Abstract
Membrane proteins operate in unique cellular environments. Once removed from their native context for the purification that is required for most types of structural or functional analyses, they are prone to denature if not properly stabilized by membrane mimetics. Detergent micelles have prominently been used to stabilize membrane proteins in aqueous environments as their amphipathic nature allows for shielding of the hydrophobic surfaces of these bio-macromolecules while supporting solubility and monodispersity in water. This study expands the utility of branched diglucoside-bearing tripod agents, designated ganglio-tripod amphiphiles, with introduction of key variations in their hydrophobic sections and shows how these latter elements can be fine-tuned to maximize membrane protein solubilization while preserving characteristics of these molecules that afford stabilization of rather fragile assemblies. Their efficacy rivals benchmark detergents heavily used today, such as n-dodecyl–β-D-maltoside.
Keywords: amphiphile, membrane protein, molecular design, protein solubilization, membrane mimetic
1. Introduction
Integral membrane proteins play central roles in many cellular processes including ion exchange, signal transduction, and material transfer between cells and their environments. It is estimated that roughly one third of human genes encode this protein class [1,2] and at least 50% of all commercially available pharmaceuticals target membrane proteins [3]. Despite such prevalence and functional importance, only a few hundred membrane protein structures are available – which is less than 1% of the soluble protein counterpart – indicating notorious difficulties in the study of their structures [4]. The discrepancy between biodiversity and sparse structural information is partly due to their low natural abundance, necessitating the development of expression systems tailored specifically for efficient membrane protein production [e.g., 5–7]. In addition, these bio-macromolecules are unstable in non-native aqueous environments due to their amphipathic characteristics [8].
In most experiments, detergents are used as complementary amphipathic agents to facilitate the solubilization, stabilization and structural and functional characterization of membrane proteins. Detergents readily self-assemble into micelles in aqueous media with a hydrophilic exterior and a hydrophobic interior – driven by the hydrophobic effect [9]. These assemblies have the ability to associate with entities bearing the complementary surfaces. In particular, detergent micelles can dismantle cellular membranes and readily bind to (or shield) the hydrophobic portions of membrane proteins [10,11]. Once solubilized, membrane proteins suspended by detergent molecules are routinely referred to as protein-detergent complexes (PDCs). As it stands currently, detergent sets may be thought to be enough for membrane protein science because more than 100 detergents are commercially available. However, only a small number of detergents are generally useful and the scope of their present application is embarrassingly narrow. Consequently, PDCs are often not stabilizing enough to prevent membrane protein denaturation and aggregation – the two main routes by which membrane protein structural and functional integrity is lost [12–14]. It is presumed that application coverage by conventional detergents is mainly limited because of their lack of structural variation [15,16]. Typical, conventional agents are commonly built biologically or synthetically from a flexible alkyl chain and a simple, single hydrophilic group (e.g. glucose, maltose or N-oxide). Finding a compatible detergent that stabilizes the membrane protein during the multi-step purification, functional characterization, and/or crystallization process(es) can prove to be a potentially painstaking trial-and-error adventure – but one that is exceedingly critical to the success of such experiments.
Alternative mimetic systems have been developed and pursued for membrane protein manipulation over the past several decades. Amphiphilic polymers (amphipols) [17,18], nanodiscs (NDs) [18–21], and lipodisqs [22] are innovative approaches to overcome the limitation of current tools for membrane protein stability. Tandem facial amphiphiles (TFAs) [23] and hemifluorinated surfactants (HFSs) [24–26] are other examples of recent inventions that have shown to be excellent in retaining the native structures of delicate membrane proteins. Amphipathic peptides such as lipopeptide detergents (LPDs) [27] and short designer peptides [28] proved to be effective for a several classes of membrane protein systems as well. Also, amphiphiles bearing rigid hydrophobic groups have shown promising behaviors in membrane protein manipulation [29–32]. However, most of these agents were not designed for extracting/solubilizing membrane proteins from the native membranes and, more importantly, have been unsuccessful in facilitating membrane protein structure determination via techniques, like X-ray crystallography, that require growth of high-quality, three-dimensional crystals. These types of membrane mimetics have been generally found more useful in structural characterization by solution-based approaches, such as NMR, EPR, or complementary optical spectroscopies.
The development of tripod amphiphiles (TPAs) as membrane mimetics has worked to coordinate the chemical and physical properties of these molecules and their associated micelles to ensure utility in membrane protein research [33–37]. Since one cannot a priori predict these properties, they need to be tested empirically. Improvements are made in an iterative process, balancing experimental efficacy with efficiency in synthesis. The TPA architecture is unique in terms of the presence of a quaternary carbon with three hydrophobic appendages [33]. This carbon in the lipophilic region limits the conformational freedom of this class of molecules, thereby making them rigid relative to conventional detergents. This rigidification likely enabled us to solve the crystal structures of several N-oxide TPAs themselves [37] and has the potential to facilitate the crystallization of a wide array of membrane proteins; TPA-solubilized bR and potassium channel from Streptomyces lividans have been crystallized, although their structures have not yet been solved [34,36]. Recent TPA advances had led to a series of molecules with bifurcated glucose headgroups with favorable solubilization and stabilization efficacy [35]. With a successful lead molecule identified and a large number of alternative, aromatic-group-containing, Grignard reagents available, the current variations on the central theme of a ganglio-TPA template were envisioned. Herein, we show that some of these new analogues also display favorable (even superior) properties in terms of membrane protein solubilization as compared to the previously described TPAs and conventional agents, while preserving their ability to stabilize membrane proteins and membrane protein complexes. The systematic variation of the hydrophobic groups in combination with an efficient, inexpensive, information-rich solubility/stability screen enables us to contemplate detergent structure-property relationships that have been, to date, experimentally inaccessible.
2. Materials and Methods
2.1. Detergent design and synthesis
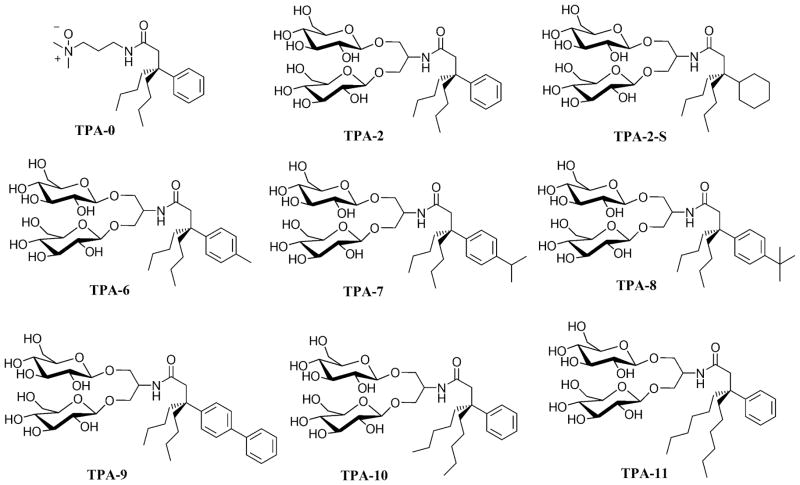
The structures of the hydrophobic group variations of the ganglio-TPA template (TPA-2) are illustrated in the six examples, TPA-6, TPA-7, TPA-8, TPA-9, TPA-10 and TPA-11 (Fig. 1). These amphiphiles share a quaternary carbon projecting three hydrophobic entities and a branched diglucoside headgroup. These agents vary in either aromatic hydrocarbons: TPA-6 (methylphenyl), TPA-7 (isopropylphenyl), TPA-8 (t-butylphenyl) and TPA-9 (biphenyl), or in the chain length of the two alkyl groups: TPA-10 (pentyl) and TPA-11 (hexyl). These hydrophobic groups were introduced into the lipophilic region via straightforward chemical reactions according to Scheme 1. Briefly, an alkylidene (A) with an alkyl group (R = butyl, pentyl or hexyl) was reacted with an Grignard reagent (R1MgBr) in the presence of Cu(I)CN, producing dinitrile-functionalized tripodal derivative (B). These dinitrile products were then subjected to hydrolysis in strong basic conditions at high temperature (200 °C). The resulting carboxylic acids (C) were activated using 1-hydroxybenzotriazole (HOBt) and 1-(3-(dimethylamino)propyl)-3-ethylcarbodiimide (EDCI) and were coupled with serinol to give amide coupling products (D). The two hydroxyl groups of these products were utilized for glycosylation with perbenzoyl-protected glucosyl bromide, followed by the removal of the protecting groups under Zemplén’s conditions [38], providing the final diglucoside-containing tripod amphiphiles (F). Additional synthetic details can be found in the expanded text of the supplementary information.
Figure 1.
Chemical structures of TPAs used as lead compounds (TPA-0, TPA-2 and TPA-2-S) and ganglio-tripod amphiphiles with the hydrophobic variations (TPA-6, TPA-7, TPA-8, TPA-9, TPA-10, and TPA-11).
Scheme 1.
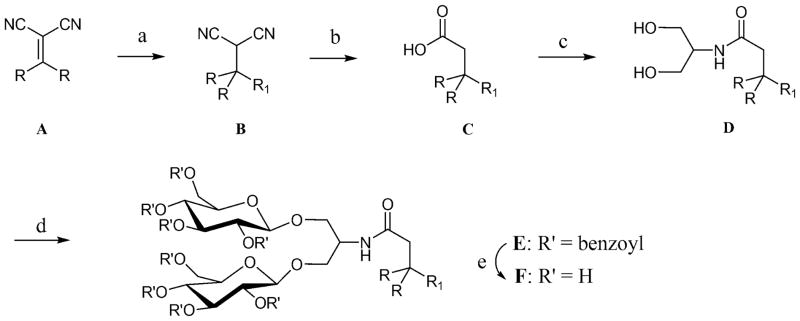
Synthetic route for the preparation of tripod amphiphiles with different lipophilic groups (R= alkyl group; R1 = aromatic group). (a) ArMgBr, Cu(I)CN, THF, 0°C; (b) KOH, ethylene glycol, 200°C; (c) serinol, EDC • HCl, HOBt, DMF, room temperature; (d) perbenzoylated glucosylbromide, AgOTf, CH2Cl2, −45°C → room temperature; (e) NaOMe, MeOH, room temperature. See details for the main text and supporting information.
2.2. Physical characterization of detergents and detergent micelles
Critical micelle concentrations (CMCs) were evaluated using a UV-absorbing probe, Orange OT [39] with modifications that streamlined the process [35]. Briefly, a concentration series of the various detergents was prepared by dissolving TPAs in distilled and deionized water. A small, but saturating, amount of Orange OT was added into these TPA solutions as a solid powder. After 30-minute incubation at room temperature, insoluble Orange OT was removed by filtration (0.42 μm pore size). The UV-Visible spectra of the flowthroughs were taken. The CMCs of new amphiphiles were determined from the point of inflection on a plot of absorbance values at 493 nm versus detergent concentration.
The hydrodynamic radii (Rh) of the micelles were determined via dynamic light scattering (DLS) as described previously [40] with slight variation. Here, each TPA was dissolved distilled and deionized water to prepare the surfactants at 0.5 wt % or 1.0 wt % concentration. A filter (0.24 μm pore size) was used to remove any particulate matter that might have been introduced during sample preparation. The Rh values of TPA micelles were calculated by software routines, integrated into the DLS control program, that analyzed the time scale of the fluctuations in the scattered-light intensity as an autocorrelation function. Measurements were run in triplicate, with the Rh values expressed as mean ± SD (n = 3).
2.3. Membrane protein solubilization and purificaition
Small-scale solubilization screens and immobilized-metal-affinity-chromatography-(IMAC)-based purification of the R. capsulatus superassembly and its components proceeded as previously described [35] with minor modification. For consistency between amphiphiles, intracytoplasmic membranes enriched in LHI-RC complexes were treated with the same concentration (1% detergent) of individual TPAs (TPA-2, TPA-6, TPA-7, TPA-8, TPA-9, TPA-10 and TPA-11) or conventional detergents (DDM and LDAO) for 30 min at 32°C. Following ultracentrifugation to pellet and remove insoluble membrane debris, detergent-solubilized LHI-RC complexes were collected as solubilized supernatants. The absorbance spectra of these two fractions were used to investigate the extraction yield and stabilization efficacy of detergents during the protein solubilization process. For longer term stabilization analysis, the detergent-solubilized superassemblies were subjected to Ni-NTA resin (Qiagen) for binding of the polyhistidine tag on the C-terminus of the M-subunit of the RC. Washing and elution (1 M imidazole) proceeded with buffers containing the individual detergents at their experimentally-derived CMC. Expanded solubilization and purification protocols are found in the supplementary information.
3. Results
3.1. Physical properties of new TPAs and their micelles
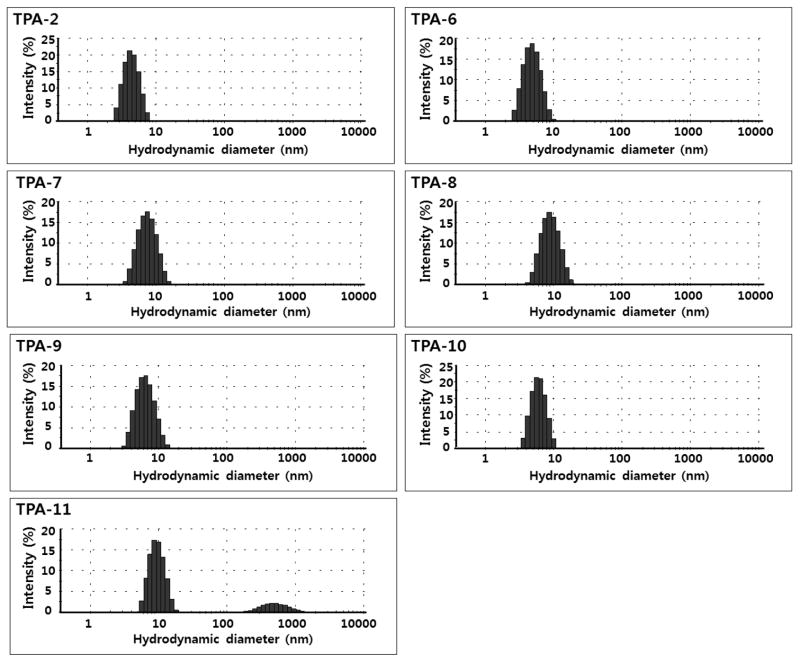
As controls, we prepared the hydrophobic variations of a conventional TPA template (TPA-0) along with those of ganglio-TPA template via the similar synthetic strategy (Fig. S1). Most of the TPA-0 variants were found to be water-insoluble, suggesting that the hydrophobicity of TPA-0 is already approaching the solubility limit. In contrast, all ganglio-TPA variations introduced here were highly soluble in water up to ~ 20% (w/v), underscoring the favorable solubility characteristics of the branched diglucoside headgroup compared to the N-oxide group and driving the design of the ganglio-TPA variations. Data for the new agents along with DDM and TPA-2 are presented in Table 1. The CMCs of the TPA series were found to decrease in value with the carbon number or chain length of the lipophilic groups, which is consistent with the general notion that a longer chain-containing detergent has a lower CMC value. The CMC value of TPA-2 was the highest (3.6 mM; 0.24 wt %) while TPA-9 was found to have the lowest (0.14 mM; 0.010 wt %). We note that the CMC of TPA-9 is comparable to that of DDM (0.17 mM; 0.0087 wt %). On the other hand, the micelles formed by the TPAs tend to increase in their hydrodynamic radii with the carbon number or chain length of the tail groups. Thus, the TPA-2 micelles are smallest while TPA-11 micelles are largest. Interestingly, TPA-9 with the longest alkyl chain and the largest carbon number among the set of molecules was measured to form micelles with the intermediate Rh value. In analysis of DLS experiments, all TPAs, except TPA-11, demonstrated monodispersed micelle distributions similar to that for DDM. In contrast, TPA-11 forms at least two kinds of molecular aggregates in solution with hydrodynamic radii of ~4.5 nm and ~270 nm (Fig. 2); the ratio of two aggregates is calculated to be > 1010 in the number distribution-based calculation, indicating much more favorable formation of smaller micelles. The larger aggregate was not a precipitate and remained in solution after prolonged high-speed centrifugation with no change in abundance relative to the dominant species. Actually, no forms of precipitation were observed for any of these molecules at these concentrations in aqueous environments.
Table 1.
Molecular weights (MW), critical micelle concentration (CMC), micellar hydrodynamic radii (Rh; mean ± SD, n = 3) and protein solubilization yields (SYs) for TPA-2, TPA-2 analogues and DDM.
| Detergent | MWa | CMC (mM) | CMC (wt %) | Rh (nm)b | SY (%)c |
|---|---|---|---|---|---|
| TPA-2 | 659.8 | ~3.6 | ~0.24 | 2.0 ± 0.06d | ~50 |
| TPA-6 | 673.8 | ~2.4 | ~0.16 | 2.3 ± 0.01d | ~70 |
| TPA-7 | 701.8 | ~1.3 | ~0.091 | 3.2 ± 0.06 | ~70 |
| TPA-8 | 715.9 | ~0.42 | ~0.030 | 4.1 ± 0.05 | ~80 |
| TPA-9 | 735.9 | ~0.14 | ~0.010 | 3.0 ± 0.01 | ~10 |
| TPA-10 | 687.8 | ~1.2 | ~0.083 | 2.7 ± 0.09 | ~50 |
| TPA-11 | 715.9 | ~0.56 | ~0.040 | 4.7 ± 0.05e | ~40 |
| DDM | 510.1 | ~0.17 | ~0.0087 | 3.5 ± 0.04 | ~70 |
Molecular weights
Hydrodynamic radius of micelles as determined at 0.5 wt % .
Solubilization yield of LHI-RC complex from the membrane.
TPA-2 and TPA-6 were used at 1.0 wt % to obtain strong signals.
Two forms of aggregates were observed with hydrodynamic radii of ~4.5 nm and ~270 nm (see Fig. 2)
Figure 2.
Representative size distributions of the micelles formed by TPA-2 and its analogs, obtained by dynamic light scattering. All TPAs were studied at 0.5 wt % except TPA-2 and TPA-11, which were used at 1.0 wt % for improved scattering signal intensity.
3.2. Membrane protein solubilization and stabilization
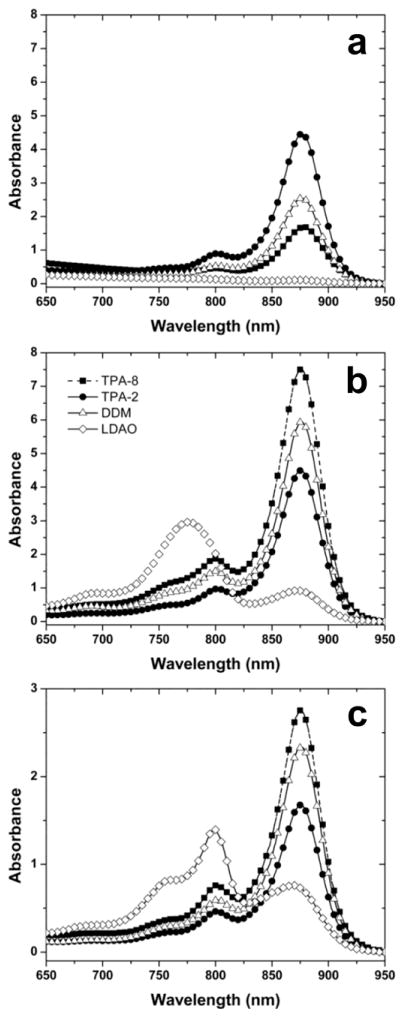
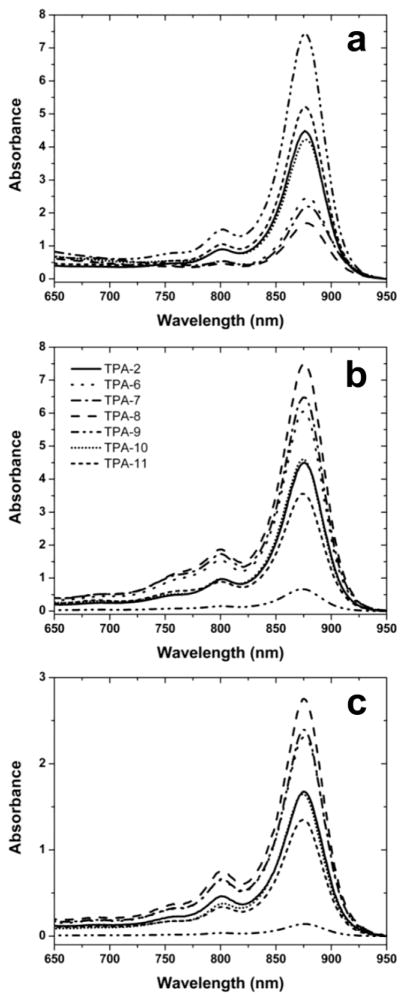
The evaluation of solubilization and stabilization properties of the new TPAs utilized the LHI-RC superassembly of the photosynthetic bacterium Rhodobacter (R.) capsulatus [41,42]. This transmembrane pigment-protein complex consists of the relatively resilient RC and the more labile LHI that surrounds the RC. The LHI-RC complex contains dozens of protein subunits (multiple copies of LHI-α, LHI-β, and single copies of RC-L, RC-M and RC-H) and a myriad of pigmented cofactors, making it challenging to preserve its native functional organization [35]. The structural integrity of the superassembly was readily assessed by pronounced absorption bands in the visible and near-IR regions of the spectra. The native conformation of the superassembly was indicated by a very strong absorbance at 875 nm. Progressively denatured forms (e.g., denatured LHI/intact RC, or denatured LHI/denatured RC) can be discerned by rather broad and intense peaks at ~800 nm and/or ~760 nm, respectively. Based upon previous studies [35,40], conventional detergents DDM and LDAO were chosen as positive and negative controls, respectively, in the assay. DDM excelled in both solubilization and stabilization of the LHI-RC superassembly (Fig. 3; Table 1), which is in a good agreement with a wide use of the agent in membrane protein science [43]. In contrast, LDAO extracted the LHI-RC complexes quantitatively and maintained the structure of the RC while proving to be too harsh for LHI. Severe degradation of this antenna complex was evident from the appearance of the rather intense peak at 760 nm that represents degraded bacteriochlorophylls lacking central Mg2+ ions (Fig. 3). All ganglio-TPAs except TPA-9 were found to stabilize the intact superassembly (Figs. 3 & 4). The lead ganglio-TPA, TPA-2, extracted roughly half of LHI-RC complexes from the intracytoplasmic membrane [35] (Fig. 3). The best solubilization efficiency (80%) was observed for TPA-8 with the t-butyl appendage on the phenyl ring (Figs. 3 & 4). Similarly, the introduction of other alkyl substituents onto the aromatic hydrocarbon at the para position leads to improved solubilization efficacy relative to TPA-2. In particular, TPA-6 and TPA-7 bearing a methylphenyl or an isopropylphenyl group, respectively, solubilized the protein in an amount comparable to that of DDM (~ 70%) (Fig. 4). Other hydrocarbon variants performed similarly or worse than TPA-2. For example, TPA-10 with two pentyl chains solubilized 50% of the protein complexes while TPA-11 with two hexyl chains solubilized only 40% of the superassemblies. TPA-9 bearing a biphenyl unit turned out to be minimally efficient (~10%). It is notable that LHI-RC complexes solubilized with branched diglucoside TPAs maintained the native structure during the protein solubilization phase (Fig 4). As a purification exercise, the solubilized proteins were subjected to immobilized metal affinity chromatography, utilizing the heptahistidine tag on the C-terminus of the M-subunit of the RC, and eluting with imidazole-containing buffers that included the respective detergents at 1xCMC (Table 1). The shape of the absorption spectrum of the protein purified in the presence of each ganglio-TPA was identical to that of respective TPA-solubilized protein (Figs. 3 & 4), as seen for DDM and TPA-2.
Figure 3.
The absorbance spectra of R. capsulatus superassembly during solubilization and purification in the best hydrophobic variant of the ganglio-TPAs (TPA-8), the lead ganglio-TPA (TPA-2), or two conventional detergents (DDM and LDAO) serving as positive or negative controls. (a) Resuspended membrane debris pelleted during ultracentrifugation, (b) solubilized supernatant from same procedure, and (c) IMAC-purified protein. Each detergent was used at 1.0 wt % for solubilization and at 1xCMC during IMAC steps. Data was collected on diluted samples (OD < 1.5), and spectra at the concentrations shown here were calculated using the dilution factor (normally 1:5 or 1:10).
Figure 4.
The spectra of R. capsulatus superassembly preparations during solubilization and purification in the presence of ganglio-TPAs with hydrophobic variants that were introduced in this study. (a) Resuspended membrane debris containing LHI-RC material which was not solubilized by detergent treatment during the experiments and was collected in the form of a pellet using ultracentrifugation. These signals are from complexes that remain embedded in the membrane and provide data complementary to that from complexes suspended by detergent micelles in the supernatant. Protein solubilization efficiencies in individual detergents were estimated based on the total amount of photosynthetic superassembly added to the solubilization reaction minus the amount still remaining in the homogenized pellets. (b) Solubilized supernatant from same procedure (c) IMAC-purified protein. Note data of TPA-2 and TPA-10 overlap in all three panels.
4. Discussion
4.1. Branched components and TPA design criteria
Nature has repeatedly invented rather simple and elegant chemical structures by which biological machinery is differentiated and stabilized. As one such example, branched oligosaccharides are ubiquitous in eukaryotes and many archae and are commonly located on the extracellular surfaces of cells or on the periphery of organelles [44–46]. Primarily serving as specific determinants in cellular recognition and communication, they are also well known to participate in cellular differentiation, to influence receptor function, and to defend against external invasion. These oligosaccharide structures are stabilizing, modulate protein activity, promote folding and conformational rearrangements, and, somewhat controversially, promote the concentration of specific lipids into rafts [47–49]. When connected to lipid – as in the gangliosides that make up a large fraction of the membranes of nerve cells – they enhance the bilayer permeability of these molecules and can be readily taken up and mobilized throughout the cell [45,46]. The structure of the glycan extensions that are readily found as protein, lipid, or peptide extensions bear a stark resemblance to the hydrophilic entities that adorn some of the more stabilizing detergents found in recent introductions [35] and in our current molecule sets. Although their existence and structural diversity was not a driving force in the design of the molecules in this study, the similarities and wealth of knowledge known about these branched oligosaccharides may serve as a rich database of chemical substituents that can be explored and/or exploited in the future design of amphipathic agents used for membrane protein solubilization and stabilization.
Detergents with branched hydrophilic designs have only begun to be explored as useful tools for preparation of membrane protein samples. Recently, we reported several tripod amphiphiles with hydrophilic group variations, including three commercially available examples (TPA-0, TPA-2 and TPA-2-S; Fig. 1) [35]. TPA-0 with an N-oxide headgroup was very powerful in solubilizing membrane proteins, but turned out to be too harsh to preserve the quaternary structures of a large, multi-subunit membrane protein assembly. On the other hand, two ganglio-TPAs (TPA-2 and TPA-2-S) were shown to be mild enough to retain such subtle structures. These amphiphiles are rather easy to prepare with TPA-2 having an advantage over TPA-2-S because an aromatic hydrocarbon rather than a cyclohexane ring can be more conveniently introduced into the congested region that is inevitably involved in producing a quaternary carbon in a TPA architecture.
Detergents with branched lipophilic components have been more extensively evaluated and have proven extremely effective for membrane protein research. For instance, tripod amphiphiles (TPAs) including the current set and neopentyl glycol (NG) class amphiphiles [50,51] have generally displayed favorable behaviors toward membrane protein solubilization in addition to stabilization. Use of some of these agents has produced high-quality crystals of various membrane proteins, providing the structures solved to near atomic resolution [34,36,52–61]. It is particularly noteworthy that a MNG agent has been used to produce the crystal structures of several GPCRs, including GPCR:G protein complexes, and a GNG agent has facilitated the determination of the crystal structure of a sodium-pumping pyrophosphatase [52–61]. These outcomes using novel agents indicate that a detergent with favorable properties in both membrane protein solubilization and stabilization can be suited for generation of a well-diffracting protein crystal. A similar trend can be found in the behaviors of conventional detergents, as exemplified by OG (n-octyl-β-D-glucopyranoside), DDM and LDAO (lauryldimethylamine-N-oxide) [51,62]– although these conventional agents with no branched lipophilic group were generally less stabilizing the native conformation of membrane proteins relative to well-behaving TPA and NG class members.
On the basis of such a hypothesis, it was reasoned that membrane protein solubilization efficiency, in addition to stabilization efficacy, could be a detergent property crucial for the success in crystal-structure determination of membrane proteins. Most detergent research has focused on the latter with little attention to the former, which may explain slow progress in the development of novel agents useful for membrane protein crystallization. Thus, it would be beneficial to develop novel detergents that display promising behaviors in both aspects - solubilization efficiency and stabilization efficacy - towards a large, diverse set of membrane proteins. As a strongly-solubilizing detergent is generally unfavorable for protein stabilization, it is a challenging design task to incorporate such conflicting attributes into novel amphiphiles. Our new datasets suggested that one effective way to achieve this goal is to emphasize the branched lipophilic groups in detergent architectures, which are the prominent features of the current and previously-described TPAs [33,35].
4.2. Effects of hydrophobic chain length on membrane protein solubilization with ganglio-TPAs
Membrane protein solubilization efficiency is determined by the combination of a number of detergent characteristics such as the hydrophobicity of the lipophilic group, the properties of head groups, and the overall geometry of the molecules. Within a set of detergents sharing the same hydrophilic group but varying in the structure and size of the hydrophobic group, the importance of the alkyl chain length – a determinant of detergent hydrophobicity – can be isolated and examined. The properties of these chains are crucial as they directly interact with the hydrophobic surfaces of membrane proteins and determine how strongly detergent molecules bind to them. Accordingly, a conventional detergent with a long carbon chain (e.g., DDM) solubilizes membrane proteins more efficiently than does a detergent with short carbon chain (e.g., n-decyl–β-D-maltoside). This expected trend was observed for the largest, structurally-related set of ganglio-TPAs in this study; the solubilized levels of the RC superassembly increased with alkyl chain length for those ganglio-TPAs with an alkyl pendant on the aromatic hydrocarbon (e.g., TPA-2, TPA-6, TPA-7 and TPA-8), with TPA-2 lowest (~50%) and TPA-8 highest (~80%). However, the opposite was found for a separate set of TPAs with non-aromatic chain extensions (e.g., TPA-2, TPA-10 and TPA-11). In this case, increases in alkyl chain length did not improve solubilization efficiency. This latter set demonstrates the interplay between lipophilic group properties (e.g., structure versus absolute carbon number versus balance in length of branches) that influence membrane protein solubilization, although it is clear that chain length is – in general – a key determinant.
Within the limits imposed by the current molecule and data sets, it is impossible to determine the exact origin of the departure from generality observed in the TPA-2, TPA-10, and TPA-11 comparison set, but the match/mismatch in chain length among lipophilic groups may be responsible. In TPA-2, neither of the two butyl chains is longer than the phenyl group and thus, the extension of those alkyl chains in TPA-10 and TPA-11 will exaggerate the chain length differences. These differences were mitigated by the addition of short alkyl groups onto phenyl ring of TPA-2, as was the case with TPA-6 through TPA-8. We hypothesize that such chain-length matching among three lipophilic groups enables those chains to interact simultaneously with the alkyl groups on the hydrophobic surfaces of the superassembly, leading to the increased protein solubilization. The biphenyl group in TPA-9, in this context, appears to be too long relative to its two other butyl-chain partners to promote such multiple interactions. In this specific case, the larger polarity of the aromatic group relative to the alkyl chains has the added potential to impact negatively the overall solubilization propensity. It is noteworthy that simultaneous, multiple-branch interactions can be only attained with amphiphiles bearing multiple alkyl chains (e.g., TPAs) and are impossible to observe for conventional detergents with single hydrophobic groups such as OG, DDM and LDAO. The favorable effect of hydrophobic groups with multiple alkyl chains increasing membrane protein solubilization relative to hydrophobic groups with a single alkyl chain was also observed in a previous TPA study exploring variations in the size and properties of branched hydrophilic headgroups ([35]; Fig S2). Based on these combined observations, protein solubilization efficiency in the case of non-conventional detergents with multiple alkyl chains appears to depend pivotally on the matching of properties amongst the branched chains.
4.3. Delineation of detergent properties that dictate membrane protein solubilization versus stabilization
Despite large variation in the hydrophobic groups, the current TPA results (as well as those from previous studies) show that all TPAs with a branched diglucoside headgroup (TPA-2, TPA-2-S and several new TPA-2 variants of this study) extracted the LHI-RC complexes and allowed for facile purification without structural degradation (Figs. 3& 4). Similar stabilization was also observed for other ganglio-TPAs sharing the same headgroup (unpublished results). These studies on hydrophobic variants suggest that ganglio-TPAs with branched di-glucose headgroups may represent a favorable ‘slot’ in detergent space (Fig. 5). In this schematic, alteration of the hydrophilicity of the headgroup are depicted on the in-plane (x) axis, systematic variation of the hydrophobicity of the lipophilic segments are displayed on the out-of-plane (y) axis, and the degree to which the amphiphile and its micelles are effective for both solubilization and stabilization are displayed on the vertical (z) axis (the latter with lower values more effective). Amphiphiles with di-glucose headgroups and a wide range of branched hydrophobic groups represent a set of effective molecules (likely among many) that appear useful for membrane protein manipulation. Many other such favorable slots are expected exist in detergent-structure-parameter space, but it is difficult to generate synthetically a related set of molecules with controlled variation in a limited number of parameters. Thus, this set is one of the first of its kind where a large number of the amphiphile variants generated proved effective.
Figure 5.
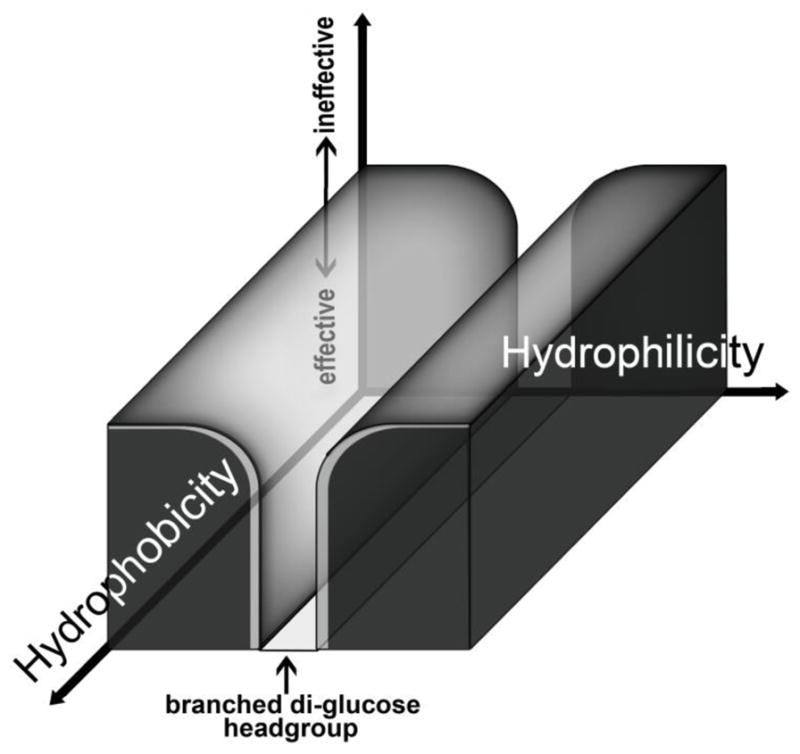
Results accumulated for variations of TPAs with branched hydrophilic and hydrophobic domains has led to a collection of molecules (TPA-2, TPA-2-S, TPA-6, TPA-7, TPA-8, TPA-10 and TPA-11) with favorable solubilization and stabilization efficacies. These promising TPAs share the branched diglucoside headgroup with large variations in the hydrophobic group. Thus, detergents with branched, di-glucose hydrophilic units appear to represent optimized tools in the search for amphipathic agents with increased utility in experiments which manipulate membrane proteins. Variants on this theme with branched hydrophobic appendages represent a ‘slot’ of effectiveness in detergent space that was surveyed in this study.
These collective results suggest that the hydrophobic group has minimal influence on the stabilization of these membrane protein complexes. Such favorable stabilizing effects of the branched diglucoside headgroup with minimal impact of the hydrophobic group variations can be observed in the preserved quality of the spectra, especially relative to those resulting from solubilization and purification with LDAO, in Figs. 4b and 4c. In contrast, the hydrophilic group variations of simpler TPAs have triggered substantial change in protein stability [34]. TPA-0 with an N-oxide headgroup destroyed most of the protein complexes whereas TPA-4 with a single, non-branched maltoside headgroup gave substantial loss in protein integrity [35]; other N-oxide or maltose-containing TPAs showed similar behaviors (unpublished results). Theoretically, detergent solubilization and stabilization efficacy should be determined by the interplay between the hydrophobic and hydrophilic group of the agents. However, based on data for the previously-reported TPAs and the current TPAs, protein solubilization efficiency appears to be determined mainly by the hydrophobic group of detergents whereas protein stabilization is effectively tuned by the properties of the hydrophilic headgroup. Although more extensive studies – with other membrane protein systems and with expanded classes of amphiphiles – are needed to prove the generality of such a result, this clear-cut delineation in our data is enough to serve as a testable hypothesis and will significantly impact membrane protein research by serving as a structure-property-relationship guideline for use in the design of novel classes of detergents for membrane protein research.
4.4. Branched ganglio-tripod amphiphile properties that may promote membrane protein crystallization
Small PDC-forming detergents such as LDAO and OG are often used favorably for the crystallization of membrane proteins, presumably because of the formation of PDCs with the large hydrophilic surface that promote crystal lattice formation [62,63]. The short head and/or tail groups of these agents could be responsible for such favorable experimental behavior. These detergents, however, have rather strong tendencies to destabilize membrane proteins relative to DDM which contains extended head and tail groups. Since small PDC-forming detergents are not generally favorable in membrane protein stabilization, it is noteworthy that designing novel agents that balance both characteristics is very challenging. At this time, it is not clear whether or not ganglio-TPAs form small PDCs relative to those formed with DDM. However, our previous study may shed light on the topic [37]. We recently reported the crystal structures of several N-oxide TPAs and found that these amphiphiles form thinner nonpolar layers than do conventional detergents in their crystalline lattice [37]. The means by which these TPAs arrange themselves therein show how the hydrophobic surface displayed by one TPA layer can accommodate that of an adjacent TPA layer. These compact, crystal-packing arrangements provide insights into the ways in which these agents might prefer to assemble around hydrophobic surfaces displayed by membrane proteins in solution. This idea is strengthened by the knowledge that glucose-containing detergents tend to form smaller PDCs relative to maltose-containing detergents [51]. Therefore, the current ganglio-TPAs, with short alkyl-chain-based lipophilic groups and a short branched diglucoside headgroup, could form small PDCs and hold potential as useful tools for membrane protein crystallogenesis experiments.
5. Conclusions
The modular scaffold of the unique class of amphipathic compounds discussed here allowed for efficient synthesis of variants that could be screened for potential utility in membrane protein studies. Previous work focused on hydrophilic variations of a promising branched hydrophobic lead structure whereas the current study analyzes hydrophobic variations of the most promising hydrophilic headgroup that emerged. The current study shows that ganglio-tripod amphiphiles with a branched diglucoside headgroup tend to display a balance of detergent properties that make them useful for membrane protein stabilization. These observations lend strength to the previous conclusion that identified the synergistic benefit of placing branching points in both the hydrophilic and lipophilic regions. The variations of the lipophilic region resulted in improved solubilization efficiency to the point where overall properties are now superior to the conventional detergent DDM, used widely today [43]. The current data on hydrophobic variants suggest that ganglio-TPAs with branched di-glucose headgroups may represent a favorable ‘slot’ (among likely many) in detergent space where a wide range of effective molecules can be generated and proved useful for membrane protein manipulation). The utility of ganglio-TPAs, especially TPA-8 of this study, is enhanced by their convenient routes of synthesis and their relatively rigid conformations. The latter feature may lead to success in membrane protein crystallization experiments. These agents are promising alternatives to conventional detergents. More significantly, the general detergent structure-property relationships that have been uncovered by systematic analysis of a set of related TPAs have identified design principles dictating detergent efficacy on membrane protein solubilization and stabilization. These principles will contribute to future amphiphile development efforts, which should enable further advances in membrane protein science.
Supplementary Material
Highlights.
This study introduces hydrophobic variations to branched glyco-tripod amphiphiles.
New agents solubilize membrane proteins effectively from a lipid bilayer.
New agents stabilize the native structure of a membrane protein in an aqueous environment.
New agents mimic flexibility and molecular architectures found naturally in gangliosides and glycosylated proteins.
An important detergent structure-property relationship was uncovered that will guide synthesis efforts in the future.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIP) (grant number 2008-0061891 and 2012R1A1A1040964 to P.S.C., K.H.C., H.E.B.) and NIH grant P01 GM75913 (S.H.G., P.D.L., M.J.W.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Pil Seok Chae, Email: pchae@hanyang.ac.kr.
Samuel H. Gellman, Email: gellman@chem.wisc.edu.
Philip D. Laible, Email: laible@anl.gov.
References
- 1.Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean, and eukaryotic organisms. Protein Sci. 1998;7:1029–1038. doi: 10.1002/pro.5560070420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 3.Overington JP, Al-Lazikani B, Hopkins AL. Opinion-How many drug targets are there? Nat Rev Drug Discovery. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 4.http://blanco.biomol.uci.edu/Membrane_Proteins_xtal.html
- 5.Junge F, Schneider B, Reckel S, Schwarz D, Dotsch V, Bernhard F. Large-scale production of functional membrane proteins. Cell Mol Life Sci. 2008;65:1729–1755. doi: 10.1007/s00018-008-8067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Midgett CR, Madeen DR. Breaking the bottleneck: Eukaryotic membrane protein expression for high-resolution structural studies. J Struct Biol. 2007;160:265–274. doi: 10.1016/j.jsb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Laible PD, Mielke DL, Hanson DK. Foreign gene expression in photosynthetic bacteria. In: Hunter CN, Daldal F, Thurnauer MC, Beatty JT, editors. The purple photosynthetic bacteria. Springer-Verlag; New York: 2009. pp. 839–860. [Google Scholar]
- 8.Lacapere JJ, Pebay-Peyroula E, Neumann JM, Etchebest C. Determining membrane protein structures: still a challenge! Trends Biochem Sci. 2007;32:259–270. doi: 10.1016/j.tibs.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Tanford C. The hydrophobic effect: Formation of micelles and biological membranes. John Wiley & Sons; New York: 1973. [Google Scholar]
- 10.White SH, Wimley WC. Membrane protein folding and stability: Physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. doi: 10.1146/annurev.biophys.28.1.319. [DOI] [PubMed] [Google Scholar]
- 11.Moller JV, le Maire M. Detergent binding as a measure of hydrophobic surface area of integral membrane proteins. J Biol Chem. 1993;268:18659–18672. [PubMed] [Google Scholar]
- 12.Garavito RM, Ferguson-Miller S. Detergents as tools in membrane biochemistry. J Biol Chem. 2001;276:32403–32406. doi: 10.1074/jbc.R100031200. [DOI] [PubMed] [Google Scholar]
- 13.Bowie JU. Stabilizing membrane proteins. Curr Opin Struct Biol. 2001;11:397–402. doi: 10.1016/s0959-440x(00)00223-2. [DOI] [PubMed] [Google Scholar]
- 14.Serrano-Vega MJ, Magnani F, Shibata Y, Tate CG. Conformational thermostabilization of the β1-adrenergic receptor in a detergent-resistant form. Proc Natl Acad Sci U S A. 2008;105:877–882. doi: 10.1073/pnas.0711253105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newstead S, Ferrandon S, Iwata S. Rationalizing alpha-helical membrane protein crystallization. Protein Sci. 2008;17:466–472. doi: 10.1110/ps.073263108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newstead S, Hobbs J, Jordan D, Carpenter EP, Iwata S. Insights into outer membrane protein crystallization. Mol Membr Biol. 2008;25:631–638. doi: 10.1080/09687680802526574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tribet C, Audebert R, Popot JL. Amphipols: Polymers that keep membrane proteins soluble in aqueous solutions. Proc Natl Acad Sci U S A. 1996;93:15047–15050. doi: 10.1073/pnas.93.26.15047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popot JL, Althoff T, Bagnard D, Baneres JL, Bazzacco P, Billon-Denis E, Catoire LJ, Champeil P, Charvolin D, Cocco MJ, Cremel G, Dahmane T, de la Maza LM, Ebel C, Gabel F, Giusti F, Gohon Y, Goormaghtigh E, Guittet E, Kleinschmidt JH, Kuhlbrandt W, Le Bon C, Martinez KL, Picard M, Pucci B, Sachs JN, Tribet C, van Heijenoort C, Wien F, Zito F, Zoonens M. Amphipols from A to Z. Annu Rev Biophys. 2011;40:379–408. doi: 10.1146/annurev-biophys-042910-155219. [DOI] [PubMed] [Google Scholar]
- 19.Nath A, Atkins WM, Sligar SG. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry. 2007;46:2059–2069. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- 20.Ritchie TK, Grinkova YV, Bayburt TH, Denisov IG, Zolnerciks JK, Atkins WM, Sligar SG. Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–231. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayburt TH, Sligar SG. Membrane protein assembly into nanodiscs. FEBS Lett. 2010;584:1721–1727. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orwick-Rydmark M, Lovett JE, Graziadei A, Lindholm L, Hicks MR, Watts A. Detergent-free incorporation of a seven-transmembrane receptor protein into nanosized bilayer lipodisq particles for functional and biophysical studies. Nano Lett. 2012;12:4687–4692. doi: 10.1021/nl3020395. [DOI] [PubMed] [Google Scholar]
- 23.Chae PS, Gotfryd K, Pacyna J, Miercke LJW, Rasmussen SGF, Robbins RA, Rana RR, Loland CJ, Kobilka B, Stroud R, Byrne B, Gether U, Gellman SH. Tandem facial amphiphiles for membrane protein stabilization. J Am Chem Soc. 2010;132:16750–16752. doi: 10.1021/ja1072959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breyton C, Chabaud E, Chaudier Y, Pucci B, Popot JL. Hemifluorinated surfactants: a non-dissociating environment for handling membrane proteins in aqueous solutions? FEBS Lett. 2004;564:312–318. doi: 10.1016/S0014-5793(04)00227-3. [DOI] [PubMed] [Google Scholar]
- 25.Abla M, Durand G, Pucci B. Propyl ended hemifluorinated surfactants: synthesis and self-assembling properties. J Org Chem. 2011;76:2084–2093. doi: 10.1021/jo102245c. [DOI] [PubMed] [Google Scholar]
- 26.Cho KH, Byrne B, Chae PS. Hemifluorinated maltose-neopentyl glycol (HF-MNG) amphiphiles for membrane protein stabilisation. ChemBioChem. 2013;14:452–455. doi: 10.1002/cbic.201200759. [DOI] [PubMed] [Google Scholar]
- 27.McGregor CL, Chen L, Pomroy NC, Hwang P, Go S, Chakrabartty A, Privé GG. Lipopeptide detergents designed for the structural study of membrane proteins. Nat Biotechnol. 2003;21:171–176. doi: 10.1038/nbt776. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Nagai Y, Reeves PJ, Kiley P, Khorana HG, Zhang S. Designer short peptide surfactants stabilize G protein-coupled receptor bovine rhodopsin. Proc Natl Acad Sci U S A. 2006;103:17707–17712. doi: 10.1073/pnas.0607167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SC, Bennett BC, Hong WX, Fu Y, Baker KA, Marcoux J, Robinson CV, Ward AB, Halpert JR, Stevens RC, Stout CD, Yeager MJ, Zhang Q. Steroid-based facial amphiphiles for stabilization and crystallization of membrane proteins. Proc Natl Acad Sci U S A. 2013;110:E1203–1211. doi: 10.1073/pnas.1221442110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell SC, Mittal R, Huang L, Travis B, Breyer RM, Sanders CR. CHOBIMALT: A cholesterol-based detergent. Biochemistry. 2010;49:9572–9583. doi: 10.1021/bi101334j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hovers J, Potschies M, Polidori A, Pucci B, Raynal S, Bonneté F, Serrano-Vega MJ, Tate CG, Picot D, Pierre Y, Popot J-L, Nehmé R, Bidet M, Mus-Veteau I, Busskamp H, Jung KH, Marx A, Timmins PA, Welte W. A class of mild surfactants that keep integral membrane proteins water-soluble for functional studies and crystallization. Mol Membr Biol. 2011;28:171–181. doi: 10.3109/09687688.2011.552440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chae PS, Rasmussen SGF, Rana RR, Gotfryd K, Kruse AC, Manglik A, Cho KH, Nurva S, Gether U, Guan L, Loland CJ, Byrne B, Kobilka BK, Gellman SH. A new class of amphiphiles bearing rigid hydrophobic groups for solubilization and stabilization of membrane proteins. Chem-Eur J. 2012;18:9485–9490. doi: 10.1002/chem.201200069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McQuade DT, Quinn MA, Yu SM, Polans AS, Krebs MP, Gellman SH. Rigid amphiphiles for membrane protein manipulation. Angew Chem Int Ed. 2000;39:758–761. [PubMed] [Google Scholar]
- 34.Theisen MJ, Potocky TB, McQuade DT, Gellman SH, Chiu ML. Crystallization of bacteriorhodopsin solubilized by a tripod amphiphile. Biochim Biophys Acta. 2005;1751:213–216. doi: 10.1016/j.bbapap.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Chae PS, Wander MJ, Bowling AP, Laible PD, Gellman SH. Glyco-tripod amphiphiles for solubilisation and stabilization of a membrane-protein superassembly: Importance of branching in the hydrophilic portion. ChemBioChem. 2008;9:1706–1709. doi: 10.1002/cbic.200800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chae PS, Laible PD, Gellman SH. Tripod amphiphiles for membrane protein manipulation. Mol BioSyst. 2010;6:89–94. doi: 10.1039/b915162c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chae PS, Guzei IA, Gellman SH. Crystallographic characterization of N-Oxide tripod amphiphiles. J Am Chem Soc. 2010;132:1953–1959. doi: 10.1021/ja9085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashton PR, Boyd SE, Brown CL, Jayaraman N, Nepogodiev SA, Stoddart JF. A convergent synthesis of carbohydrate-containing dendrimers. Chem-Eur J. 1996;2:1115–1128. [Google Scholar]
- 39.Schott H. Solubilization of a water-insoluble dye as a method for determining micellar molecular weights. J Phys Chem. 1966;70:2966–2973. [Google Scholar]
- 40.Chae PS, Wander MJ, Cho KH, Laible PD, Gellman SH. Carbohydrate-containing Triton X-100 analogues for membrane protein solubilization and stabilization. Mol BioSyst. 2013;9:626–629. doi: 10.1039/c3mb25584k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youvan DC, Ismail S, Bylina EJ. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38:19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- 42.Laible PD, Kirmaier C, Udawatte CS, Hofman SJ, Holten D, Hanson DK. Quinone reduction via secondary B-branch electron transfer in mutant bacterial reaction centers. Biochemistry. 2003;42:1718–1730. doi: 10.1021/bi026959b. [DOI] [PubMed] [Google Scholar]
- 43.Caffrey M, Li D, Dukkipati A. Membrane protein structure determination using crystallography and lipidic mesophases: recent advances and successes. Biochemistry. 2012;51:6266–6288. doi: 10.1021/bi300010w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghazarian H, Idoni B, Oppenheimer SB. A glycobiology review: Carbohydrates, lectins and implications in cancer therapeutics. Acta Histochem. 2011;113:236–247. doi: 10.1016/j.acthis.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varki A. Biological roles of oligosaccharides – all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gagneux P, Varki A. Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology. 1999;9:747–755. doi: 10.1093/glycob/9.8.747. [DOI] [PubMed] [Google Scholar]
- 47.Lingwood D, Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 48.Pike LJ. Lipid rafts: bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 49.Gallala HD, Sandhoff K. Principles of microdomain formation in biological membranes-Are there lipid liquid ordered domains in living cellular membranes? Trends Glycosci Glycotechnol. 2008;20:277–295. [Google Scholar]
- 50.Chae PS, Rasmussen SGF, Rana RR, Gotfryd K, Chandra R, Goren MA, Kruse AC, Nurva S, Loland CJ, Pierre Y, Drew D, Popot JL, Picot D, Fox BG, Guan L, Gether U, Byrne B, Kobilka BK, Gellman SH. Maltose-neopentyl glycol (MNG) amphiphiles for solubilization, stabilization and crystallization of membrane proteins. Nat Methods. 2010;7:1003–1008. doi: 10.1038/nmeth.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chae PS, Rana RR, Gotfryd K, Rasmussen SGF, Kruse AC, Cho KH, Capaldi S, Carlsson E, Kobilka BK, Loland CJ, Gether U, Banerjee S, Byrne B, Lee JK, Gellman SH. Glucose-neopentyl glycol (GNG) amphiphiles for membrane protein study. Chem Commun. 2013;49:2287–2289. doi: 10.1039/c2cc36844g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rasmussen SGF, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, DeVree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SGF, Choi HJ, DeVree BT, Sunahara RK, Chae PS, Gellman SH, Dror RO, Shaw DE, Weis WI, Caffrey M, Gmeiner P, Kobilka BK. Structure and function of an irreversible agonist-β2 adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rasmussen SGF, DeVree BT, Zou Y, Kruse AC, Chung KY, Kobilka TS, Thian FS, Chae PS, Pardon E, Calinski D, Mathiesen JM, Shah STA, Lyons JA, Caffrey M, Gellman SH, Steyaert J, Skiniotis G, Weis WI, Sunahara RK, Kobilka BK. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kruse AC, Hu J, Pan AC, Arlow DH, Rosenbaum DM, Rosemond E, Green HF, Liu T, Chae PS, Dror RO, Shaw DE, Weis WI, Wess J, Kobilka BK. Structure and dynamics of the M3 muscarinic acetylcholine receptor. Nature. 2012;482:552–556. doi: 10.1038/nature10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haga K, Kruse AC, Asada H, Kobayashi TY, Shiroishi M, Zhang C, Weis WI, Okada T, Kobilka BK, Haga T, Kobayashi T. Structure of the human M2 muscarinic acetylcholine receptor bound to an antagonist. Nature. 2012;482:547–551. doi: 10.1038/nature10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manglik A, Kruse AC, Kobilka TS, Thian FS, Mathiesen JM, Sunahara RK, Pardo L, Weis WI, Kobilka BK, Granier S. Crystal structure of the μ-opioid receptor bound to a morphinan antagonist. Nature. 2012;485:321–326. doi: 10.1038/nature10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Granier S, Manglik A, Kruse AC, Kobilka TS, Thian FS, Weis WI, Kobilka BK. Structure of the δ-opioid receptor bound to naltrindole. Nature. 2012;485:400–404. doi: 10.1038/nature11111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kellosalo J, Kajander T, Kogan K, Pokharel K, Goldman A. The structure and catalytic cycle of a sodium-pumping pyrophosphatase. Science. 2012;337:473–476. doi: 10.1126/science.1222505. [DOI] [PubMed] [Google Scholar]
- 60.White JF, Noinaj N, Shibata Y, Love J, Kloss B, Xu F, Gvozdenovic-Jeremic J, Shah P, Shiloach J, Tate CG, Grisshammer R. Structure of the agonist-bound neurotensin receptor. Nature. 2012;490:508–513. doi: 10.1038/nature11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rollauer SE, Tarry MJ, Graham JE, ääskeläinen MJ, äger FJ, Johnson S, Krehenbrink M, Liu SM, Lukey MJ, Marcoux J, McDowell MA, Rodriguez F, Roversi P, Stansfeld PJ, Robinson CV, Sansom MS, Palmer T, Högbom M, Berks BC, Lea SM. Structure of the TatC core of the twin-arginine protein transport system. Nature. 2012;492:210–214. doi: 10.1038/nature11683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Privé GG. Detergents for the stabilization and crystallization of membrane proteins. Methods. 2007;41:388–397. doi: 10.1016/j.ymeth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q, Tao H, Hong WX. New amphiphiles for membrane protein structural biology. Methods. 2011;55:318–323. doi: 10.1016/j.ymeth.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.