Abstract
As a result of their unique electronic, optical, and mechanical properties, carbon nanotubes (CNTs) have been implemented in therapeutic and imaging applications. In an idealized situation, CNTs would be disposed of after they transport their theranostic payloads. Biodegradation represents an attractive pathway for the eliminating of CNT carriers post-delivery and may be integral in catalyzing the release of the cargo from the delivery vehicle. Accordingly, recent research efforts have focused on peroxidase-driven biodegradation of CNTs. In this review, we not only summarize recent efforts to biodegrade CNTs in the test tube, in vitro, and in vivo, but also attempt to explore the fundamental parameters underlying degradation. Encouraged by the in vivo results obtained to date, we envision a future, where carbon-based nano-containers, which are specifically designed to target organs/cells, deliver their cargo, and biodegrade via peroxidase-driven mechanism, will represent an attractive therapeutic delivery option in nanomedicine.
Keywords: Biodegradation, carbon nanotubes, graphene, nanoparticles, peroxidases, drug delivery
1. Introduction
Structurally, single-walled carbon nanotubes (SWCNT) can be envisioned as a single sheet of graphene rolled seamlessly into a cylinder; multiwalled carbon nanotubes (MWCNTs) are comprised of concentrically nestled SWCNTs of increasing diameter. Because their geometries are characterized by nanometer-sized diameters (i.e. from 0.4 to 3 nm and 2 to 100 nm for SWCNTs and MWCNTs, respectively) and a length of several micrometers [1], carbon nanotubes (CNTs) possess a high aspect ratio. Moreover, CNTs are endowed with unique electronic [2], optical [3], and mechanical properties [4], which enable this sp2-hybridized allotrope of carbon to be employed for diverse applications such as electronics [5], sensors [6, 7], composite materials [8], energy conversion devices [9], and medicine [10, 11]. This review article is related to their drug delivery application and will examine issues related to in vitro and in vivo biodegradation of CNTs catalyzed by peroxidases.
1.1. CNTs and Drug Delivery
A vast variety of nanoparticles (NPs) composed of metals, metal oxides, quantum dots (QDs), polymers, and carbon (e.g. carbon nanotubes and graphene) are receiving increasing interest over the recent years due to their potential in vivo therapeutic or imaging applications [12–14]. As a result of their high surface area and unique optical and electronic properties, CNTs have the potential of becoming a highly efficient delivery vehicle for drugs and biomolecules [14]. Because as-prepared CNTs are hydrophobic, to enhance their dispersibility in aqueous environments and thereby implement this carbon-based nanomaterial for biomedical applications [15], CNTs must be modified by either covalent or noncovalent means; this action will not only enhance biodispersibility but also biocompatibility of CNTs. Once rendered biodispersible and biocompatible, the high surface area of the CNTs presents an attractive platform for therapeutic delivery, where peptides, nucleic acids, proteins, and drugs could be (1) stored in the large inner-tubular volume; (2) π-π stacked to the walls; and/or (3) covalently conjugated to defect sites on the walls/edges of this carbon-based nanomaterial [16]. Moreover, CNTs are proficient at entering the nuclei of cells [17], which may render this nanomaterial integral for gene therapy applications. Interestingly, the near-infrared (NIR) biological transparency window, which occurs in the range of 800 to 1400 nm, falls within the region where SWCNTs strongly absorb and emit light (i.e. 800 to 1600 nm) [14]. As a result, SWCNTs have been employed for photothermal therapy, photoacoustic imaging, and Raman detection/imaging [14].
The primary hindrance to employing CNTs for biomedical applications entails potential toxicity issues that include oxidative stress and inflammatory pathways [18]. By controlling physicochemical properties such as length and degree of functionalization and pharmacological parameters such as dosing, the toxicological properties of CNTs can be mitigated [19]. Moreover, in vitro and in vivo studies have been implemented to acquire insight into the biological transformation and long-run fate of CNTs; these studies have demonstrated that there exist pathways to mitigate toxicity through the biodegradation of CNTs. These findings are applicable to CNT drug delivery vehicle, where biodegradation may either catalyze the release of the cargo conjugated to the CNT carrier or destroy the CNT delivery vehicle post-release thereby mitigating potential toxicity. Accordingly, this article will guide the reader through these recent in vitro and in vivo efforts to biodegrade CNTs.
2. In vitro Degradation of CNTs Outside Living Systems
Recent reports have demonstrated that the plant peroxidase, horseradish peroxidase (HRP) [20–25], and the animal peroxidases, myeloperoxidase (MPO) [25–28] and eosinophil peroxidase (EPO) [29], catalyze the degradation/biodegradation of carbon nanomaterials. In this section, we investigate in test tube examples of and provide plausible explanations of enzyme-catalyzed degradation of CNTs. This section will function as a tutorial for later sections in which cellular and in vivo studies that demonstrated biodegradation of CNTs are explored.
2.1. HRP – The Model Peroxidase System for CNT Degradation
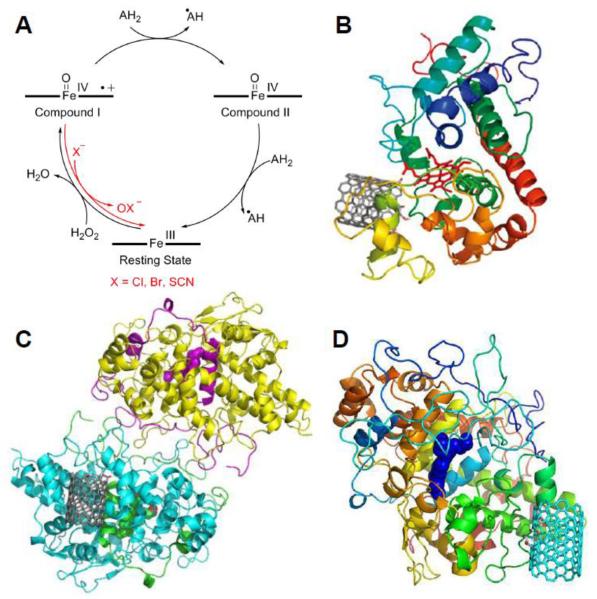
In an early study, we demonstrated that HRP catalyzed the degradation of carboxylated SWCNTs in the presence of H2O2 [20]. HRP, which has been employed in applications ranging from biotechnology to bioremediation [30], represents a classical secretory plant peroxidase that is derived from the horseradish plant (Armoracia rusticana) [31]. This monomeric enzyme consists of a noncovalently bound ferriprotoporphyrin IX prosthetic heme group [31] as its active site. When HRP is in its native, resting state, this group is in the ferric, Fe(III), form. The heme center is converted into an oxo-ferryl iron (Fe4+=O) and a porphyrin π cation radical referred to as Compound I via a protein-assisted mechanism upon reaction with hydrogen peroxide (H2O2), which is reduced back to the ferric resting state by the peroxidase cycle (Figure 1A) [30].
Figure 1.
(A) The catalytic peroxidase cycle for HRP, MPO and EPO. For MPO and EPO, compound I is reduced directly to the resting state via conversion of halide to hypohalite (red path). (B, C, D) Molecular modeling of carboxylated SWCNTs binding to the active sites of (B) HRP, (C) MPO and (D) EPO. Panel B reproduced from [21] Copyright American Chemical Society 2009, Panel C reproduced from [26] Copyright Macmillan Publishers, Ltd. 2010, and Panel D reproduced from [29] Copyright Wiley-VCH 2013.
In a following mechanistic investigations, we demonstrated that only SWCNTs that contain oxygen moieties/defect sites underwent degradation; pristine SWCNTs were not observed to degrade over the same time period [21]. The introduction of defect site that consist of oxygen functionalities (e.g. carboxyl, carbonyl, and hydroxyl groups) by strong oxidizing agents, represents one common methodology employed to enhance the biocompatibility of CNTs [32, 33]. Carboxyl groups are attached to the graphitic backbone by one bond [34]; therefore, because it is necessary to cleave only a single bond, CNTs functionalized with these groups would therefore appeared most susceptible to enzymatic oxidation versus other functional groups. Moreover, the ability of HRP to preferentially react on carboxylated CNTs was supported by the different degradation behavior of carboxylated MWCNTs [22, 23]. As the carboxylation process can only impart functional groups to the outer layers of MWCNTs, the more pristine inner layers failed to be completely degraded after the exfoliation of outer layers.
As previously mentioned, pristine CNTs were not observed to degrade over the period of analysis [25]. This may be attributed to two reasons. The first explanation is related to the overall dispersity of CNTs in an aqueous media; because pristine CNTs are intrinsically hydrophobic, there exists a strong tendency for nanotubes to aggregate into bundles, ropes, and networks as a result of van der Waals forces. To counteract these effects, CNTs have been modified by numerous covalent and noncovalent modifications to render this nanomaterial more dispersible in an aqueous environment [15]. To this end, CNTs have been functionalized employing several methodologies, including: (1) the creation of defect-sites via strong oxidant chemistry, (2) covalent chemistry performed on sidewall, (3) attachment of pyrene derivatives via π-π interactions, and (4) noncovalent wrapping utilizing surfactants/amphiphiles, polymers, and biopolymers [35]. These techniques often yield a high-quality dispersion, which is composed mainly of individualized nanotubes with the remainder consisting of small bundles; Coleman and coworkers demonstrated that the quality of these dispersions scale with the ζ-potential of the dispersion [36]. As the ζ-potential increases, the electrostatic repulsion of the functional groups imparted by the dispersing agent/via covalent modification is sufficient to overcome the attractive van der Waals interactions of the CNTs [37]. While the direct role of bundling on degradation has not yet been examined, all studies that reported degradation were conducted on functionalized CNTs that demonstrate minimal bundling.
The second factor, which is also related to dispersibility, entails nanotube-enzyme interactions. When dispersed in an aqueous media, the negative charge of carboxyl groups on the covalently functionalized SWCNTs may facilitate degradation by encouraging the proper orientation/proximity of the peroxidase enzyme's heme active site to the SWCNT substrate, which has been demonstrated by molecular modeling studies. Due to electrostatic interactions, the negatively charged carboxylated CNTs would orient themselves toward a positively charged arginine residue, Arg178, which is positioned near HRP's heme site (Figure 1B) [21]. On the other hand, pristine SWCNTs were predicted to orient themselves towards the distal end of the enzyme away from the active heme site thereby failing to undergo degradation [21].
Even if the functionalized CNTs are well dispersed and properly oriented relative to the enzyme's active site, oxidation/degradation is not expected to proceed unless the standard potential of the reactive intermediates of HRP exceed the value for CNTs. For example, the reactive intermediates of HRP have a mean standard potential (E0) of +0.884 V (Table 1) [38] while SWCNTs have a work function (V) under vacuum of ~5 eV and a corresponding standard potential (E0) of +0.5 V [39, 40]. Therefore, the reactive intermediates of HRP possess more positive redox potentials and are stronger oxidants than SWCNTs [25]. Consequently, SWCNTs should theoretically be spontaneously oxidized through the donation of electrons from their valence band to these mediators.
Table 1.
Standard Reduction Potentials (at pH 7) Along the Peroxidase Cycle for Peroxidase Involved in CNT Degradation/Biodegradation
HRP represents a promising model system for fundamental studies and may be relevant for environmental applications relating to carbon nanotube bioremediation. For applications relating to nanotoxicity and drug delivery, more physiologically relevant peroxidases were examined.
2.2. MPO and EPO – Peroxidases that Generate Hypohalous Acids
In collaboration with others, we have further expanded the in vitro degradation of CNTs to a series of peroxidases derived from animals including humans. A representative study demonstrated that human myeloperoxidase (hMPO) degraded carboxylated SWCNTs [26]. MPO consists of a homodimeric protein that is predominately expressed in granules of neutrophils (i.e. a type of leukocytes). In response to the phagocytosis of bacterium, MPO is released from these granules primarily into phagolysosomal compartment during which this peroxidase enzyme functions as a bactericide through the generation of both reactive intermediates (i.e. formed via the peroxidase cycle) and oxidants that are formed during the halogenation cycle (Figure 1A) [31, 41–43]. During the halogenation cycle, MPO demonstrates the ability to oxidize Cl−, Br−, and the pseudohalide, SCN− at high rates; during the process, these substrates donate two electrons to Compound I to generate the ferric form of the enzyme and, in turn, are converted to the corresponding (pseudo)hypohalous acids (HXO, such that X = Cl, Br, SCN) [44].
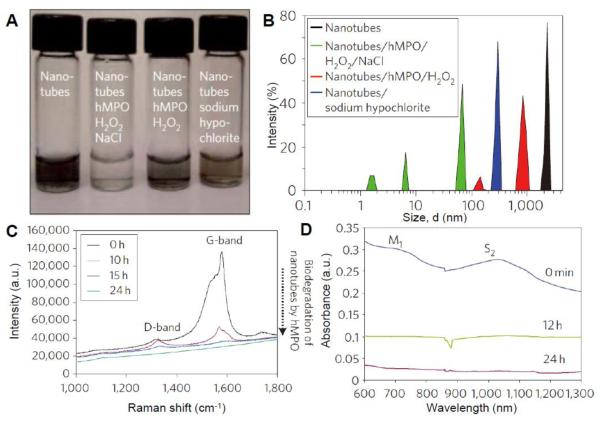
After incubating carboxylated SWCNTs with hMPO in the presence of both H2O2 and Cl− for 24 h, the dispersion appeared almost clear in color (Figure 2A). Moreover, the SWCNTs underwent structural deformation including both a decrease in size as revealed by dynamic light scattering (DLS) (Figure 2B) and an increase in defects as evidenced by the increase in the D/G band ratio, which was determined by Raman spectroscopy (Figure 2C). Degradation of the SWCNTs was also confirmed by the suppression of the metallic (M1) and semiconducting (S2) transition bands that characterize the vis-NIR absorption spectra for SWCNTs (Figure 2D). The control experiment, which excluded Cl− and therefore relied only on the reactive intermediates formed during the peroxidase cycle, demonstrated markedly less SWCNT degradation thereby suggesting the important role of the strong oxidant, hypochlorous acid (HClO), in the degradation process; the in vitro study by Vlasova et al. confirmed this finding [27]. Presumably, the synergetic effects of hypochlorite and the reactive intermediates of MPO facilitated a much higher efficiency of CNT degradation for the MPO/H2O2/Cl− system versus the HRP/H2O2 system. Of importance, small oxidants like HClO has the ability to diffuse from active site and oxidize CNTs; the more bulky reactive intermediates, on the other hand, must be in close enough proximity of the CNT to promote degradation. Moreover, the enzyme-catalyzed oxidation of CNTs may be in competition with other substances that have lower redox potential (i.e. more reductive), as shown in our recent study, where the enzymatic degradation of SWCNTs was mitigated by antioxidants added to the system [45].
Figure 2.
(A) Photographs of carboxylated single-walled carbon nanotubes (SWCNTs) incubated with or without degradative reagents after 24 h. (B) Dynamic light scattering data of different samples showing decreasing sizes of degraded nanotubes. (C) Raman spectra of SWCNTs before and after 24 h of degradation by MPO/H2O2/Cl−. (D) Visible-near infrared (Vis-NIR) absorption spectra of SWCNTs being degraded during 24 h. Adapted from [26] Copyright Macmillan Publishers, Ltd. 2010.
For MPO, amino acid residues, Glu242, Asp94 and Met243, are covalently bound to the ferriprotoporphyrin IX heme group [31], and molecular simulations suggested the existence of two potential interaction sites for CNT binding depending on their functionality [26]. For acid treated SWCNTs, the positively charged arginine residues (i.e. Arg 294, 307, and 507), which reside near the proximal end of the heme group, strongly interacted with the negatively charged carboxylated nanotubes; this area also contained the catalytically active tyrosine residues, Tyr293 and 313 (Figure 1C) [26]. Alternatively, pristine SWCNTs appeared to favor a second binding site, which exists at the distal end of the heme group away from the tyrosine residues [26].
EPO also facilitated the degradation of CNTs. EPO, which is expressed by eosinophils (i.e. a type of leukocytes), assists in the destruction of invading parasites. Because, in general, parasites are larger than bacteria, eosinophils attach to these species and exocytose their granule contents, which is composed of EPO at 40% by mass. Interestingly, EPO possesses a 70% amino acid homology with MPO but weighs roughly half of latter enzyme (i.e. 69.8 kDa versus 144 kDa, respectively) [44], and inflammation of the human lung is characterized by the presence of EPO [29]. Like MPO, EPO contains a modified heme groups that are composed of ferriprotoporphyrin IX as its active site and therefore can generate reactive intermediates by the peroxidase cycle and hypohalous acids by the halogenation cycle [44]. It is important to note that because of the differences in the active-site topology and binding sites between MPO and EPO, the redox potentials of the enzymes (Table 1) does not always correlate with the rate constants that the enzymes exhibit for a given (pseudo)halide [44]. For example, at neutral pH and given physiological concentrations of Cl−, Br−, and, SCN−, MPO primarily generates HClO and HSCNO although it has the ability to oxidize Br−. HBrO and HOSCN and primarily produced by EPO despite this enzyme being a strong enough oxidant to convert Cl− to HClO, [44]. The reduction potentials for these (pseudo)hypohalous acids at pH 7 are denoted by Table 2 [44].
Table 2.
Two-electron Reduction Potentials of (Pseudo)hypohalous Acids at pH 7 in Water [45]
| Standard Reduction Potentials (V) | ||
|---|---|---|
| HClO/Cl− | HBrO/Br− | HSCNO/SCN− |
| 1.28 | 1.13 | 0.56 |
For the in vitro experiment, carboxylated SWCNTs were incubated with human EPO (hEPO) under physiological condition with constant addition of H2O2 and NaBr. During the 96 h incubation, degradation of the SWCNTs was visually observed by a gradual color fading of the SWCNT suspension. A decrease in both the S2 characteristic band for SWCNTs in the NIR spectra and the Raman-derived D/G band ratio was observed, which suggested the degradation of SWCNTs under EPO oxidation, which was also confirmed by transmission electron microscopy (TEM). Therefore, the reactive intermediates and HBrO acid generated by EPO through the peroxidase and halogenation cycles, respectively, successfully resulted in the degradation of oxidized nanotubes. Significantly, when lactoperoxidase (LPO) was incubated with H2O2 and Br−, CNT degradation was observed confirming the strong oxidizing role of HBrO [27].
For EPO, two binding sites were identified both of which are stabilized by the electrostatic interactions between the positively charged arginine residues (i.e. Arg205, Arg207 and Arg209 for binding site 1 and Arg94 and Arg99 for binding site 2) and the negatively charged oxygen-containing functionalities on oxidized SWCNTs (Figure 1D) [29]. Of these two sites, binding site 1 was identified to be located on the same side as the catalytic site, appeared to be closer to the entrance of the catalytic site versus binding site 2, and overlapped with one of the bromide ion binding sites. Consequently, these cumulative results indicate that binding site 1 of EPO was the preferred location for biodegradation of oxidized SWCNTs [29].
3. In vitro Degradation of CNTs in Cells
3.1. Factors that Facilitate in vitro Degradation in Cellular Culture
Having had success with enzyme-catalyzed degradation of CNTs in the test tube, cellular studies were performed to elucidate the effect of innate degradative enzymes of the cell on CNTs. To this end, the in vitro degradation of CNTs in cell culture has been thus far investigated [26, 29]. The results demonstrated that significant degradation of CNTs was typically observed in granulocytes such as neutrophils and eosinophils, and the degradation was associated with active oxidases and peroxidases that are rich in the aforementioned cells together with reactive intermediates, which are considered to be key factors for CNT degradation by cells.
3.1.1. Oxidative Stress Induced by CNTs
It is commonly accepted that the cytotoxicity of nanoparticles such as CNTs is largely derived from oxidative stress resulting from their small size and large specific surface area [46–48]. Moreover, the oxidative stress also arises from increased levels of reactive oxygen species (ROS) that include superoxide radical anions and hydroxyl radicals. As a natural byproduct of cellular respiration process in mitochondria, the ROS are normally balanced by a series of anti-oxidant enzymes. However, the cellular homeostasis can be disrupted when the intracellular ROS levels are significantly levitated by environmental stress (e.g. exposure to nanoparticles), thereby leading to oxidative stress. It is believed that nanoparticles can trigger the formation of ROS through two different pathways [49]. Some transition metal-based nanoparticles can catalyze decomposition of peroxides and form ROS directly such as the Fenton reaction triggered by iron catalytic nanoparticles from unpurified SWCNTs [50]; while in more general cases, the ROS are generated by phagocytic cells of the immune system (i.e. neutrophils, eosinophils, and macrophages) in response to the extrinsic nanoparticles. When phagocytes actively internalize nanoparticles, the massive ROS generation process is considered to be NADPH oxidase-dependent [49, 51], where upon activation, NADPH oxidase ensembles at the phagolysosomal membrane and transfers electrons to oxygen to form superoxide in a process known as “oxidative burst” [52]. In turn, superoxide anions will dismutate to H2O2 [53], which is an initiator of the aforementioned peroxidase cycle of MPO in neutrophils and EPO in eosinophils. CNTs, in particular, because of their large surface activity, are highly likely to induce oxidative burst.
3.1.2. Role of Oxidative Stress to the in vitro Degradation of CNTs
It is commonly thought that oxidative stress triggers cell damage and death, and this phenomenon may be the cause of many diseases and cancers [54, 55]. However, the beneficial effects of oxidative stress are receiving more and more attention in recent years as studies revealed the important immune functions of ROS in “oxidative signaling,” which activates the inflammatory response in phagocytic cells, as well as in the attack and degradation of pathogens [49, 56]. The massive generation of strong ROS in response to pathogen engulfment has a large enough oxidative potential to break C-C and C-H bonds in bacteria and viruses thereby leading to their degradation. This oxidative clearance of pathogens also applies to degradation of carbonaceous nanomaterials including CNTs as they share similar sizes and elemental compositions with bacteria or viruses.
The in vitro degradation of CNTs is more likely to occur in the activated phagocytic cells with high oxidative stress. As demonstrated by in test tube results, innate peroxidases, such as MPO in neutrophils and EPO in eosinophils, play a pivotal role in oxidative degradation of CNTs. Under natural inflammatory response, neutrophils are attracted and activated by chemoattractants at the inflammatory sites, and MPO is released thus being able to act both intra-and extra-cellularly [57]. H2O2, which originated from NADPH oxidase, forms inside the phagosome, activates MPO, and leads to the production of reactive enzymatic intermediates and oxidants (e.g. hydroxyl radicals, HClO, etc.) [53], both of which are responsible for CNT degradation. As a result, the phagosomes of the phagocytes provide the exact environment facilitating the degradation of CNTs as simulated under test tube conditions. Both H2O2-generating enzyme (e.g. NADPH oxidase) and oxidative peroxidase are essential factors for the degradation of CNTs inside phagocytes under oxidative stress. The presence of halide ions also facilitates the degradation process by forming strong oxidative hypohalous acids. Finally, in the in vitro experiment, chemoattractants and degranulation promoting agent must be added to promote degradation. While chemoattractants activate the phagocytic cells, and degranulation promoting agent (e.g. cytochalasin B) trigger the release of peroxidase.
3.1.3. Cellular Internalization of CNTs
The in vitro degradation of CNTs may occur either inside or outside of the cells depending on the distribution of the peroxidase upon cellular activation. If the peroxidase is localized mainly inside of the cells, then CNTs must be pre-opsonized for efficient internalization. Opsonization can be achieved by functionalization of CNTs with immunoglobulins, which specifically target receptors on phagocytes [58]. For example, in our previous study of CNT degradation in neutrophils [26], most of the MPO was translocated into the intracellular phagolysosomal space, where degradation ensued. Therefore, the nanotubes were targeted with IgG for enhanced cellular uptake. A significant increase in uptake and degradation of IgG-nanotubes by neutrophils occurred relative to non-functionalized nanotubes. Additionally, coating with specialized “eat-me” signals such as phosphatidylserine also makes CNTs recognizable by different professional phagocytes [59]. In contrast, in the study of CNT degradation by eosinophils [29], opsonization of CNTs was not required as the biodegradative peroxidase EPO is exocytosed upon cellular activation.
3.2. Examples of in vitro Degradation
Although a plethora of studies have investigated the cellular uptake of CNTs and their subsequent effects [60–62], only a few studies have addressed the potential cellular clearance of CNTs through biodegradation. Neves, et al. reported that the RNA-coated oxidized double-walled CNTs taken up by human prostate adenocarcinoma cells in vitro underwent possible defect-induced structural change due to increasing D to G band ratios from the Raman spectra [63]. However, the potential degradation mechanism and the eventual fate of CNTs were not discussed. In order to understand the CNT degradation process in actual living systems, we and others have investigated the in vitro degradation in both neutrophils and eosinophils conducted by MPO and EPO, respectively.
3.2.1. The in vitro Degradation of CNTs by Neutrophils
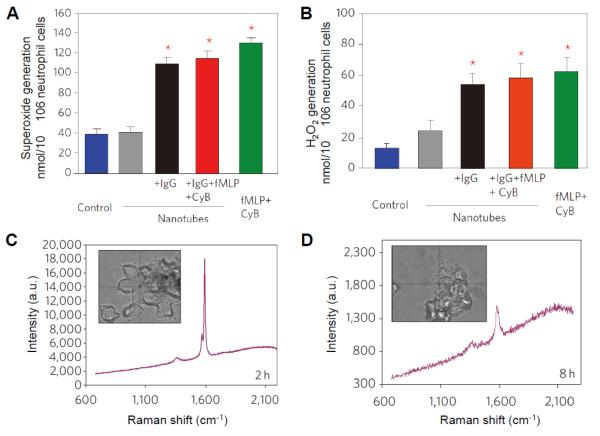
Human neutrophils, which are rich in MPO, are a type of professional phagocytes that are activated in response of inflammation and engulf pathogens. In an in vitro experiment [26], the neutrophils were first treated with fMLP and cytochalasin B to increase the activity of the cells and trigger the release of hMPO. Next, the short, oxidized SWCNTs were functionalized with IgG for their efficient internalization into neutrophils, and they were subsequently incubated with activated neutrophils. The activated neutrophils were shown to undergo an oxidative burst thus generating increased levels of superoxide and H2O2 (Figure 3A, B). Raman microscopy confirmed that IgG-nanotubes were completely degraded by neutrophils during a period of 12 h; on the other hand, only 30% of the non-IgG-treated nanotubes were degraded (Figure 3C, D). It was found that both active hMPO and NADPH oxidase were essential for CNT degradation by neutrophils. Moreover, the in vitro degradation of CNTs was found to be more profound in neutrophils than in macrophages, which contain much lower level of hMPO versus neutrophils. Recently, Vlasova et al. demonstrated that PEGylated SWCNTs activated isolated human neutrophils to produce HClO, and both oxidized and PEGylated SWCNTs were determined to activate neutrophils in whole blood samples [64]. Therefore, the mechanism of CNT degradation by neutrophils stems from hMPO associated oxidative stress triggered by activated cells.
Figure 3.
(A, B) Quantitative assay of extracellular superoxide radicals (A) and H2O2 (B) generated by activated neutrophils. *Significant different from control (P<0.05); error bars represent standard deviations (s.d.).) (C, D) Raman spectra of neutrophils containing IgG-nanotubes after 2 h (C) and 8 h (D). Adapted from [26] Copyright Macmillan Publishers, Ltd. 2010.
3.2.2. The in vitro Degradation of CNTs by Eosinophils
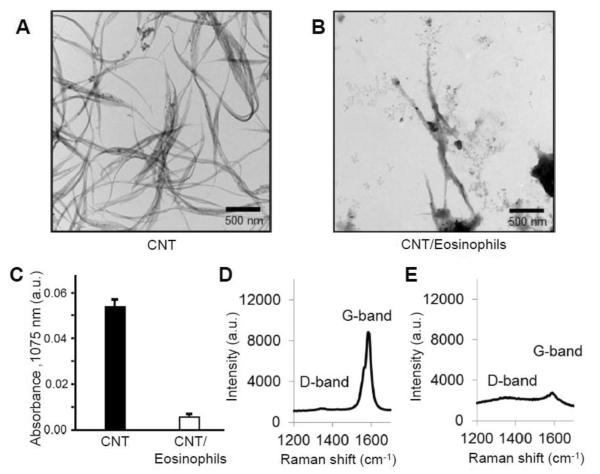
When in an inflammatory state, eosinophils are an additional type of professional phagocytes that generate oxidative species via EPO. In order to simulate the inflammatory conditions that are induced by CNT exposure, CNT degradation experiments were carried out in a cellular culture system with highly purified murine eosinophils that were obtained from mouse bone marrow progenitors [29]. Different from MPO, which is expressed inside neutrophils for killing bacterial cells, EPO is exocytosed from activated eosinophils and is mainly responsible for destroying parasites [44]. Degranulation and exocytosis of murine EPO (mEPO) were triggered by cytochalasin B and a platelet-activating factor (PAF). Upon 48 h incubation of carboxylated SWCNTs with activated mEPO, TEM images revealed that the bulk of SWCNT bundles were degraded leaving residual CNTs and carbonaceous materials (Figure 4A, B). The degradation was also confirmed by a decrease in both the S2 band for the NIR spectra and the G-band for the Raman spectra of SWCNTs (Figure 4C–E). It was noted that the degradation of CNTs by eosinophils occurs extracellularly in contrast of degradation by neutrophils, as the mEPO is exocytosed upon cellular activation and functions by forming reactive intermediates and HBrO as described in Section 2.2.
Figure 4.
(A, B) TEM images of SWCNTs alone (A) and after 48 h of incubation with activated eosinophils (B). (C) The absorbance of S2 band at 1075 nm from SWCNTs alone and SWCNTs after 48 h of incubation with activated eosinophils. (D, E) Raman spectra of SWCNTs unincubated (D) and SWCNTs incubated with eosinophils (E) showing the diminishing D and G bands. Reproduced from [29] Copyright Wiley-VCH 2013.
4. Biodistribution, Clearance, and Fate of CNTs in vivo
The biodistribution, clearance, and fate of CNTs in vivo are influenced by administration method, length, and functionalization of this nanomaterial. The term “clearance” mostly refers to the excretion of CNTs through lymphatic, biliary, or renal pathways [65, 66]; an alternative mechanism of CNT clearance via enzyme-catalyzed biodegradation in vivo is rarely discussed in the literature and will be the focus of Section 5. Importantly, the method by which CNTs are administered influences their biodistribution and clearance [67]. CNTs that are injected intravenously demonstrate low toxicity as a result of an opsonization regulated reticuloendothelial system (RES) capture, which minimizes the interaction of SWCNTs with parenchyma cells and restricts the toxicity to primarily the Kupffer cells (KC) and alveolar macrophages (AM) [68]. Therefore, CNT clearance mechanisms from AM and KC include either departing the lung via mucus through mucociliary transport [69] or being slowly excreted from KCs via bile [65]. On the other hand, intratracheally instilled SWCNTs and SWCNTs that were injected into the peritoneal cavity of mice induce more serious injury including inflammation and the formation of granuloma [70–72].
Depending on their length, CNTs may exhibit different pathogenic behaviors. Long fibers and large aggregates of CNTs, which are difficult for macrophages to phagocytose, typically induce asbestos-like pathogenicity including chronic inflammation and formation of granulomas and fibrosis in the lungs [72, 73]. After administration, these bulky materials often have a much longer retention time within the tissues and are not cleared. In contrast, short, functionalized CNTs tend to be more easily phagocytosed by the mononuclear phagocyte system or cleared through different pathways [66, 74, 75]. This was exemplified by the work of Kolosnjaj-Tabi et al. [76]. After administering SWCNTs of different lengths into Swiss mice via intraperitoneal injection, their data revealed that: large aggregates of SWCNTs (>10 μm) irremediably induce granuloma formation; smaller aggregates (<10 μm) were engulfed by phagocytes but remain persistent in cells for several months; and small, well-functionalized SWCNTs (<300 nm) were eliminated via the kidneys and bile ducts.
The surface modification of a CNT has been reported to directly affect its inflammatory response and facilitate its clearance. To this end, numerous strategies are available to reduce the size of CNTs and tune their surface properties. For example, functionalization with DNA, protein, or polymers may improve the dispersion and biocompatibility of CNTs [77–79]. Dai's group demonstrated that short SWCNTs (~100 ± 50 nm) functionalized with branched PEGylated phospholipids chains exhibited a significant increase in circulation time (≈15 h) versus non-branched PEGylated SWCNTs; they hypothesized that branched PEG structures on SWCNTs provided optimal biological inertness and resistance to opsonization/nonspecific binding of proteins [65]. In turn, this limited the rapid RES uptake of the SWCNTs and thus prolonged their circulation time in the blood. After 1 day of circulation, SWCNTs were predominantly located in the RES organs of the liver and spleen with uptake inversely depended on the degree of SWCNT surface PEGlyation. Meanwhile, over a three month period, the concentration of SWCNTs remained very low in most of the organs except for the liver and spleen in which the concentration of SWCNTs steadily decreased over the 3-month period with retention in the spleen and liver inversely proportional to the degree of PEGlyation. Because excretion of SWCNTs via the renal pathway was restricted to nanotubes that were very short in length (i.e. <50 nm in length, diameter 1–2 nm, less then the renal excretion threshold), Dai and coworkers determined that the primary mechanism for the removal of SWCNTs involved the biliary pathway as evidenced by SWCNTs being found in both the intestine and feces.
In contrast to Dai's work [65], Singh et al. observed rapid, first-order clearance of functionalized/water dispersible 111In-labeled DTPA-SWCNTs that were between 300 and 1,000 nm in length from the blood through the renal excretion pathway without any toxic side effects or mortality [80]. The difference between the observations of Dai and Singh may have stemmed from the fact that PEGylated nanotubes possess unique properties that effect pharmacokinetics, biodistribution, and clearance/fate. Recently, Sacchetti et al. completed a study, where they examined the effects of surface charge and PEG conformation on the adsorption of plasma proteins to SWCNTs and the resulting biodistribution [81]. Since the conformation of the PEG group affects the adsorption of the corona's major components, it was determined that the conformation of PEG had greater influence over the composition of the protein corona versus the surface charge imparted on a nanotube. This is important because the adsorption of certain proteins may directly influence the biodistribution of PEGylated SWCNTs.
5. In vivo Biodegradation of CNTs
5.1. Setting the State for Biodegradation: Inflammatory Response and Recruitment of Professional Phagocytes
Depending on their dimension, functionalization and administration methods, CNTs may introduce different levels of acute or chronic inflammation [61, 73, 82], causing fibrosis, oxidative stress, and mutagenesis [83]. CNT-induced inflammatory response is usually associated with increasing levels of cytokines and professional phagocytes such as macrophages and neutrophils [61]. For example, Muller et al. demonstrated that, compared with un-ground MWCNTs, MWCNTs ground with an oscillatory ball mill induced more significant cytotoxicity and inflammatory response in lungs, accompanied with increased levels of neutrophils and eosinophils [84]. Therefore, the accumulation of professional phagocytes during inflammation is postulated to be responsible for this clearance by engulfment of CNTs [73] and/or creating oxidative environment that facilitates the biodegradation of CNTs in vivo [27]. This behavior was corroborated by Vlasova et al., who observed both an increase in the percentage of circulating neutrophils and activation of neutrophils and macrophages in the peritoneal cavity thereby suggesting an inflammatory response after an intraperitoneal injection of PEGylated SWCNTs into mice [64]. In turn, the activated neutrophils are capable of producing high, local concentrations of HOCl, which represent favorable conditions for biodegrading the nanotubes.
5.2. The in vivo Biodegradation of CNTs in the Lung
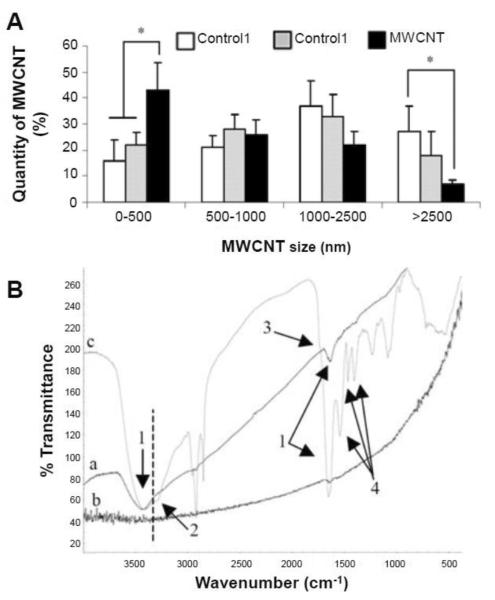
The in vivo degradation of CNTs was possibly observed in a study by Elgrabli et al. [85]. Oxidized MWCNTs were intratracheally instilled into rat lungs at different doses. The injected MWCNTs were found to be predominantly distributed in the lungs and gradually diminished from the lung during 180 days. A significant increase in the number of alveolar macrophages (AMs), which are responsible for engulfing MWCNTs, was observed. The phagocytosis of MWCNTs by AMs was directly dependent on dosage and inversely related to time. To this end, the number of AMs that contained MWCNTs increased with an increasing dosage of MWCNTs, and as time progressed, this value gradually decreased. It was postulated that the AM cells underwent apoptosis after phagocytosis of MWCNTs and were subsequently engulfed by other AMs. The interesting finding of this study was that MWCNTs underwent both physical and chemical modification 15 days after instillation. Significant diminution of MWCNT length was noticed suggesting the cleavage of MWCNTs by rat lungs, along with the introduction of different functional groups on MWCNTs (Figure 5). Although detailed characterization and mechanism of this structural change remained vague, the authors proposed that the AMs might modify the MWCNTs upon phagocytosis and facilitate their further elimination from the lung.
Figure 5.
(A) Length distribution of MWCNTs from different samples performed on at least 100 MWCNTs per sample. Control 1: MWCNTs along; Control 2: MWCNTs incubated for 15 days with suspension of lysed cells from broncheoalveolar lavage (BAL); MWCNT: MWCNTs injected to rats after 15 days. *Significant from control (p<0.05); error bars represent s.d. (B) Infrared spectra of samples from (a) Control 1, (b) Control 2, and (c) MWCNTs injected to rats after 15 days. Possible peak assignment: 1. OH from H2O, 2. OH from alcohol group, 3. low quantity of carbonyl group, 4. nitrogen functional group. Reproduced from [85].
In a recent collaborative study, we investigated the in vivo degradation of oxidized SWCNTs instilled into the lung via pharyngeal aspiration of either wild-type (w/t) or myeloperoxidase knockout (MPO k/o) mice (i.e. mice with MPO deficiency) [28]. For both types of mice, a pulmonary inflammatory response and the accumulation of neutrophils was observed as early as 1 day after exposure. While the phagocytized SWCNTs remained persistent in the neutrophils of MPO k/o mice and induced a stronger fibrogenic response, SWCNTs underwent significant diminution in the lungs of w/t mice versus MPO k/o mice as evidenced through quantitative imaging (Figure 6). Twenty-eight days after exposure, TEM and Raman analysis also confirmed that the oxidized SWCNTs underwent biodegradation as evidenced by the shortening of the CNTs and an increase in the ratio of the D to G bands for the respective techniques; this effect, however, was more apparent in the w/t mice, which indicated the crucial role of MPO in oxidatively biodegrading SWCNTs in vivo [26]. Since the degradation was also observed in MPO k/o mice at a much lower rate, other hemeperoxidases (e.g. EPO and LPO) may also contribute to the in vivo biodegradation process. In agreement with our previous in vitro study, the in vivo results strengthened the importance of MPO containing neutrophils in the biodegradation of CNTs.
Figure 6.
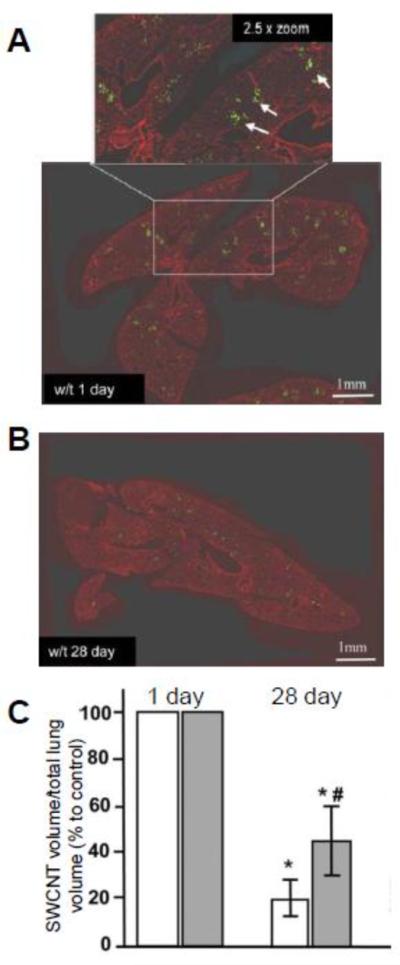
(A, B) Quantitative imaging of the lung tissue sections from w/t mice treated with SWCNTs illustrated with green pseudo-color after 1 day (A) and 28 days (B). Inset in A - higher magnification of a field with the presence of SWCNT (green punctuate spots pointed by white arrows). (C) Ratio of the volume of SWCNTs to the total lung volume for w/t and MPO k/o mice 1 day and 28 days after injection, respectively. Adapted from [28].
5.3. The in vivo biodegradation of CNTs in the Brain
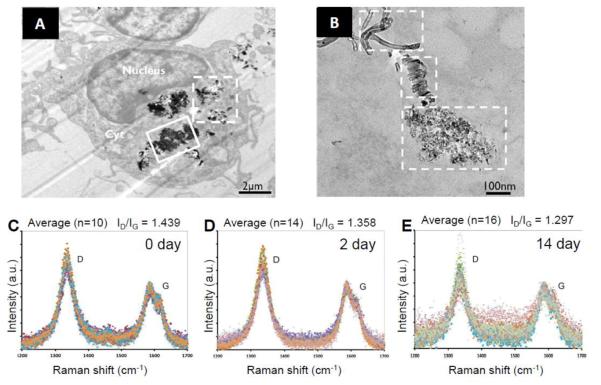
Nunes et al. recently investigated the fate of MWCNTs in mouse neuronal tissue [86]. Amine-functionalized MWCNTs were stereotactically injected into the motor cortex of a mouse brain, where microglia functioned as the primary professional phagocytes for the brain's immune system. TEM imaging was performed on parenchyma samples near the injection site 2 days post-injection. While widespread microglia internalization of MWCNTs was considered to be the predominant mechanism of early tissue response, the micrographs revealed that this nanomaterial was also internalized into different types of brain cells including neurons (Figure 7A). Aggregates of MWCNTs were localized in the possible phagolysosomal vesicles through active endocytosis or phagocytosis, and individualized MWCNTs were mainly internalized in the cytoplasm through a direct membrane translocation pathway. Besides the intact nanotubes, many internalized MWCNTs were observed to undergo severe structural deformation yielding amorphous debris (Figure 7B), which indicated the widespread initiation of the degradation process in microglia on day 2 after injection. This structural deformation was confirmed by Raman spectra that were taken of the injected brain tissue on days 2 and 14 after injection (Figure 7C, D, E), which demonstrated an overall reduction in the intensity of the D and G peaks coupled with an increase in background noise thereby indicating diminishing CNT content. A decrease in the D to G band ratio was observed, however, which might indicate the incomplete degradation of MWCNTs in microglia as was observed in another degradation experiment conducted on MWCNTs [23]. While the authors attributed the possible in vivo degradation of MWCNTs to the highly efficient phagocytosis capacity of microglia cells that possess both an oxidative lysosomal environment with low pH and an abundance of hydrolytic enzymes, the actual mechanism of degradation remained hypothetical.
Figure 7.
(A) Low resolution TEM image of microglia with amine-functionalized MWCNTs localized intracellularly, both intact individualized MWCNTs (dash box) and structurally deformed MWCNT debris (solid) is exhibited. (B) TEM image with higher resolution showing co-existing intact MWCNTs (top), carbon sheets unraveled form MWCNTs (middle) and deformed MWCNT debris. (C, D, E) Raman spectra taken from the starting MWCNTs before injection and brain sections 2 days and 14 days after MWCNT injection. The corresponding D to G band ratios (ID/IG) are recorded. Reproduced from [86] Copyright Future Medicine 2012.
6. Comparison to Other Nanomaterials
Unlike peroxidase-catalyzed degradation of CNTs and graphene oxide [24], the biodegradation of other nanoparticles may undergo completely different pathways depending on their chemical properties. The most inert gold nanoparticles (NPs) are rarely reported to undergo biodegradation in cells [87] with their eventual in vivo fate likely to include retention inside organs such as the liver and spleen [88]. In contrast, iron oxide NPs can be gradually degraded in the acidic environment of endosomes or lysosomes after cellular uptake thereby releasing ferric ions [89]. Quantum dots (QDs) such as CdSe and CdTe were also reported to undergo a pH-dependent degradation [90], and the presence of intracellular hypochlorous acid and hydrogen peroxide may facilitate the degradation process [91]. The degradation of QDs is not desired because the released Cd2+ ions are highly cytotoxic, which, in turn, greatly limits the biomedical applications of QDs. Different types of inorganic NPs, which include semiconductor, metal, metal oxide, and lanthanide-doped QDs are commonly encapsulated in polymers or coated with lipids or surfactants to preserve the as-synthesized properties of the nanomaterial [92]. The surface properties of these carbonaceous coatings, which are highly vulnerable to oxidative biodegradation, will impact the interactions of NPs with cells and components of biofluids thus defining their fate and effectiveness in the body. Therefore, peroxidase-catalyzed biodegradation reactions can affect not only carbonaceous NPs but also many other types of NPs that utilize carbon-based coatings as a part of their formulation.
A large group of biocompatible polymeric NPs has been developed as novel drug delivery vehicles such as poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), etc., which can be degraded through either enzymatic or hydrolytic pathways [93, 94]. Along these lines, de Gracia Lux et al. developed polymeric capsules that contained aryl boronic ester protecting groups integrated into their polymer design [95]. Due to the cleavage of the boronic ester by biological levels of H2O2 (i.e. 50–100 μM), which resulted in the degradation of the polymer backbone, these nanoparticle demonstrated the ability to release cargo when incubated in physiological environments such as with activated neutrophils.
7. Outlook
Ideally, delivery vehicles (e.g. CNTs or graphene derivatives) that transport theranostic payloads should be disposed of after arriving at their target destination sites. To this end, while many chemical methodologies have been developed to “oxidatively cut” and degrade CNT, the aggressive nature of these oxidants precludes their possible employment in physiologically relevant environments (i.e. tissues and body biofluids). Consequently, this prompted an active search for possible enzymatic mechanisms and pathways whereby mild and controlled oxidation reactions would effectively biodegrade CNTs. The discovery of peroxidase-driven biodegradation processes is an important milestone on the path to regulated and targeted spatiotemporal degradation of CNT. Through either noncovalent functionalization or covalent attachment through stimuli-cleavable groups, specialized signaling molecules of lipid and/or protein nature will facilitate the internalization of CNTs. Also, one can envision the attachment of chemical/biological species that will activate the professional phagocytes. Acute and resolution phases of the body's inflammatory response are characterized by the predominant accumulation of myeloperoxidase (MPO)-rich PMNs and macrophages, respectively. One can envision that MPO-driven oxidative reaction will represent the dominant pathways for the CNTs biodegradation during the acute phase. In contrast, macrophages, which are relatively poor in MPO, may utilize a different oxidizing system such as peroxynitrite generators to be involved in the CNT biodegradation process. In this regard, NADPH-oxidase and NO synthases (e.g. iNOS) may be particularly important as sources of superoxide radicals and NO•, respectively. By producing these reactive oxygen and nitrogen species (i.e. ROS and RNS), macrophages are involved in the production of peroxynitrite, whose high oxidizing potential is sufficient to trigger CNT oxidative degradation. Therefore, by tailoring functionalization, one can envision a possible tool for regulating the biodegradation of CNTs coated with important payloads. Alternatively, nano-containers with encapsulated freight can be utilized in ways where oxidative biodegradation of the vehicle will facilitate the release of the inner contents as they reach the desired targets. While different types of nano-containers have been designed and fabricated [96–98], approaches towards the utilization of peroxidase-catalyzed oxidative biodegradation on these types of delivery nano-devices in target organs/cells have not yet been developed and represent an exciting future area of research.
Acknowledgments
The project described was supported by NIEHS R01ES019304, NIOSH OH008282, and NIH U19AI068021. GPK acknowledges support from the EPA STAR Graduate Fellowship FP-91713801. YZ acknowledges a graduate student fellowship through Bayer MaterialScience.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kim SN, Rusling JF, Papadimitrakopoulos F. Carbon nanotubes for electronic and electrochemical detection of biomolecules. Adv. Mater. 2007;19:3214–3228. doi: 10.1002/adma.200700665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Odom TW, Huang J-L, Kim P, Lieber CM. Structure and electronic properties of carbon nanotubes. J. Phys. Chem. B. 2000;104:2794–2809. [Google Scholar]
- [3].Dresselhaus MS, Dresselhaus G, Jorio A. Unusual properties and structure of carbon nanotubes. Annu. Rev. Mater. Res. 2004;34:247–278. [Google Scholar]
- [4].Ruoff RS, Lorents DC. Mechanical and thermal properties of carbon nanotubes. Carbon. 1995;33:925–930. [Google Scholar]
- [5].Avouris P, Appenzeller J, Martel R, Wind SJ. Carbon nanotube electronics. Proc. IEEE. 2003;91:1772–1784. [Google Scholar]
- [6].Kauffman DR, Star A. Carbon nanotube gas and vapor sensors. Angew. Chem., Int. Ed. 2008;47:6550–6570. doi: 10.1002/anie.200704488. [DOI] [PubMed] [Google Scholar]
- [7].Feigel IM, Vedala H, Star A. Biosensors based on one-dimensional nanostructures. J. Mater. Chem. 2011;21:8940–8954. [Google Scholar]
- [8].Coleman JN, Khan U, Blau WJ, Gun'ko YK. Small but strong: A review of the mechanical properties of carbon nanotube-polymer composites. Carbon. 2006;44:1624–1652. [Google Scholar]
- [9].Baughman RH, Zakhidov AA, de Heer WA. Carbon nanotubes--the route toward applications. Science. 2002;297:787–792. doi: 10.1126/science.1060928. [DOI] [PubMed] [Google Scholar]
- [10].Bianco A, Kostarelos K, Prato M. Applications of carbon nanotubes in drug delivery. Curr. Opin. Chem. Biol. 2005;9:674–679. doi: 10.1016/j.cbpa.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [11].Liu Z, Tabakman S, Welsher K, Dai H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Research. 2009;2:85–120. doi: 10.1007/s12274-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538–544. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].De M, Ghosh PS, Rotello VM. Applications of nanoparticles in biology. Adv. Mater. 2008;20:4225–4241. [Google Scholar]
- [14].Vashist SK, Zheng D, Pastorin G, Al-Rubeaan K, Luong JHT, Sheu F-S. Delivery of drugs and biomolecules using carbon nanotubes. Carbon. 2011;49:4077–4097. [Google Scholar]
- [15].Bianco A, Kostarelos K, Prato M. Making carbon nanotubes biocompatible and biodegradable. Chem. Commun. 2011;47:10182–10188. doi: 10.1039/c1cc13011k. [DOI] [PubMed] [Google Scholar]
- [16].Kamalha E, Shi X, Mwasiagi J, Zeng Y. Nanotechnology and carbon nanotubes; a review of potential in drug delivery. Macromol. Res. 2012;20:891–898. [Google Scholar]
- [17].Qureshi SR, Sahni YP, Singh SK, Bhat MA, Dar AA, Quadri SA. Nanotechnology based drug delivery system. J. Pharm. Res. Opin. 2011;1:161–165. [Google Scholar]
- [18].Rothen-Rutishauser B, Brown DM, Piallier-Boyles M, Kinloch IA, Windle AH, Gehr P, Stone V. Relating the physicochemical characteristics and dispersion of multiwalled carbon nanotubes in different suspension media to their oxidative reactivity in vitro and inflammation in vivo. Nanotoxicology. 2010;4:331–342. doi: 10.3109/17435390.2010.489161. [DOI] [PubMed] [Google Scholar]
- [19].Zhang W, Zhang Z, Zhang Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011;6:555. doi: 10.1186/1556-276X-6-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Allen BL, Kichambare PD, Gou P, Vlasova, Kapralov AA, Konduru N, Kagan VE, Star A. Biodegradation of single-walled carbon nanotubes through enzymatic catalysis. Nano Lett. 2008;8:3899–3903. doi: 10.1021/nl802315h. [DOI] [PubMed] [Google Scholar]
- [21].Allen BL, Kotchey GP, Chen Y, Yanamala NVK, Klein-Seetharaman J, Kagan VE, Star A. Mechanistic investigations of horseradish peroxidase-catalyzed degradation of single-walled carbon nanotubes. J. Am. Chem. Soc. 2009;131:17194–17205. doi: 10.1021/ja9083623. [DOI] [PubMed] [Google Scholar]
- [22].Russier J, Menard-Moyon C, Venturelli E, Gravel E, Marcolongo G, Meneghetti M, Doris E, Bianco A. Oxidative biodegradation of single- and multi-walled carbon nanotubes. Nanoscale. 2011;3:893–896. doi: 10.1039/c0nr00779j. [DOI] [PubMed] [Google Scholar]
- [23].Zhao Y, Allen BL, Star A. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A. 2011;115:9536–9544. doi: 10.1021/jp112324d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kotchey GP, Allen BL, Vedala H, Yanamala N, Kapralov AA, Tyurina YY, Klein-Seetharaman J, Kagan VE, Star A. The enzymatic oxidation of graphene oxide. ACS Nano. 2011;5:2098–2108. doi: 10.1021/nn103265h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kotchey GP, Hasan SA, Kapralov AA, Ha SH, Kim K, Shvedova AA, Kagan VE, Star A. A natural vanishing act: The enzyme-catalyzed degradation of carbon nanomaterials. Acc. Chem. Res. 2012;45:1770–1781. doi: 10.1021/ar300106h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kagan VE, Konduru NV, Feng W, Allen BL, Conroy J, Volkov Y, Vlasova II, Belikova NA, Yanamala N, Kapralov A, Tyurina YY, Shi J, Kisin ER, Murray AR, Franks J, Stolz D, Gou P, Klein-Seetharaman J, Fadeel B, Star A, Shvedova AA. Carbon nanotubes degraded by neutrophil myeloperoxidase induce less pulmonary inflammation. Nat. Nanotechnol. 2010;5:354–359. doi: 10.1038/nnano.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Vlasova I, Sokolov A, Chekanov A, Kostevich V, Vasilyev V. Myeloperoxidase-induced biodegradation of single-walled carbon nanotubes is mediated by hypochlorite. Russ. J. Bioorg. Chem. 2011;37:453–463. doi: 10.1134/s1068162011040157. [DOI] [PubMed] [Google Scholar]
- [28].Shvedova AA, Kapralov AA, Feng WH, Kisin ER, Murray AR, Mercer RR, St. Croix CM, Lang MA, Watkins SC, Konduru NV, Allen BL, Conroy J, Kotchey GP, Mohamed BM, Meade AD, Volkov Y, Star A, Fadeel B, Kagan VE. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS One. 2012;7:e30923. doi: 10.1371/journal.pone.0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Andón FT, Kapralov AA, Yanamala N, Feng W, Baygan A, Chambers BJ, Hultenby K, Ye F, Toprak MS, Brandner BD, Fornara A, Klein-Seetharaman J, Kotchey GP, Star A, Shvedova AA, Fadeel B, Kagan VE. Biodegradation of single-walled carbon nanotubes by eosinophil peroxidase. Small. 2013 doi: 10.1002/smll.201202508. DOI: 10.1002/smll.201202508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Veitch NC. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry. 2004;65:249–259. doi: 10.1016/j.phytochem.2003.10.022. [DOI] [PubMed] [Google Scholar]
- [31].Azevedo AM, Martins VC, Prazeres DMF, Vojinović V, Cabral JMS, Fonseca LP. Biotechnol. Annu. Rev. Elsevier; 2003. Horseradish peroxidase: A valuable tool in biotechnology; pp. 199–247. [DOI] [PubMed] [Google Scholar]
- [32].Wepasnick KA, Smith BA, Schrote KE, Wilson HK, Diegelmann SR, Fairbrother DH. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon. 2011;49:24–36. [Google Scholar]
- [33].Yang J-C, Yen C-H, Wang W-J, Horng J-J, Tsai Y-P. Assessment of adequate sodium hypochlorite concentration for pre-oxidization of multi-walled carbon nanotubes. J. Chem. Technol. Biotechnol. 2010;85:699–707. [Google Scholar]
- [34].Liu X, Hurt RH, Kane AB. Biodurability of single-walled carbon nanotubes depends on surface functionalization. Carbon. 2010;48:1961–1969. doi: 10.1016/j.carbon.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tasis D, Tagmatarchis N, Bianco A, Prato M. Chemistry of carbon nanotubes. Chem. Rev. 2006;106:1105–1136. doi: 10.1021/cr050569o. [DOI] [PubMed] [Google Scholar]
- [36].Sun Z, Nicolosi V, Rickard D, Bergin SD, Aherne D, Coleman JN. Quantitative evaluation of surfactant-stabilized single-walled carbon nanotubes: Dispersion quality and its correlation with zeta potential. J. Phys. Chem. C. 2008;112:10692–10699. [Google Scholar]
- [37].Hu H, Yu AP, Kim E, Zhao B, Itkis ME, Bekyarova E, Haddon RC. Influence of the zeta potential on the dispersability and purification of single-walled carbon nanotubes. J. Phys. Chem. B. 2005;109:11520–11524. doi: 10.1021/jp050781w. [DOI] [PubMed] [Google Scholar]
- [38].Farhangrazi ZS, Fossett ME, Powers LS, Ellis WR. Variable-temperature spectroelectrochemical study of horseradish peroxidase. Biochemistry. 1995;34:2866–2871. doi: 10.1021/bi00009a017. [DOI] [PubMed] [Google Scholar]
- [39].Gratzel M. Photoelectrochemical cells. Nature. 2001;414:338–344. doi: 10.1038/35104607. [DOI] [PubMed] [Google Scholar]
- [40].Choi HC, Shim M, Bangsaruntip S, Dai H. Spontaneous reduction of metal ions on the sidewalls of carbon nanotubes. J. Am. Chem. Soc. 2002;124:9058–9059. doi: 10.1021/ja026824t. [DOI] [PubMed] [Google Scholar]
- [41].Nauseef WM. How human neutrophils kill and degrade microbes: An integrated view. Immunol. Rev. 2007;219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- [42].Hansson M, Olsson I, Nauseef WM. Biosynthesis, processing, and sorting of human myeloperoxidase. Arch. Biochem. Biophys. 2006;445:214–224. doi: 10.1016/j.abb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- [43].Arnhold J. Properties, functions, and secretion of human myeloperoxidase. Biochemistry. 2004;69:4–9. doi: 10.1023/b:biry.0000016344.59411.ee. [DOI] [PubMed] [Google Scholar]
- [44].Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: From molecular mechanisms to health implications. Antioxid. Redox Signaling. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- [45].Kotchey GP, Gaugler JA, Kapralov AA, Kagan VE, Star A. Effect of antioxidants on enzyme-catalysed biodegradation of carbon nanotubes. J. Mater. Chem. B. 2013;1:302–309. doi: 10.1039/C2TB00047D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. Cytotoxicity of carbon nanomaterials: Single-wall nanotube, multi-wall nanotube, and fullerene. Environ. Sci. Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- [47].Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, Rice-Ficht AC, Ramesh GT. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κb in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Auffan M, Rose J, Bottero J-Y, Lowry GV, Jolivet J-P, Wiesner MR. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009;4:634–641. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- [49].Shvedova AA, Pietroiusti A, Fadeel B, Kagan VE. Mechanisms of carbon nanotube-induced toxicity: Focus on oxidative stress. Toxicol. Appl. Pharmacol. 2012;261:121–133. doi: 10.1016/j.taap.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, Kisin ER, Schwegler-Berry D, Mercer R, Castranova V, Shvedova AA. Direct and indirect effects of single walled carbon nanotubes on raw 264.7 macrophages: Role of iron. Toxicol. Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- [51].Shvedova AA, Kisin ER, Murray AR, Kommineni C, Castranova V, Fadeel B, Kagan VE. Increased accumulation of neutrophils and decreased fibrosis in the lung of nadph oxidase-deficient c57bl/6 mice exposed to carbon nanotubes. Toxicol. Appl. Pharmacol. 2008;231:235–240. doi: 10.1016/j.taap.2008.04.018. [DOI] [PubMed] [Google Scholar]
- [52].Chanock SJ, El Benna J, Smith RM, Babior BM. The respiratory burst oxidase. J. Biol. Chem. 1994;269:24519–24522. [PubMed] [Google Scholar]
- [53].Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- [54].Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- [55].Halliwell B. Oxidative stress and cancer: Have we moved forward? Biochem. J. 2007;401:1–11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- [56].Segal AW. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zipfel M, Carmine TC, Gerber C, Niethammer D, Bruchelt G. Evidence for the activation of myeloperoxidase by f-meth-leu-phe prior to its release from neutrophil granulocytes. Biochem. Biophys. Res. Commun. 1997;232:209–212. doi: 10.1006/bbrc.1997.6257. [DOI] [PubMed] [Google Scholar]
- [58].McKenzie SE, Schreiber AD. Fc gamma receptors in phagocytes. Curr. Opin. Hematol. 1998;5:16. doi: 10.1097/00062752-199801000-00003. [DOI] [PubMed] [Google Scholar]
- [59].Konduru NV, Tyurina YY, Feng W, Basova LV, Belikova NA, Bayir H, Clark K, Rubin M, Stolz D, Vallhov H. Phosphatidylserine targets single-walled carbon nanotubes to professional phagocytes in vitro and in vivo. PLoS One. 2009;4:e4398. doi: 10.1371/journal.pone.0004398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cherukuri P, Bachilo SM, Litovsky SH, Weisman RB. Near-infrared fluorescence microscopy of single-walled carbon nanotubes in phagocytic cells. J. Am. Chem. Soc. 2004;126:15638–15639. doi: 10.1021/ja0466311. [DOI] [PubMed] [Google Scholar]
- [61].Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, Tyurina YY, Gorelik O, Arepalli S, Schwegler-Berry D. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- [62].Kam NWS, Liu Z, Dai H. Carbon nanotubes as intracellular transporters for proteins and DNA: An investigation of the uptake mechanism and pathway. Angew. Chem. 2006;118:591–595. doi: 10.1002/anie.200503389. [DOI] [PubMed] [Google Scholar]
- [63].Neves V, Heister E, Costa S, Tîlmaciu C, Borowiak- Palen E, Giusca CE, Flahaut E, Soula B, Coley HM, McFadden J. Uptake and release of double- walled carbon nanotubes by mammalian cells. Adv. Funct. Mater. 2010;20:3272–3279. [Google Scholar]
- [64].Vlasova II, Vakhrusheva TV, Sokolov AV, Kostevich VA, Gusev AA, Gusev SA, Melnikova VI, Lobach AS. Pegylated single-walled carbon nanotubes activate neutrophils to increase production of hypochlorous acid, the oxidant capable of degrading nanotubes. Toxicol. Appl. Pharmacol. 2012;264:131–142. doi: 10.1016/j.taap.2012.07.027. [DOI] [PubMed] [Google Scholar]
- [65].Liu Z, Davis C, Cai W, He L, Chen X, Dai H. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by raman spectroscopy. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1410–1415. doi: 10.1073/pnas.0707654105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Murphy FA, Poland CA, Duffin R, Al-Jamal KT, Ali-Boucetta H, Nunes A, Byrne F, Prina-Mello A, Volkov Y, Li S. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011;178:2587–2600. doi: 10.1016/j.ajpath.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Yang ST, Wang X, Jia G, Gu Y, Wang T, Nie H, Ge C, Wang H, Liu Y. Long-term accumulation and low toxicity of single-walled carbon nanotubes in intravenously exposed mice. Toxicol. Lett. 2008;181:182–189. doi: 10.1016/j.toxlet.2008.07.020. [DOI] [PubMed] [Google Scholar]
- [68].Owens Iii DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006;307:93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [69].Yang S.-t., Guo W, Lin Y, Deng X.-y., Wang H.-f., Sun H.-f., Liu Y.-f., Wang X, Wang W, Chen M, Huang Y.-p., Sun Y-P. Biodistribution of pristine single-walled carbon nanotubes in vivo†. The Journal of Physical Chemistry C. 2007;111:17761–17764. [Google Scholar]
- [70].Lam C-W, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol. Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- [71].Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GAM, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in rats. Toxicol. Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- [72].Poland CA, Duffin R, Kinloch I, Maynard A, Wallace WAH, Seaton A, Stone V, Brown S, MacNee W, Donaldson K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008;3:423–428. doi: 10.1038/nnano.2008.111. [DOI] [PubMed] [Google Scholar]
- [73].Smart S, Cassady A, Lu G, Martin D. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047. [Google Scholar]
- [74].Murphy FA, Poland CA, Duffin R, Donaldson K. Length-dependent pleural inflammation and parietal pleural responses after deposition of carbon nanotubes in the pulmonary airspaces of mice. Nanotoxicology. doi: 10.3109/17435390.2012.713527. DOI: 10.3109/17435390.17432012.17713527. [DOI] [PubMed] [Google Scholar]
- [75].Ali-Boucetta H, Nunes A, Sainz R, Herrero MA, Tian B, Prato M, Bianco A, Kostarelos K. Asbestos-like pathogenicity of long carbon nanotubes alleviated by chemical functionalization. Angew. Chem., Int. Ed. 2013;52:2274–2278. doi: 10.1002/anie.201207664. [DOI] [PubMed] [Google Scholar]
- [76].Kolosnjaj-Tabi J, Hartman KB, Boudjemaa S, Ananta JS, Morgant G, Szwarc H, Wilson LJ, Moussa F. In vivo behavior of large doses of ultrashort and full-length single-walled carbon nanotubes after oral and intraperitoneal administration to swiss mice. ACS Nano. 2010;4:1481–1492. doi: 10.1021/nn901573w. [DOI] [PubMed] [Google Scholar]
- [77].Singh R, Pantarotto D, McCarthy D, Chaloin O, Hoebeke J, Charalambos D, Briand JP, Prato M, Bianco A, Kostarelos K. Binding and condensation of plasmid DNA onto functionalized carbon nanotubes: Toward the construction of nanotube-based gene delivery vectors. J. Am. Chem. Soc. 2005;127:4388–4396. doi: 10.1021/ja0441561. [DOI] [PubMed] [Google Scholar]
- [78].Kam NWS, Jessop TC, Wender PA, Dai H. Nanotube molecular transporters: Internalization of carbon nanotube-protein conjugates into mammalian cells. J. Am. Chem. Soc. 2004;126:6850–6851. doi: 10.1021/ja0486059. [DOI] [PubMed] [Google Scholar]
- [79].Schipper ML, Nakayama-Ratchford N, Davis CR, Kam NWS, Chu P, Liu Z, Sun X, Dai H, Gambhir SS. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008;3:216–221. doi: 10.1038/nnano.2008.68. [DOI] [PubMed] [Google Scholar]
- [80].Singh R, Pantarotto D, Lacerda L, Pastorin G, Klumpp C, Prato M, Bianco A, Kostarelos K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3357–3362. doi: 10.1073/pnas.0509009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sacchetti C, Motamedchaboki K, Magrini A, Palmieri G, Mattei M, Bernardini S, Rosato N, Bottini N, Bottini M. Surface polyethylene glycol conformation influences the protein corona of polyethylene glycol-modified single-walled carbon nanotubes: Potential implications on biological performance. ACS Nano. 2013;7:1974–1989. doi: 10.1021/nn400409h. [DOI] [PubMed] [Google Scholar]
- [82].Huizar I, Malur A, Midgette Y, Kukoly C, Chen P, Ke P, Podila R, Rao A, Wingard C, Dobbs L. Novel murine model of chronic granulomatous lung inflammation elicited by carbon nanotubes. Am. J. Respir. Cell Mol. Biol. 2011;45:858. doi: 10.1165/rcmb.2010-0401OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Shvedova AA, Kisin E, Murray AR, Johnson VJ, Gorelik O, Arepalli S, Hubbs AF, Mercer RR, Keohavong P, Sussman N. Inhalation vs. Aspiration of single-walled carbon nanotubes in c57bl/6 mice: Inflammation, fibrosis, oxidative stress, and mutagenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008;295:L552–L565. doi: 10.1152/ajplung.90287.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, Arras M, Fonseca A, Nagy JB, Lison D. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol. Appl. Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- [85].Elgrabli D, Floriani M, Abella-Gallart S, Meunier L, Gamez C, Delalain P, Rogerieux F, Boczkowski J, Lacroix G. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part. Fibre Toxicol. 2008;5:20. doi: 10.1186/1743-8977-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nunes A, Bussy C, Gherardini L, Meneghetti M, Herrero MA, Bianco A, Prato M, Pizzorusso T, Al-Jamal KT, Kostarelos K. In vivo degradation of functionalized carbon nanotubes after stereotactic administration in the brain cortex. Nanomedicine. 2012;7:1485–1494. doi: 10.2217/nnm.12.33. [DOI] [PubMed] [Google Scholar]
- [87].Alkilany AM, Murphy CJ. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010;12:2313–2333. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chen YS, Hung YC, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res. Lett. 2009;4:858–864. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Arbab AS, Wilson LB, Ashari P, Jordan EK, Lewis BK, Frank JA. A model of lysosomal metabolism of dextran coated superparamagnetic iron oxide (spio) nanoparticles: Implications for cellular magnetic resonance imaging. NMR Biomed. 2005;18:383–389. doi: 10.1002/nbm.970. [DOI] [PubMed] [Google Scholar]
- [90].Mahendra S, Zhu H, Colvin VL, Alvarez PJ. Quantum dot weathering results in microbial toxicity. Environ. Sci. Technol. 2008;42:9424–9430. doi: 10.1021/es8023385. [DOI] [PubMed] [Google Scholar]
- [91].Mancini MC, Kairdolf BA, Smith AM, Nie S. Oxidative quenching and degradation of polymer-encapsulated quantum dots: New insights into the long-term fate and toxicity of nanocrystals in vivo. J. Am. Chem. Soc. 2008;130:10836–10837. doi: 10.1021/ja8040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliver. Rev. doi: 10.1016/j.addr.2012.08.015. DOI: http://dx.doi.org/10.1016/j.addr.2012.1008.1015. [DOI] [PubMed] [Google Scholar]
- [93].Soppimath KS, Aminabhavi TM, Kulkarni AR, Rudzinski WE. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release. 2001;70:1–20. doi: 10.1016/s0168-3659(00)00339-4. [DOI] [PubMed] [Google Scholar]
- [94].Li J, Rothstein SN, Little SR, Edenborn HM, Meyer TY. The effect of monomer order on the hydrolysis of biodegradable poly(lactic-co-glycolic acid) repeating sequence copolymers. J. Am. Chem. Soc. 2012;134:16352–16359. doi: 10.1021/ja306866w. [DOI] [PubMed] [Google Scholar]
- [95].de Gracia Lux C, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chun H, Hahm MG, Homma Y, Meritz R, Kuramochi K, Menon L, Ci L, Ajayan PM, Jung YJ. Engineering low-aspect ratio carbon nanostructures: Nanocups, nanorings, and nanocontainers. ACS Nano. 2009;3:1274–1278. doi: 10.1021/nn9001903. [DOI] [PubMed] [Google Scholar]
- [97].Oyama TG, Oshima A, Washio M, Tagawa S. Fabrication of nanobeads from nanocups by controlling scission/crosslinking in organic polymer materials. Nanotechnology. 2012;23:495307. doi: 10.1088/0957-4484/23/49/495307. [DOI] [PubMed] [Google Scholar]
- [98].Zhao Y, Tang Y, Chen Y, Star A. Corking carbon nanotube cups with gold nanoparticles. ACS Nano. 2012;6:6912–6921. doi: 10.1021/nn3018443. [DOI] [PubMed] [Google Scholar]