Abstract
Major depressive disorder (MDD) has been associated with reduced leukocyte telomere length (LTL). It is not known, however, whether psychosocial and behavioral protective factors moderate this association. In the current study, we examine whether multisystem resiliency – defined by healthy emotion regulation, strong social connections, and health behaviors (sleep and exercise) – predicts LTL and mitigates previously demonstrated associations between depression diagnosis and LTL. LTL was measured, using a quantitative PCR assay, in 954 patients with stable cardiovascular disease in the Heart and Soul Study. In a fully adjusted model, high multisystem resiliency predicted longer LTL (b = 80.00, SE = 27.17, p = .003), whereas each individual factor did not. Multisystem resiliency significantly moderated the MDD-LTL association (p = .02). Specifically, MDD was significantly related to LTL at 1 SD below the mean of multisystem resiliency (b = −142.86, SE = 56.46, p = .01), but not at 1 SD above the mean (b = 49.07, SE = 74.51, p = .51). This study suggests that MDD associations with biological outcomes should be examined within a psychosocial–behavioral context, because this context shapes the nature of the direct relationship. Further research should explore the cognitive, neural, and other physiological pathways through which multisystem resiliency may confer biological benefit.
Keywords: Major depressive disorder, Telomeres, Cell aging, Resiliency, Social connections, Emotion regulation, Physical activity
1. Introduction
Major depressive disorder (MDD) plays a significant role in the development of aging related disorders (Evans et al., 2005; Kiecolt-Glaser and Glaser, 2002), including cardiovascular disease (CVD) (Musselman et al., 1998), immune disorders (Irwin and Miller, 2007), and early mortality (Arfken et al., 1999; Schulz et al., 2000). Key biological pathways in the pathogeneses of these diseases have also been linked to depression, including insulin resistance (Winokur et al., 1988), glucocorticoid resistance (Pariante and Miller, 2001), inflammation (Miller et al., 2009), oxidative stress (Robert, 2000), and cellular aging (Wolkowitz et al., 2010).
In recent years, increasing attention has been directed to the role of cellular aging in health, indexed in part by leukocyte telomere length (LTL) (Epel, 2009; Wolkowitz et al., 2010). Telomeres are protective DNA-protein complex caps at the ends of chromosomes, and shortening of the telomeric DNA repeat tracts is associated with increased risk for aging-related diseases. The cellular enzyme telomerase adds the telomeric TTAGGG repeat sequences onto the ends of telomeres, counteracting or delaying shortening during cell division. If telomeres become critically short, normal cells commonly enter a senescent state, marked by overproduction and release of pro-inflammatory cytokines (Blackburn, 2010). Telomeres are not solely biomarkers of disease, as experiments in rodents implicate telomere shortening and lower telomerase activity as causes of mitochondrial damage, increased oxidative stress, and damage to tissues (Jaskelioff et al., 2011; Perez-Rivero et al., 2006; Sahin et al., 2011).
To date, several studies have associated either current MDD (Hartmann et al., 2010; Simon et al., 2006; Wikgren et al., 2012) or history of MDD (Wolkowitz et al., 2011) with shorter telomeres in healthy patients. Additionally, our recent work suggests that both diagnosed depression and depressive symptomatology are related to shorter telomeres in patients with coronary heart disease (Hoen et al., 2011). Protective intrapersonal, interpersonal, and behavioral factors that mitigate the associations between MDD and LTL remain unexplored. A large literature highlights key psychological, social, and behavioral features that predict physical health. In the current study, we examine the independent and combined associations of such key features with telomere length and in moderating the depression–telomere length association. We’ve recently proposed that emotion regulation, social connections, and healthy behaviors can combine to moderate associations between psychological distress and cellular aging (Puterman and Epel, 2012), and we test this proposal in this study.
1.1. Emotion regulation
There is significant work demonstrating the links between psychological traits such as optimism/pessimism (Brummett et al., 2006; Giltay et al., 2004; Maruta et al., 2002; Scheier and Carver, 1987), hostility (Smith et al., 2004; Tindle et al., 2009), and mastery (Mausbach et al., 2007; Pudrovska et al., 2005) with physical health outcomes, including telomere length (Brydon et al., 2011; Epel et al., 2006; O’Donovan et al., 2009). One important candidate pathway linking psychological traits to health outcomes is emotion regulation (John and Gross, 2007). It is well established that poor emotion regulation strategies, such as emotion suppression, are associated with autonomic arousal and cardiovascular disease (Gross and Levenson, 1993, 1997; Kubzansky et al., 2011) and thus relevant constructs for links with cellular aging. However, no studies to date have examined emotion regulation and telomeres.
1.2. Social connections
Social connections are important to health (Cohen, 2004; Uchino, 2009). Socially isolated individuals are at increasing risk of cardiovascular disease (Barth et al., 2010; Shankar et al., 2011) and early mortality (Steptoe et al., 2013). Social connections especially benefit those experiencing elevated psychosocial distress (Cohen and Wills, 1985; Hawkley and Cacioppo, 2004; Uchino, 2006). Recent studies suggest that married individuals (Mainous et al., 2011), those with healthy social ties (Uchino et al., 2012), and those with greater perceived social support (Carroll et al., 2013) have longer telomeres. Whether social connections are related to telomeres in individuals with heart disease or whether it can moderate the relationship between depression and telomere length remains unexplored.
1.3. Healthy behaviors
Forty percent of mortality is attributable to health behaviors (McGinnis and Foege, 1993; Mokdad et al., 2004). Health behaviors include multiple domains, such as physical activity, diet, sleep, smoking, alcohol intake, seatbelt use – to name a few. Of interest to the current study of depression and telomere length, physical activity and sleep have individually been shown to compound or mitigate the effects of psychological distress on health-related outcomes. For example, psychological distress (i.e. depressed or stressed) is related to higher circulating interleukin-6 levels (Rethorst et al., 2011), shorter telomeres (Puterman et al., 2010b), and higher fasting glucose (Puterman et al., 2012) in physically inactive participants but not in those who are active. Research in rodents also suggests that sleep deprivation alters biological stress responses that shape biological health (Meerlo et al., 2002; Sgoifo et al., 2006) and these effects appear to extend to humans as well (Meerlo et al., 2008). While current research demonstrates that physical activity (Cherkas et al., 2008; Ludlow et al., 2008; Werner et al., 2009) and sleep quality (Liang et al., 2011; Prather et al., 2011) are directly related to telomere length, little is known about how these health behaviors combine with other resiliency factors to moderate the relationship between psychological distress and telomere length.
Examining these relationships in isolation may enhance understanding of the specific biological mechanisms through which each factor protects individuals from disease development. However, examining multisystem resiliency – how these factors that span systems (intrapersonal, interpersonal, and behavioral) work in concert – may prove to be of equal or more important clinical significance and utility (Puterman and Epel, 2012). Resiliency comes in many intrapersonal and interpersonal forms, including ways of coping and responding to emotions, health behaviors such as physical activity, sleep, and diet, and social features of connections with others (Ryff et al., 2012). These factors tend to cluster together naturally in people (Low et al., 2011; Sun et al., in press; Taylor and Seeman, 1999), which makes the cluster perhaps more phenotypically representative. There is growing interest in examining a combination of psychosocial and lifestyle factors as resiliency that decreases disease risk (Agrigoroaei and Lachman, 2011; Low et al., 2011; Taylor et al., 2000). Recent work by Lachman and colleagues (Agrigoroaei and Lachman, 2011) found that combining psychosocial and behavioral resiliency factors, specifically social connections, physical activity and sense of control, moderates the relationship between socioeconomic position and cognitive functioning.
No studies have examined how emotional regulation, social connections, and healthy behaviors combine and how they are related biological outcomes, or how they confer biological benefit to those with MDD. Thus, in the current study of patients with stable heart disease (Whooley et al., 2008), we test whether these three factors, independently or combined, are directly associated with longer leukocyte telomere length. In addition, we test whether the previously demonstrated link between MDD and LTL (Hoen et al., 2011) can be moderated by multisystem resiliency.
2. Methods
2.1. Participants
The Heart and Soul Study is a prospective cohort study designed to examine the mechanisms through which psychological factors predict risk of CVD events and mortality in patients with stable CVD. Administrative data were used to identify outpatients with documented CVD at two Department of Veterans Affairs Medical Centers (San Francisco VA Medical Center and the VA Palo Alto Health Care System, California), one University medical center (University of California, San Francisco), and nine public health clinics in the Community Health Network of San Francisco. The protocol was approved by the appropriate institutional review boards (Committee on Human Research, University of California, San Francisco; Research and Development Committee, VA Medical Center, San Francisco; Medical Human Subjects Committee, Stanford University, Stanford, California; Human Subjects Committee, Veterans Affairs Palo Alto Health Care System, Palo Alto, California; and the Data Governance Board of the Community Health Network, San Francisco). All participants provided written informed consent. Eligible patients had known CVD documented by at least one of the following: a history of myocardial infarction, angiographic evidence of ≥50% stenosis in one of more coronary vessels, prior evidence of inducible ischemia by treadmill or nuclear testing, or a history of coronary revascularization. A total of 1024 participants were enrolled (age range: 45–90 years) and methods are described elsewhere (Whooley et al., 2008). Telomere length was assayed in 954 participants. Of these, two did not have a structured clinical interview, and four did not have complete data on predictors of interest. This left 948 participants for the present analyses.
2.2. Procedure
Study participants were instructed to (1) not use aspirin for 1 week, (2) not eat for 12 h (except for medications, which they were instructed to take with water), and (3) not smoke for 5 h before their study appointments. At their appointments, participants donated morning blood samples, completed standardized questionnaires and medical histories, and underwent a resting echocardiogram to assess cardiac function.
3. Materials
3.1. Outcome
Telomere Length
Methods of the telomere length assay in The Heart and Soul Study have been described previously (Farzaneh-Far et al., 2010). According to standard procedures, genomic DNA was isolated from the peripheral blood leukocytes that were stored at −70 °C. Purified DNA samples were diluted to a fixed concentration of 3 ng/ul. A quantitative polymerase chain reaction-based assay measured the relative mean LTL. This assay compares the mean telomere repeat sequence copy number (T) to a reference single copy gene copy number (S) in each sample. Standard curves were derived from serially diluted reference DNA. The T/S ratio was calculated from the average quantity of the reference DNA found to match with each experimental sample for the concentration of the targeted template (for T: telomere repeats, and for S: beta-globin gene). The inter-assay coefficient of variability for LTL measurement was 3.7%, and the intra-assay coefficient of variability was 2.5%. Staff conducting assays were blinded to knowledge of depression status. The equation for conversion from T/S ratio to base pairs, derived from comparing T/S and telomeric restriction fragment (TRF) by Southern blot analysis of the human primary fibroblast cell line IMR 90, was base pairs = 3274 + 2413* (T/S).
3.2. Predictor variables
(a) Emotion Suppression
In the current study, emotion regulation was assessed with the four-item Emotion Suppression Scale from the Emotion Regulation Questionnaire (Gross and John, 2003). Example items are “When I am feeling negative emotions, I make sure not to express them” and “I control my emotions by not expressing them,” rated on a 5-point scale from 1 = strongly agree to 5 = strongly disagree. Lower levels of suppression are thought to reflect a more adaptive emotion regulation profile. Items were summed and the composite score was standardized (Cronbach’s alpha was .79).
(b) Social Connections
The widely used 12-item short form of the Interpersonal Support Evaluation List (Cohen et al., 1985) measures levels of perceived social support on a scale from 1 = definitely false to 4 = definitely true. Higher summed values suggest greater social connections (Cronbach’s alpha was .86).
(c) Health behaviors
The Heart and Soul Study evaluated physical activity and sleep quality, and these are included in the present conceptualization of health behaviors that may combine to promote behavioral resiliency. An averaged score of standardized physical activity and sleep quality was computed. (1) Physical Activity. Participants rated a one-item question describing how active they were over the previous month, including activities, such as brisk walking, swimming, general conditioning, or recreational sports, that were done for at least 15–20 min. Participants selected one of six options, from 0 = not at all active, 1 = a little active (1–2 times per month), 2 = fairly active (3–4 times per month), 3 = quite active (1–2 times per week), 4 = very active (3–4 times per week), 5 = extremely active (5 or more times per week). This single item physical activity measure has previously been shown to be a strong predictor of subsequent cardiovascular events among cardiac patients with high depressive symptoms (Whooley et al., 2008). (2) Sleep quality. Sleep quality was assessed with a one item question, “During the last month, how would you rate your sleep quality overall?” on a five point scale from 0 = “Very bad” to 4 = “very good”. Physical activity and sleep quality were standardized and averaged for a Healthy Behaviors factor.
(d) Multisystem Resiliency
The standardized scores for emotion suppression, social connections scale, and healthy behaviors were averaged to capture multisystem resiliency. Higher values for multisystem resiliency indicate low emotion suppression, stronger social connections, and healthier behaviors.
(e) Assessment of Depression
Presence of MDD in the past month was diagnosed according to Diagnostic and Statistical Manual, Fourth Edition, criteria. We used the modified Computerized National Institute of Mental Health Diagnostic Interview Schedule (CDIS-IV), a structured and validated interview designed to yield psychiatric diagnosis (Robins et al., 1981). Trained research assistants administered the interview during the appointment.
(f) Covariates
Confounders included: (1) sociodemographic factors (age, education level achieved, sex, race; completed with standardized questionnaires); (2) medication use (statins, aspirin, antidepressants, angiotensin receptor blockers, angiotensin converting enzyme inhibitors; participants were asked to bring their medication bottles to the study visit and current medications were recorded by study staff); (3) self-reported comorbid conditions (hypertension, congestive heart failure, stroke, diabetes mellitus, asthma); (4) body mass index (kg/m2; measured by trained technicians according to a standardized protocol); (5) current smoking status (yes/no); and (6) resting left ventricular ejection fraction (echocardiography using an Acuson Sequoia Ultrasound System with a 3.5 MHz transducer (Siemens, Mountain View, California).
4. Statistical approach
We used Pearson correlations, t-tests, and Chi square tests to compare covariates in those with versus without current MDD. We also used Pearson correlations and t-tests to examine the association of the covariates with LTL. Next, we regressed LTL on multisystem resiliency, first in an unadjusted model and then in a model accounting for covariates [Step 1: sociodemographics (age, gender, education and race); Step 2: health conditions (self reported morbidities of hypertension, congestive heart failure, stroke, diabetes mellitus, asthma); Step 3: medication use (statins, aspirin, antidepressants, angiotensin receptor blockers, and/or angiotensin converting enzyme inhibitors); and Step 4: BMI, smoking status and resting left ventricular ejection fraction]. We also tested the independent effects of standardized emotion suppression, social connections, physical activity, and sleep quality as independent variables in the same model, both in an unadjusted model and a model including the four groups of covariates. Next, we tested whether mean-centered multisystem resiliency moderated the association between MDD diagnosis and LTL by following the method outlined by Cohen et al. (2003), and followed the analyses with simple slope tests at one standard deviation above (+1 SD) and one standard deviation below (−1 SD) the mean of multisystem resiliency. We further tested the log-odds of being in the bottom 25% of leukocyte telomere length compared to top 75% for those with a MDD diagnosis compared to no diagnosis at −1 and +1 SD of the mean of multisystem resiliency. All analyses were performed with SPSS version 20.0.
5. Results
Participant characteristics for those with versus without a MDD diagnosis are presented in Table 1. Participants with MDD were more likely to be younger, female, diagnosed with diabetes, current smokers, taking anti-depressants, and to have a higher resting left ventricular ejection fraction (all p-values <.05). Depressed participants also had significantly shorter telomeres (adjusted for age and sex), significantly weaker social connections, poorer sleep quality, and less physical activity (whether examined as categorical with chi square statistic or considered continuous with t-test), and had a lower multisystem resiliency than those who were not depressed. Longer telomere length was significantly associated with younger age (r(948) = −.18, p < .001), higher resting left ventricular ejection fraction (r(948) = .09, p = .005), no history of stroke (t(944) = 2.77, p = .006), and use of daily aspirin (t(934) = −3.17, p = .002). Evaluation of correlations among resiliency variables revealed those with greater social connections had higher levels of physical activity (r(948) = .21, p < .001), better sleep (r(948) = .27, p < .001), and lower emotion suppression (r(948) = .25, p < .001). Better sleep quality was associated with greater physical activity (r(948) = .12, p < .001) and lower emotion suppression (r(948) = .14, p < .001). Greater physical activity and low emotion suppression were marginally correlated with a small effect size (r(948) = .06, p = .09). Zero-order correlations between each factor and the combined resiliency factor ranged from .43 to .77.
Table 1.
Characteristics of participants in Heart and Soul Study sample by major depressive disorder diagnosis.
| MDD diagnosis
|
Test statistic | P | ||
|---|---|---|---|---|
| No (N = 743; % = 78.4) | Yes (N = 205; % = 21.6) | |||
| Age, mean (SD) | 68.2 (10.5) | 61.7 (10.8) | t = 7.76 | <.001 |
| Male sex, no. (%) | 629 (84.7) | 143 (69.8) | χ2 = 23.60 | <.001 |
| White, no. (%) | 448 (60.3) | 123 (60.0) | χ2 = 0.01 | 0.94 |
| Body mass index (kg/m2) mean (SD) | 28.29 (5.31) | 29.01 (5.68) | t = −1.70 | .09 |
| Smoking status, current no. (%) | 131 (17.6) | 58 (28.4) | χ2 = 11.69 | .001 |
| Ejection fraction mean (SD) | 0.61 (0.10) | 0.63 (0.07) | t = −2.80 | .005 |
| Comorbid conditions | ||||
| Diabetes no. (%) | 184 (24.8) | 66 (32.2) | χ2 = 4.52 | .04 |
| Stroke no. (%) | 104 (14.0) | 28 (13.7) | χ2 = 0.01 | 1.00 |
| Congestive heart failure no. (%) | 125 (16.9) | 37 (18.1) | χ2 = .17 | .68 |
| Hypertension no. (%) | 525 (70.8) | 145 (70.7) | χ2 = .00 | 1.00 |
| Asthma no. (%) | 111 (14.9) | 42 (20.6) | χ2 = 3.77 | .07 |
| Medications | ||||
| Statins no. (%) | 490 (66.8) | 119 (58.6) | χ2 = 4.74 | .03 |
| Aspirin no. (%) | 534 (72.9) | 150 (73.9) | χ2 = 0.09 | .79 |
| Angiotensin receptor blockers/angiotensin converting enzyme inhibitors; no. (%) | 385 (52.5) | 102 (50.2) | χ2 = 0.33 | .58 |
| Antidepressants no. (%) | 78 (10.6) | 99 (48.8) | χ2 = 150.71 | <.001 |
| Physical activity mean (SD) | 2.49 (1.68) | 2.05 (1.67) | t = 3.28 | .001 |
| Not at all active, no. (%) | 129 (17.4) | 47 (22.9) | χ2 = 13.43 | .02 |
| A little active, no. (%) | 121 (16.3) | 46 (22.4) | ||
| Fairly active, no. (%) | 108 (14.5) | 33 (16.1) | ||
| Quite active, no. (%) | 119 (16.0) | 28 (13.7) | ||
| Very active, no. (%) | 175 (23.6) | 30 (14.6) | ||
| Extremely active, no. (%) | 91 (12.2) | 21 (10.2) | ||
| Sleep quality (mean, SD) | 2.30 (1.08) | 1.74 (1.08) | t = 6.59 | <.001 |
| Very good, no (%) | 38 (5.1) | 27 (13.1) | χ2 = 42.77 | <.001 |
| Fairly good, no (%) | 133 (17.9) | 61 (42.7) | ||
| Good, no (%) | 248 (33.3) | 67 (32.5) | ||
| Fairly bad, no (%) | 217 (29.1) | 40 (19.4) | ||
| Very bad, no (%) | 109 (14.6) | 11 (5.3) | ||
| Emotion suppression (reversed) mean (%) | 12.52 (3.32) | 12.08 (3.53) | t = 1.64 | .10 |
| Social connections mean (%) | 38.52 (7.05) | 33.16 (7.43) | t = 9.55 | <.001 |
| Multisystem resiliency mean (%) | 0.05 (0.62) | −0.32 (0.66) | t = 8.35 | <.001 |
| Leukocyte telomere length+ (base pairs), mean (SD) | 5453 (538) | 5411 (489 | F = 5.42 | .02 |
Adjusted for sex and age.
In the unadjusted model, greater multisystem resiliency was associated with significantly longer LTL (B = 0.10, p = .003), and this result was unchanged in the fully adjusted model (B = 0.10, p = .003). Multisystem resiliency contributes approximately 1% to the variation in telomere length, similar to that of resting left ventricular ejection fraction but less than age (4% of variation in the current model). As seen in Table 2, Model A, in the fully adjusted model, a one standard deviation increase in multisystem resiliency was significantly associated with a 80 base pairs increase in LTL. We further examined the odds of being categorized in the bottom 25% versus top 75% of LTL in the sample as a function of multisystem resiliency. A one-unit increase in multisystem resiliency predicted a 36% greater likelihood of being in the top 75% of LTL (B = .31, odds ratio = 1.36, p = .02, 95% CI = 1.06, 1.74).
Table 2.
Adjusted associations of telomere length and reverse coded emotion suppression, social connections, and physical activity combined as multisystem resiliency (Model A) and as independent factors (Model B).
| Leukocyte telomere length (base pairs)
|
||||||
|---|---|---|---|---|---|---|
| Model A
|
Model B
|
|||||
| b | SE | 95% C.I. | b | SE | 95% C.I. | |
| Multisystem resiliency | 80.00 | 27.17 | 26.68, 133.31 | – | – | – |
| Emotion suppressiona | – | – | – | 12.07 | 18.22 | −23.68, 47.83 |
| Social connections | – | – | – | 22.48 | 18.60 | −14.02, 58.97 |
| Physical activity | – | – | – | 27.70 | 17.97 | −7.57, 62.97 |
| Sleep quality | – | – | – | 30.49 | 17.97 | −4.78, 65.77 |
Note: Models are adjusted for sociodemographics (age, race, sex, education level), chronic conditions (hypertension, congestive heart failure, stroke, diabetes mellitus, asthma), medication use (statins, aspirin, antidepressants, angiotensin receptor blockers, the angiotensin converting enzyme inhibitors), smoking status, BMI, and resting left ventricular ejection fraction. Z-scored emotion suppression, social connections, physical activity, and sleep quality were included in Model B.
Emotion Suppression is reverse coded such that a positive relationship with telomere length suggests that low emotion suppression is associated with longer telomeres.
When examining each variable independently in an unadjusted model, however, social connections and sleep quality were not significantly associated with LTL (B = 0.02, p = .62 and B = 0.03, p = .35, respectively), whereas low emotion suppression (reversed; B = 0.06, p = .08) and physical activity (B = 0.06, p = .06) were marginally associated with LTL. Table 2, Model B presents the results of the fully adjusted regression model examining the associations of LTL with emotion suppression (reverse scored), social connections, physical activity and sleep quality. Emotion suppression (B = 0.02, p = .51), social connections (B = 0.04, p = .23), physical activity (B = 0.05, p = .12) and sleep quality (B = 0.06, p = .09) were not significantly independently associated with LTL in the model adjusted for demographics, comorbid medical conditions, medication use, and other health behaviors/status.
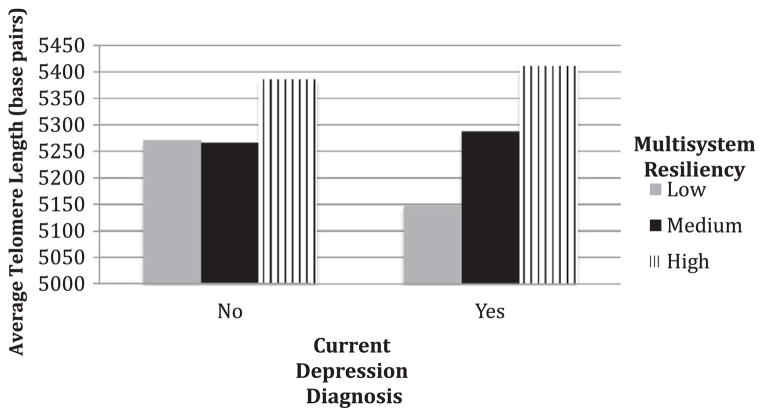
Next, we tested whether the relationship between MDD and LTL was moderated by multisystem resiliency. We included all covariates from the previous regression models in the moderation analysis. Results indicated a significant moderation effect of MDD-LTL association by multisystem resiliency (interaction B = 148.12, SE = 63.06, p = .02). At high levels of multisystem resiliency (1 SD above the mean), MDD was unrelated to LTL (b = 49.07, SE = 74.51, p = .51). At low levels of multisystem resiliency (1 SD below the mean), a diagnosis of MDD was related to an average 142 less base pairs compared to no MDD diagnosis (b = −142.86, SE = 56.46, p = .01). Additionally, at low levels of multisystem resiliency, MDD diagnosis was associated with a 91% increase in the odds of being in being in the bottom quartile of LTL (odds ratio = 1.91, p = .01, 95% CI = 1.15, 3.16). At +1 SD, MDD was not associated with the odds of having short telomeres (odds ratio = 0.83, p = .63, 95% CI = 0.39, 1.77). Fig. 1 illustrates the moderating effects of multisystem resiliency of those with and without a depression diagnosis. To examine whether these effects were driven by one particular factor, we followed with a set of four final analyses predicting the log-odds of being in the bottom 25% of LTL as a function of MDD at −1 SD and +1 SD of the mean of each factor alone. Similar to the models with the multisystem protective profile score, at −1 SD of low emotion suppression, social connections, physical activity and sleep quality, MDD significantly predicted increased likelihood of being categorized in the bottom quartile of LTL (odds ratios = 2.50, 1.72, 1.77, and 1.67 respectively, p ≤ .05). At +1 SD above the mean of each factor alone, MDD was unassociated with the odds for being in the lowest quartile of telomere length, suggesting that each factor is contributing to the moderation effect of the multisystem resiliency, with the strongest apparent effect for emotion regulation.
Fig. 1.
Predicted telomere length in base pairs as a function of multisystem resiliency for depressed and non-depressed patients.
6. Discussion
In the current study, we found that multisystem resiliency, defined by lower emotion suppression, stronger social connections, greater physical activity, and better sleep quality, was related to longer telomeres, while the individual factors were not by themselves associated with telomere length. Multisystem resiliency also moderated the association between MDD and LTL, with current depression associated with significantly shorter telomeres in those with lower resiliency, but not in those with higher. This suggests that a combination of psychosocial and behavioral factors may protect against cellular aging, particularly in those with depression.
6.1. A multisystem approach
The findings from the current study support a multisystem approach to understanding biological resiliency to distress and adversity. As seen in Fig. 1, multisystem resiliency is especially important to those with a current diagnosis of depression. Our results provide evidence that emotion regulation, social connections, and healthy behaviors are integral components of resiliency that can potentially guard against the deleterious physical effects of distress (Puterman and Epel, 2012). Key salutary aspects of the emotional, social, and physical selves promote healthy physiology, but in particular buffer from adversity of stress and depression. While our results are cross-sectional in nature, a large literature exists demonstrating the moderating effects of social connections on biological outcomes in distressed individuals (Cohen, 2004; Uchino, 2009). And while the benefits of physical activity have long been established (Hamer et al., 2012; Haskell et al., 2007; Penedo and Dahn, 2005), only more recent findings indicate that physical activity buffers the effects of psychological distress and depression on biological regulatory processes, such as immune function and insulin resistance (Puterman et al., 2010b, 2011; Rethorst et al., 2011). Similarly, good sleep is integral to adaptation to stress (Meerlo et al., 2002, 2008; Sgoifo et al., 2006). To our knowledge, this is the first study to demonstrate the moderating potential of healthy emotion regulation on a biological marker of stress in depressed individuals.
Multisystem resiliency may alter a myriad of pathways, interrupting a cascade of harmful effects that accelerate cellular aging. Multisystem resiliency may counter the effects of distress by impeding stress responsivity at various levels, from the cognitive and emotional through neural activation, and more downstream physiological responses. Emotion regulation, social connections, physical activity, and sleep may enhance the experience of positive affect, reduce negative affect, and modify cognitive processes such as rumination that are directly linked to depression (Nolen-Hoeksema and Davis, 1999; Puterman et al., 2010a). Physically active depressed and non-depressed individuals also experience increased positive affect after exercise (Wichers et al., 2012). Good sleep buffers the relationship between stress and negative affect (Hamilton et al., 2007) and adaptive emotion regulation is related to increased positive affect, decreased negative affect, and less perseverative thinking (Davidson et al., 2000; Gross and John, 2003). Negative cognitions and emotions can cause neurobiological changes that lead to activation of the hypothalamic–pituitary–adrenal axis and sympathetic–adrenal–medullary axis. This increases inflammation and reactive oxygen species, accelerating loss of telomeres by increasing cell turnover and DNA damage (Wolkowitz et al., 2010).
From a neurologic perspective, adaptive emotion regulation and social connections also reduce activity in brain regions associated with depression that induce autonomic, neuroendocrine, and inflammatory responses (Eisenberger et al., 2007; Lieberman et al., 2007; Slavich et al., 2010). Engagement in a single episode of physical exercise and maintenance of a physically active lifestyle are also related to neurologic changes that can reduce the stress response (Colcombe et al., 2004; Wong et al., 2007). Furthermore, physical activity leads to increases in the expression of genes that encode brain-derived neurotrophic factor, a promoter of neural plasticity (Dishman, 2006; Vaynman et al., 2004) that is typically decreased in depressed individuals (Martinowich et al., 2007). Examining studies of downstream biomarkers, depressed individuals have greater levels of reactive oxidized species (Szuster-Ciesielska et al., 2008), insulin resistance (Winokur et al., 1988), and circulating pro-inflammatory cytokines (Schiepers et al., 2005). In contrast, individuals with greater social connectivity (Kiecolt-Glaser et al., 2010), healthy emotional regulation (Kiecolt-Glaser et al., 2002; Kinnunen et al., 2005), physical activity (Goldhammer et al., 2005; Leeuwenburgh and Heinecke, 2001), and good sleep quality (Prather et al., 2009; Simpson and Dinges, 2007) have lower levels of these harmful biomarkers. These studies suggest that this combination of protective factors could prevent the chain of stress-induced changes that lead to decreased telomere length.
While in general, people with depression likely have low levels of these positive buffering factors (68% of depressed participants were in the bottom half of multisystem resiliency), it is notable that a significant percentage (32%) of depressed individuals were still able to maintain average or high resiliency. Severity and frequency of stressors and social connections early in life may shape the expression and development of these resiliency factors in adulthood (Repetti et al., 2002; Shonkoff et al., 2009). Yet, it may be feasible to promote this protective triad in people with depression. Interventions have already tested components of this model. A physical activity intervention can reduce depression to the same extent as antidepressants (Blumenthal et al., 2007; Brosse et al., 2002). Adaptive emotion regulation is a goal of mindfulness based cognitive therapy for depression, and has promise for, at least, psychological well being (Zautra et al., 2008). Yet, a psychosocial intervention directed towards enhancing social support and psychosocial functioning in cardiac patients has proven unsuccessful (Berkman et al., 2003). Perhaps focusing on all of these factors in one intervention might provide the most benefit for depression.
6.2. Limitations
The findings from this study should be considered within the context of several limitations. First, and most importantly, the current study is based on a cross-sectional examination of the data, thus directionality is unable to be determined. This is especially important as genetic factors and early life experiences may contribute to depression, the resiliency factors, and telomere length.
Second, physical activity and sleep quality measurement in the current study are each based on single-item assessments. While the distributions of both factors across the sample were not skewed, participants only rated these items with categorical anchors. Previous work indicates that the one item measure of physical activity largely mediated the association of depressive symptoms and subsequent cardiovascular events in patients with CHD (Whooley et al., 2008). Multisystem resiliency may be bolstered with a more thorough measure of physical activity and sleep, or, perhaps, objective measurement with accelerometers.
Third, we included physical activity and sleep quality in our measure of healthy behaviors, however, poor diet is also evidenced to exacerbate the negative health effects of stress (Dallman et al., 2005; Epel et al., 2000, 2011). Diet information was not available in the current study. We suggest that, when data is available, a composite for healthy behaviors should include diet in addition to physical activity and sleep, especially when examining the moderation effects of healthy behaviors on the relationship between psychosocial distress and health.
Fourth, our adaptive emotion regulation factor of “low emotion suppression” was derived from a validated emotion suppression scale (Gross and John, 2003). Low emotion suppression does not necessarily suggest elevated emotion expression. Furthermore, there are other equally important emotion regulation strategies that were not included in the parent study, such as cognitive reappraisal (Gross and John, 2003; John and Gross, 2004). Psychological resilience has also been conceptualized as a tendency to experience elevated positive emotions (Tugade et al., 2004) and a greater sense of control (Agrigoroaei and Lachman, 2011; Lachman and Agrigoroaei, 2010). Thus understanding how emotion regulation ties in with these components of psychological resilience is an important direction for research.
Fifth, while significant, social connections, emotion regulation and health behaviors contribute minimally to the variation in telomere length (1% of total variation). However, given that in most studies, as expected, the major predictor of telomere shortening is chronological age, and that age accounts for only 4% of the variation in the current study, it is not surprising that resiliency accounts for less than age. Thus, multisystem resiliency accounts for 25% of the effect of age. Furthermore, perhaps these combined effects are stronger in a healthy population.
Sixth, while the effect of current depression on telomere length was moderated by multisystem resiliency it is unknown if those with recurrent depression over the lifetime are also protected by such a profile.
Finally, the Heart and Soul Study included older, predominantly male, population with heart disease and the results may not be generalizable to other populations.
7. Summary
Major depressive disorder is often associated with diseases of aging and various biomarkers associated with risk for disease development, such as shorter LTL. The current study suggests that associations of MDD and biological outcomes should be examined within a psychosocial and behavioral context that encompasses multiple indicators of resiliency. We found that multisystem resiliency, comprised of the essential features of emotion regulation, social connections, and health behaviors, was significantly associated with longer telomere length and moderated the association of depression and telomere length. Many studies show that psychosocial and behavioral factors alter relationships between depression and biology, and our findings suggest that our current understanding of depression-disease links may be stronger or only present in those with unhealthy lifestyles, poor social ties, and maladaptive emotion regulatory skills. Previously obscured results may be revealed by considering such a multisystem, moderation analysis, and may propel us to better understand the biology of aging.
Acknowledgments
We thank the study participants for their support and generous contribution of time. This research was supported in part by NIH/NHLBI Grant K99HL109247 and the Robert Wood Johnson Foundation to Dr. Puterman and NIH/NHLBI Grant K23 HL 094765-01and a Grant from the Irene Perstein Foundation to Dr. Cohen. The Heart and Soul Study was funded by the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), Bethesda, MD, the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, the Ischemia Research and Education Foundation, South San Francisco, CA, and the Nancy Kirwan Heart Research Fund, San Francisco, CA. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Medical Center, San Francisco, CA. The funding organizations had no role in the design or conduct of the study; data collection, management, analysis or interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
Financial disclosure
Drs. Elizabeth Blackburn, Elissa Epel and Jue Lin are co-founders of Telome Health Inc., a diagnostics company related to telomere biology, and they own stock in the company.
References
- Agrigoroaei S, Lachman ME. Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. J Gerontol Ser B, Psychol Sci Soc Sci. 2011:i130–i140. doi: 10.1093/geronb/gbr017. http://dx.doi.org/10.1093/geronb/gbr017. [DOI] [PMC free article] [PubMed]
- Arfken CL, Lichtenberg PA, Tancer ME. Cognitive impairment and depression predict mortality in medically ill older adults. J Gerontol A Biol Sci Med Sci. 1999;54 (3):M152–M156. doi: 10.1093/gerona/54.3.m152. [DOI] [PubMed] [Google Scholar]
- Barth J, Schneider S, von Känel R. Lack of social support in the etiology and the prognosis of coronary heart disease: a systematic review and meta-analysis. Psychosom Med. 2010;72 (3):229–238. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Schneiderman N. Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. J Am Med Assoc. 2003;289 (23):3106–3116. doi: 10.1001/jama.289.23.3106. http://dx.doi.org/10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: the means to the end (Nobel Lecture) Angew Chem Int Ed. 2010;49 (41):7405–7421. doi: 10.1002/anie.201002387. http://dx.doi.org/10.1002/anie.201002387. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69 (7):587–596. doi: 10.1097/PSY.0b013e318148c19a. http://dx.doi.org/10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosse AL, Sheets ES, Lett HS, Blumenthal JA. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med. 2002;32 (12):741–760. doi: 10.2165/00007256-200232120-00001. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Helms MJ, Dahlstrom WG, Siegler IC. Prediction of all-cause mortality by the Minnesota multiphasic personality inventory optimism-pessimism scale scores: study of a college sample during a 40 year follow-up period-in a model that. Mayo Clinic Proceedings. 2006;81 (12):1541. doi: 10.4065/81.12.1541. [DOI] [PubMed] [Google Scholar]
- Brydon L, Lin J, Butcher L, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Hostility and cellular aging in men from the Whitehall II Cohort. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.08.020. http://dx.doi.org/10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed]
- Carroll JE, Diez Roux AV, Fitzpatrick AL, Seeman T. Low social support is associated with shorter leukocyte telomere length in late life: multi-ethnic study of atherosclerosis. Psychosom Med. 2013 doi: 10.1097/PSY.0b013e31828233bf. http://dx.doi.org/10.1097/PSY.0b013e31828233bf. [DOI] [PMC free article] [PubMed]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168 (2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. Am Psychol. 2004;59 (8):676–684. doi: 10.1037/0003-066X.59.8.676. http://dx.doi.org/10.1037/0003-066x.59.8.676. [DOI] [PubMed] [Google Scholar]
- Cohen S, Wills TS. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98 (2):310–357. [PubMed] [Google Scholar]
- Cohen S, Memelstein R, Kamarck T, Hoberman H. Measuring the functional components of social support. In: Sarason IG, Sarason B, editors. Social Support: Theory, Research and Application. Martinus Nijhoff; The Hague: 1985. pp. 73–94. [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. 3. Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Nat Acad Sci USA. 2004;101 (9):3316–3321. doi: 10.1073/pnas.0400266101. http://dx.doi.org/10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19 (4):275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126 (6):890–909. doi: 10.1037/0033-2909.126.6.890. http://dx.doi.org/10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Dishman RK. The new emergence of exercise neurobiology. Scand J Med Sci Sports. 2006;16 (6):379–380. doi: 10.1111/j.1600-0838.2006.00609.x. http://dx.doi.org/10.1111/j.1600-0838.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35 (4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. http://dx.doi.org/10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Horm Int J Endocrinol Metab. 2009;8 (1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62 (5):623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31 (3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. http://dx.doi.org/10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Epel ES, Tomiyama AJ, Dallman M. Stress and Reward Neural Networks, Eating, and Obesity: Handbook of Food Addiction. Oxford University Press; Oxford: 2011. [Google Scholar]
- Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KRR, Valvo WJ. Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry. 2005;58 (3):175–189. doi: 10.1016/j.biopsych.2005.05.001. http://dx.doi.org/10.1016/j.biopsych.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PLoS ONE. 2010;5 (1):e8612. doi: 10.1371/journal.pone.0008612. http://dx.doi.org/10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giltay EJ, Geleijnse JM, Zitman FG, Hoekstra T, Schouten EG. Dispositional optimism and all-cause and cardiovascular mortality in a prospective cohort of elderly Dutch men and women. Arch Gen Psychiatry. 2004;61 (11):1126. doi: 10.1001/archpsyc.61.11.1126. [DOI] [PubMed] [Google Scholar]
- Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol. 2005;100 (1):93–99. doi: 10.1016/j.ijcard.2004.08.073. http://dx.doi.org/10.1016/j.ijcard.2004.08.073. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. http://dx.doi.org/10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: physiology, self-report, and expressive behavior. J Pers Soc Psychol. 1993;64:970. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: the acute effects of inhibiting negative and positive emotion. J Abnorm Psychol. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Hamer M, Ingle L, Carroll S, Stamatakis E. Physical activity and cardiovascular mortality risk: possible protective mechanisms? Med Sci Sports Exercise. 2012;44 (1):84–88. doi: 10.1249/MSS.0b013e3182251077. http://dx.doi.org/10.1249/MSS.0b013e3182251077 (Research Support, Non-US Gov’t) [DOI] [PubMed] [Google Scholar]
- Hamilton NA, Catley D, Karlson C. Sleep and the affective response to stress and pain. Health Psychol. 2007;26 (3):288. doi: 10.1037/0278-6133.26.3.288. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere length of patients with major depression is shortened but independent from therapy and severity of the disease. Depress Anxiety. 2010;27 (12):1111–1116. doi: 10.1002/da.20749. http://dx.doi.org/10.1002/Da.20749. [DOI] [PubMed] [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exercise. 2007;39 (8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. (1410.1249/mss.1420b1013e3180616b3180627) [DOI] [PubMed] [Google Scholar]
- Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain Behav Immun. 2004;18 (2):114–119. doi: 10.1016/j.bbi.2003.09.005. http://dx.doi.org/10.1016/j.bbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel ES, Lin J, Whooley MA. Depression and leukocyte telomere length in patients with coronary heart disease: data from the heart and soul study. Psychosom Med. 2011;73 (7):541–547. doi: 10.1097/PSY.0b013e31821b1f6e. http://dx.doi.org/10.1097/Psy.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21 (4):374–383. doi: 10.1016/j.bbi.2007.01.010. http://dx.doi.org/10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, DePinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469 (7328):102–U1700. doi: 10.1038/nature09603. http://dx.doi.org/10.1038/Nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. J Pers. 2004;72:1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. http://dx.doi.org/10.1111/j.1467-6494.2004.00298.x (Special Issue: Emotions, Personality, and Health) [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ. Individual differences in emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. The Guilford Press; NY: 2007. pp. 351–372. [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Depression and immune function: central pathways to morbidity and mortality. J Psychosom Res. 2002;53 (4):873–876. doi: 10.1016/s0022-3999(02)00309-4. http://dx.doi.org/10.1016/s0022-3999(02)00309-4. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. http://dx.doi.org/10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neurosci Biobehav Rev. 2010;35 (1):33–38. doi: 10.1016/j.neubiorev.2009.09.003. http://dx.doi.org/10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen ML, Kokkonen M, Kaprio J, Pulkkinen L. The associations of emotion regulation and dysregulation with the metabolic syndrome factor. J Psychosom Res. 2005;58 (6):513–521. doi: 10.1016/j.jpsychores.2005.02.004. http://dx.doi.org/10.1016/j.jpsychores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Park N, Peterson C, Vokonas P, Sparrow D. Healthy psychological functioning and incident coronary heart disease: the importance of self-regulation. Arch Gen Psychiatry. 2011;68 (4):400. doi: 10.1001/archgenpsychiatry.2011.23. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S. Promoting functional health in midlife and old age: long-term protective effects of control beliefs, social support, and physical exercise. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013297. http://dx.doi.org/10.1371/journal.pone.0013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwenburgh C, Heinecke JW. Oxidative stress and antioxidants in exercise. Curr Med Chem. 2001;8 (7):829–838. doi: 10.2174/0929867013372896. http://dx.doi.org/10.2174/0929867013372896. [DOI] [PubMed] [Google Scholar]
- Liang GY, Schernhammer E, Qi L, Gao X, De Vivo I, Han JL. Associations between rotating night shifts, sleep duration, and telomere length in women. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023462. http://dx.doi.org/10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words. Psychol Sci. 2007;18 (5):421–428. doi: 10.1111/j.1467-9280.2007.01916.x. http://dx.doi.org/10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Low CA, Matthews KA, Kuller LH, Edmundowicz D. Psychosocial predictors of coronary artery calcification progression in postmenopausal women. Psychosom Med. 2011;73 (9):789–794. doi: 10.1097/PSY.0b013e318236b68a. http://dx.doi.org/10.1097/Psy.0b013e318236b68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between physical activity level, telomere length, and telomerase activity. Med Sci Sports Exercise. 2008;40 (10):1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainous AG, Everett CJ, Diaz VA, Baker R, Mangino M, Codd V, Samani NJ. Leukocyte telomere length and marital status among middle-aged adults. Age Ageing. 2011;40 (1):73–78. doi: 10.1093/ageing/afq118. http://dx.doi.org/10.1093/ageing/afq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10 (9):1089–1093. doi: 10.1038/nn1971. http://dx.doi.org/10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- Maruta T, Colligan RC, Malinchoc M, Offord KP. Optimism-pessimism assessed in the 1960s and self-reported health status 30 years later. Mayo Clin Proc. 2002;77 (8):748–753. doi: 10.4065/77.8.748. http://dx.doi.org/10.4065/77.8.748. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Patterson TL, Känel RV, Mills PJ, Dimsdale JE, Ancoli-Israel S, Grant I. The attenuating effect of personal mastery on the relations between stress and Alzheimer caregiver health: a five-year longitudinal analysis. Aging Ment Health. 2007;11 (6):637–644. doi: 10.1080/13607860701787043. http://dx.doi.org/10.1080/13607860701787043. [DOI] [PubMed] [Google Scholar]
- McGinnis JM, Foege WH. Actual causes of death in the United States. J Am Med Assoc. 1993;270 (18):2207–2212. [PubMed] [Google Scholar]
- Meerlo P, Koehl M, Van der Borght K, Turek F. Sleep restriction alters the hypothalamic–pituitary–adrenal response to stress. J Neuroendocrinol. 2002;14 (5):397–402. doi: 10.1046/j.0007-1331.2002.00790.x. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev. 2008;12 (3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65 (9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States. J Am Med Assoc. 2004;291 (10):1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry. 1998;55 (7):580–592. doi: 10.1001/archpsyc.55.7.580. http://dx.doi.org/10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Davis CG. “Thanks for sharing that”: ruminators and their social support networks. J Pers Soc Psychol. 1999;77 (4):801–814. doi: 10.1037//0022-3514.77.4.801. [DOI] [PubMed] [Google Scholar]
- O’Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn EH, Epel ES. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav Immun. 2009;23 (4):446–449. doi: 10.1016/j.bbi.2008.11.006. http://dx.doi.org/10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49 (5):391–404. doi: 10.1016/s0006-3223(00)01088-x. http://dx.doi.org/10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18 (2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Perez-Rivero G, Ruiz-Torres MP, Rivas-Elena JV, Jerkic M, Diez-Marques ML, Lopez-Novoa JM, Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114 (4):309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. http://dx.doi.org/10.1161/Circulationaha.105.611111. [DOI] [PubMed] [Google Scholar]
- Prather AA, Marsland AL, Hall M, Neumann SA, Muldoon MF, Manuck SB. Normative variation in self-reported sleep quality and sleep debt is associated with stimulated pro-inflammatory cytokine production. Biol Psychol. 2009;82 (1):12–17. doi: 10.1016/j.biopsycho.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather AA, Puterman E, Lin J, O’Donovan A, Krauss J, Tomiyama AJ, Blackburn E. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011 doi: 10.4061/2011/721390. http://dx.doi.org/10.4061/2011/721390. [DOI] [PMC free article] [PubMed]
- Pudrovska T, Schieman S, Pearlin LI, Nguyen K. The sense of mastery as a mediator and moderator in the association between economic hardship and health in late life. J Aging Health. 2005;17 (5):634–660. doi: 10.1177/0898264305279874. http://dx.doi.org/10.1177/0898264305279874. [DOI] [PubMed] [Google Scholar]
- Puterman E, Epel ES. An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc Pers Psychol Compass. 2012;6 (11):807–825. doi: 10.1111/j.1751-9004.2012.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, DeLongis A, Pomaki G. Protecting us from ourselves: social support as a buffer of trait and state rumination. J Soc Clin Psychol. 2010a;29 (7):797–820. http://dx.doi.org/10.1521/jscp.2010.29.7.797. [Google Scholar]
- Puterman E, Lin J, Blackburn EH, O’Donovan A, Adler NE, Epel ES. The power of exercise: buffering the effect of chronic stress on telomere length. PLoS ONE. 2010b;5(5) doi: 10.1371/journal.pone.0010837. http://dx.doi.org/10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, O’Donovan A, Adler NE, Tomiyama AJ, Kemeny M, Wolkowitz OM, Epel ES. Physical activity moderates effects of stressor-induced rumination on cortisol reactivity. Psychosom Med. 2011;73 (7):604–611. doi: 10.1097/PSY.0b013e318229e1e0. http://dx.doi.org/10.1097/Psy.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Adler NE, Matthews KA, Epel ES. Financial strain and impaired fasting glucose: the moderating role of physical activity in the coronary artery risk development in young adults study. Psychosom Med. 2012;74 (2):187–192. doi: 10.1097/PSY.0b013e3182448d74. http://dx.doi.org/10.1097/PSY.0b013e3182448d74. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002;128 (2):330–366. http://dx.doi.org/10.1037//0033-2909.128.2.330. [PubMed] [Google Scholar]
- Rethorst CD, Moynihan J, Lyness JM, Heffner KL, Chapman BP. Moderating effects of moderate-intensity physical activity in the relationship between depressive symptoms and interleukin-6 in primary care patients. Psychosom Med. 2011;73 (3):265–269. doi: 10.1097/PSY.0b013e3182108412. http://dx.doi.org/10.1097/Psy.0b013e3182108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert MS. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biol Psychiatry. 2000;48 (8):755–765. doi: 10.1016/s0006-3223(00)00971-9. http://dx.doi.org/10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National institute of mental health diagnostic interview schedule: its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38 (4):381–389. doi: 10.1001/archpsyc.1981.01780290015001. http://dx.doi.org/10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Friedman EM, Fuller-Rowell T, Love GD, Miyamoto Y, Morozink J, Tsenkova V. Varieties of resilience in MIDUS. Soc Pers Psychol Compass. 2012;6 (11):792–806. doi: 10.1111/j.1751-9004.2012.00462.x. http://dx.doi.org/10.1111/j.1751-9004.2012.00462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;475(7355) doi: 10.1038/nature09787. http://dx.doi.org/10.1038/Nature10223(470, 359, 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier ME, Carver CS. Dispositional optimism and physical well-being: the influence of generalized outcome expectancies on health. J Pers. 1987;55 (2):169–210. doi: 10.1111/j.1467-6494.1987.tb00434.x. http://dx.doi.org/10.1111/j.1467-6494.1987.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Schiepers OJG, Wichers MC, Maes M. Cytokines and major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29 (2):201–217. doi: 10.1016/j.pnpbp.2004.11.003. http://dx.doi.org/10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults – the Cardiovascular Health Study. Arch Intern Med. 2000;160 (12):1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Buwalda B, Roos M, Costoli T, Merati G, Meerlo P. Effects of sleep deprivation on cardiac autonomic and pituitary–adrenocortical stress reactivity in rats. Psychoneuroendocrinology. 2006;31 (2):197–208. doi: 10.1016/j.psyneuen.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30 (4):377–385. doi: 10.1037/a0022826. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities building a new framework for health promotion and disease prevention. J Am Med Assoc. 2009;301 (21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Wong KK. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60 (5):432–435. doi: 10.1016/j.biopsych.2006.02.004. http://dx.doi.org/10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Simpson N, Dinges DF. Sleep and inflammation. Nutr Rev. 2007;65 (s3):S244–S252. doi: 10.1111/j.1753-4887.2007.tb00371.x. [DOI] [PubMed] [Google Scholar]
- Slavich GM, Way BM, Eisenberger NI, Taylor SE. Neural sensitivity to social rejection is associated with inflammatory responses to social stress. Proc Nat Acad Sci USA. 2010;107 (33):14817–14822. doi: 10.1073/pnas.1009164107. http://dx.doi.org/10.1073/pnas.1009164107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TW, Glazer K, Ruiz JM, Gallo LC. Hostility, anger, aggressiveness, and coronary heart disease: an interpersonal perspective on personality, emotion, and health. J Pers. 2004;72 (6):1217–1270. doi: 10.1111/j.1467-6494.2004.00296.x. http://dx.doi.org/10.1111/j.1467-6494.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Nat Acad Sci. 2013;110 (15):5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, Rexrode KM. Healthy lifestyle and leukocyte telomere length in US women. 2012;7(5):e38374. doi: 10.1371/journal.pone.0038374. http://dx.doi.org/10.1371/journal.pone.0038374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuster-Ciesielska A, Słotwińska M, Stachura A, Marmurowska-Michałowska H, Dubas-Œlemp H, Bojarska-Junak A, Kandefer-Szerszeń M. Accelerated apoptosis of blood leukocytes and oxidative stress in blood of patients with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32 (3):686–694. doi: 10.1016/j.pnpbp.2007.11.012. http://dx.doi.org/10.1016/j.pnpbp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE. Psychosocial resources and the ses-health relationship. Ann N Y Acad Sci. 1999;896 (1):210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. http://dx.doi.org/10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Kemeny ME, Reed GM, Bower JE, Gruenewald TL. Psychological resources, positive illusions, and health. Am Psychol. 2000;55 (1):99–109. doi: 10.1037//0003-066x.55.1.99. [DOI] [PubMed] [Google Scholar]
- Tindle HA, Chang YF, Kuller LH, Manson JE, Robinson JG, Rosal MC, Matthews KA. Optimism, cynical hostility, and incident coronary heart disease and mortality in the women’s health initiative. Circulation. 2009;120 (8):656–662. doi: 10.1161/CIRCULATIONAHA.108.827642. http://dx.doi.org/10.1161/circulationaha.108.827642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tugade MM, Fredrickson BL, Feldman Barrett L. Psychological resilience and positive emotional granularity: examining the benefits of positive emotions on coping and health. J Pers. 2004;72 (6):1161–1190. doi: 10.1111/j.1467-6494.2004.00294.x. http://dx.doi.org/10.1111/j.1467-6494.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29 (4):377–387. doi: 10.1007/s10865-006-9056-5. http://dx.doi.org/10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Understanding the links between social support and physical health: a life-span perspective with emphasis on the separability of perceived and received support. Perspect Psychol Sci. 2009;4 (3):236–255. doi: 10.1111/j.1745-6924.2009.01122.x. http://dx.doi.org/10.1111/j.1745-6924.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Cawthon RM, Smith TW, Light KC, McKenzie J, Carlisle M, Bowen K. Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres? Health Psychol. 2012 doi: 10.1037/a0026836. http://dx.doi.org/10.1037/a0026836. [DOI] [PMC free article] [PubMed]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20 (10):2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. http://dx.doi.org/10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Laufs U. Physical exercise prevents cellular senescence in circulating leukocytes and in the vessel wall. Circulation. 2009;120 (24):2438–2447. doi: 10.1161/CIRCULATIONAHA.109.861005. http://dx.doi.org/10.1161/circulationaha.109.861005. [DOI] [PubMed] [Google Scholar]
- Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. J Am Med Assoc. 2008;300 (20):2379–2388. doi: 10.1001/jama.2008.711. http://dx.doi.org/10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichers M, Peeters F, Rutten BPF, Jacobs N, Derom C, Thiery E, van Os J. A time-lagged momentary assessment study on daily life physical activity and affect. Health Psychol. 2012;31:135–144. doi: 10.1037/a0025688. [DOI] [PubMed] [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjäll K, Bergdahl J, Hultdin J, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71 (4):294–300. doi: 10.1016/j.biopsych.2011.09.015. http://dx.doi.org/10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Winokur A, Maislin G, Phillips JL, Amsterdam JD. Insulin resistance after oral glucose tolerance testing in patients with major depression. Am J Psychiatry. 1988;145 (3):325–330. doi: 10.1176/ajp.145.3.325. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27 (4):327–338. doi: 10.1002/da.20686. http://dx.doi.org/10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su YL, Blackburn EH. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress – preliminary findings. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017837. http://dx.doi.org/10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SW, Kimmerly DS, Massé N, Menon RS, Cechetto DF, Shoemaker JK. Sex differences in forebrain and cardiovagal responses at the onset of isometric handgrip exercise: a retrospective fMRI study. J Appl Physiol. 2007;103 (4):1402–1411. doi: 10.1152/japplphysiol.00171.2007. http://dx.doi.org/10.1152/japplphysiol.00171.2007. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Davis MC, Reich JW, Tennen H, Irwin MR, Nicassio P, Parrish B. Comparison of cognitive behavioral and mindfulness meditation interventions on adaptation to rheumatoid arthritis for patients with and without history of recurrent depression. J Consult Clin Psychol. 2008;76 (3):408–421. doi: 10.1037/0022-006X.76.3.408. http://dx.doi.org/10.1037/0022-006x.76.3.408. [DOI] [PubMed] [Google Scholar]