Abstract
This protocol permits rapid isolation (in less than 1 hr) of murine pancreatic acini, making it possible to maintain them in culture for more than one week. More than 20 x 106 acinar cells can be obtained from a single murine pancreas. This protocol offers the possibility to independently process as many as 10 pancreases in parallel. Because it preserves acinar architecture, this model is well suited for studying the physiology of the exocrine pancreas in vitro in contrast to cell lines established from pancreatic tumors, which display many genetic alterations resulting in partial or total loss of their acinar differentiation.
Keywords: Cancer Biology, Issue 78, Cellular Biology, Molecular Biology, Biomedical Engineering, Medicine, Anatomy, Physiology, Surgery, Oncology, Pancreas, Exocrine, Cells, Cultured, Mice, Primary Cell Culture, Exocrine pancreas, Cell culture, Primary acinar cells, Mouse, pancreatic cancer, cancer, tumor, tissue, animal model
Introduction
A frequently encountered problem for research laboratories working on exocrine pancreatic tissue is the difficulty of cultivating acinar cells in vitro for a period of time long enough to allow a long-term experiment.
One factor impeding development of such culture systems is the intrinsic sensitivity of pancreatic tissue to experimental manipulation due to the high content in glycolytic, proteolytic, and lipolytic enzymes, which literally digest the pancreatic tissue when they are released during the isolation of pancreatic cells.
A second factor is the remarkable in vitro plasticity of acinar cells, which tend to lose their secretory characteristics and transdifferentiate to other mature cells, such as pancreatic ductal cells or hepatocyte-like cells 1. In vitro, this cell plasticity varies with the experimental conditions (such as culture medium composition) 2 and introduces a degree of complexity into the design of appropriate culture conditions for exocrine pancreatic cells 1.
Several methods have been developed for the isolation and culture of acinar cells, first from the guinea pig pancreas 3-5. Initially, those protocols involved digestion of pancreatic tissue with collagenase, chymotrypsin, and a protease cocktail, with ultimate isolation by vigorous mechanical dissociation. The pancreatic cells isolated in this way displayed abnormal structural and functional characteristics, notably a loss of apical structures and significant damage to their membrane receptors. Isolated cells remained viable for only 1 or 2 days.
Preparation of dispersed acini maintains their intra- and intercellular architecture, preserving cell membranes, limiting damage to surface receptors, and thus improving exocrine secretion in response to secretagogues 6-8. As a result, this method offers the major advantage of extending acinar cell viability to 7-10 days in vitro. Furthermore, this method is currently preferred to acinar cell isolation 9-12 because maintenance of intercellular contacts, including cell coupling by gap junctions, is an essential determinant of the exocrine pancreatic acinar cell phenotype 13.
As the dedifferentiation of acinar cells and their transdifferentiation to ductal cells is one of the proposed mechanisms for the genesis of aggressive exocrine pancreatic cancers 14, the dispersed acini model is also an adequate system to study pancreatic plasticity and its subsequent molecular mechanisms. Furthermore, in combination with the use of genetically modified animals 15,16 and the development of gene transfer techniques (adenoviral 2 or lentiviral transduction, use of nanoparticles, etc), this in vitro primary acinar cell model can be very useful in determining how various genetic dysfunctions affect the regulation of acinar cell differentiation or dedifferentiation and should provide better understanding of the molecular events responsible for the onset of pancreatitis, precancerous lesions, and changes in cell plasticity.
Isolation of dispersed acini is the approach we use in our laboratory to culture pancreatic acinar cells. We here describe and discuss the method used. It involves enzymatic dissociation of pancreatic tissue (with a bacterial collagenase) coupled to mechanical disruption without dissociation of acinar cells. While most protocols involve culturing the acini, either in suspension or on specially treated plastic substrates, we grow them in suspension only briefly (for 24 hr), seeding them afterwards onto matrix scaffolds if prolonged cell culture is required.
This protocol allows rapid isolation (in less than 1 hr) of dispersed pancreatic acini, sustainable for more than one week in culture. It allows isolation of more than 20 x 106 acinar cells per mouse pancreas. Its simplicity makes it possible to process independently as many as 10 pancreases in parallel. By maintaining the intra- and intercellular architecture of acini and thus the acinar phenotype of isolated primary cells, this model constitutes a system of choice for the study of transdifferentiation mechanisms, as all other exocrine pancreatic models currently available are derived from pancreatic tumors displaying many genetic alterations leading to cellular transformation.
Protocol
All procedures were approved by an ethic committee under regulatory of governmental authority ("Comité d'Evaluation Commun au Centre Léon Bérard, à l'Animalerie de transit de l'ENS, au PBES et au laboratoire P4" (CECCAPP)). Mice were maintained in a specific pathogen-free animal facility at the "Plateforme AniCan, Centre Léon Bérard" (Lyon, France) and handled in compliance with the institutional guidelines.
A schematic representation of the procedure is shown in Figure 1.
1. Pancreas Dissection and Dilaceration (Day 0)
A very rapid dissection is critical for an optimal yield of extraction and to insure a good viability of cells in culture. In order to reduce the time needed for pancreas isolation, all instruments and equipment must be ready before the mouse euthanasia.
Euthanize mouse by CO2 asphyxiation or cervical dislocation.
From this step, all procedures have to be performed under a sterile atmosphere (microbiological safety cabinet, level II) with sterile dissection equipment.
Fix the mouse and spray the mouse abdomen with 70% ethanol. With any dissecting scissors and forceps, make a V-shaped incision at the genital area and continue it up to the diaphragm to open completely the abdominal cavity.
Position the liver lobes against the diaphragm; they should remain there if the body cavity is open far enough. Pull the gut and the colon outside the abdominal cavity to your left, and find the rectum. With a pair of curved forceps and dissecting scissors, grab and section the rectum.
With the same pair of forceps, carefully unroll entirely the bowel from the rectum to the stomach by pulling the intestine on your left.
At this step, the pancreas can be distinguished as a small strip between the stomach and the beginning of the bowel. Its ligations with the spleen remain intact.
Using Noyes scissors and a pair of forceps, carefully cut the pancreas along the bowel and liberate it with the spleen from the rest of the digestive tract.
Grab the spleen and section the pancreas attached to it (Figure 2).
At this step, be sure that no mesenteric fat tissue and/or other adjacent tissue (spleen, bowel, etc) could be collected with the pancreas, to avoid cellular contamination.
For the rest of the procedure, all buffers must be prepared without calcium ion Ca2+ chelators to avoid the complete dissociation of the exocrine pancreatic tissue in single acinar cells.
Rinse the pancreas twice in Hank's Balanced Salt Solution (HBSS) 1x.
At this step, as fat tissue will float contrary to pancreas that will sink, it is easily possible to visualize and rapidly remove the contaminant white adipose tissue still attached to pancreas.
If the pancreas needs to be transported to the cell culture facility, it must be kept on ice in HBSS 1x.
Transfer the pancreas in a sterile Petri dish containing 5 ml of HBSS 1x. Using Noyes scissors and a scalpel, slice the pancreas in small pieces of 1 to 3 mm3 (Figure 3A).
2. Enzymatic and Mechanical Dissociations of Pancreas (Day 0)
Transfer them into a sterile 50 ml polypropylene tube.
Centrifuge for 2 min at 450 x g and 4 °C. Aspirate and discard the supernatant to remove cell fragments and blood cells.
Add 10 ml of collagenase IA solution (HBSS 1x containing 10 mM HEPES, 200 U/ml of collagenase IA, and 0.25 mg/ml of trypsin inhibitor) to pancreas sections. Using a 25 ml serological pipette, transfer them to a 25 cm2 flask. Incubate it for 20-30 min at 37 °C. During this time (every 5 min), perform a mechanical dissociation by energetically moving back-and-forth the pancreas fragments about ten times, in sterile pipettes of decreasing size (25, 10, and 5 ml serological pipettes).
At this step, it is essential to frequently monitor the extent of the enzymatic dissociation of pancreatic sections.
When the pancreatic tissue seems to be well-dissociated (according to the disappearance of pancreatic fragments and to the increased turbidity of the solution) (Figure 3B), stop the enzymatic reaction by adding 10 ml of cold buffered washing solution (HBSS 1x containing 5 % Fetal Bovine Serum (FBS) and 10 mM HEPES).
Transfer it into a sterile 50 ml polypropylene tube and centrifuge for 2 min at 450 x g and 4 °C. Carefully aspirate and discard the supernatant to remove the collagenase IA solution.
Resuspend and wash the pellet with 10 ml of buffered washing solution. Centrifuge for 3 min at 450 x g and 4 °C. Carefully aspirate and discard the supernatant. Repeat this step two more times.
3. Filtration and Seeding of Dispersed Acini (Day 0)
Resuspend the cell pellet in 7 ml of Waymouth's medium containing 2.5 % FBS, 1 % Penicillin-Streptomycin mixture (PS), 0.25 mg/ml of trypsin inhibitor, and 25 ng/ml of recombinant human Epidermal Growth Factor (EGF).
Filtrate the cell mixture by allowing it to pass through a 100 μm filter to retain the non-digested fragments (ducts, blood vessels, and Langerhans islets). Pancreatic acinar structures (acinus of 10-15 cells) pass through.
Rinse the filter with 6 ml of Waymouth's medium containing FBS, PS, trypsin inhibitor, and EGF.
After this step, the cells have to be treated very carefully, to avoid any acini dissociation.
Seed the isolated acini in a 6-well culture dish (2 ml per well) (Figure 3C). Culture them at 37 °C under 5% (v/v) CO2 atmosphere.
After this step, the acinar cells are cultured in suspension.
4. Acinar Cell Culture (Day 1 to 10)
Twenty-four hours after, transfer the acini (in suspension) into a new 6-well culture dish, to eliminate the contaminant cells and cellular remnants that have adhered overnight (Figure 4).
If the cell culture needs to be extended for several days or if the experimental conditions require cells grown in monolayer, it is recommended to transfer and seed acini on matrix scaffolds.
The day before seeding on matrix support (Day 0), coat a 6-well culture dish with type I collagen (5 μg/cm2). Add 1 ml of type I collagen solution (50 μg/ml in 0.02 M acetic acid, 0.2 μm-filtered) to each well and allow it to passively adsorb on plastic, during 1 hr at 37 °C (or overnight at 4 °C).
Aspirate the type I collagen solution and rinse the coated well twice with Phosphate-Buffered Saline 1x.
Allow the coated well to dry (under a microbiological safety cabinet) at least 12 hr before use.
Transfer the isolated primary acini (obtained at Step 4.1) into the type I collagen-coated 6-well culture dish and culture them in the same conditions as previously described (at 37 °C under 5% (v/v) CO2 atmosphere). The cells adhere to the type I collagen substrate for 2 days.
On Day 3, change the culture medium to eliminate non-viable cells that have not adhered. With time in culture, the cells will progressively spread on the collagen-containing support. Change the culture medium every 3 days (Figure 5).
The isolated acinar cells obtained can be counted, after a complete mechanical dissociation by using a Thoma cell counting chamber. Note that isolated acinar cells cannot be maintained in culture afterwards.
The quality of the acinar culture obtained can be controlled by checking the expression of acinar specific markers such as Trypsinogen, Pancreas Transcription Factor 1 subunit Alpha, or Carboxypeptidase A1 (by immunocytochemistry or immunofluorescence experiments).
Representative Results
Figure 1 schematizes the "dispersed" acini method for primary acinar cells isolation. The critical steps, which have to be strictly respected during the protocol, are described in the discussion part.
To facilitate its removal, the pancreas has to be collected from the abdomen along with the attached spleen (Figure 2). Both organs need to be cut apart, and the residual fat tissue that could be still attached to the pancreas must be removed (Step 1.6).
The macro- and microscopic pictures, which are shown in Figure 3, represent the result after every single step of the enzymatic and mechanical dissociations of the pancreas. After slicing, the pancreas is divided into small parts (Figure 3A; Step 1.8). The Figure 3B shows the aspect of the pancreas following a successful enzymatic dissociation relying on a careful temporal monitoring of the on-going digestion (Step 2.4). This step is a crucial step to determine the exact time that is needed. The Figure 3C shows the obtained material after the filtration of the pancreatic mixture (Step 3.4). Only the well-separated acini are kept after this step.
The Figure 4 is a typical Day 1 illustration of pancreatic acini isolated using our protocol. The transfer of acinar cells into a new culture dish permits to eliminate the cellular fragments and the adherent contaminant cells (Step 4.1).
As shown in Figure 5, when cultured on type I collagen, the acinar cells spread and lose their acinar differentiation (morphology and 3D organization), giving rise to a monolayer of spindle-shaped cells (Step 4.6).
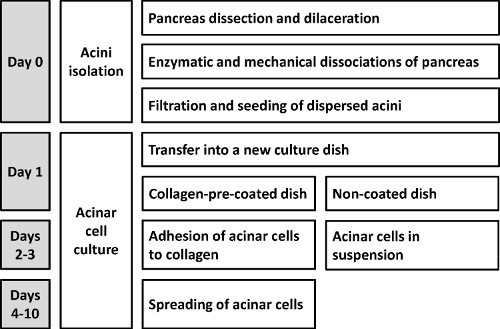 Figure 1. Schematic representation of the protocol allowing the isolation of mouse primary dispersed acini.
Figure 1. Schematic representation of the protocol allowing the isolation of mouse primary dispersed acini.
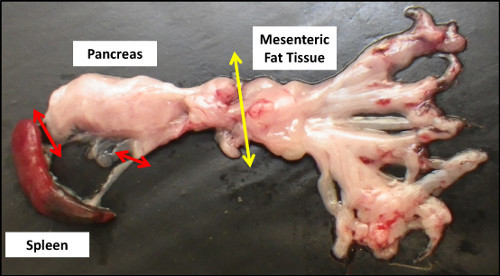 Figure 2. Gross anatomy of the pancreas after dissection. Red and yellow arrows respectively indicate the remaining anatomical connections with the spleen and mesenteric fat tissue that need to be cut for pancreas collection.
Figure 2. Gross anatomy of the pancreas after dissection. Red and yellow arrows respectively indicate the remaining anatomical connections with the spleen and mesenteric fat tissue that need to be cut for pancreas collection.
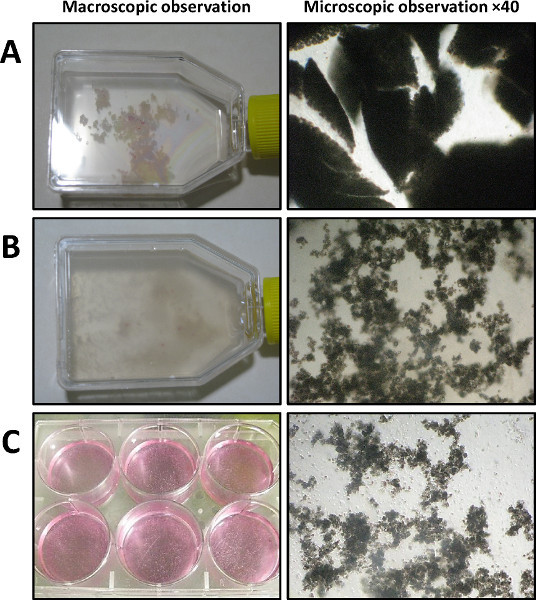 Figure 3. Dispersed acini isolation (Day 0) visualized by macroscopic observation (left panel) and phase-contrast microscopy (right panel, 40X magnification). A) After dilaceration (shown in a 25 cm2 flask). B) After collagenase IA digestion and vigorous mechanical dissociation. C) After filtration and transfer in a 6-well culture dish.
Figure 3. Dispersed acini isolation (Day 0) visualized by macroscopic observation (left panel) and phase-contrast microscopy (right panel, 40X magnification). A) After dilaceration (shown in a 25 cm2 flask). B) After collagenase IA digestion and vigorous mechanical dissociation. C) After filtration and transfer in a 6-well culture dish.
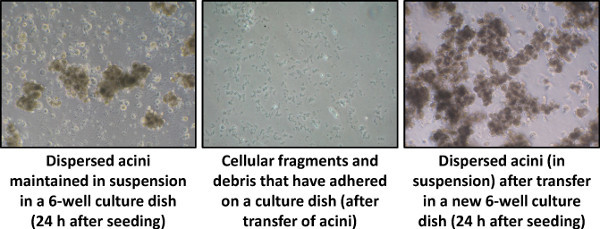 Figure 4. Dispersed acini culture, 24 hr after seeding (Day 1), visualized by phase-contrast microscopy (40X magnification).
Figure 4. Dispersed acini culture, 24 hr after seeding (Day 1), visualized by phase-contrast microscopy (40X magnification).
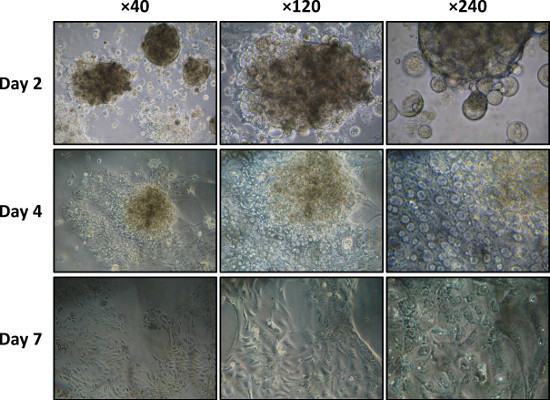 Figure 5. Dispersed acini culture on a type I collagen-coated dish on days 2, 4, and 7 visualized by phase-contrast microscopy (40X, 120X, and 240X magnifications).
Figure 5. Dispersed acini culture on a type I collagen-coated dish on days 2, 4, and 7 visualized by phase-contrast microscopy (40X, 120X, and 240X magnifications).
Discussion
In this protocol, we describe a procedure for isolating pancreatic acinar cells. This method makes possible to isolate more than 20 x 106 acinar cells per animal in less than 1 hr. Thanks to its rapid and simple implementation (as many as 10 pancreases can be independently processed per experiment in parallel), this protocol appears as a good compromise between existing isolation methods 3-5,9-12,17 .
Critical steps/Trouble-shooting
The efficiency of this method relies on precautions taken at a few critical points. The first step of pancreas dilaceration (Step 1.8) is essential to subsequent pancreas enzymatic digestion. Insufficient cutting-up will decrease the yield of enzymatic dissociation and the number of acinar cells obtained. This makes it necessary to prolong incubation with collagenase IA, inevitably causing an excessive dissociation of acinar cells and consecutively increasing cell death.
As cautioned above, the second critical step is digestion by collagenase IA (Step 2.3). Too much enzymatic dissociation leads to a high rate of cell death. The extent of pancreas digestion must be frequently monitored during this critical step (Figure 3B). After mechanical and enzymatic dissociations, special attention needs to be paid in order to very gently handle the dispersed acini in order to preserve their intercellular structures.
Limitations
Even if the addition of Epidermal Growth Factor (EGF) to the Waymouth's culture may precipitate the progressive acinar-to-ductal transdifferentiation, it is essential to maintain the cells alive.
As described in the protocol and if needed, the in vitro culture period of the acinar cells can be extended to up to 10 days by seeding the cells on matrix scaffolds such as type I collagen. Yet importantly, and as shown by others 1, culturing cells on type I collagen will induce the progressive transdifferentiation of acinar cells to ductal cells. This process starts after 4 days of culture on collagen and can be complete after 7 days. It may therefore be necessary to check for the presence of a specific ductal marker such as the Cystic Fibrosis Transmembrane Conductance Regulator or Cytokeratin-19 (by immunocytochemistry or immunofluorescence experiments) if the acinar cell culture needs to be extended to 1 week. The alternative use of Matrigel as a matrix scaffold, by reducing the adherence of dispersed acini, makes it possible to extend the maintenance of their acinar phenotype for 2 days.
Possible modifications
Sectioning the rectum during the dissection step increases the risk of bacterial contamination. We have never experienced such a situation. However, if needed, there is another procedure to circumvent this possible issue. It consists of finding the stomach, spleen and the first part of duodenum, and sectioning attached pancreas. Note that this alternate procedure needs to be perfectly mastered. Otherwise, the duration of the dissection will get longer, jeopardizing then the quality of the removed biological material.
The long-term monolayer culture of acinar cells can be optimized, notably by modifying the support matrix. If required by the experimental conditions, type I collagen can be replaced with another scaffold, such as Matrigel. In this case, Matrigel must be freshly prepared at 400 μg/ml concentration in cold Phosphate-Buffered Saline 1x. Thereafter, wells are incubated with Matrigel (40 μg/cm2) overnight at 4 °C. This point must be strictly respected. The culture procedure remains the same as described in Step 4.
The method can be further optimized empirically. For example, the amount of amino acids in the culture medium of exocrine pancreatic cells can regulate protein synthesis by these cells 18. Sphyris et al. 2 and Bläuer et al. 19 have elaborated a complete culture medium containing a higher amount of amino acids (essential and non-essential), promoting maintenance of acinar cells in the differentiated state. Such a medium might be very useful if one wishes to culture acinar cells isolated by this method for more than 10 days.
Another parameter that could be modified if long-term culture is required, is the pH of the culture medium. Physiologically, the apical pole of acinar cells is in permanent contact with a bicarbonate-rich, slightly alkaline liquid giving rise to the pancreatic juice. It is tempting to speculate that the pH of the acinar cell environment could be important in maintaining the acinar differentiation state. Some protocols previously reported the use of a culture medium with a pH adjusted to 7.8 to mimic the "initial" physiology of acinar cells 19.
Significance of the technique
It is important to mention that there exist other protocols for culturing acinar cells, using pancreas explants and organotypic cultures 19. Their application, based on pancreatic cell migration from the explant to the membrane on which they are cultured, is more difficult. Isolation of these pancreatic cells notably requires a first week of pancreatic explant culture. The major advantage of this method is that no enzymatic dissociation is needed, so that both the cell membrane integrity and cell-to-cell interactions are preserved. Under these conditions, acinar cells can be maintained in vitro for up to 14 days. Yet the acinar cell culture obtained is not pure, and contaminant fibroblasts, ductal cells, and endothelial cells are inevitably present, which could be incompatible with some kinds of experiments. In contrast, our procedure rapidly yields a pure population of acinar cells, conserving their initial architecture of acini.
Dorrell et al. described another method in order to isolate the different mouse pancreatic cell types (including acinar, duct, and endocrine cells), using Fluorescence-Activated Cell Sorting (FACS) 17. This method is very efficient to obtain a pure (or specific) population of acinar cells. However, it requires a fluorescent labeling with specific antibodies, prior sorting. Moreover, this procedure also requires an expertise in FACS and a flow cytometer. Besides, this technique does not permit an extended culture of acinar cells, due to the loss of their initial architecture of acini. Our rapid method allows an extended in vitro culture of dispersed acinar cells with a quality and a purity that are compatible with most of the further routine applications.
Future applications
Once mastered, this technique for isolating/culturing acinar cells should prove very useful in addressing a range of questions and notably for investigating the mechanisms involved in pancreatic plasticity and transdifferentiation, which are well known but poorly understood. By preserving some inter- and intracellular communications, this dispersed acini model remains more physiologically relevant than immortalized cell lines.
In the field of pancreatic tumorigenesis, this primary cell model provides an adequate system for studying acinar cell transdifferentiation, one of the mechanisms proposed to generate aggressive pancreatic cancers. Although immortalized human or murine cell lines (such as Colo357, Panc-1, or BxPC3) might be more flexible to use than primary acinar cells, both their origin and their complex genetic status (as transformed cell lines initially isolated from pancreatic tumors or even pancreatic metastases) constitute major drawbacks in studying such mechanisms.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
We thank the staff of AniCan (CRCL, Lyon) for their technical assistance with animal care. This work was supported by the Institut National de la Santé Et de la Recherche Médicale (INSERM Avenir Program), the Ligue Nationale Contre le Cancer, by the Association pour la Recherche sur le Cancer, by the Institut National du Cancer, and by fellowships from the Ligue Nationale Contre le Cancer (JG), from the Institut National du Cancer (JG), from the Ministère de l'Enseignement Supérieur et de la Recherche of France (RMP and DFV) and from the Association pour la Recherche sur le Cancer (DFV).
References
- Lardon J, Bouwens L. Metaplasia in the pancreas. Differentiation. 2005;73:278–286. doi: 10.1111/j.1432-0436.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- Sphyris N, Logsdon CD, Harrison DJ. Improved retention of zymogen granules in cultured murine pancreatic acinar cells and induction of acinar-ductal transdifferentiation in vitro. Pancreas. 2005;30:148–157. doi: 10.1097/01.mpa.0000147086.15867.ab. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Jamieson JD. Structural and functional characterization of isolated pancreatic exocrine cells. Proc. Natl. Acad. Sci. U.S.A. 1972;69:3028–3032. doi: 10.1073/pnas.69.10.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Jamieson JD. Studies on dispersed pancreatic exocrine cells. I. Dissociation technique and morphologic characteristics of separated cells. J. Cell. Biol. 1974;63:1037–1056. doi: 10.1083/jcb.63.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A, Jamieson JD. Studies on dispersed pancreatic exocrine cells. II. Functional characteristics of separated cells. J. Cell. Biol. 1974;63:1057–1073. doi: 10.1083/jcb.63.3.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz GS, et al. Guinea pig pancreatic acini prepared with purified collagenase. Exp. Cell Res. 1980;130:49–62. doi: 10.1016/0014-4827(80)90041-5. [DOI] [PubMed] [Google Scholar]
- Williams JA, Korc M, Dormer RL. Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am. J. Physiol. 1978;235:517–524. doi: 10.1152/ajpendo.1978.235.5.E517. [DOI] [PubMed] [Google Scholar]
- Logsdon CD, Williams JA. Epidermal growth factor binding and biologic effects on mouse pancreatic acini. Gastroenterology. 1983;85:339–345. [PubMed] [Google Scholar]
- Han B, Logsdon CD. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-kappaB activation. Am. J. Physiol. 1999;277:74–82. doi: 10.1152/ajpcell.1999.277.1.C74. [DOI] [PubMed] [Google Scholar]
- Ji B, Kopin AS, Logsdon CD. Species differences between rat and mouse CCKA receptors determine the divergent acinar cell response to the cholecystokinin analog JMV-180. J. Biol. Chem. 2000;275:19115–19120. doi: 10.1074/jbc.M001685200. [DOI] [PubMed] [Google Scholar]
- Ji KA, Yang MS, Jou I, Shong MH, Joe EH. Thrombin induces expression of cytokine-induced SH2 protein (CIS) in rat brain astrocytes: involvement of phospholipase A2, cyclooxygenase, and lipoxygenase. Glia. 2004;48:102–111. doi: 10.1002/glia.20059. [DOI] [PubMed] [Google Scholar]
- Gaiser S, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon CD, Williams JA. Pancreatic acinar cells in monolayer culture: direct trophic effects of caerulein in vitro. Am. J. Physiol. 1986;250:440–447. doi: 10.1152/ajpgi.1986.250.4.G440. [DOI] [PubMed] [Google Scholar]
- Stanger BZ, Dor Y. Dissecting the cellular origins of pancreatic cancer. Cell Cycle. 2006;5:43–46. doi: 10.4161/cc.5.1.2291. [DOI] [PubMed] [Google Scholar]
- Vincent DF, et al. Tif1gamma suppresses murine pancreatic tumoral transformation by a smad4-independent pathway. Am. J. Pathol. 2012;180:2214–2221. doi: 10.1016/j.ajpath.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent DF, et al. Inactivation of TIF1gamma cooperates with Kras to induce cystic tumors of the pancreas. PLoS Genet. 2009;5:e1000575. doi: 10.1371/journal.pgen.1000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrell C, et al. Isolation of mouse pancreatic alpha, beta, duct and acinar populations with cell surface markers. Mol. Cell Endocrinol. 2011;339:144–150. doi: 10.1016/j.mce.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case RM. Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol. Rev. Camb. Philos. Soc. 1978;53:211–354. doi: 10.1111/j.1469-185x.1978.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Blauer M, Nordback I, Sand J, Laukkarinen J. A novel explant outgrowth culture model for mouse pancreatic acinar cells with long-term maintenance of secretory phenotype. Eur. J. Cell. Biol. 2011;90:1052–1060. doi: 10.1016/j.ejcb.2011.07.004. [DOI] [PubMed] [Google Scholar]


