P. Fibrosis in regressing melanoma versus nonfibrosis in halo nevus upon melanocyte disappearance: could it be related to a different cytokine microenvironment? J Cutan Pathol. 2007;34:301–8. doi: 10.1111/j.1600-0560.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- MORRIS KT, BUSAM KJ, BERO S, PATEL A, BRADY MS. Primary cutaneous melanoma with regression does not require a lower threshold for sentinel lymph node biopsy. Ann Surg Oncol. 2008;15:316–22. doi: 10.1245/s10434-007-9675-2. [DOI] [PubMed] [Google Scholar]
- MUSETTE P, BACHELEZ H, FLAGEUL B, DELARBRE C, KOURILSKY P, DUBERTRET L, GACHELIN G. Immune-mediated destruction of melanocytes in halo nevi is associated with the local expansion of a limited number of T cell clones. J Immunol. 1999;162:1789–94. [PubMed] [Google Scholar]
- NAZARIAN RM, PRIETO VG, ELDER DE, DUNCAN LM. Melanoma biomarker expression in melanocytic tumor progression: a tissue microarray study. J Cutan Pathol. 2010;37(Suppl 1):41–7. doi: 10.1111/j.1600-0560.2010.01505.x. [DOI] [PubMed] [Google Scholar]
- NORDLUND JJ, KIRKWOOD JM, FORGET BM, MILTON G, ALBERT DM, LERNER AB. Vitiligo in patients with metastatic melanoma: a good prognostic sign. J Am Acad Dermatol. 1983;9:689–96. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- OLAH J, GYULAI R, KOROM I, VARGA E, DOBOZY A. Tumour regression predicts higher risk of sentinel node involvement in thin cutaneous melanomas. Br J Dermatol. 2003;149:662–3. doi: 10.1046/j.1365-2133.2003.05502.x. [DOI] [PubMed] [Google Scholar]
- OVERWIJK WW, RESTIFO NP. Autoimmunity and the immunotherapy of cancer: targeting the “self” to destroy the “other”. Crit Rev Immunol. 2000;20:433–50. [PMC free article] [PubMed] [Google Scholar]
- QUAGLINO P, MARENCO F, OSELLA-ABATE S, CAPPELLO N, ORTONCELLI M, SALOMONE B, FIERRO MT, SAVOIA P, BERNENGO MG. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational cohort study. Ann Oncol. 2010;21:409–14. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- QUAGLINO P, ORTONCELLI M, COMESSATTI A, PONTI R, NOVELLI M, BERGALLO M, COSTA C, CICCHELLI S, SAVOIA P, BERNENGO MG. Circulating CD4+CD25 bright FOXP3+ T cells are up-regulated by biological therapies and correlate with the clinical response in psoriasis patients. Dermatology. 2009;219:250–8. doi: 10.1159/000238305. [DOI] [PubMed] [Google Scholar]
- RAM M, SHOENFELD Y. Harnessing autoimmunity (vitiligo) to treat melanoma: a myth or reality? Ann N Y Acad Sci. 2007;1110:410–25. doi: 10.1196/annals.1423.043. [DOI] [PubMed] [Google Scholar]
- RAO UN, LEE SJ, LUO W, MIHM MC, JR, KIRKWOOD JM. Presence of tumor-infiltrating lymphocytes and a dominant nodule within primary melanoma are prognostic factors for relapse-free survival of patients with thick (t4) primary melanoma: pathologic analysis of the e1690 and e1694 intergroup trials. Am J Clin Pathol. 2010;133:646–53. doi: 10.1309/AJCPTXMEFOVYWDA6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REQUENA C, BOTELLA-ESTRADA R, TRAVES V, NAGORE E, ALMENAR S, GUILLEN C. Problems in defining melanoma regression and prognostic implication. Actas Dermosifiliogr. 2009;100:759–66. [PubMed] [Google Scholar]
- RIBAS A, GLASPY JA, LEE Y, DISSETTE VB, SEJA E, VU HT, TCHEKMEDYIAN NS, OSEGUERA D, COMIN-ANDUIX B, WARGO JA, AMARNANI SN, MCBRIDE WH, ECONOMOU JS, BUTTERFIELD LH. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27:354–67. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- RIMOLDI D, MUEHLETHALER K, SALVI S, VALMORI D, ROMERO P, CEROTTINI JC, LEVY F. Subcellular localization of the melanoma-associated protein Melan-AMART-1 influences the processing of its HLA-A2-restricted epitope. J Biol Chem. 2001;276:43189–96. doi: 10.1074/jbc.M103221200. [DOI] [PubMed] [Google Scholar]
- RONGIOLETTI F, CECCHI F, REBORA A. Halo phenomenon in melanocytic nevi (Sutton’s nevi). Does the diameter matter? J Eur Acad Dermatol Venereol. 2011;25:1231–2. doi: 10.1111/j.1468-3083.2010.03790.x. [DOI] [PubMed] [Google Scholar]
- ROSENBERG SA, WHITE DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–4. [PubMed] [Google Scholar]
- RUBEGNI P, NAMI N, RISULO M, TATARANNO D, FIMIANI M. Melanoma with halo. Clin Exp Dermatol. 2009;34:749–50. doi: 10.1111/j.1365-2230.2008.03030.x. [DOI] [PubMed] [Google Scholar]
- SCHALLREUTER KU, LEVENIG C, BERGER J. Vitiligo and cutaneous melanoma. A case study. Dermatologica. 1991;183:239–45. doi: 10.1159/000247693. [DOI] [PubMed] [Google Scholar]
- SOSMAN JA, KIM KB, SCHUCHTER L, GONZALEZ R, PAVLICK AC, WEBER JS, MCARTHUR GA, HUTSON TE, MOSCHOS SJ, FLAHERTY KT, HERSEY P, KEFFORD R, LAWRENCE D, PUZANOV I, LEWIS KD, AMARAVADI RK, CHMIELOWSKI B, LAWRENCE HJ, SHYR Y, YE F, LI J, NOLOP KB, LEE RJ, JOE AK, RIBAS A. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–14. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEECKAERT R, VAN GEEL N, LUITEN RM, VAN GELE M, SPEECKAERT M, LAMBERT J, VERMAELEN K, TJIN EP, BROCHEZ L. Melanocyte-specific immune response in a patient with multiple regressing nevi and a history of melanoma. Anticancer Res. 2011;31:3697–703. [PubMed] [Google Scholar]
- TEULINGS HE, TJIN EP, WILLEMSEN KJ, KREBBERS G, VAN NOESEL CJ, KEMP EH, NIEUWEBOER-KROBOTOVA L, VAN DER VEEN JP, LUITEN RM. Radiation-induced melanoma-associated leucoderma, systemic antimelanoma immunity and disease-free survival in a patient with advanced-stage melanoma: a case report and immunological analysis. Br J Dermatol. 2013 doi: 10.1111/bjd.12136. [DOI] [PubMed] [Google Scholar]
- THEOS AC, TRUSCHEL ST, RAPOSO G, MARKS MS. The Silver locus product Pmel17/gp100/Silv/ME20: controversial in name and in function. Pigment Cell Res. 2005;18:322–36. doi: 10.1111/j.1600-0749.2005.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOPALIAN SL, HODI FS, BRAHMER JR, GETTINGER SN, SMITH DC, MCDERMOTT DF, POWDERLY JD, CARVAJAL RD, SOSMAN JA, ATKINS MB, LEMING PD, SPIGEL DR, ANTONIA SJ, HORN L, DRAKE CG, PARDOLL DM, CHEN L, SHARFMAN WH, ANDERS RA, TAUBE JM, MCMILLER TL, XU H, KORMAN AJ, JURE-KUNKEL M, AGRAWAL S, MCDONALD D, KOLLIA GD, GUPTA A, WIGGINTON JM, SZNOL M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAO H, MILLMAN P, LINETTE GP, HODI FS, SOBER AJ, GOLDBERG MA, HALUSKA FG. Hypopigmentation associated with an adenovirus-mediated gp100/MART-1-transduced dendritic cell vaccine for metastatic melanoma. Arch Dermatol. 2002;138:799–802. doi: 10.1001/archderm.138.6.799. [DOI] [PubMed] [Google Scholar]
- VAN GEEL N, SPEECKAERT R, LAMBERT J, MOLLET I, DE KEYSER S, DE SCHEPPER S, BROCHEZ L. Halo naevi with associated vitiligo-like depigmentations: pathogenetic hypothesis. J Eur Acad Dermatol Venereol. 2012;26:755–61. doi: 10.1111/j.1468-3083.2011.04160.x. [DOI] [PubMed] [Google Scholar]
- WOLCHOK JD, KLUGER H, CALLAHAN MK, POSTOW MA, RIZVI NA, LESOKHIN AM, SEGAL NH, ARIYAN CE, GORDON RA, REED K, BURKE MM, CALDWELL A, KRONENBERG SA, AGUNWAMBA BU, ZHANG X, LOWY I, INZUNZA HD, FEELY W, HORAK CE, HONG Q, KORMAN AJ, WIGGINTON JM, GUPTA A, SZNOL M. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Y, LIN X, FU W, LUO X, KANG K. An approach to the correlation between vitiligo and autoimmune thyroiditis in Chinese children. Clin Exp Dermatol. 2010;35:706–10. doi: 10.1111/j.1365-2230.2009.03671.x. [DOI] [PubMed] [Google Scholar]
- ZEFF RA, FREITAG A, GRIN CM, GRANT-KELS JM. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620–4. doi: 10.1016/s0190-9622(97)70181-6. [DOI] [PubMed] [Google Scholar]
- ZIPPELIUS A, PITTET MJ, BATARD P, RUFER N, DE SMEDT M, GUILLAUME P, ELLEFSEN K, VALMORI D, LIENARD D, PLUM J, MACDONALD HR, SPEISER DE, CEROTTINI JC, ROMERO P. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J Exp Med. 2002;195:485–94. doi: 10.1084/jem.20011658. [DOI] [PMC free article] [PubMed] [Google Scholar]