Abstract
Implantable microdevices are gaining significant attention for several biomedical applications1-4. Such devices have been made from a range of materials, each offering its own advantages and shortcomings5,6. Most prominently, due to the microscale device dimensions, a high modulus is required to facilitate implantation into living tissue. Conversely, the stiffness of the device should match the surrounding tissue to minimize induced local strain7-9. Therefore, we recently developed a new class of bio-inspired materials to meet these requirements by responding to environmental stimuli with a change in mechanical properties10-14. Specifically, our poly(vinyl acetate)-based nanocomposite (PVAc-NC) displays a reduction in stiffness when exposed to water and elevated temperatures (e.g. body temperature). Unfortunately, few methods exist to quantify the stiffness of materials in vivo15, and mechanical testing outside of the physiological environment often requires large samples inappropriate for implantation. Further, stimuli-responsive materials may quickly recover their initial stiffness after explantation. Therefore, we have developed a method by which the mechanical properties of implanted microsamples can be measured ex vivo, with simulated physiological conditions maintained using moisture and temperature control13,16,17.
To this end, a custom microtensile tester was designed to accommodate microscale samples13,17 with widely-varying Young's moduli (range of 10 MPa to 5 GPa). As our interests are in the application of PVAc-NC as a biologically-adaptable neural probe substrate, a tool capable of mechanical characterization of samples at the microscale was necessary. This tool was adapted to provide humidity and temperature control, which minimized sample drying and cooling17. As a result, the mechanical characteristics of the explanted sample closely reflect those of the sample just prior to explantation.
The overall goal of this method is to quantitatively assess the in vivo mechanical properties, specifically the Young's modulus, of stimuli-responsive, mechanically-adaptive polymer-based materials. This is accomplished by first establishing the environmental conditions that will minimize a change in sample mechanical properties after explantation without contributing to a reduction in stiffness independent of that resulting from implantation. Samples are then prepared for implantation, handling, and testing (Figure 1A). Each sample is implanted into the cerebral cortex of rats, which is represented here as an explanted rat brain, for a specified duration (Figure 1B). At this point, the sample is explanted and immediately loaded into the microtensile tester, and then subjected to tensile testing (Figure 1C). Subsequent data analysis provides insight into the mechanical behavior of these innovative materials in the environment of the cerebral cortex.
Keywords: Bioengineering, Issue 78, Biophysics, Biomedical Engineering, Molecular Biology, Cellular Biology, Electrical Engineering, Materials Science, Nanotechnology, Nanocomposites, Electrodes, Implanted, Neural Prostheses, Micro-Electrical-Mechanical Systems, Implants, Experimental, mechanical properties (composite materials), Dynamic materials, polymer nanocomposite, Young's modulus, modulus of elasticity, intracortical microelectrode, polymers, biomaterials
Protocol
1. Sample Preparation
Prepare PVAc-NC film of thickness in the range of 25-100 μm using a solution casting and compression technique10-12.
Adhere film to a silicon wafer by heating on a hot plate for two min at 70 °C (above the glass transition temperature) to promote intimate contact between the film and the wafer. This step ensures that the prepared film remains flat and fixed to the Si wafer, which is necessary for planar micromachining processes.
Pattern the film into the test sample geometries by laser-micromachining (VLS 3.50, VersaLASER). Set the CO2 direct-write laser micromachining parameters to 1.0% power (0.5 W), 4.0% speed (56 mm/s), and 1,000 pulses per inch13,16.
Pattern samples that will be used to establish environmental conditions ("setup samples") into dogbone-shaped structures with lateral pad dimensions 1.5 x 1.5 mm2, and lateral beam dimensions 300 x 3,000 μm2, with a thickness matching that of the film throughout (Figure 2).
Pattern the samples for ex vivo experiments ("implant sample") into beams 300 μm x 6 mm, with a thickness matching that of the film.
Carefully release the samples from the wafer using a razor blade and tweezers.
For sample handling, prepare custom-machined acrylic holders designed to serve as part of the grip system in the microtensile tester. Laser-etched markings show the centerline of the holder and 1.5 mm from the end. Place a small amount of cyanoacrylate gel-based adhesive on the centerline of the acrylic holder and carefully adhere a 1.5 mm length of the implant sample to the holder and overlap the marked centerline (Figure 3). Each implant sample requires one acrylic holder. Be careful to ensure that the adhesive gel remains only along the 1.5 mm length of PVAc-NC being adhered to the acrylic holder. Otherwise, the adhesive gel can interfere with the mechanical behavior of the sample.
Remove moisture from all samples by placing them in a desiccator for at least 24 hr.
Measure the length, width, and thickness dimensions of the samples using an optical microscope.
2. Establish Environmental Conditions
Load a dry setup sample into the microtensile tester (see Figure 4), first clamping between the mobile grips, then between the fixed grips.
Mount an air brush with a water-filled reservoir into a fixed position, with the nozzle directed toward the microtensile sample. Connect the air brush to an air compressor via plastic tubing. With the air brush nozzle completely closed, turn on the air compressor.
Begin cyclic microtensile testing procedure, alternating between tensile strain (positive strain) and compressive strain (negative strain) applied to the sample, remaining within the linear elastic region of the stress-strain plot. For PVAc-NC, the applied strain is limited to less than 2%. In the custom microtensile tester used in these experiments, the strain rate was controlled while the required force to achieve that strain was measured. Alternatively, a different setup could involve controlling the applied force while measuring the resultant strain.
Gradually increase the flow from the air brush nozzle, and monitor the slope of the stress-strain plot as a function of the amount of flow from the air brush. The maximum flow that does not cause a significant (>10%) reduction in Young's modulus over a period of 60 sec is the level that will be used for the ex vivo experiments. At this point, the humidity conditions that will not wet a dry sample (and thus contribute to a reduction in Young's modulus), and will also minimize sample drying after being exposed to biological fluids in vivo have been established.
Measure the temperature near the sample. An ideal setup would include a thermocouple with digital readout, and be performed while the airbrush is operating. Set the intensity and distance of the radiant heat source such that the sample temperature is held to 37 °C, to match physiological conditions.
3. Compare Environmental Control to Non-Environmental Control
Immerse setup samples for at least 30 min in phosphate buffered saline. After this amount of time, the sample is completely saturated and has been reduced to its minimum Young's modulus at a given temperature.
Quickly load a sample into the microtensile tester and begin cyclic microtensile testing, with the air brush off, while the sample dries. This will determine how quickly the sample dries under non-controlled conditions.
Load a second PBS-saturated setup sample into the microtensile tester, and begin cyclic microtensile testing with the air brush on. This will determine how quickly the sample dries under controlled environmental conditions.
4. Probe Implantation and Explantation
Attach implant sample to a micromanipulator clamp and position orthogonal to the cortical tissue.
Prior to insertion, keep tissue sufficiently moist with saline to ensure homogeneity of tissue mechanics.
Lower the polymer sample into the cortex using the micromanipulator hand controls. Leave sample in cortical tissue until the target implant time, generally between 1 and 30 min. To prevent tissue from drying for time points over 5 min, lightly dab the tissue every 5 min using a saline-soaked cotton swab.
While the probe is implanted in the cortex, prepare the microtensile tester for loading the currently implanted sample by setting the drive rod to the zero-displacement position of 3.0 mm from the stationary sample clamp. Also, set the air brush nozzle to the flow setting and the radiant heat source to the proper intensity determine in Step 2.4.
At the end of the specified implant time, raise the probe out of the cortex using the micromanipulator hand controls. Immediately, and carefully, remove the sample from the micromanipulator clamp and load into the microtensile tester, as described in more detail in Step 5.2.
5. Microtensile Testing of Implant Samples
To save time after explantation, ensure that the microtensile tester is completely ready to accept the implant sample prior to implantation, as described in Step 4.4.
Immediately after explantation, load the sample between the two sets of microtensile tester clamps. Since the sample is mounted to an acrylic holder designed to serve as the top half of one clamp, place the implant sample assembly on the mobile grip, sample side down. It is important to ensure that the sample is mounted such that strain is applied only along the length of the probe to avoid applying torque to the sample during testing. As such, the sample must be mounted to the center of each clamp, and the clamps must be level with respect to each other.
Adjust the sample position such that the distance between the clamps is 3.0 mm, and the end of the probe is placed into the fixed clamp. This 3.0 mm length between clamps is the gauge length for the sample, and will be used in later calculations to determine the strain on the sample.
Immediately after securing the sample between both clamps, and within 2 min of explantation from the neural tissue, activate the motor in the tensile direction to elongate the sample at a constant rate (10 μm/sec used here), while simultaneously measuring and recording the elongation of the sample (using a displacement indicator, Mitutoyu 543-561) and associated force (using a load cell, Transducer Techniques MDB-2.5) required to strain the sample.
Halt the microtensile test upon mechanical failure of the sample, or when the end of the drive rod range is reached. Export the collected data, and perform data analysis.
Repeat the microtensile testing for each sample and/or each set of conditions (i.e. insertion time).
6. Data Analysis
Convert the raw elongation data to engineering strain applied to the implant sample by dividing the distance of elongation by the initial gauge length, as described in Equation 1, where ε is the applied strain, t is time, d is the displacement measured by the micrometer indicator, and L0 is the initial gauge length of the sample:
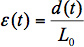 (1)
(1) Convert the raw force data to the engineering stress on the sample by dividing the force (in Newtons), by the transverse cross-sectional area, as described in Equation 2:
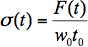 (2) where σ is the stress on the sample, F is the force measured by the load cell (in Newtons), w0 is the initial width of the sample, and t0 is the initial thickness of the sample.
(2) where σ is the stress on the sample, F is the force measured by the load cell (in Newtons), w0 is the initial width of the sample, and t0 is the initial thickness of the sample.Plot the stress (σ[t]) vs. strain (ε[t]) curve for each sample using a computer program, such as Microsoft Excel.
Isolate the linear elastic portion of the plot and use software-based curve fitting tools to find the best fit line to this portion. The slope of the best fit line corresponds to the Young's modulus of the sample. The isolated portion of the plot should include at least 10 stress-strain points, and should be taken from the portion of the plot where the slope is greatest.
For cyclic tests, the Young's modulus will need to be determined for each cycle. This may be automated or performed manually.
For the cyclic tests, plot the Young's modulus of each cycle versus time. This indicates how the measured modulus changes with time, which is indicative of how quickly a setup sample is wetting or drying.
For implant samples, each sample and time of implant corresponds to a single cycle of the cyclic tests. Measure the Young's modulus using the procedure described above for each implant sample.
Plot the Young's modulus versus implant time. At this point, comparisons can be made to benchtop investigations, etc.
Representative Results
The mechanical properties of nearly all polymeric materials, including our PVAc-NC, are dependent upon exposure to environmental conditions. Most notably, these include the exposure to heat and moisture. When a material is plasticized due to the uptake of moisture, or undergoes a thermal transition, it displays a reduction in Young's modulus. In preparing the moisture- and temperature-controlled environment for ex vivo sample mechanical characterization, it is important to ensure that there is minimal change in the moisture content of the sample while loading the sample into the microtensile tester, as well as during mechanical testing. This is evaluated using the control setup sample experiments to ensure that the sample is not influenced by the moisture generated by the air brush, nor does it quickly dry in the external environment. Figure 5 shows an example plot demonstrating the mechanical behavior of a dry setup sample during cyclical tensile testing for an appropriate air brush moisture setting. Any change in Young's modulus while the air brush is turned on is minimal. This is important as the external environment should not contribute to a reduction or increase in stiffness. When the flow from the air brush is set too high, the Young's modulus of the sample will significantly decrease within approximately 60 sec.
Control over the mechanical testing environment can also ensure that the materials do not prematurely dry out. For example, the use of our moisture controlled environment increases the time required for an explanted sample to dry and recover its pre-implantation mechanical properties. Figure 6 demonstrates the drying behavior of two control setup samples soaked to saturation then subjected to cyclical tensile testing under both controlled and non-controlled environmental conditions. Under a non-controlled environment, the samples recover a Young's modulus exceeding 400 MPa in the 150 sec during which the sample was loaded into the microtensile tester. This Young's modulus increase of 20-40 times that of a saturated sample resulted from the rapid drying of the sample13. Under environmental control, an appreciable increase in Young's modulus is not measured until 240 sec after removal from the immersion bath. This period of time is sufficient to both load the sample and perform enough of the mechanical testing to enable extraction of the Young's modulus.
The design for the implant samples for ex vivo testing (Figure 3) includes consideration of a number of factors. First, the samples need to be implanted into the tissue of interest, which is the cerebral cortex in this investigation. As a result, the sample should have a needle-inspired geometry, which is represented by the narrow PVAc-NC beam. In addition, the sample should be designed with regard to the force required to penetrate the tissue of interest without buckling. Euler's buckling formula takes into account the Young's modulus of the material, as well as the length, width, and thickness of the beam to provide a critical force at which a beam-type probe is expected to buckle17. In this study, the beam dimensions were chosen such that the probe would penetrate through the neural tissue without risk of buckling. Given previous studies showing an insertion force less than 15 mN, a chosen probe length of 4.5 mm to allow a 3 mm test beam and a 1.5 mm length for gripping, and a known film thickness exceeding 75 μm, we could calculate that the probe width should exceed 107 μm. To ensure maximum repeatability with the laser micromachining tool, a width of 300 μm was chosen for the samples. An additional point of concern is handling of the microprobe sample during insertion into the tissue and removal from the tissue. As a simple beam can be damaged during handling, attaching the beam to a more substantial structure (i.e. the acrylic holder) enables safer transfer to implantation and to mechanical testing. Finally, this assembly should be optimized to allow for loading into the tensile tester as quickly as possible.
A representative plot showing the stress-strain curves for a dry sample and a wet sample that had been implanted in the rat cortex for 30 min is shown in Figure 7. The Young's modulus, which corresponds to the slope of the stress-strain plot in the linear elastic region, is clearly much greater for the dry sample than for the implanted sample. Both samples were strained to break. However, the Young's modulus is derived from the linear elastic portion of the plot that is collected early in the tensile test, before entering plastic deformation and sample failure, as shown in Figure 8. Figure 9 demonstrates that after approximately 5 min of implantation, the sample displays little change in the Young's modulus, suggesting that the sample reaches saturation and minimum stiffness within this period of time.
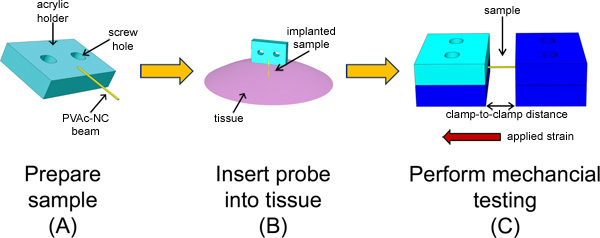 Figure 1. Schematic overview of the experimental method to characterize the in vivo mechanical behavior of a stimuli-responsive, mechanically-adaptive polymer nanocomposite microprobe.(A) First, the sample is prepared by patterning the PVAc-NC film into a beam and mounting onto an acrylic holder. (B) The probe is then implanted into the cerebral cortex for a specified period of time. (C) Finally, the sample is explanted and subjected to microtensile testing using a custom-built microtensile tester.
Figure 1. Schematic overview of the experimental method to characterize the in vivo mechanical behavior of a stimuli-responsive, mechanically-adaptive polymer nanocomposite microprobe.(A) First, the sample is prepared by patterning the PVAc-NC film into a beam and mounting onto an acrylic holder. (B) The probe is then implanted into the cerebral cortex for a specified period of time. (C) Finally, the sample is explanted and subjected to microtensile testing using a custom-built microtensile tester.
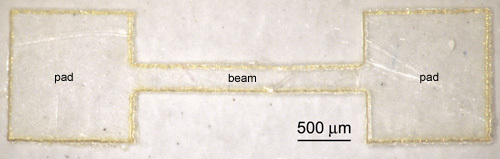 Figure 2. Laser-micromachined PVAc-NC setup sample for establishing necessary environmental conditions for maintaining the in vivo mechanical behavior of the PVAc-NC implant samples after explantation.
Figure 2. Laser-micromachined PVAc-NC setup sample for establishing necessary environmental conditions for maintaining the in vivo mechanical behavior of the PVAc-NC implant samples after explantation.
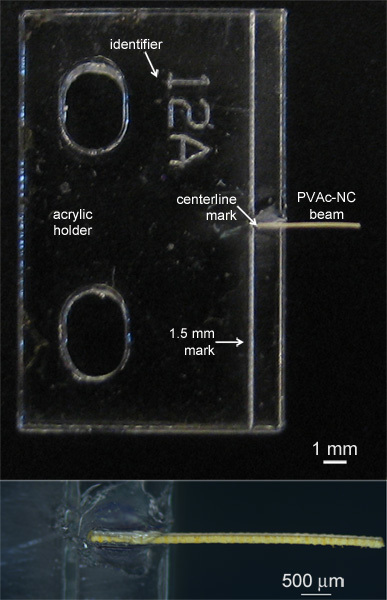 Figure 3. Photographs of implant sample, consisting of a laser-patterned PVAc-NC beam mounted to an acrylic holder.
Figure 3. Photographs of implant sample, consisting of a laser-patterned PVAc-NC beam mounted to an acrylic holder.
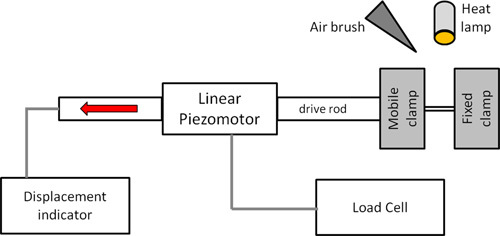 Figure 4. Block diagram of the microtensile tester. The sample is clamped between a fixed clamp and a mobile clamp that is attached to the drive rod of the linear piezomotor. The strain rate of the linear piezomotor is controlled and the strain is measured using the displacement indicator. The load required to strain the sample is measured by a load cell. The environmental conditions in the vicinity of the sample are controlled by an air brush and a heat lamp.
Figure 4. Block diagram of the microtensile tester. The sample is clamped between a fixed clamp and a mobile clamp that is attached to the drive rod of the linear piezomotor. The strain rate of the linear piezomotor is controlled and the strain is measured using the displacement indicator. The load required to strain the sample is measured by a load cell. The environmental conditions in the vicinity of the sample are controlled by an air brush and a heat lamp.
 Figure 5. Young's modulus (E) as a function of time, as measured during cyclical tensile tests to determine the correct air brush settings for controlling the moisture in the test environment. The shaded region is the time during which the air brush was turned on. At the air brush settings used, the Young's modulus does not change significantly over time, suggesting that the amount of water absorbed by the setup sample from the air brush is not enough to contribute to a reduction in stiffness.
Figure 5. Young's modulus (E) as a function of time, as measured during cyclical tensile tests to determine the correct air brush settings for controlling the moisture in the test environment. The shaded region is the time during which the air brush was turned on. At the air brush settings used, the Young's modulus does not change significantly over time, suggesting that the amount of water absorbed by the setup sample from the air brush is not enough to contribute to a reduction in stiffness.
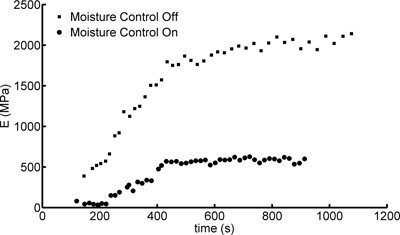 Figure 6. Young's modulus (E) versus time for water-saturated samples in both moisture controlled and non-controlled tensile testing environments. The recovery of the initial Young's modulus is much slower in the controlled environment.
Figure 6. Young's modulus (E) versus time for water-saturated samples in both moisture controlled and non-controlled tensile testing environments. The recovery of the initial Young's modulus is much slower in the controlled environment.
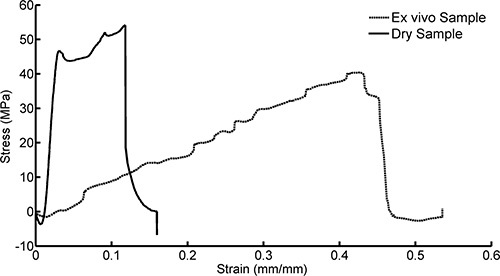 Figure 7. Example of stress-strain plots for PVAc-NC samples that were dry (never implanted) and wet (ex vivo, explanted from tissue after 30 min in vivo).
Figure 7. Example of stress-strain plots for PVAc-NC samples that were dry (never implanted) and wet (ex vivo, explanted from tissue after 30 min in vivo).
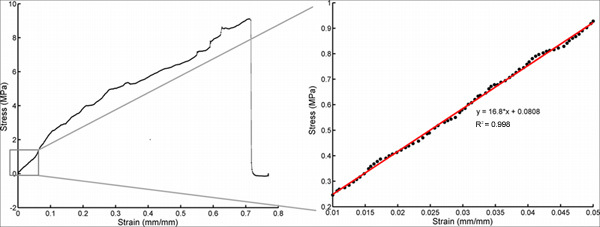 Figure 8. Additional set of stress-strain plots to demonstrate that the linear elastic portion of the plot is isolated from the overall stress-strain plot (left), and extracted and fit to a line (right). For this particular measurement, the Young's modulus is 16.8 MPa. Click here to view larger figure.
Figure 8. Additional set of stress-strain plots to demonstrate that the linear elastic portion of the plot is isolated from the overall stress-strain plot (left), and extracted and fit to a line (right). For this particular measurement, the Young's modulus is 16.8 MPa. Click here to view larger figure.
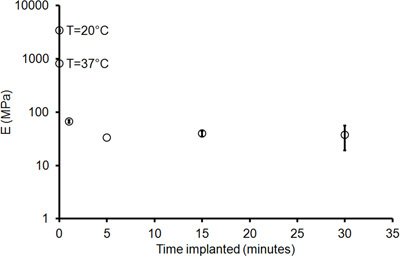 Figure 9. Young's modulus, E, versus implant time for PVAc-NC samples implanted in cortex. The error bars represent standard error with n = 4, with the exception of the 5 min implant, with n = 2.
Figure 9. Young's modulus, E, versus implant time for PVAc-NC samples implanted in cortex. The error bars represent standard error with n = 4, with the exception of the 5 min implant, with n = 2.
Discussion
The advancement of implantable biomedical microelectromechanical systems (bioMEMS) for interacting with biological systems is motivating the development of new materials with highly-tailored properties. Some of these materials are designed to exhibit a change in material properties in response to a stimulus found in the physiological environment. One recently-developed class of materials responds to the presence of hydrogen bond-forming liquids (e.g. water) and elevated temperatures to reduce the Young's modulus, a measure of the material stiffness, by three orders of magnitude10,11,18. These polymer nanocomposite materials have a soft polymer matrix (i.e. poly(vinyl acetate) ) with cellulose nanofibers as the nanofiller phase. Interactions between the cellulose nanofibers dictate the mechanical properties of the material as a whole, and are turned "on" when dry and turned "off" when wet. In addition, water plasticizes the polymer nanocomposite, thereby reducing the glass transition temperature to below body temperature (37 °C), resulting in a further reducing in Young's modulus. One application for this class of materials is to serve as a bio-adaptive substrate for intracortical probes to interfacing with individual neurons 13,17. However, the benefits of a mechanically-adaptive material are not limited to interfacing with the nervous system.
Presented here is a method by which the mechanical behavior of PVAc-NC-based microprobes can be assessed after implantation in neural tissue for a specified amount of time. Using this method, ex vivo mechanical data can be collected for comparison to benchtop studies. Further, the timescale of changes in mechanical properties can be assessed. The environmental control enabled by the highly tunable air brush and radiant heat settings provides a mechanism by which the implanted samples can be tested ex vivo with minimal change in mechanical properties resulting from the change in environment. As such, the in vivo behavior of the material can be inferred, providing superior information compared to benchtop experiments with samples completely immersed in artificial cerebrospinal fluid (ACSF). The complex physiological environment demands the availability of such methods, but experimental methods for this assessment are limited.
There are several advantages to our method for mechanical characterization of implanted, mechanically-adaptive polymer nanocomposite samples. The custom microtensile tester is suitable for testing samples with dimensions comparable to a typical neural probe (1.5-8 mm-long, 50-500 μm-wide, 15-100 μm-thick3,19-21). Other mechanical characterization methods are suited for either larger, bulk samples or nanoscale samples. Utilizing a mechanical testing tool of the appropriate scale removes the unknown of property scalability. In addition, the microtensile tester has open access to the sample under test, enabling moisture and temperature control of the test environment. Further, even with environmental control, it is necessary to begin tensile testing quickly after removing the sample from the neural tissue. Ex vivo sample drying, and thus stiffening, has been minimized here using test sample and microtensile tester designs that facilitate rapid (generally within 120 sec) loading and commencement of mechanical testing. Finally, this microtensile tester accommodates samples that do not have pads on both ends, facilitating use of probe-like samples for mechanical testing that can be implanted into animals in the identical manner as for biological evaluation.
Removal of the test sample from the neural tissue presents a new environment, which can lead to changes in mechanical behavior after explantation because the stimuli-responsive behavior of the material is reversible and potentially fast acting. When using this environmentally-controlled tensile testing method to assess the change in mechanical behavior after sample implantation into the brain for a given period of time, the potential discrepancies with respect to that actual Young's modulus in vivo should be considered. First, by testing the samples ex vivo, they are, by definition, removed from the physiological environment and subjected to an alternative environment. For a sample with mechanical properties dependent upon the environmental conditions, removing a sample from the environment will alter its mechanical properties. The timescale with which this change occurs depends upon to the material properties, as well as the degree to which the external environment is controlled.
This approach to characterization and quantification of stimuli-responsive mechanical behavior is best suited for samples with needle-like geometries, with a length much larger than the width or thickness of the device. Additionally, the stiffness of the material and the specific motor and its maximum force should be considered when choosing device dimensions. Given a set of sample dimensions, a more rigid material will require a larger pull-force to apply the same amount of strain as a material with a smaller Young's modulus. Reducing the width and/or thickness, or increasing the sample length, will reduce the amount of force required to elongate the sample a given amount. For the custom tensile testing setup, the linear piezomotor has a maximum pull force of 6 N, which allows for samples with Young's modulus of 5 GPa and cross-sectional area up to a 24,000 μm2 to be strained 5% without reaching the maximum pull force of the motor. The load cell used to measure the force in the microtensile tester has a resolution of less than 1 mN, so that the smallest Young's modulus that can be measured in the samples used in our study (width 300 μm, thickness 100 μm) approximately 1 MPa. This lower limit can be further reduced with the use of samples with larger cross-sectional area, however. The displacement indicator has a resolution of 0.5 μm, which is adequate for the materials with elastic behavior limited to 0.2% strain (at an initial length of 3 mm), which is an order-of-magnitude smaller than the elastic region for PVAc-NC even in the dry state.
One limitation to this method of ex vivo characterization is that it may not be effective for very rigid or brittle materials. Practically speaking, as the sample must be quickly mounted into the microtensile tester, a brittle material is at risk of breaking during the mounting procedure. Additionally, the beam-like samples (with dimensions matching those of our experiments) with one end adhered to the acrylic holder and the other end free cannot be used for materials exceeding approximately 2.5 GPa as the force required to strain the sample exceeds the force of the clamps holding the sample in place, resulting in slippage of the sample through the clamps and inaccurate results. This problem was overcome with the use of dogbone-shaped samples with pads on each end. This use of this method for measurement and analysis of the in vivo mechanical behavior of microprobes is not limited to the PVAc-NC class of materials. Additional potential applications include monitoring the degradation rate of biodegradable materials22 and characterizing the mechanical behavior of biological tissues23,24, as well as characterization of microscale structures for non-biological applications. Further, additional environmental controls can be added (e.g. pH, wavelength of ambient light, electrical field, magnetic field) for materials that are responsive to different stimuli25,26. One of the main advantages of this method is its versatility and applicability to many different materials and applications.
Disclosures
We have nothing to disclose.
Acknowledgments
This work was supported by the Department of Biomedical Engineering at Case Western Reserve University through both lab start-up funds (J. Capadona), and the Medtronic Graduate Fellowship (K. Potter). Additional funding on this research was supported in part by NSF grant ECS-0621984 (C. Zorman), the Case Alumni Association (C. Zorman), the Department of Veterans Affairs through a Merit Review Award (B7122R), as well as the Advanced Platform Technology Center (C3819C).
References
- Chen PJ, Saati S, Varma R, Humayun MS, Tai YC. Wireless intraocular pressure sensing using microfabricated minimally invasive flexible-coiled LC sensor implant. Journal of Microelectromechanical Systems. 2010;19:721–734. [Google Scholar]
- Ren X, Zheng N, Gao Y, Chen T, Lu W. Biodegradable three-dimension micro-device delivering 5-fluorouracil in tumor bearing mice. Drug Delivery. 2012;19:36–44. doi: 10.3109/10717544.2011.635720. [DOI] [PubMed] [Google Scholar]
- Bai Q. Single-unit neural recording with active microelectrode arrays. IEEE Transactions on Biomedical Engineering. 2001;48:911. doi: 10.1109/10.936367. [DOI] [PubMed] [Google Scholar]
- Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, kirke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Transactions on Biomedical Engineering. 2001;48:361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- Hassler C, Boretius T, Stieglitz T. Polymers for neural implants. Journal of Polymer Science Part B: Polymer Physics. 2011;49:18–33. [Google Scholar]
- Mercanzini A, Colin P, Bensadoun JC, Bertsch A, Renaud P. In Vivo Electrical Impedance Spectroscopy of Tissue Reaction to Microelectrode Arrays. IEEE Transactions on Biomedical Engineering. 2009;56:1909–1918. doi: 10.1109/TBME.2009.2018457. [DOI] [PubMed] [Google Scholar]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. Journal of Neuroscience Methods. 2005;148:1–18. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Subbaroyan J, Kipke D, editors. Engineering in Medicine and Biology Society, 2006. EMBS'06. 28th Annual International Conference of the IEEE; IEEE; 2006. pp. 3588–3591. [DOI] [PubMed] [Google Scholar]
- Harris J, Capadona J, Miller R, Healy B, Shanmuganathan K, Rowan S, Weder C, Tyler D. Mechanically adaptive intracortical implants improve the proximity of neuronal cell bodies. Journal of Neural Engineering. 2011;8:066011. doi: 10.1088/1741-2560/8/6/066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capadona JR, Shanmuganathan K, Tyler DJ, Rowan SJ, Weder C. Stimuli-Responsive Polymer Nanocomposites Inspired by the Sea Cucumber Dermis. Science. 2008;319:1370–1374. doi: 10.1126/science.1153307. [DOI] [PubMed] [Google Scholar]
- Shanmuganathan K, Capadona JR, Rowan SJ, Weder C. Stimuli-Responsive Mechanically Adaptive Polymer Nanocomposites. ACS Applied Materials & Interfaces. 2009;2:165–174. doi: 10.1021/am9006337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmuganathan K, Capadona JR, Rowan SJ, Weder C. Bio-inspired mechanically-adaptive nanocomposites derived from cotton cellulose whiskers. Journal of Materials Chemistry. 2010;20:180. [Google Scholar]
- Hess A, Capadona J, Shanmuganathan K, Hsu L, Rowan S, Weder C, Tyler D, Zorman C. Development of a stimuli-responsive polymer nanocomposite toward biologically optimized, MEMS-based neural probes. Journal of Micromechanics and Microengineering. 2011;21:054009. [Google Scholar]
- Capadona JR, Tyler DJ, Zorman CA, Rowan SJ, Weder C. Mechanically adaptive nanocomposites for neural interfacing. Materials Research Society Bulletin. 2012;37:581–589. [Google Scholar]
- Ophir J, Cespedes I, Garra B, Ponnekanti H, Huang Y. Elastography: ultrasonic imaging of tissue strain and elastic modulus in vivo. European journal of ultrasound. 1996;3:49–70. [Google Scholar]
- Hess A, Shanmuganathan K, Capadona J, Hsu L, Rowan S, Weder C, Tyler D, Zorman C, editors. Micro Electro Mechanical Systems (MEMS). IEEE 24th International Conference on; IEEE; 2011. pp. 453–456. [Google Scholar]
- Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR. In vivo deployment of mechanically adaptive nanocomposites for intracortical microelectrodes. Journal of Neural Engineering. 2011;8:046010. doi: 10.1088/1741-2560/8/4/046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmuganathan K. Bio-inspired Stimuli-responsive Mechanically Dynamic Nanocomposites. Case Western Reserve University; 2010. [Google Scholar]
- Rousche PJ, Pellinen DS, Pivin DP, Williams JC, Vetter RJ, Kipke DR. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Transactions on Biomedical Engineering. 2001;48:361–371. doi: 10.1109/10.914800. [DOI] [PubMed] [Google Scholar]
- Norlin P, Kindlundh M, Mouroux A, Yoshida K, Hofmann UG. A 32-site neural recording probe fabricated by DRIE of SOI substrates. Journal of Micromechanics and Microengineering. 2002;12:414. [Google Scholar]
- Ward MP, Rajdev P, Ellison C, Irazoqui PP. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Research. 2009;1282:183–200. doi: 10.1016/j.brainres.2009.05.052. [DOI] [PubMed] [Google Scholar]
- Lin JM, Chang PK. A Novel Remote Health Monitor with Replaceable Non-Fragile Bio-Probes on RFID Tag. Applied Mechanics and Materials. 2012;145:415–419. [Google Scholar]
- Kunzelman KS, Cochran R. Stress/strain characteristics of porcine mitral valve tissue: parallel versus perpendicular collagen orientation. Journal of Cardiac Surgery. 1992;7:71–78. doi: 10.1111/j.1540-8191.1992.tb00777.x. [DOI] [PubMed] [Google Scholar]
- Snedeker J, Niederer P, Schmidlin F, Farshad M, Demetropoulos C, Lee J, Yang K. Strain-rate dependent material properties of the porcine and human kidney capsule. Journal of Biomechanics. 2005;38:1011–1021. doi: 10.1016/j.jbiomech.2004.05.036. [DOI] [PubMed] [Google Scholar]
- Ahn S, Kasi RM, Kim SC, Sharma N, Zhou Y. Stimuli-responsive polymer gels. Soft Matter. 2008;4:1151–1157. doi: 10.1039/b714376a. [DOI] [PubMed] [Google Scholar]
- Stuart MAC, et al. Emerging applications of stimuli-responsive polymer materials. Nature Materials. 2010;9:101–113. doi: 10.1038/nmat2614. [DOI] [PubMed] [Google Scholar]


