Abstract
The serotonin1A receptor is an important member of the G protein-coupled receptor (GPCR) family. It is involved in the generation and modulation of a variety of cognitive and behavioral functions, and serves as a drug target. Previous work from our laboratory has established the sensitivity of the function of the serotonin1A receptor to membrane cholesterol. Solubilization of the hippocampal serotonin1A receptor utilizing the zwitterionic detergent CHAPS is accompanied by loss of cholesterol and results in reduction in specific ligand binding. Replenishment of cholesterol to solubilized membranes restores specific ligand binding to the receptor. We utilized this strategy of sterol replenishment of solubilized membranes to explore the stereospecific stringency of cholesterol for receptor function. We used two stereoisomers of cholesterol, ent-cholesterol (enantiomer of cholesterol) and epi-cholesterol (a diastereomer of cholesterol) for this purpose. Importantly, we show here that while ent-cholesterol could replace cholesterol in supporting receptor function, epi-cholesterol could not. These results imply that the requirement of membrane cholesterol for the serotonin1A receptor function is diastereospecific, yet not enantiospecific. Our results extend and help define specificity of the interaction of membrane cholesterol with the serotonin1A receptor, and represent the first report utilizing ent-cholesterol to examine stereospecificity of GPCR-cholesterol interaction.
Keywords: Serotonin1A receptor, cholesterol, ent-cholesterol, epi-cholesterol, ligand binding, fluorescence anisotropy
1. Introduction
The G protein-coupled receptor (GPCR) superfamily comprises the largest and most diverse group of proteins in mammals, and is involved in information transfer (signal transduction) from outside the cell to the cellular interior [1–3]. GPCRs are typically seven transmembrane domain proteins and regulate physiological responses to a diverse array of stimuli, and mediate multiple physiological processes. Due to this reason, GPCRs have emerged as major drug targets in all clinical areas [4]. It is estimated that ~50% of clinically prescribed drugs target GPCRs [5].
The serotonin1A (5-HT1A) receptor is a representative member of the GPCR family and is implicated in the generation and modulation of various cognitive, behavioral, and developmental functions [6–8]. Ligands that bind to the serotonin1A receptor are reported to possess potential therapeutic effects in anxiety or stress-related disorders [6]. As a consequence, the serotonin1A receptor serves as an important target in the development of therapeutic agents for neuropsychiatric disorders such as anxiety and depression [9]. Since GPCRs are integral membrane proteins with multiple transmembrane passes, the interaction of GPCRs with membrane lipids is an important determinant in their structure and function [10–14]. In fact, an important feature observed in recently solved high resolution crystal structures of GPCRs (such as rhodopsin [15], β1-adrenergic receptor [16], β2-adrenergic receptor [17,18] and A2A adenosine receptor [19]), is the close association of cholesterol molecules to the receptor. Previous work from our laboratory has comprehensively demonstrated the requirement of membrane cholesterol in the organization, dynamics, and function of the serotonin1A receptor ([20–22]; reviewed in [11,12,14]).
Cholesterol is an essential and representative membrane lipid in higher eukaryotes and is crucial in membrane organization, dynamics, function, and sorting [23,24]. A hallmark of membrane cholesterol is its nonrandom distribution in domains (or pools) in biological and model membranes [25–28]. These domains are believed to be crucial since various cellular processes such as membrane sorting and trafficking [29], signal transduction [30], and the entry of pathogens [31,32] have been attributed to these types of domains. The role of cholesterol in the function and organization of membrane proteins and receptors constitutes an emerging and exciting area of research [10–14]. The detailed mechanism underlying the effect of membrane cholesterol on the structure and function of membrane proteins and receptors is not clear and appears to be complex [12,33,34]. A possible mechanism by which membrane cholesterol has been proposed to modulate the function of membrane receptors is by a direct (specific) interaction, which could induce a conformational change in the receptor. An alternative mechanism envisages an indirect way by altering the membrane physical properties in which the receptor is embedded. Yet another possibility could be a combination of both. A particular kind of proposed specific interaction is based on the concept of ‘nonannular’ binding sites of membrane lipids in membrane proteins [34,35]. Nonannular sites are characterized by lack of accessibility to the annular lipids, i.e., these sites cannot be displaced by competition with annular lipids [36,37].
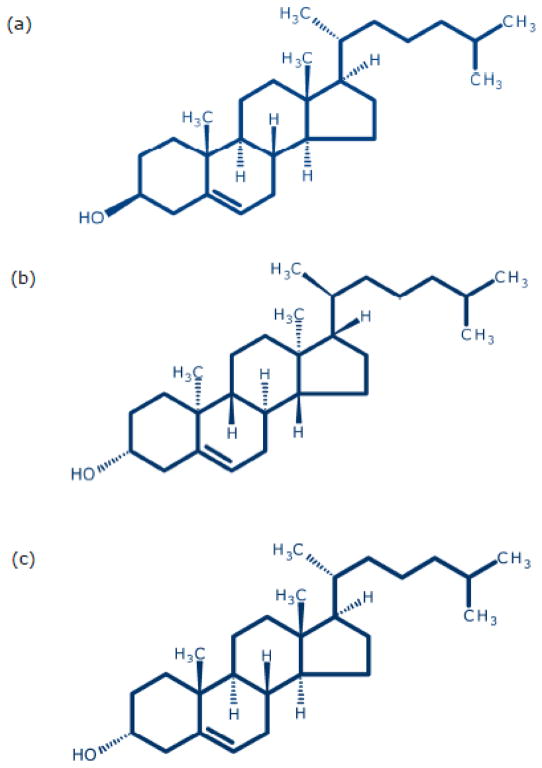
As mentioned above, earlier work from our laboratory has comprehensively demonstrated the requirement of membrane cholesterol in the function of the serotonin1A receptor [11,12,14]. An important aspect of our results is that the interaction between cholesterol and the serotonin1A receptor was shown to be considerably stringent since immediate biosynthetic precursors of cholesterol (differing with cholesterol merely in a double bond) were not able to maintain receptor function [21,38,39]. In order to further explore the degree of structural (stereospecific) stringency necessary for the ligand binding function of the serotonin1A receptor, we examined whether stereoisomers of cholesterol [enantiomer of cholesterol (ent-cholesterol), or diastereomer of cholesterol (epi-cholesterol); see Fig. 1] could support the ligand binding function of the receptor. We show that while ent -cholesterol could replace cholesterol in supporting receptor function, epi-cholesterol could not.
Fig. 1.
Chemical structures of (a) cholesterol, (b) ent-cholesterol and (c) epi-cholesterol. Both epi-cholesterol and epi-cholesterol are stereoisomers of cholesterol. ent-Cholesterol is the enantiomer of cholesterol. Enantiomers are non-superimposable mirror images of one another. epi-Cholesterol, on the other hand, is a diastereomer and is not a mirror image of cholesterol. ent-Cholesterol, but not epi-cholesterol, share identical physicochemical properties with cholesterol. See text for more details.
2. Materials and methods
2.1. Materials
CHAPS, cholesterol, MβCD, DMPC, DPH, EDTA, EGTA, MgCl2, MnCl2, iodoacetamide, PEG, PMSF, serotonin, sucrose, polyethylenimine, sodium azide, and Tris were obtained from Sigma Chemical Co. (St. Louis, MO). 3-epicholesterol (5-cholesten-3′-ol), to be denoted as epi-cholesterol, was obtained from Steraloids (Newport, RI). The enantiomer of cholesterol (ent-cholesterol) was synthesized as previously described [40,41]. BCA reagent for protein estimation was from Pierce (Rockford, IL). [3H]8-OH-DPAT (sp. activity 106 Ci/mmol) was purchased from DuPont New England Nuclear (Boston, MA). GF/B glass microfiber filters were from Whatman International (Kent, U.K.). All solvents used were of analytical grade. All other chemicals used were of the highest purity available. Water was purified through a Millipore (Bedford, MA) Milli-Q system and used throughout. Fresh bovine brains were obtained from a local slaughterhouse within 10 min of death and the hippocampal region was carefully dissected out. The hippocampi were immediately flash frozen in liquid nitrogen and stored at −70 °C till further use.
2.2. Methods
2.2.1. Preparation of native hippocampal membranes
Native hippocampal membranes were prepared as described previously [42]. Bovine hippocampal tissue (~50 g) was homogenized as 10% (w/v) in a polytron homogenizer in 2.5 mM Tris, 0.32 M sucrose, 5 mM EDTA, 5 mM EGTA, 0.02% sodium azide, 0.24 mM PMSF, 10 mM iodoacetamide, pH 7.4 buffer. The homogenate was centrifuged at 900×g for 10 min at 4 °C. The resultant supernatant was filtered through four layers of cheesecloth and centrifuged at 50,000×g for 20 min at 4 °C. The pellet obtained was suspended in 10 vol of 50 mM Tris, 1 mM EDTA, 0.24 mM PMSF, 10 mM iodoacetamide, pH 7.4 buffer using a hand-held Dounce homogenizer and centrifuged at 50,000×g for 20 min at 4 °C. This procedure was repeated until the supernatant was clear. The final pellet (native hippocampal membranes) was suspended in a minimum volume of 50 mM Tris, pH 7.4 (buffer A), homogenized using a hand-held Dounce homogenizer, flash frozen in liquid nitrogen and stored at −70 °C. Protein concentration was assayed using the BCA reagent [43].
2.2.2. Solubilization of native membranes
Hippocampal membranes (HM) were solubilized as described previously using the zwitterionic detergent CHAPS [44–46]. CHAPS-solubilized membrane was precipitated using PEG in order to remove NaCl from the solubilized extract, since agonist binding of the serotonin1A receptor is inhibited by NaCl [42]. This procedure also removes detergent. The PEG-precipitated CHAPS-solubilized membrane (referred to as solubilized membrane (SM)) was suspended in buffer A and used immediately for sterol replenishment and radioligand binding assays.
2.2.3. Sterol replenishment of solubilized membranes
Solubilized membranes were replenished with ent-cholesterol, epi-cholesterol or cholesterol using water soluble complexes of MβCD and the respective sterol. The complex was prepared by dissolving required amounts of the sterol (ent-cholesterol, epi-cholesterol or cholesterol) and MβCD in a ratio of 1:10 (mol/mol) in buffer A by constant vortexing at room temperature (~23 °C). Stock solutions (typically 2 mM of ent-cholesterol, epi-cholesterol or cholesterol:20 mM MβCD) of this complex were freshly prepared prior to each experiment. Sterol replenishments were carried out at a protein concentration of ~2 mg/ml by incubating solubilized membranes with 1 mM sterol: 10 mM MβCD complex for 30 min in buffer A at 25 °C under constant shaking. Membranes were then spun down at 100,000×g for 1 h at 4 °C, suspended in the same buffer, and immediately used for radioligand binding assays.
2.2.4. Radioligand binding assays
Receptor binding assays were carried out as described earlier [20] with some modifications. Tubes in duplicate with ~0.8 mg protein in a total volume of 1 ml of 50 mM Tris, 1 mM EDTA, 10 mM MgCl2, 5 mM MnCl2, pH 7.4 buffer were incubated with the radiolabeled agonist [3H]8-OH-DPAT (final concentration in assay tube being 0.29 nM) for 1 h at 25 °C. Nonspecific binding was determined by performing the assay in the presence of 10 μM serotonin. The binding reaction was terminated by rapid filtration under vacuum in a Millipore multiport filtration apparatus through Whatman GF/B 2.5 cm diameter glass microfiber filters (1.0 μm pore size), which were presoaked in 0.15% polyethylenimine for 1 h [47]. Filters were then washed three times with 3 ml of cold water (4 °C), dried and the retained radioactivity was measured in a Packard Tri-Carb 1500 liquid scintillation counter using 5 ml of scintillation fluid.
2.2.5. Estimation of inorganic phosphate
The concentration of lipid phosphate was determined subsequent to total digestion by perchloric acid [48] using Na2HPO4 as standard. DMPC was used as an internal standard to assess lipid digestion. Samples without perchloric acid digestion produced negligible readings.
2.2.6. Fluorescence anisotropy measurements
Fluorescence anisotropy experiments were carried out using the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene (DPH) as described previously [49]. Steady state fluorescence was measured in a Hitachi F-4010 spectrofluorometer using 1 cm path length quartz cuvettes at room temperature (~23 °C). Excitation and emission wavelengths were set at 358 and 430 nm. Excitation and emission slits with bandpasses of 1.5 and 20 nm were used. The optical density of the samples measured at 358 nm was always less than 0.15. Fluorescence anisotropy measurements were performed using a Hitachi polarization accessory. Anisotropy (r) values were calculated from the equation [50]:
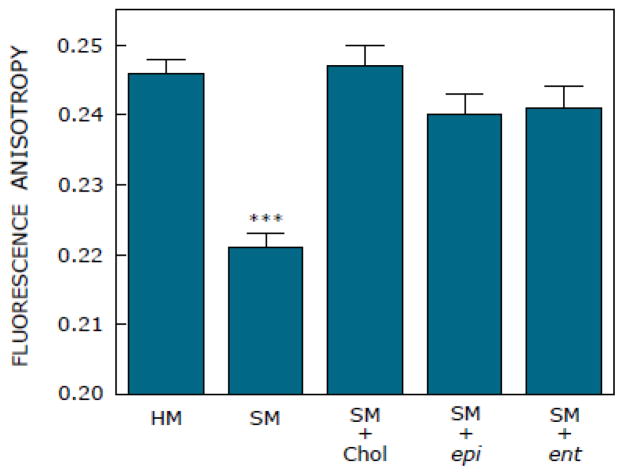
where IVV and IVH are the measured fluorescence intensities (after appropriate background subtraction) with the excitation polarizer vertically oriented and the emission polarizer vertically and horizontally oriented, respectively. G is the grating correction factor and is the ratio of the efficiencies of the detection system for vertically and horizontally polarized light and is equal to IHV/IHH. All experiments were done with multiple sets of samples and average values of fluorescence anisotropy are shown in Fig. 3.
Fig. 3.
Effect of replenishment of cholesterol, ent-cholesterol and epi-cholesterol into solubilized membranes (SM) on steady state fluorescence anisotropy of the membrane probe DPH. Solubilized membranes were replenished with cholesterol, ent-cholesterol or epi-cholesterol using 1 mM of the respective sterol: 10 mM MβCD complex. Fluorescence anisotropy measurements were performed with membranes containing 50 nmol phospholipid at a probe to phospholipid ratio of 1:100 (mol/mol) at room temperature (~23 °C). Values represent means ± S.E. of duplicate points from at least four independent experiments [*** corresponds to p<0.0001; the change in fluorescence anisotropy was tested against the corresponding value obtained with native hippocampal membranes (HM)]. See Materials and methods for other details.
2.2.7. Statistical analysis
Significance levels were estimated using Student’s two-tailed unpaired t-test using Graphpad Prism software version 4.0 (San Diego, CA).
3. Results and discussion
The enantiomer of cholesterol (ent-cholesterol) is the non-superimposable mirror image of native (natural) cholesterol (see Fig. 1b). Enantiomers have identical physicochemical properties (except for the direction of rotation of plane-polarized light). As a consequence, the membrane biophysical properties (such as compressibility and phase behavior) remain same when native cholesterol is replaced with ent-cholesterol [40,51–53]. In addition, both native cholesterol and ent-cholesterol support normal growth of a mutant mammalian cell line [54]. ent-Cholesterol is often utilized to distinguish specific interaction of cholesterol from nonspecific effects [53,55–57]. epi-Cholesterol is a diastereomer of cholesterol in which only the orientation of the hydroxyl group at carbon-3 is inverted relative to native cholesterol (Fig. 1c). Previous studies have shown that the biophysical properties of epi-cholesterol and native cholesterol are different in membranes [40,53 and references therein]. For example, epi-cholesterol and native cholesterol have been reported to differ in their tilt angles, condensing ability, and phase transition properties in membranes [58–61].
Purified membrane proteins are ideally suited for studying lipid-protein interactions. However, purification of membrane proteins poses a considerable challenge. A necessary criterion for purification of an integral membrane protein is that the protein must be carefully removed from the native membrane and dispersed in solution. This process, termed solubilization, is most efficiently accomplished utilizing amphiphilic detergents [62,63]. In this process, proteins and lipids held together in native membranes, are dissociated in the presence of a suitable detergent. This results in the formation of small protein and lipid clusters that remain dissolved (solubilized) in the aqueous solution. In our previous work, we partially purified the hippocampal serotonin1A receptor by solubilizing the receptor in a functionally active form using CHAPS, a synthetic zwitterionic detergent, which is mild and non-denaturing [44,64]. The solubilization conditions were highly optimized so as to prevent dissociation and depletion of trimeric G-proteins, which could result from high concentrations of CHAPS [65,66], and therefore helpful in effectively solubilizing GPCRs in a functionally active form. Hippocampal membranes, solubilized this way, contain the serotonin1A receptor in a relatively pure (enriched) form. Interestingly, it has been previously shown by us [67] and others [68] that solubilization of the serotonin1A receptor by CHAPS leads to a reduction in membrane cholesterol and specific ligand binding to the receptor. More importantly, we previously demonstrated that upon replenishment of solubilized membranes with cholesterol, specific ligand binding of the serotonin1A receptor could be restored [67]. In this paper, we utilized this strategy of sterol replenishment to the solubilized receptor to explore the stereospecific stringency of cholesterol for receptor function utilizing stereoisomers of cholesterol (ent-cholesterol and epi-cholesterol).
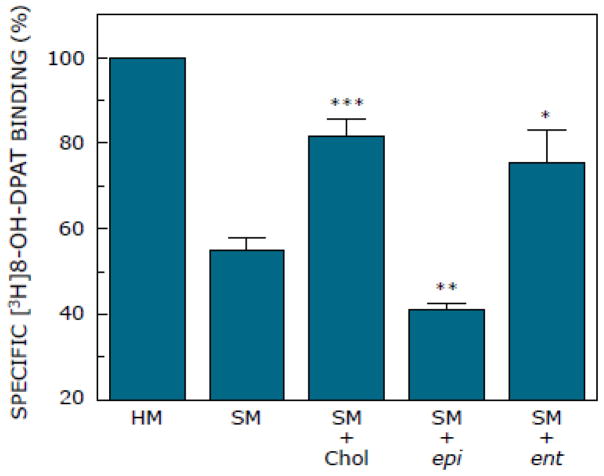
Fig. 2 shows specific binding of the agonist [3H]8-OH-DPAT to serotonin1A receptors in solubilized hippocampal membranes, and upon replenishment of solubilized membranes with either epi-cholesterol or cholesterol. Specific [3H]8-OH-DPAT binding to native hippocampal membranes served as a control for these experiments. The figure shows that the specific [3H]8-OH-DPAT binding to the serotonin1A receptor is reduced upon solubilization to ~55% of the control (native membranes). We attribute this reduction in binding to the loss of membrane cholesterol accompanying solubilization [67]. Subsequent treatment of solubilized membranes with MβCD-cholesterol complex led to considerable recovery (~82%) of specific [3H]8-OH-DPAT binding, due to replenishment of cholesterol. Interestingly, replenishment of solubilized membranes with epi-cholesterol could not restore specific [3H]8-OH-DPAT binding to the receptor and remained at ~41% relative to control (native membranes). These results show that epi-cholesterol is unable to support the ligand binding function of the serotonin1A receptor.
Fig. 2.
Effect of replenishment of epi-cholesterol (epi) and ent-cholesterol (ent) into solubilized membranes (SM) on specific binding of the agonist [3H]8-OH-DPAT to the serotonin1A receptor. Solubilized hippocampal membranes were replenished with cholesterol, epi-cholesterol or ent-cholesterol using 1 mM of respective sterol: 10 mM MβCD complex. Values are expressed as percentages of specific binding obtained in native hippocampal membranes (HM). Data shown are means ± S.E. of at least four independent experiments [*, ** and *** correspond to significant (p<0.05, p<0.01 and p<0.0001, respectively) difference in specific ligand binding to cholesterol, epi-cholesterol or ent-cholesterol-replenished membranes relative to solubilized membranes]. See Materials and methods for other details.
In order to explore the enantioselectivity of cholesterol in its interaction with the serotonin1A receptor, we carried out replenishment of solubilized membranes with ent-cholesterol. As mentioned earlier, ent-cholesterol is often utilized to distinguish specific interaction of cholesterol from nonspecific effects [40,53,55–57]. The effect of replenishment of solubilized membranes with ent-cholesterol is shown in Fig. 2. Interestingly, replenishment with ent-cholesterol resulted in recovery of specific [3H]8-OH-DPAT binding to ~75% of native membranes, comparable to that of cholesterol-replenished membranes (Fig. 2). Taken together, these results suggest that the requirement of membrane cholesterol for the serotonin1A receptor function is diastereospecific, but not enantiospecific.
The above difference between epi-cholesterol and ent-cholesterol in their ability to restore specific ligand binding to the serotonin1A receptor, in principle, could be due to a change in membrane order. In order to examine this possibility, we carried out fluorescence anisotropy measurements with the membrane probe DPH. DPH is a rod-like molecule and partitions into the interior of the membrane. The membrane partitioning of DPH has previously been shown to be independent of the phase state of the membrane [69]. Fluorescence anisotropy is correlated to the rotational diffusion of membrane embedded probes such as DPH [50], which is sensitive to the packing of lipid acyl chains. Fig. 3 shows that the fluorescence anisotropy of DPH exhibits a significant reduction upon solubilization. Upon replenishment of solubilized membranes with ent-cholesterol, epi-cholesterol or cholesterol, fluorescence anisotropy was found to increase and to be similar to that of native (control) membranes in all cases.
epi-Cholesterol has been earlier reported to differ with cholesterol in several biophysical properties [40,58–61]. However, our results show that the overall membrane order of hippocampal membranes, monitored by fluorescence anisotropy of DPH, is more or less invariant, irrespective of whether the sterol in the membrane is cholesterol or epi-cholesterol (Fig. 3). A possible reason for this could be that previous work on biophysical properties of epi-cholesterol was carried out in binary mixtures of lipids in model membranes where the consequences of stereospecific sterol-lipid interactions are readily observable due to membrane homogeneity. In contrast, we used hippocampal membranes of neuronal origin which have a complex lipid composition [70] that could mask stereospecific sterol-lipid interactions. Similar results were observed upon replenishment of HEK-293 cell membranes with epi-cholesterol following cholesterol depletion [71]. In addition, the same authors reported that specific ligand binding to the oxytocin receptor (the specific requirement of membrane cholesterol for the function of this GPCR has been demonstrated [71]) exhibits significant reduction upon replacement of cholesterol with epi-cholesterol. Taken together, our present results with the serotonin1A receptor and previous results of Gimpl et al. with the oxytocin receptor [71] point to the stringent requirement of cholesterol structure (the equatorial orientation of the 3-hydroxyl group in particular) in the function of these important GPCRs.
The selectivity of natural cholesterol and its enantiomer on the function of several peptides and proteins such as gramicidin ion channel [55], nicotinic acetylcholine receptor [72], epidermal growth factor receptor [51], inward rectifier K+ channel [56], and the sterol regulatory element-binding protein [57] have been previously studied. In addition, the stereospecific requirement of cholesterol for bacterial toxins such as Vibrio cholerae cytolysin and streptococcal streptolysin O [73], a polyene antibiotic amphotericin B [55,74] and the growth, behavior and viability of Caenorhabditis elegans [75] have been studied utilizing ent-cholesterol. Interestingly, requirement of cholesterol has been reported to be enantioselective in the case of inward rectifier K+ channel [56], Vibrio cholerae cytolysin [73], amphotericin B [74] and Caenorhabditis elegans [75]. On the other hand, it was reported that the effect of cholesterol on the protein function is not enantioselective for proteins such as the nicotinic acetylcholine receptor [72], epidermal growth factor receptor [51], streptococcal streptolysin O [73], and the sterol regulatory element-binding protein [57]. In these cases, ent-cholesterol has been particularly utilized to differentiate the specific and general role of cholesterol in the protein function, solely on the expectation that a specific protein binding site for cholesterol will be geometrically stringent enough to differentiate between enantiomers. This stringency of interaction requires more than two specific interactions between the ligand and its receptor [40,53]. A possibility of a non-enantioselective pattern of binding in a non-geometrically constrained protein cleft (such as a nonannular lipid binding site, as discussed above) could therefore explain our results and is consistent with what has been proposed previously [40,53]. It is therefore prudent to be cognizant of this alternative explanation when interpreting a finding of lack of enantioselectivity.
In conclusion, our results show that ent-cholesterol, but not epi-cholesterol, could replace cholesterol in supporting the function of the serotonin1A receptor (see Fig. 4), although the overall membrane order appears to be comparable in all cases. These results therefore show that the requirement of membrane cholesterol for the serotonin1A receptor function is diastereospecific, but not enantiospecific. We have previously shown that immediate biosynthetic precursors of cholesterol, differing with cholesterol in merely a double bond, were not able to support the function of the serotonin1A receptor [21,38,39,45]. In addition, we have shown that the serotonin1A receptor is more compact [76] and stable [49] in the presence of membrane cholesterol. We have very recently shown by coarse-grain molecular dynamics simulation that membrane cholesterol binds preferentially to certain sites on the receptor [77]. A prominent site among these is the cholesterol recognition/interaction amino acid consensus (CRAC) motif, recently identified by us in GPCRs [78].
Fig. 4.
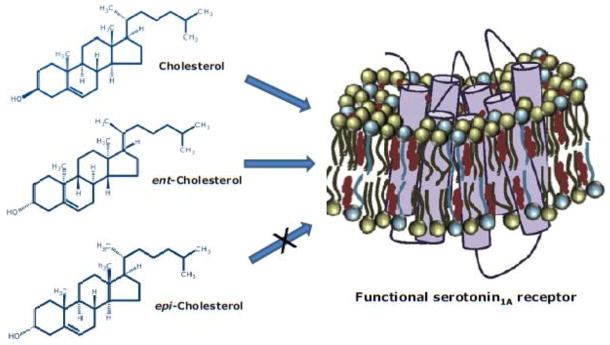
A schematic representation of the reconstituted serotonin1A receptor replenished with various sterols. The serotonin1A receptor is shown in purple, and the replenished sterol molecules are shown in maroon. Replenishment with cholesterol and ent-cholesterol supports the function of the receptor, whereas replenishment with epi-cholesterol is unable to support the function of the receptor. See text for more details.
We show here that a key structural feature of cholesterol for its ability to affect the function of the serotonin1A receptor is the equatorial configuration of the 3-hydroxyl group. epi-Cholesterol, differing with cholesterol solely in the axial orientation of the 3-hydroxyl group, could not support receptor function, whereas ent-cholesterol which maintains the 3-hydroxy group in the equatorial configuration supports receptor function. Our present results therefore further extend the degree of specificity of the interaction between the serotonin1A receptor and membrane cholesterol. Yet, these results show that this specificity of interaction falls short of achieving enantioselectivity. We conclude that membrane lipid interactions of GPCRs could be of varying specificity and envisage that this type of regulated specificity affects the efficacy of the receptor-ligand interaction and is physiologically important. To the best of our knowledge, our results constitute the first report utilizing ent-cholesterol to explore the stereospecific requirement of cholesterol for GPCR function.
Highlights.
Stereospecificity of cholesterol in supporting the serotonin1A receptor function
Cholesterol enantiomer supports ligand binding function of serotonin1A receptors
The equatorial configuration of the 3-hydroxyl group of cholesterol is crucial
Cholesterol requirement is diastereospecific, but not enantiospecific
Acknowledgments
This work was supported by the Council of Scientific and Industrial Research, Govt. of India. A.C. is an Adjunct Professor at the Special Centre for Molecular Medicine of Jawaharlal Nehru University (New Delhi) and Indian Institute of Science Education and Research (Mohali), and Honorary Professor at the Jawaharlal Nehru Centre for Advanced Scientific Research (Bangalore). A.C. gratefully acknowledges support from J.C. Bose Fellowship (Department of Science and Technology, Govt. of India). Work in the laboratory of D.F.C. was supported by NIH grant GM47969. We thank Durba Sengupta, Sourav Haldar, and members of our laboratory for critically reading the manuscript.
Abbreviations
- 5-HT1A receptor
5-hydroxytryptamine-1A receptor
- 8-OH-DPAT
8-hydroxy-2(di-N-propylamino)tetralin
- BCA
bicinchoninic acid
- CHAPS
3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate
- DMPC
dimyristoyl-sn-glycero-3-phosphocholine
- DPH
1,6-diphenyl-1,3,5-hexatriene
- ent-cholesterol
enantiomer of cholesterol
- epi-cholesterol (3-epicholesterol)
diastereomer of cholesterol
- GPCR
G protein-coupled receptor
- HM
hippocampal membranes
- MβCD
methyl-β-cyclodextrin
- PEG
polyethylene glycol
- PMSF
phenylmethylsulfonyl fluoride
- SM
solubilized membranes
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 2.Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]
- 4.Heilker R, Wolff M, Tautermann CS, Bieler M. G-protein-coupled receptor-focused drug discovery using a target class platform approach. Drug Discov Today. 2009;14:231–240. doi: 10.1016/j.drudis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 5.Schlyer S, Horuk R. I want a new drug: G-protein-coupled receptors in drug development. Drug Discov Today. 2006;11:481–493. doi: 10.1016/j.drudis.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Pucadyil TJ, Kalipatnapu S, Chattopadhyay A. The serotonin1A receptor: a representative member of the serotonin receptor family. Cell Mol Neurobiol. 2005;25:553–580. doi: 10.1007/s10571-005-3969-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Müller CP, Carey RJ, Huston JP, De Souza Silva MA. Serotonin and psychostimulant addiction: focus on 5-HT1A-receptors. Prog Neurobiol. 2007;81:133–178. doi: 10.1016/j.pneurobio.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Kalipatnapu S, Chattopadhyay A. Membrane organization and function of the serotonin1A receptor. Cell Mol Neurobiol. 2007;27:1097–1116. doi: 10.1007/s10571-007-9189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celada P, Bortolozzi A, Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 2013 doi: 10.1007/s40263-013-0071-0. in press. [DOI] [PubMed] [Google Scholar]
- 10.Burger K, Gimpl G, Fahrenholz F. Regulation of receptor function by cholesterol. Cell Mol Life Sci. 2000;57:1577–1592. doi: 10.1007/PL00000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pucadyil TJ, Chattopadhyay A. Role of cholesterol in the function and organization of G-protein coupled receptors. Prog Lipid Res. 2006;45:295–333. doi: 10.1016/j.plipres.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Paila YD, Chattopadhyay A. Membrane cholesterol in the function and organization of G-protein coupled receptors. Subcell Biochem. 2010;51:439–466. doi: 10.1007/978-90-481-8622-8_16. [DOI] [PubMed] [Google Scholar]
- 13.Oates J, Watts A. Uncovering the intimate relationship between lipids, cholesterol and GPCR activation. Curr Opin Struct Biol. 2011;21:802–807. doi: 10.1016/j.sbi.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Jafurulla M, Chattopadhyay A. Membrane lipids in the function of serotonin and adrenergic receptors. Curr Med Chem. 2013;20:47–55. [PubMed] [Google Scholar]
- 15.Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GF. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23:3609–3620. doi: 10.1038/sj.emboj.7600374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, Schertler GF, Tate CG. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature. 2011;469:241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherezov V, Rosenbaum DM, Hanson MA, Rasmussen SGF, Thian FS, Kobilka TS, Choi HJ, Kuhn P, Weis WI, Kobilka BK, Stevens RC. High-resolution crystal structure of an engineered human β2-adrenergic G protein-coupled receptor. Science. 2007;318:1258–1265. doi: 10.1126/science.1150577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson MA, Cherezov V, Griffith MT, Roth CB, Jaakola VP, Chien EYT, Velasquez J, Kuhn P, Stevens RC. A specific cholesterol binding site is established by the 2.8 Å structure of the human β2-adrenergic receptor. Structure. 2008;16:897–905. doi: 10.1016/j.str.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, IJzerman AP, Cherezov V, Stevens RC. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pucadyil TJ, Chattopadhyay A. Cholesterol modulates the ligand binding and G-protein coupling to serotonin1A receptors from bovine hippocampus. Biochim Biophys Acta. 2004;1663:188–200. doi: 10.1016/j.bbamem.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Paila YD, Murty MRVS, Vairamani M, Chattopadhyay A. Signaling by the human serotonin1A receptor is impaired in cellular model of Smith-Lemli-Opitz Syndrome. Biochim Biophys Acta. 2008;1778:1508–1516. doi: 10.1016/j.bbamem.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Shrivastava S, Pucadyil TJ, Paila YD, Ganguly S, Chattopadhyay A. Chronic cholesterol depletion using statin impairs the function and dynamics of human serotonin1A receptors. Biochemistry. 2010;49:5426–5435. doi: 10.1021/bi100276b. [DOI] [PubMed] [Google Scholar]
- 23.Simons K, Ikonen E. How cells handle cholesterol. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 24.Mouritsen OG, Zuckermann MJ. What’s so special about cholesterol? Lipids. 2004;39:1101–1113. doi: 10.1007/s11745-004-1336-x. [DOI] [PubMed] [Google Scholar]
- 25.Xu X, London E. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry. 2000;39:843–849. doi: 10.1021/bi992543v. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee S, Maxfield FR. Membrane domains. Annu Rev Cell Dev Biol. 2004;20:839–866. doi: 10.1146/annurev.cellbio.20.010403.095451. [DOI] [PubMed] [Google Scholar]
- 27.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhuri A, Chattopadhyay A. Transbilayer organization of membrane cholesterol at low concentrations: implications in health and disease. Biochim Biophys Acta. 2011;1808:19–25. doi: 10.1016/j.bbamem.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 30.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 31.Pucadyil TJ, Chattopadhyay A. Cholesterol: a potential therapeutic target in Leishmania infection? Trends Parasitol. 2007;23:49–53. doi: 10.1016/j.pt.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Chattopadhyay A, Jafurulla M. Role of membrane cholesterol in leishmanial infection. Adv Exp Med Biol. 2012;749:201–213. doi: 10.1007/978-1-4614-3381-1_14. [DOI] [PubMed] [Google Scholar]
- 33.Paila YD, Chattopadhyay A. The function of G-protein coupled receptors and membrane cholesterol: specific or general interaction? Glycoconj J. 2009;26:711–720. doi: 10.1007/s10719-008-9218-5. [DOI] [PubMed] [Google Scholar]
- 34.Lee AG. Biological membranes: the importance of molecular detail. Trends Biochem Sci. 2011;36:493–500. doi: 10.1016/j.tibs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Paila YD, Tiwari S, Chattopadhyay A. Are specific nonannular cholesterol binding sites present in G-protein coupled receptors? Biochim Biophys Acta. 2009;1788:295–302. doi: 10.1016/j.bbamem.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Simmonds AC, East JM, Jones OT, Rooney EK, McWhirter J, Lee AG. Annular and non-annular binding sites on the (Ca2+ + Mg2+)-ATPase. Biochim Biophys Acta. 1982;693:398–406. doi: 10.1016/0005-2736(82)90447-3. [DOI] [PubMed] [Google Scholar]
- 37.Jones OT, McNamee MG. Annular and nonannular binding sites for cholesterol associated with the nicotinic acetylcholine receptor. Biochemistry. 1988;27:2364–2374. doi: 10.1021/bi00407a018. [DOI] [PubMed] [Google Scholar]
- 38.Singh P, Paila YD, Chattopadhyay A. Differential effects of cholesterol and 7-dehydrocholesterol on the ligand binding activity of the hippocampal serotonin1A receptor: implications in SLOS. Biochem Biophys Res Commun. 2007;358:495–499. doi: 10.1016/j.bbrc.2007.04.135. [DOI] [PubMed] [Google Scholar]
- 39.Singh P, Saxena R, Paila YD, Jafurulla M, Chattopadhyay A. Differential effects of cholesterol and desmosterol on the ligand binding function of the hippocampal serotonin1A receptor: implications in desmosterolosis. Biochim Biophys Acta. 2009;1788:2169–2173. doi: 10.1016/j.bbamem.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Westover EJ, Covey DF. The enantiomer of cholesterol. J Membr Biol. 2004;202:61–72. doi: 10.1007/s00232-004-0714-7. [DOI] [PubMed] [Google Scholar]
- 41.Jiang X, Covey DF. Total synthesis of ent-cholesterol via a steroid C,D-ring side-chain synthon. J Org Chem. 2002;67:4893–4900. doi: 10.1021/jo025535k. [DOI] [PubMed] [Google Scholar]
- 42.Harikumar KG, Chattopadhyay A. Metal ion and guanine nucleotide modulations of agonist interaction in G-protein coupled serotonin1A receptors from bovine hippocampus. Cell Mol Neurobiol. 1998;18:535–553. doi: 10.1023/A:1026383527092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 44.Chattopadhyay A, Harikumar KG, Kalipatnapu S. Solubilization of high affinity G-protein-coupled serotonin1A receptors from bovine hippocampus using pre-micellar CHAPS at low concentration. Mol Membr Biol. 2002;19:211–220. doi: 10.1080/09687680210149586. [DOI] [PubMed] [Google Scholar]
- 45.Chattopadhyay A, Paila YD, Jafurulla M, Chaudhuri A, Singh P, Murty MRVS, Vairamani M. Differential effects of cholesterol and 7-dehydrocholesterol on ligand binding of solubilized hippocampal serotonin1A receptors: implications in SLOS. Biochem Biophys Res Commun. 2007;363:800–805. doi: 10.1016/j.bbrc.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 46.Singh P, Jafurulla M, Paila YD, Chattopadhyay A. Desmosterol replaces cholesterol for ligand binding function of the serotonin1A receptor in solubilized hippocampal membranes: support for nonannular binding sites for cholesterol? Biochim Biophys Acta. 2011;1808:2428–2434. doi: 10.1016/j.bbamem.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 47.Bruns RF, Lawson-Wendling K, Pugsley TA. A rapid filtration assay for soluble receptors using polyethylenimine-treated filters. Anal Biochem. 1983;132:74–81. doi: 10.1016/0003-2697(83)90427-x. [DOI] [PubMed] [Google Scholar]
- 48.McClare CWF. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971;39:527–530. doi: 10.1016/0003-2697(71)90443-x. [DOI] [PubMed] [Google Scholar]
- 49.Saxena R, Chattopadhyay A. Membrane cholesterol stabilizes the human serotonin1A receptor. Biochim Biophys Acta. 2012;1818:2936–2942. doi: 10.1016/j.bbamem.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 50.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3. Springer; New York: 2006. [Google Scholar]
- 51.Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects. Studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mannock DA, McIntosh TJ, Jiang X, Covey DF, McElhaney RN. Effects of natural and enantiomeric cholesterol on the thermotropic phase behavior and structure of egg sphingomyelin bilayer membranes. Biophys J. 2003;84:1038–1046. doi: 10.1016/S0006-3495(03)74920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Covey DF. ent-Steroids: novel tools for studies of signaling pathways. Steroids. 2009;74:577–585. doi: 10.1016/j.steroids.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu F, Rychnovsky SD, Belani JD, Hobbs HH, Cohen JC, Rawson RB. Dual roles for cholesterol in mammalian cells. Proc Natl Acad Sci USA. 2005;102:14551–14556. doi: 10.1073/pnas.0503590102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mickus DE, Levitt DG, Rychnovsky SD. Enantiomeric cholesterol as a probe of ion-channel structure. J Am Chem Soc. 1992;114:359–360. [Google Scholar]
- 56.D’Avanzo N, Hyrc K, Enkvetchakul D, Covey DF, Nichols CG. Enantioselective protein-sterol interactions mediate regulation of both prokaryotic and eukaryotic inward rectifier K+ channels by cholesterol. PLoS One. 2011;6:e19393. doi: 10.1371/journal.pone.0019393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kristiana I, Luu W, Stevenson J, Cartland S, Jessup W, Belani JD, Rychnovsky SD, Brown AJ. Cholesterol through the looking glass: ability of its enantiomer also to elicit homeostatic responses. J Biol Chem. 2012;287:33897–33904. doi: 10.1074/jbc.M112.360537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demel RA, Bruckdorfer KR, van Deenen LLM. Structural requirements of sterols for the interaction with lecithin at the air-water interface. Biochim Biophys Acta. 1972;255:311–320. doi: 10.1016/0005-2736(72)90030-2. [DOI] [PubMed] [Google Scholar]
- 59.Duforurc EJ, Perish EJ, Chitrakorn S, Smith ICP. Structural and dynamical details of cholesterol-lipid interaction as revealed by deuterium NMR. Biochemistry. 1984;23:6062–6071. [Google Scholar]
- 60.Murari R, Murari MP, Baumann WJ. Sterol orientations in phosphatidylcholine liposomes as determined by deuterium NMR. Biochemistry. 1986;25:1062–1067. doi: 10.1021/bi00353a017. [DOI] [PubMed] [Google Scholar]
- 61.Cheetham JJ, Wachtel E, Bach D, Epand RM. Role of the stereochemistry of the hydroxyl group of cholesterol and the formation of nonbilayer structures in phosphatidylethanolamines. Biochemistry. 1989;28:8928–8934. doi: 10.1021/bi00448a036. [DOI] [PubMed] [Google Scholar]
- 62.Kalipatnapu S, Chattopadhyay A. Membrane protein solubilization: recent advances and challenges in solubilization of serotonin1A receptors. IUBMB Life. 2005;57:505–512. doi: 10.1080/15216540500167237. [DOI] [PubMed] [Google Scholar]
- 63.Seddon AM, Curnow P, Booth PJ. Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 64.Chattopadhyay A, Harikumar KG. Dependence of critical micelle concentration of a zwitterionic detergent on ionic strength: implications in receptor solubilization. FEBS Lett. 1996;391:199–202. doi: 10.1016/0014-5793(96)00733-8. [DOI] [PubMed] [Google Scholar]
- 65.Jones MB, Garrison JC. Instability of the G-protein (β5 subunit in detergent. Anal Biochem. 1999;268:126–133. doi: 10.1006/abio.1998.3064. [DOI] [PubMed] [Google Scholar]
- 66.Bayewitch ML, Nevo I, Avidor-Reiss T, Levy R, Simonds WF, Vogel Z. Alterations in detergent solubility of heterotrimeric G proteins after chronic activation of Gi/o-coupled receptors: changes in detergent solubility are in correlation with onset of adenylyl cyclase superactivation. Mol Pharmacol. 2000;57:820–825. doi: 10.1124/mol.57.4.820. [DOI] [PubMed] [Google Scholar]
- 67.Chattopadhyay A, Jafurulla M, Kalipatnapu S, Pucadyil TJ, Harikumar KG. Role of cholesterol in ligand binding and G-protein coupling of serotonin1A receptors solubilized from bovine hippocampus. Biochem Biophys Res Commun. 2005;327:1036–1041. doi: 10.1016/j.bbrc.2004.12.102. [DOI] [PubMed] [Google Scholar]
- 68.Banerjee P, Joo JB, Buse JT, Dawson G. Differential solubilization of lipids along with membrane proteins by different classes of detergents. Chem Phys Lipids. 1995;77:65–78. doi: 10.1016/0009-3084(95)02455-r. [DOI] [PubMed] [Google Scholar]
- 69.London E, Feigenson GW. Fluorescence quenching in model membranes: an analysis of the local phospholipid environments of diphenylhexatriene and gramicidin A′. Biochim Biophys Acta. 1981;649:89–97. [Google Scholar]
- 70.Wen Z, Kim HY. Alterations in hippocampal phospholipid profile by prenatal exposure to ethanol. J Neurochem. 2004;89:1368–1377. doi: 10.1111/j.1471-4159.2004.02433.x. [DOI] [PubMed] [Google Scholar]
- 71.Gimpl G, Burger K, Fahrenholz F. Cholesterol as modulator of receptor function. Biochemistry. 1997;36:10959–10974. doi: 10.1021/bi963138w. [DOI] [PubMed] [Google Scholar]
- 72.Addona GH, Sandermann HJ, Kloczewiak MA, Miller KW. Low chemical specificity of the nicotinic acetylcholine receptor sterol activation site. Biochim Biophys Acta. 2003;1609:177–182. doi: 10.1016/s0005-2736(02)00685-5. [DOI] [PubMed] [Google Scholar]
- 73.Zitzer A, Westover EJ, Covey DF, Palmer M. Differential interaction of the two cholesterol-dependent, membrane-damaging toxins, streptolysin O and Vibrio cholerae cytolysin, with enantiomeric cholesterol. FEBS Lett. 2003;553:229–231. doi: 10.1016/s0014-5793(03)01023-8. [DOI] [PubMed] [Google Scholar]
- 74.Richter RK, Mickus DE, Rychnovsky SD, Molinski TF. Differential modulation of the antifungal activity of amphotericin B by natural and ent-cholesterol. Bioorg Med Chem Lett. 2004;14:115–118. doi: 10.1016/j.bmcl.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Crowder CM, Westover EJ, Kumar AS, Ostlund RE, Covey DF. Enantiospecificity of cholesterol function in vivo. J Biol Chem. 2001;276:44369–44372. doi: 10.1074/jbc.C100535200. [DOI] [PubMed] [Google Scholar]
- 76.Paila YD, Tiwari S, Sengupta D, Chattopadhyay A. Molecular modeling of the human serotonin1A receptor: role of membrane cholesterol in ligand binding of the receptor. Mol Biosyst. 2011;7:224–234. doi: 10.1039/c0mb00148a. [DOI] [PubMed] [Google Scholar]
- 77.Sengupta D, Chattopadhyay A. Identification of cholesterol binding sites in the serotonin1A receptor. J Phys Chem B. 2012;116:12991–12996. doi: 10.1021/jp309888u. [DOI] [PubMed] [Google Scholar]
- 78.Jafurulla M, Tiwari S, Chattopadhyay A. Identification of cholesterol recognition amino acid consensus (CRAC) motif in G-protein coupled receptors. Biochem Biophys Res Commun. 2011;404:569–573. doi: 10.1016/j.bbrc.2010.12.031. [DOI] [PubMed] [Google Scholar]