Abstract
The 41st family of solute carriers (SLC41) comprises three members A1, A2 and A3, which are distantly homologous to bacterial Mg2+ channel MgtE. SLC41A1 was recently characterized as being an Na+/Mg2+ exchanger (NME; a predominant cellular Mg2+ efflux system). Little is known about the exact function of SLC41A2 and SLC41A3, although, these proteins have also been linked to Mg2+ transport in human (animal) cells. The molecular biology (including membrane topology, cellular localization, transcriptomics and proteomics) of SLC41A2 and SLC41A3 compared with SLC41A1 has only been poorly explored. Significantly more data with regard to function, functional regulation, involvement in cellular signalling, complex-forming ability, spectrum of binding partners and involvement in the pathophysiology of human diseases are available for SLC41A1. Three recent observations namely the identification of the null mutation, c.698G>T, in SLC41A1 underlying the nephronophthisis-like phenotype, the recognition of a putative link between SLC41A1 and Parkinson’s disease, and the observation that nearly 55% of preeclamptic placental samples overexpress SLC41A1, marks the protein as a possible therapeutic target of these diseases. A potential role of the SLC41 family of Mg2+ transporters in the pathophysiology of human diseases is further substantiated by the finding that SLC41A3 knockout mice develop abnormal locomotor coordination.
Introduction
The importance of magnesium (Mg) for normal cellular, tissue, organ, and body physiology has been described in many original papers and reviews. However, the molecular entities responsible for Mg2+-transport in higher eukaryotes and their intracellular distribution have started to be identified only recently. During the last decade, several genes have been shown to encode for putative or confirmed Mg2+ transport systems (homeostatic factors) in humans, mammals and other higher eukaryotes (Tab. 1). In view of the large spectrum of processes that involve Mg, this count is likely not final.
Table 1.
| Mg2+ transporter | Gene | Function | Localization | Reference |
|---|---|---|---|---|
| SLC41A1 | NU | Na+/Mg2+ exchanger (primarily Mg2+ efflux system) | CM | [5, 9, 11, 47] |
| SLC41A2 | NU | putative Mg2+ carrier Mg2+ transport mechanism with channel like properties |
CM?, IMC? | [7, 10, 13] |
| SLC41A3 | NU | putative Mg2+ carrier Mg2+ transport mechanism with channel like properties |
CM? IMC? | [8] |
| NIPA1 | NU | Mg2+ transport mechanism with channel like properties | CM | [a] |
| NIPA2 | NU | Mg2+ transport mechanism with channel like properties | CM | [b] |
| MagT1 | NU | putative Mg2+ channel | CM | [c] |
| N33/TUSC3 | NU | putative function in cellular Mg2+- homeostasis | CM | [c] |
| TRPM6 | NU | chanzyme/cation channel | CM | [d] |
| TRPM7 | NU | chanzyme/cation channel | CM | [e] |
| MRS2 | NU | Mg2+ channel | IMM | [28] |
| HIP14 | NU | Mg2+ transport mechanism with chanzyme like properties | GA, | [f] |
| HIP14L | NU | Mg2+ transport mechanism with chanzyme like properties | GA, | [f] |
| MMgT1 | NU | Mg2+ transport mechanism putative carrier | GA, PGV | [g] |
| MMgT2 | NU | Mg2+ transport mechanism with putative carrier | GA, PGV | [g] |
| CNNM2/ACDP2 | NU | putative Mg2+ or cellular Mg2+ homeostatic factor | CM, IMC, N | [h,i,j] |
Abbreviations: NU = nuclear, CM = cytoplasmic membrane, IMC = intracellular membrane compartments, IMM = inner mitochondrial membrane, GA = Golgi apparatus, PGV = post-Golgi vesicles, ? = possible, putative.
References indicated with a number are included in the main references list. Following references are specific to this table and they were not used elsewhere:
Goytain A, Hines RM, El-Husseini A, et al. NIPA1(SPG6), the basis for autosomal dominant form of hereditary spastic paraplegia, encodes a functional Mg2+ transporter. Journal of Biological Chemistry 2007, 282(11):8060–8;
Goytain A, Hines RM, Quamme GA. Functional characterization of NIPA2, a selective Mg2+ transporter. American Journal of Physiology Cell Physiology 2008, 295(4):C944-53;
Zhou H, Clapham DE. Mammalian MagT1 and TUSC3 are required for cellular magnesium uptake and vertebrate embryonic development. Proceedings of the National Academy of Sciences USA. 2009, 106(37):15750–5;
Schlingmann KP, Weber S, Peters M, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nature Genetics 2002, 31(2):166–70;
Runnels LW, Yue L, Clapham DE. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291(5506):1043–7;
Goytain A, Hines RM, Quamme GA. Huntingtin-interacting proteins, HIP14 and HIP14L, mediate dual functions, palmitoyl acyltransferase and Mg2+ transport. Journal of Biological Chemistry 2008, 283(48):33365–74;
Goytain A, Quamme GA. Identification and characterization of a novel family of membrane magnesium transporters, MMgT1 and MMgT2. American Journal of Physiology Cell Physiology 2008, 294(2):C495–502;
Wang CY, Shi JD, Yang P, et al. Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 2003, 306:37–44;
Sponder G, Svidova S, Schweigel M, et al. Splice-variant 1 of the ancient domain protein 2 (ACDP2) complements the magnesium-deficient growth phenotype of Salmonella enterica sv. typhimurium strain MM281. Magnesium Research 2010, 23(2):105–14;
de Baaij JH, Stuiver M, Meij IC, et al. Membrane topology and intracellular processing of cyclin M2 (CNNM2). Journal of Biological Chemistry 2012, 287(17):13644–55.
An exciting journey aimed at identify genes encoding for Mg2+ transporter molecules was begun in 1976 with the pioneering work of Park and colleagues who identified corA, corB and mgt mutants affecting Mg2+-transport in E. coli [1]. In 1986 the group around M. E. Maguire cloned a predominant bacterial Mg2+-transport system CorA from Salmonella typhimurium [2]. Shortly afterwards this was followed by the cloning of Mg2+-regulated MgtA and MgtB P-type ATPases (both from S. typhimurium) [3] and of MgtE, an Mg2+-channel with limited phylogenetic distribution (cloned from Prowidencia stuartii) [4]. In 2003, Wabbaken and colleagues cloned a human homologue of MgtE named solute carrier family 41 member A1 (SLC41A1; in the following, only given as A1) [5]. Goytain and Quamme demonstrated that A1 and also SLC41A2 and SLC41A3 (both also distantly homologous with MgtE; both cloned from Mus musculus; in the following, only given as A2 and A3) from the same protein family were able to conduct the electrogenic transport of Mg2+ when heterologously expressed in Xenopus laevis oocytes [6, 7, 8]. The A1 or A2-dependent electrogenic transport of Mg2+ seen in Xenopus oocytes was not observed in mammalian or avian cellular systems transfected with human A1 or A2 [9, 10]. The molecular biology and physiology of A3 remains unexplored. In regards to A2, only limited knowledge to its exact function(s) and mode of operation is currently available. However, A1, despite initial thoughts that it might represent an ion channel mechanism, has now been shown to function as an Na+/Mg2+ exchanger (NME), at least in human and mammalian cells [11]. The discovery of A1 being an NME has physically bridged a large pool of knowledge concerning the physiology and pathophysiology of Na+/Mg2+ exchange (a mechanism known for three decades as being extant) with an opportunity to perform a molecular examination of NME suspected to be directly or indirectly involved in a plethora of ailments of mankind.
Transcriptomics, Cellular Localization, Topology and Complex-Forming Abilities of SLC41A1, A2 and A3
Transcripts of the human genes SLC41A1 (1q31-32), SLC41A2 (12q23.3) and SLC41A3 (3q21.2) have been identified in various organs and tissues. The A1 transcript is abundantly expressed in heart, in testis and also in the adrenal and thyroid glands, in prostate and in ovaries whereas lower levels have been detected in all other tested tissues. To date it has also been detected in all tested cell lines such as HEK-293, Tom-1, BV173, Reh, Jurkat and JVM-13 [5, 9, 11, Kolisek et al., unpublished]. Therefore, we can assume that member A1 is ubiquitously expressed in human cells. Romanuik and colleagues [12] have identified A1-expression as being responsive to androgens. No information is available about the expression of human A2 and A3 across human tissues and organs in peer-reviewed bibliography. However, some information can be extracted from www.proteinatlas.org.
All three members of the SLC41 family (human A1, 513 aa, 56 kDa; human A2, 573 aa, 62.3 kDa; human A3, 500 aa, 54.6 kDa) were predicted and also experimentally confirmed by functional studies as being proteins integral to the cytoplasmic membrane (A1 and A2 in both human and mouse models, A3 only in the mouse model) [6, 7, 8, 9, 10, 11]. However, Sahni and Scharenberg in their recent review [13] advocate the targeting of A2 into membranes of intracellular compartments.
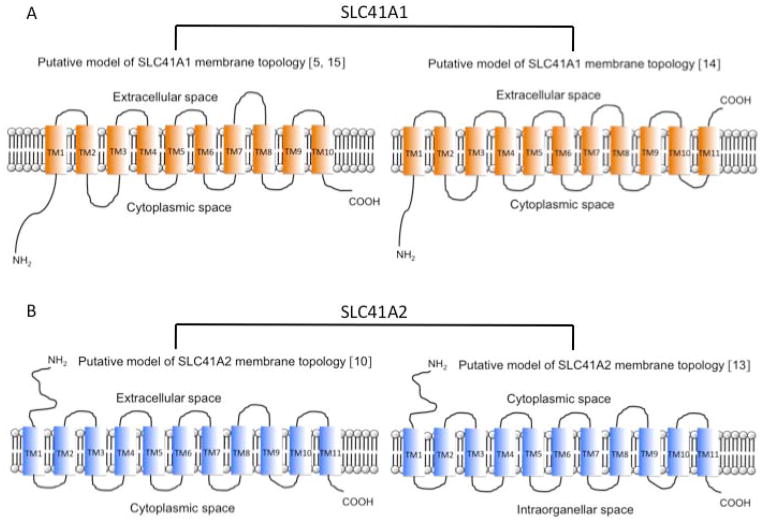
The membrane topology of A1 is controversial. Initially, the ten transmembrane helices (TM) “inside–in” model of A1 topology was predicted (Fig. 1A, Tab. 2) [5]. Results of the computer-predictions of A1 topology are summarized in table 2. The results of independent studies have lead to a consensus concerning the intracellular orientation of the N-terminus of member A1 [9, 11, 14]. However, data regarding the C-terminus orientation are contradictory. Based on their epitope-tagging studies, the group of Mandt [14] has proposed a model possessing eleven TM and a C-terminus oriented extracellularly (Fig. 1A). In contrast, Sponder and colleagues [15], by utilizing the split-ubiquitin functional assay in yeast, demonstrate that “A1 C-terminally tagged with a Cub-LexA-VP16 reporter cassette is targeted to cytoplasmic membrane and that the C-terminus is oriented such that it allows the reconstitution of functional ubiquitin and is, therefore, intracellular”. It conforms with the study of Nestler and colleagues [16], claiming that the bait vector pBT3-STE, carrying the Cub-LexA-VP16 moiety fused to the C-terminus of A1, is suitable for split-ubiquitin yeast two hybrid assay (SU-YTH) with SLC41A1 serving as the bait (intracellular/intracytosolic orientation of the reporter moiety is the precondition for performing SU-YTH assay). Thus, the data of Sponder and colleagues [15] support the initially proposed ten TM “inside-in” model (Fig. 1A). Only further research, e.g. showing that A1 C-terminally tagged with Cub-LexA-VP16 is functional, will shed more light on the exact organization of A1 topology.
Figure 1.
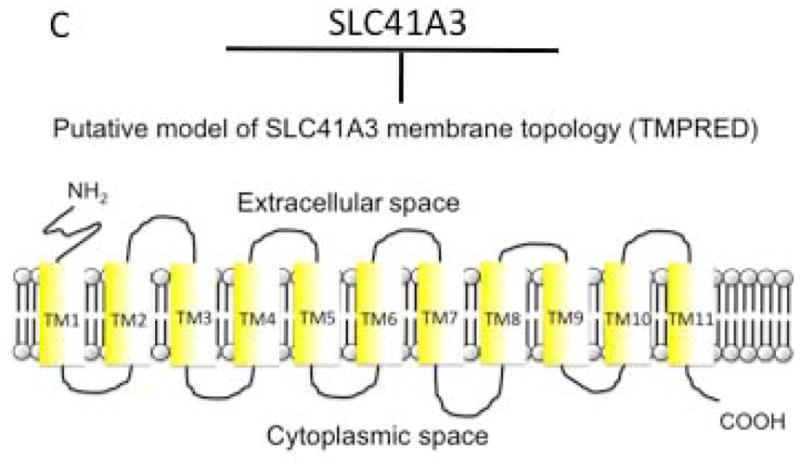
Models of membrane topology of SLC41A1 (A) and SLC41A2 (B) constructed according to available peer-reviewed bibliography. Plot C depicts a computer predicted-model (TMPRED) of SLC41A3 topology.
Table 2.
Predictions of SLC41A1 topology generated with various prediction software.
| 10 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | TM9 | TM10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | i-o | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | o-i | C- | |
| TMPred1 | in | 96 123 |
185 205 |
225 242 |
261 279 |
286 306 |
319 337 |
348 364 |
411 427 |
439 463 |
483 503 |
in |
| TopPred II2 | in | 100 120 |
185 205 |
222 242 |
259 279 |
286 306 |
318 338 |
344 364 |
411 431 |
435 455 |
486 506 |
in |
| PSORT II3 | in | 107 123 |
128 144 |
188 204 |
225 241 |
257 273 |
290 306 |
322 338 |
411 427 |
439 455 |
488 504 |
in |
| MEMSAT SVM4,5,6 | in | 98 121 |
135 150 |
181 202 |
214 241 |
258 277 |
286 306 |
315 338 |
407 431 |
436 466 |
481 502 |
in |
| TOP- CONS7 | in | 100 120 |
140 160 |
181 201 |
222 242 |
261 281 |
285 305 |
317 337 |
409 429 |
443 463 |
484 504 |
in |
| SCAMPI- seq8 | in | 102 122 |
129 149 |
185 205 |
222 242 |
261 281 |
285 305 |
317 337 |
407 427 |
437 457 |
484 504 |
in |
| 10 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | TM9 | TM10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | - | - | - | - | - | - | - | - | - | - | C- | |
| DAS c.off 2.29 | - | 100 115 |
136 143 |
189 201 |
221 241 |
264 273 |
289 304 |
325 332 |
411 425 |
437 460 |
489 502 |
- |
| Split 4.010 | - | 96 125 |
178 206 |
212 242 |
254 278 |
286 305 |
320 336 |
346 368 |
411 430 |
434 465 |
479 501 |
- |
| 10 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | TM9 | TM10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | C- | |
| TMHMM11 | out | 98 120 |
182 204 |
219 241 |
257 279 |
284 306 |
315 337 |
347 366 |
411 428 |
438 460 |
481 503 |
out |
| 9 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | TM9 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | o-i | C- | ||
| Predict Protein12 | out | 101 118 |
183 200 |
220 238 |
261 278 |
287 304 |
319 336 |
412 429 |
442 459 |
484 501 |
in | |
| Phobius13 | out | 98 123 |
180 204 |
210 241 |
253 274 |
286 305 |
317 337 |
409 427 |
439 459 |
479 504 |
in | |
| OCTO- PUS14 | out | 103 123 |
181 201 |
222 242 |
261 281 |
285 305 |
320 340 |
409 429 |
443 463 |
484 504 |
in | |
| SCAMPI- msa15 | out | 103 123 |
181 201 |
222 242 |
261 281 |
285 305 |
320 340 |
409 429 |
443 463 |
484 504 |
in |
| 8 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | C- | |||
| MEMSAT35 | out | 100 121 |
179 203 |
217 241 |
251 274 |
286 309 |
409 432 |
443 467 |
478 500 |
out |
| 11 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | TM9 | TM10 | TM11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | i-o | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | o-i | i-o | C- | |
| HMMTOP 2.016 | in | 96 120 |
135 154 |
185 204 |
217 241 |
254 273 |
286 305 |
318 337 |
346 365 |
411 430 |
439 458 |
479 498 |
out |
| PRO17 | in | 100 120 |
141 161 |
182 202 |
223 243 |
253 273 |
285 305 |
315 335 |
346 366 |
409 429 |
440 460 |
481 501 |
out |
| PRO-DIV17 | in | 99 119 |
140 160 |
181 201 |
222 242 |
254 274 |
286 306 |
316 336 |
349 369 |
407 427 |
440 460 |
481 501 |
out |
| 11 TM helices | TM1 | TM2 | TM3 | TM4 | TM5 | TM6 | TM7 | TM8 | TM9 | TM10 | TM11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N- | - | - | - | - | - | - | - | - | - | - | - | C- | |
| SOSUI18 | - | 99 121 |
128 150 |
180 202 |
219 241 |
256 278 |
285 306 |
317 339 |
346 368 |
408 430 |
437 459 |
481 503 |
- |
The predictions were generated according respective algorithms desribed in:
Hofmann K, Stoffel W. TMbase - A database of membrane spanning proteins segments. Biol. Chem. Hoppe-Seyler. 1993, 374:166;
von Heijne G. Membrane Protein Structure Prediction, Hydrophobicity Analysis and the Positive-inside Rule. J. Mol. Biol. 1992, 225:487–94;
Mitsuteru C, Nakao CM, Nakai K. Improvement of PSORT II Protein Sorting Prediction for Mammalian Proteins. Genome Informatics. 2002, 13:441–2;
Nugent T, Jones DT. Transmembrane protein topology prediction using support vector machines. BMC Bioinformatics. 2009, 10:159;
Jones DT. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics. 2007, 23:538–44;
Jones DT, Taylor WR, Thornton JM. A Model Recognition Approach to the Prediction of All-Helical Membrane Protein Structure and Topology. Biochem. 1994, 33:3038–49;
Bernsel A, Viklund H, Hennerdal A, et al. TOPCONS: consensus prediction of membrane protein topology. Nucleic Acids Res. 2009, 37:W465–8;
Cserzo M, Wallin E, Simon I, et al. Prediction of transmembrane alpha-helices in prokaryotic membrane proteins: the Dense Alignment Surface method. Prot. Eng. 1997, 10(6):673–6;
Juretic D, Zoranic L, Zucic D. Basic charge clusters and predictions of membrane protein topology J. Chem. Inf. Comput. Sci. 2002, 42:620–32;
Krogh A, Larsson B, von Heijne G, et al. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305:567–80;
Rost B, Yachdav G, Liu J. The PredictProtein server. Nucleic Acids Res. 2004, 32:W321–26;
Käll L, Krogh A, Sonnhammer ELL. A Combined Transmembrane Topology and Signal Peptide Prediction Method. Journal of Molecular Biology, 2004, 338(5):1027–36;
Viklund H, Elofsson A. A method that improves topology prediction for transmembrane proteins by using two-track ANN-based preference scores and an improved topological grammar. Bioinformatics. 2008, 24:1662–8;
Tusnády GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics. 2001, 17:849–850;
Hirokawa T, Boon-Chieng S, Mitaku S. SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics. 1998; 14(4):378–9.
The strongly preferred computer-predicted model of SLC41A2 topology (TMPred at www.ch.embnet.org) possesses ten TM with both termini being oriented extracellularly. However, Sahni and colleagues have demonstrated experimentally that the N-terminus of A2 is oriented extracellularly and the C-terminus intracellularly when overexpressed in DT40 chicken cells and, therefore, they have proposed the eleven TM “outside-in” model of membrane topology of A2 (Fig. 1B) [10]. Considering the structure-function relationship determined for MgtE, Sahni and Scharenberg have recently concluded that, in their pilot study [10], A2 has most probably been mistargeted to the cytoplasmic membrane as a result of its overexpression. Their assumption that A2 is primarily functional in the membranes of the intracellular compartments must be further tested (Fig. 1B) [13]. The online-generated prediction of A3 topology is shown at figure 1C. The computer analyses do not reveal any specific targeting-(signal)-sequences, therefore, a more specific intracellular localization of A2 or A3 may not be assumed (http://www.cbs.dtu.dk/services/SignalP/).
Kolisek and colleagues have shown that A1 forms protein complexes with high molecular mass in vivo [9]. Moreover, they propose that these complexes have a hetero-oligomeric character [9]. Recently, Nestler and colleagues have utilized SU-YTH assay and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MLDI-TOF-MS) and identified multiple binding partners of A1 [16]. The most prominent binding partners of A1 identified are 3-beta-hydroxysteroid-Δ(8),Δ(7)-isomerase (emopamil-binding protein) and B-cell receptor associated-protein 31. Other binding partner candidates include: IER3IP1, PPIB, UPF0480 protein C15orf24, SPINT2, C14orf1/PEBP28, NIFIE14, YIPF6, KCP2, SLC31A2, SLC35B1, SLC39A13, CRACM1, MTCH2, ACCA1, UBB, ATX2L, HSP7C and TBB. These are mostly proteins integral to membranes constituting the endoplasmic reticulum (ER) and Golgi apparatus (GA) (Fig. 2A) and playing a role in proteoneogenesis, proper folding, maturation, secretion, anterograde transport, and the regulation of apoptosis (Fig. 2B). Interestingly, among the binding partners of A1 other members of the SLC superfamily involved in lysosomal Cu2+-, GA Zn2+- and ER sugar-transport have been identified. Nestler and colleagues therefore speculate that these proteins undergo similar modifications with regard to their maturation (posttranslational modification in ER and GA) as A1 [16].
Figure 2.
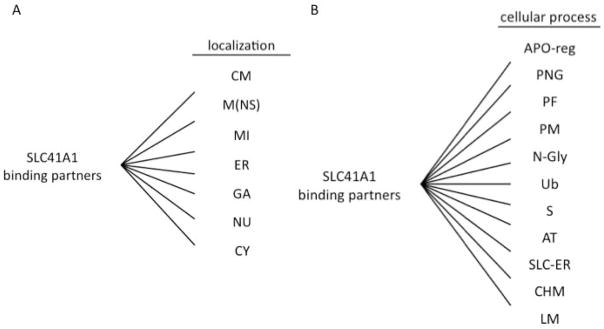
SLC41A1, networks depicting cellular localization (A) and function (B) of its interactors. (A) CM = cytoplasmic membrane, M(NS) = membranes (non-specified), MI = mitochondria, ER = endoplasmic reticulum, GA = Golgi apparatus, NU = nucleus, CY = cytoplasm; (B) APO-reg = apoptosis (regulation), PNG = proteoneogenesis, PF = protein folding, PM = protein maturation, N-Gly = N-glykosylation, Ub = ubiquitination, S = secretion, AT = anterograde transport, SLC-ER = solute transport in endoplasmic reticulum and Golgi, CHM = cholesterol metabolism (biosynthesis), LM = lipids metabolism (biosynthesis).
So far, no information is available about the complex-forming abilities of A2 and A3.
Goytain and Quamme [6] have proposed N-glycosylation as a possible posttranslational modification of A1. The NetNGlyc 1.0 prediction machine (DTU) indeed recognizes four putative glycosylation sequons within the sequence of A1. Three of them are located within the N-terminal cytoplasmic domain, and only the fourth is located in the putative extracellular loop (aa463 – aa483; TMpred at ch.EMBnet.org). Therefore, the identification of a component of oligosaccharyltransferase (KCP2) among putative A1-binding partners further substantiates N-glycosylation as a possible posttranslational modification of A1 [16].
Several functional studies have demonstrated that phosphorylation mediated via cAMP-activated protein kinase A (PKA) [5,8] and PKC [9] plays a key role in the regulation of Na+/Mg2+ exchange. Indeed, Kolisek and colleagues [11] have shown that phosphorylation mediated by cAMP-activated PKA is an important event for the activation of A1-mediated Mg2+ efflux (Na+/Mg2+ exchange). The N-terminal flanking sequence of A1, which seems to be an important regulatory region [10, 11, 15], possesses, in addition to the predicted PKC-phosphorylation sites, multiple putative phosphorylation hotspots for p38MAPK, cdc2, GSK3, cdk5, DNAPK and CKII (NetPhos 1.0; [15]). Involvement of these protein kinases in the regulation of A1 function remains to be explored.
Moreover, ubiquitination seems to play a role in the regulation of A1 turnover. Computer prediction has revealed ubiquitination hotspots at positions 4, 8, 58, 92, 146 and 339 (http://bdmpub.biocuckoo.org). The role of ubiquitination in the turnover/degradation of A1 has been substantiated by MALDI-TOF-MS analysis combined with in-gel trypsin digestion, performed on Coomassie-stained protein bands resulting from the electro-separation of affinity-purified strep-A1 (performed under native conditions) and its potential binding partners [16]. Nestler and colleagues have demonstrate that a protein band with a molecular mass well exceeding 170 kDa comprises A1, ACCA1 and ubiquitin, therefore, indicating that A1, or ACCA1 or both are prone to ubiquitination [16].
SLC41 family of Mg2+ carriers
Experimental data in favour of two mechanisms have been presented in the literature: (ion channel [6]; or ion exchanger [9, 11]). Structural data have revealed that ion channels form aqueous pores across the plasma membrane, whereas exchangers can have either large aqueous vestibules reaching deep into the bilayer of the membrane or form hourglass-like funnels with a narrow, and possibly water-excluding selectivity filter [17]. Channels and exchangers can be defined and distinguished by biophysical characteristics, such as ion flux rates (slower in exchangers), temperature-sensitivity (more so in exchangers) and dependence on both membrane voltage and concentration gradients of the transported ion species.
Several reports have presented experimental data suggesting that A1, A2 and A3 represent Mg2+ transport proteins [6, 7, 8, 9, 10, 11, 14]. A1 and A2 were originally considered to be putative Mg2+ transporters based on distant sequence homologies with the prokaryotic Mg2+ transporter MgtE [5]. Their initial functional characterizations used complementary RNA (cRNA) expression in Xenopus oocytes and membrane current recordings with two-electrode voltage-clamp (TEV) [6, 7, 8]. As the original A3 data were presented within a review article [8], only limited information is available about buffers composition and experimental procedures. Therefore, these will not be further discussed. Currents were compared between oocytes injected with H2O and oocytes injected with A1 or A2 cRNA. The membrane potential was held at −15 mV and currents were elicited with voltage steps of 2 s duration and ranging from −150 mV to +25 mV. Increasing extracellular Mg2+ concentrations from 0.2 mM to 10 mM gave rise to a saturable inward current at hyperpolarized potentials with an assessed reversal potential of about −20 mV. Outward currents also seemed to increase in parallel, although this was less clear because of the absence of recordings above +25 mV. Importantly, when using extracellular divalents other than Mg2+, the oocyte experiments delineated a decrease in inward currents, with Ca2+ being particularly ineffective in eliciting any measurable currents. A1 and A2 were concluded to represent an Mg2+ transport mechanism with channel-like properties [6, 7], as the presented data could accommodate both, a constitutively active ion channel or an electrogenic ion exchange mechanism. Unfortunately, kinetic current data that could have shed light on this issue were not provided in these publications [6, 7] and hence no definitive conclusion can be reached.
One way to differentiate between an ion channel and ion exchanger mechanism is to exploit the mandatory presence of both substrates for ion exchange to occur. Ion channels do not have this prerequisite as long as the permeating ion is available in sufficient quantities to move down its concentration gradient. The study of A1 by using Xenopus oocyte TEV tested for the possibility that A1 could be NME by removing Na+ from the extracellular solution [6]. This experimental manipulation did not alter the measured inward currents and thus favoured the concept of a constitutively active ion channel mechanism, if one assumed that the inward currents were indeed carried by A1. However, one of the major limitations of the TEV Xenopus oocyte system is the inability to control experimentally the internal milieu of the oocyte. Thus, any exogenously introduced protein could potentially activate unrelated endogenous currents such as swelling-and/or Ca2+-activated chloride (Cl−) currents. As is well known, oocytes injected with Ca2+, and to some extent with other divalent ions, induce Cl− currents [18, 19, 20] and the expression of A1 or A2 might lead to such activation. In this context, it should be mentioned that the overexpression of A1 in HEK293 cells leads to the secondary activation of endogenous ATP-sensitive Cl− currents [9]. The removal of external Cl− from the solution, as mentioned in the experimental approach for A1 [6] does not help clarify whether the measured inward currents are carried by divalent ions or Cl− ions, as Cl−is a negatively charged ion and inward currents would reflect Cl− efflux. For the same reason, the experiments in which Na+ was removed also remain open to this alternative explanation.
The first, and so far only, attempts at measuring A1 or A2 related whole-cell currents by using the patch-clamp technique were performed in tetracycline-inducible cell systems [9, 10, 14]. Whereas no obvious currents could be seen in A2-expressing DT40 chicken B cells [10], overexpression of A1 in HEK293 cells caused a secondary activation of an endogenous ATP-sensitive Cl− current that was completely inhibited by the Cl− channel blocker DIDS [9]. The latter experimental manipulation did not uncover any additional currents. Regardless, A1 or A2 expression clearly enables Mg2+ transport, as supercharging extracellular Mg2+ levels to 10 mM leads to an obvious increase in intracellular Mg2+ concentrations [9, 10, 14] and Mg2+-transporter-deficient bacteria (Salmonella sp.) survive a Mg2+-deprived environment when overexpressing A1 [9]. The greatest challenge of the ion channel hypothesis relates to the gating of the protein. If one takes the data acquired by TEV in the oocyte system at face value and if the measured currents are carried by A1/A2, one would have to consider SLC41 proteins as being constitutively active ion channels, since removal of external Na+ had no apparent effect on the currents and would therefore seem to exclude an electrogenic exchange mechanism [6, 7]. However, experiments performed using whole-cell patch-clamp conditions do not give rise to any measurable currents [9, 10], indicating that the SLC41 proteins are not constitutively active ion channels. Instead they might represent an ion channel mechanism whose gating mechanism awaits discovery or they are carriers that produce electroneutral ion exchange and remain undetectable electrophysiologically.
The most straightforward evidence implicating A1 as a Mg2+ transporter has emerged from functional complementation experiments in Mg2+-transport-deficient Salmonella sp. strain MM281 transformed with pUC18-hSLC41A1 [9]. Here, an increase in extracellular Mg2+ concentrations to 10 mM significantly enhances intracellular Mg2+ concentrations. Given an experimental Na+ concentration of 0.5 mM [Na+]e, with an estimated 3 mM [Na+]i [9, 21], the driving ion in this situation is most likely to be Mg2+ with a concentration ratio of ~20 compared with a ratio of ~6 for Na+. This allows Mg2+ flux into the bacteria, as these typically have highly negative membrane potentials when metabolically active [22]. The very negative membrane potential also helps to explain the partial rescue of Salmonella growth in only 100 μM extracellular Mg2+. Under these experimental conditions both Mg2+ and Na+ have similar concentration ratios (8 and 6, respectively) and with a theoretical reversal potential of NME around −26 mV under these conditions, the exchanger could potentially work in reverse mode, allowing Mg2+ influx and the efflux of Na+.
The situation in mammalian cells, particularly in electrically non-excitable cells, is more complicated. Here, Na+ is the relevant driving ion for Na+/Mg2+ exchange, as the ratio of its concentration across the membrane is at least 6-fold larger than that for Mg2+ (0.5 mM [Mg2+]i/1 mM [Mg2+]e versus 12 mM [Na+]i/145 mM [Na+]e). Since the direction of ion transport is defined by the equilibrium potential of the transported ions, A1 would normally extrude Mg2+ and import Na+ and reverse only when concentration gradients are experimentally manipulated to define artificially the driving ion species. This makes experiments with intact cells difficult to interpret, as the intracellular milieu is not easily manipulated, and alterations of extracellular ion conditions need to consider resulting changes in the transporter’s reversal potential, the direction of transport, the relationship between driven and driving ion species and even the ability to transport ions. Considering that resting HEK293 cells may have a membrane potential of around −15 mV [23], a resting Mg2+ concentration of 0.2 mM [9, 11] and that the external Na+ is usually high (145 mM), at the physiological level of 1 mM external Mg2+, Na+ will be the driving ion. The relatively positive reversal potential (~ +40 mV) for NME that can be assumed under these conditions would favour constant Mg2+ extrusion. Indeed, Mg2+ extrusion plays a critical role in transcellular Mg2+ transport as seen during Mg2+ reabsorption in the distal convoluted tubule of the kidney [24]. This basolateral efflux of Mg2+ is thought to be Na+-dependent [25].
Experimentally increasing external Mg2+ to high concentrations such as 10 mM would result in a shift of the driving ion from Na+ to Mg2+, which now would have an estimated concentration ratio of 50 (10 mM/0.2 mM), thus driving Mg2+ influx and transporting Na+ against its concentration gradient. This indeed has been observed in HEK293 cells with or without overexpression of SLC41A1, whereby the presence of SLC41A1 has an amplifying effect on Mg2+ influx [11]. Furthermore, high external Mg2+ concentrations (15 mM) rescue cell growth and proliferation of TRPM7-deficient DT40 chicken B cells overexpressing A1 [14] or A2 [10]. The removal of external Mg2+ while maintaining Na+ levels should also make Mg2+ the driving ion species for the exchanger, resulting in Mg2+ efflux at more positive potentials. Furthermore, this process should be inhibited by simply removing the external Na+, and this is indeed observed in HEK293 cells overexpressing A1 [11].
SLC41A1 as a Na+/Mg2+ exchanger
The basal [Mg2+]i of most cells is maintained between 0.5 to 1.2 mM and measurable Mg2+ transport has long been known to take place only when it is decreased or increased, e.g. by extracellular Mg2+ deficiency or after hormonal stimulation. Thus, to overcome this problem for Mg2+ transport studies, various methods such as Mg2+ depletion by incubation in Mg2+-free media and artificial Mg2+ loading or the application of metabolic inhibitors [26, 27] must be used to generate conditions favouring the uptake or efflux of the ion.
Because the first functional data concerning A1-related transport [6] suggested a higher influx capacity in A1-overexpressing cells, Kolisek and colleagues [9] designed their experiments to promote Mg2+ uptake. Cells were Mg2+-depleted by incubation in totally Mg2+-free media and most experiments were performed under inwardly directed Mg2+ gradients ([Mg2+]i ≪ [Mg2+]e: 2, 5, or 10 mM). This lead to an [Mg2+]i increase composed of an A1-independent linear component (most probably channel-mediated) and an A1-related temperature-sensitive component. The latter was insensitive to cobalt(III)hexaammine, a known inhibitor of bacterial/mitochondrial [28, 29] and mammalian [9] Mg2+ channels. Most interestingly, A1-overexpressing HEK293 cells, although Mg2+-deficient showed a significant loss of Mg2+ when incubated under Mg2+-free conditions ([Mg2+]i > [Mg2+]e: 0 mM). Measurements of the total [Mg] via AAS (atomic absorption spectroscopy) confirmed net Mg2+ transport showing a 12.7% increase and 25.6% loss of total [Mg] after a 20-min incubation in medium containing 5 mM or zero Mg2+, respectively. Together, these results characterize A1 as a functional Mg2+ carrier and give the first evidence for the ability of the protein to mediate the efflux of the ion.
Kolisek and colleagues [11] have recently performed a second study with A1-overexpressing HEK293 cells to test whether the protein represents the molecular correlate of one of the postulated Mg2+ efflux systems. As any efflux activity is dependent on the [Mg2+]i [30], experiments were carried out with HEK293 cells that had been Mg2+-loaded by a 20-min incubation in a 10 mM Mg2+-containing medium. The A1-related Mg2+ extrusion was not directly linked to anion (Cl−, HCO3−) transport. However, it showed typical features of NME such as 1) activation by an increased [Mg2+]i [26], 2) strict dependence on extracellular Na+ [31, 32], 3) inhibition by imipramine and quinidine [33] and, 4) regulation via cAMP-dependent PKA [33]. Thus, unquestionably, A1 represents NME, the predominant Mg2+ efflux system in mammalian cells [32, 34].
As known for other Na+-dependent exchangers (e.g. Na+/Ca2+ antiport, Na+/H+ exchanger), the driving force for A1-mediated Na+/Mg2+ exchange is, in the first instance, the inwardly directed concentration gradient for Na+ [11]. This makes the transporter directly dependent on extracellular Na+ and indirectly dependent on the Na+/K+-pump and thereby on ATP [11]. In comparison with controls, A1-overexpressing HEK293 cells have a high [Na+]i (about 40 mM) but reduced [Mg2+]i (about 0.1 mM). Therefore, increasing the extracellular Mg2+ concentration will reduce the driving force for an electroneutral Mg2+ efflux and makes it possible that the exchanger switches to the reverse mode, thereby performing Mg2+ uptake. This mode of operation has been shown in erythrocytes, heart muscle cells and rumen epithelium cells [35, 36, 37, 38] and might explain difficulties in identifying the natural function of A1 in some studies [14].
In general, for a correct interpretation of the results of functional studies, consideration of the functions of cells and/or tissues is essential. For example, in view of the function of NME for Mg2+ transport across the basolateral membrane of intestinal, renal and placental epithelia [39, 40], its transcription upregulation in specific cell types, e.g. renal distal tubule cells exposed to low Mg [8], is not surprising. Mg2+ influx systems such as TRPM6/TRPM7 or MagT1 are rapidly inactivated at normal Mg2+ concentrations. Thus an increased expression/activity of A1 could be interpreted as a mechanism to maintain a driving force for Mg2+ uptake under Mg-deficient conditions [41]. For this, only local changes of [Mg2+]i are sufficient; these can also be induced by the hormonal activation of A1 [42].
SLC41 family and its involvement in human diseases
Systemic and intracellular magnesium insufficiency has long been suspected to contribute to the development and progress of cardiovascular, metabolic, neurological (neurodegenerative) and psychiatric diseases and also conditions associated with pregnancy such as preeclampsia and eclampsia [43, 44]. Several reports have recently been published linking A1 (as a key component of cellular and perhaps also of systemic Mg2+ homeostasis) with serious human illnesses.
Among the most exciting findings has been the discovery that A1 is part of a novel Parkinson’s disease (PD) susceptibility locus PARK16. In this regard, Tucci and colleagues [45] identified a rare coding variant of A1 p.A350V putatively linked to PD. This study was shortly followed by a report of Yan and colleagues [46] identifying additional genetic variants of A1 present in a Chinese PD cohort, namely p.L146G, p.P480P and c.552 + 50G > A. Interestingly, a conditional knockout mouse line, designated as Slc41a3tm1a(KOMP)Wtsi (http://www.knockoutmouse.org/martsearch/search?query=Slc41a3), which was generated as part of the International Knockout Mouse Consortium program, displays abnormal locomotor coordination (http://en.wikipedia.org/wiki/SLC41A3).
Kolisek and colleagues [44] have performed an analysis of the transcription activities of magnesium-responsive genes in human placenta samples from the Mexican Mestizo cohorts of normoevolutive women and preeclamptic women. They found A1 to be the only magnesium responsive gene with deregulated expression in the placentas of preeclamptic woman, with A1 being significantly overexpressed in approximately 55% of tested preeclamptic samples and in only 9.5% of normoevolutive samples [44]. These findings strongly suggest an important role of A1 in the pathophysiology of preeclampsia.
A recently identified variant of A1, c.698G>T, has been associated with the nephronophthisis-like phenotype (NPHP-LP) [47]. This mutation results in the skipping of exon 6 of A1, leading to an in-frame deletion of a transmembrane helix [47]. Hurd and colleagues [47] suggest that defects in the maintenance of renal Mg2+ homeostasis caused by mutated A1 lead to tubular defects that result in NPHP-LP.
Conclusion
At the molecular ad physiological level A1 is the best characterized protein out of the three members of the SLC41 family. From the experimental evidences it is obvious that A1 is an NME, a primary Mg2+-efflux system (able to operate also in a reverse mode under specific physiological conditions), and not a cation (Mg2+) channel as was assumed in the pilot report of Goytain and Quamme [6]. Little is known about the function of A2 and A3 in the context of intracellular Mg2+ homeostasis or about the exact mode of their operation. However, based on the homology between A1 and the two other members of SLC41 family it could be assumed, that both A2 and A3 also function as Mg2+ (X+) carriers.
Cellular localization and membrane topology of A2 and A3 also awaits further examination. Consensus is lacking on the orientation of the C-terminus of A1.
Nothing is known about complex-forming abilities of A2 and A3. A1 has been demonstrated to form transient heterooligomeric complexes with proteins probably being involved in its posttranslational maturation (modification), proper folding, anterograde transport and vesicular sorting. The interactions of A1 and its binding partners identified with the split-ubiquitin yeast two-hybrid assay await further examination.
Phosphorylation plays an important role in the regulation of A1 Na+/Mg2+ exchange activity. The stimulating effect of cAMP-dependent PKA on A1 was already demonstrated. The effects of other protein kinases, which have been computer-predicted to phosphorylate A1, remain to be examined experimentally. Moreover, the predicted N-glycosylation and ubiquitination of A1 need further exploration.
No link has been established between A2 or A3 and any known disease. However, A1 has been identified to be a part of the PD susceptibility locus PARK16 and its genetic variants exclusively associated with PD have been identified, predominantly in Asian (Chinese) cohorts. Moreover, recently, a link between preeclamsia and elevated expression of A1 in placental tissues has been demonstrated. The A1 null mutation c.698G>T has been associated with the nephronophthisis-like phenotype. Further research will be needed to elucidate the exact role of A1 (or its variants) in the pathophysiology of these diseases. Disturbed Na+/Mg2+ exchange has been associated with cardiovascular and metabolic diseases. Therefore, any linkage of A1 with such ailments in the future will be unsurprising. The wide spectrum of diseases involving A1 (or Na+/Mg2+ exchange) might also indicate an important role of A1 in regulation of systemic Mg homeostasis.
Acknowledgments
Our gratitude is due to Dr. Theresa Jones for linguistic corrections. This work was supported by the research grant from the German Research Foundation (DFG) KO-3586/3-2 to MK and by NIH Grant P01 GM078195 to AF. MSR and MK are inventors on the pending patent EP2011/065979 “Na+/Mg2+ exchanger”.
Footnotes
Contributions
AF, MSR & MK wrote the manuscript. All authors read and approved the manuscript.
Contributor Information
Andrea Fleig, Laboratory of Cell and Molecular Signalling, Center for Biomedical Research at The Queen’s Medical Center, Honolulu, HI USA.
Monika Schweigel-Röntgen, Institute for Nutritional Physiology “Oskar Kellner”, Leibniz Institute for Farm Animal Biology, Dummerstorf, Germany.
Martin Kolisek, Email: martink@zedat.fu-berlin.de, Institute für Veterinär-Physiologie, Freie Universität Berlin, Oertzenweg 19b, 14163 Berlin, Germany; tel: +49-30-838-62598.
References
- 1.Park MH, Wong BB, Lusk JE. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. Journal of Bacteriology. 1976;126(3):1096–103. doi: 10.1128/jb.126.3.1096-1103.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hmiel SP, Snavely MD, Miller CG, Maguire ME. Magnesium transport in Salmonella typhimurium: characterization of magnesium influx and cloning of a transport gene. Journal of Bacteriology. 1986;168(3):1444–50. doi: 10.1128/jb.168.3.1444-1450.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hmiel SP, Snavely MD, Florer JB, Maguire ME, Miller CG. Magnesium transport in Salmonella typhimurium: genetic characterization and cloning of three magnesium transport loci. Journal of Bacteriology. 1989;171(9):4742–51. doi: 10.1128/jb.171.9.4742-4751.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend DE, Esenwine AJ, George J, 3rd, Bross D, Maguire ME, Smith RL. Cloning of the mgtE Mg2+ transporter from Providencia stuartii and the distribution of mgtE in gram-negative and gram-positive bacteria. Journal of Bacteriology. 1995;177(18):5350–4. doi: 10.1128/jb.177.18.5350-5354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wabakken T, Rian E, Kveine M, Aasheim HC. (2003) The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochemical and Biophysical Research Communications. 2003;306:718–24. doi: 10.1016/s0006-291x(03)01030-1. [DOI] [PubMed] [Google Scholar]
- 6.Goytain A, Quamme GA. Functional characterization of human SLC41A1, a Mg2+ transporter with similarity to prokaryotic MgtE Mg2+ transporters. Physiological Genomics. 2005;21(3):337–42. doi: 10.1152/physiolgenomics.00261.2004. [DOI] [PubMed] [Google Scholar]
- 7.Goytain A, Quamme GA. Functional characterization of the mouse solute carrier, SLC41A2. Biochemical and Biophysical Research Communications. 2005;330(3):701–5. doi: 10.1016/j.bbrc.2005.03.037. Erratum in: Biochemical and Biophysical Research Communications 2007, 356(3), 822. [DOI] [PubMed] [Google Scholar]
- 8.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. American Journal of Physiology Cell Physiology. 2010;298(3):C407–29. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 9.Kolisek M, Launay P, Beck A, Sponder G, Serafini N, Brenkus M, Froschauer EM, Martens H, Fleig A, Schweigel M. SLC41A1 is a novel mammalian Mg2+ carrier. The Journal of Biological Chemistry. 2008;283:16235–16247. doi: 10.1074/jbc.M707276200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sahni J, Nelson B, Scharenberg AM. SLC41A2 encodes a plasma-membrane Mg2+ transporter. Biochemical Journal. 2007;401:505–513. doi: 10.1042/BJ20060673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolisek M, Nestler A, Vormann J, Schweigel-Rontgen M. Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. American Journal of Physiology Cell Physiology. 2012;302:C318–326. doi: 10.1152/ajpcell.00289.2011. [DOI] [PubMed] [Google Scholar]
- 12.Romanuik TL, Wang G, Holt RA, Jones SJ, Marra MA, Sadar MD. Identification of novel androgen-responsive genes by sequencing of LongSAGE libraries. BMC Genomics. 2009;10:476. doi: 10.1186/1471-2164-10-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sahni J, Scharenberg AM. The SLC41 family of MgtE-like magnesium transporters. Molecular Aspects of Medicine. 2013;34(2–3):620–8. doi: 10.1016/j.mam.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandt T, Song Y, Scharenberg AM, Sahni J. SLC41A1 Mg2+ transport is regulated via Mg2+-dependent endosomal recycling through its N-terminal cytoplasmic domain. Biochemical Journal. 2011;439(1):129–39. doi: 10.1042/BJ20110807. [DOI] [PubMed] [Google Scholar]
- 15.Sponder G, Rutschmann K, Kolisek M. Split-ubiquitin functional assay supports ten transmembrane “inside-in” topology of Na+/Mg2+ exchanger SLC41A1. Submitted. [Google Scholar]
- 16.Nestler A, Sponder S, Rutschmann K, Mastrototaro L, Weise C, Vormann J, Schweigel-Röntgen M, Kolisek M. Nature of SLC41A1 complexes: report on split-ubiquitin yeast two hybrid assay. Magnesium Research. 2013;26(2):56–66. doi: 10.1684/mrh.2013.0339. [DOI] [PubMed] [Google Scholar]
- 17.Gouaux E, Mackinnon R. Principles of selective ion transport in channels and pumps. Science. 2005;310:1461–1465. doi: 10.1126/science.1113666. [DOI] [PubMed] [Google Scholar]
- 18.Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. The Journal of Physiology. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miledi R, Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. The Journal of Physiology. 1984;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dascal N. Voltage clamp recordings from Xenopus oocytes. Current Protocols in Neuroscience. 2001;Chapter 6(Unit 6):12. doi: 10.1002/0471142301.ns0612s10. [DOI] [PubMed] [Google Scholar]
- 21.Lo CJ, Leake MC, Berry RM. Fluorescence measurement of intracellular sodium concentration in single Escherichia coli cells. Biophysical Journal. 2006;90:357–365. doi: 10.1529/biophysj.105.071332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro HM. Membrane potential estimation by flow cytometry. Methods. 2000;21:271–279. doi: 10.1006/meth.2000.1007. [DOI] [PubMed] [Google Scholar]
- 23.Sogaard R, Ljungstrom T, Pedersen KA, Olesen SP, Jensen BS. KCNQ4 channels expressed in mammalian cells: functional characteristics and pharmacology. American Journal of Physiology Cell Physiology. 2001;280:C859–866. doi: 10.1152/ajpcell.2001.280.4.C859. [DOI] [PubMed] [Google Scholar]
- 24.Alexander RT, Hoenderop JG, Bindels RJ. Molecular determinants of magnesium homeostasis: insights from human disease. Journal of the American Society of Nephrology. 2008;19:1451–1458. doi: 10.1681/ASN.2008010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gunther T. Mechanisms and regulation of Mg2+ efflux and Mg2+ influx. Mineral and Electrolyte Metabolism. 1993;19:259–265. [PubMed] [Google Scholar]
- 26.Kubota T, Tokuno K, Nakagawa J, Kitamura Y, Ogawa H, Suzuki Y, Suzuki K, Oka K. Na+/Mg2+ transporter acts as a Mg2+ buffering mechanism in PC12 cells. Biochemical and Biophysical Research Communications. 2003;303(1):332–6. doi: 10.1016/s0006-291x(03)00346-2. [DOI] [PubMed] [Google Scholar]
- 27.Flatman PW, Smith LM. Magnesium transport in magnesium-loaded ferret red blood cells. Pflugers Archive. 1996;432(6):995–1002. doi: 10.1007/s004240050227. [DOI] [PubMed] [Google Scholar]
- 28.Kolisek M, Zsurka G, Samaj J, Weghuber J, Schweyen RJ, Schweigel M. Mrs2p is an essential component of the major electrophoretic Mg2+ influx system in mitochondria. EMBO Journal. 2003;22(6):1235–44. doi: 10.1093/emboj/cdg122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kucharski LM, Lubbe WJ, Maguire ME. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. Journal of Biological Chemistry. 22(22):16767–73. doi: 10.1074/jbc.M001507200. [DOI] [PubMed] [Google Scholar]
- 30.Büttner S, Günther T, Schäfer A, Vormann J. Magnesium metabolism in erythrocytes of various species. Magnesium-Bulletin. 1998;20:101–9. [Google Scholar]
- 31.Yoshimura M, Oshima T, Matsuura H, Watanabe M, Higashi Y, Ono N, Hiraga H, Kambe M, Kajiyama G. Effect of the transmembrane gradient of magnesium and sodium on the regulation of cytosolic free magnesium concentration in human platelets. Clinical Science (London) 1995;89(3):293–8. doi: 10.1042/cs0890293. [DOI] [PubMed] [Google Scholar]
- 32.Schweigel M, Park HS, Etschmann B, Martens H. Characterization of the Na+-dependent Mg2+ transport in sheep ruminal epithelial cells. American Journal of Physiology Gastrointestinal and Liver Physiology. 2006;290(1):G56–65. doi: 10.1152/ajpgi.00014.2005. [DOI] [PubMed] [Google Scholar]
- 33.Schweigel M, Kuzinski J, Deiner C, Kolisek M. Rumen epithelial cells adapt magnesium transport to high and low extracellular magnesium conditions. Magnesium Research. 2009;22(3):133–50. [PubMed] [Google Scholar]
- 34.Fagan TE, Romani A. alpha(1)-Adrenoceptor-induced Mg2+ extrusion from rat hepatocytes occurs via Na+-dependent transport mechanism. American Journal of Physiology Gastrointestinal and Liver Physiology. 2001;280(6):G1145–56. doi: 10.1152/ajpgi.2001.280.6.G1145. [DOI] [PubMed] [Google Scholar]
- 35.Schweigel M, Vormann J, Martens H. Mechanisms of Mg2+ transport in cultured ruminal epithelial cells. American Journal of Physiology Gastrointestinal and Liver Physiology. 2000;278(3):G400–8. doi: 10.1152/ajpgi.2000.278.3.G400. [DOI] [PubMed] [Google Scholar]
- 36.Günther T, Vormann J. Reversibility of Na+/Mg2+ antiport in rat erythrocytes. Biochimica et Biophysica Acta. 1995;1234(1):105–10. doi: 10.1016/0005-2736(94)00267-s. [DOI] [PubMed] [Google Scholar]
- 37.Flatman PW, Smith LM. Sodium-dependent magnesium uptake by ferret red cells. Journal of Physiology. 1991;443:217–30. doi: 10.1113/jphysiol.1991.sp018831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tashiro M, Tursun P, Konishi M. Intracellular and extracellular concentrations of Na+ modulate Mg2+ transport in rat ventricular myocytes. Biophysical Journal. 2005;89(5):3235–47. doi: 10.1529/biophysj.105.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quamme GA. Control of magnesium transport in the thick ascending limb. American Journal of Physiology. 1989;256(2 Pt 2):F197–210. doi: 10.1152/ajprenal.1989.256.2.F197. [DOI] [PubMed] [Google Scholar]
- 40.Leonhard-Marek S, Stumpff F, Brinkmann I, Breves G, Martens H. Basolateral Mg2+/Na+ exchange regulates apical nonselective cation channel in sheep rumen epithelium via cytosolic Mg2+ American Journal of Physiology Gastrointestinal and Liver Physiology. 2005;288(4):G630–45. doi: 10.1152/ajpgi.00275.2004. [DOI] [PubMed] [Google Scholar]
- 41.He Y, Yao G, Savoia C, Touyz RM. Transient receptor potential melastatin 7 ion channels regulate magnesium homeostasis in vascular smooth muscle cells: role of angiotensin II. Circulation Research. 2005;96(2):207–15. doi: 10.1161/01.RES.0000152967.88472.3e. [DOI] [PubMed] [Google Scholar]
- 42.Fatholati M, LaNoue K, Romani A, Scarpa A. Relationship between total and free cellular Mg2+ during metabolic stimulation of rat cardiac myocytes and perfused hearts. Archives of Biochemistry and Biophysics. 2000;374:395–401. doi: 10.1006/abbi.1999.1619. [DOI] [PubMed] [Google Scholar]
- 43.Nishizawa Y, Morii H, Durlach J. New Perspectives in Magnesium Research (Nutrition and Health) Springer-Verlag London Ltd; 2007. [Google Scholar]
- 44.Kolisek M, Galaviz-Hernández C, Vázquez-Alaniz F, Sponder G, Javaid S, Kurth K, Nestler A, Rodríguez-Moran M, Verlohren S, Guerrero-Romer F, Aschenbach JR, Vormann J. SLC41A1 is the only magnesium responsive gene significantly overexpressed in placentas of preeclamptic women. Hypertension in Pregnancy. 2013 doi: 10.3109/10641955.2013.810237. Ahead of print. [DOI] [PubMed] [Google Scholar]
- 45.Tucci A, Nalls MA, Houlden H, Revesz T, Singleton AB, Wood NW, Hardy J, Paisán-Ruiz C. Genetic variability at the PARK16 locus. European Journal of Human Genetics. 2010;18(12):1356–9. doi: 10.1038/ejhg.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Y, Tian J, Mo X, Zhao G, Yin X, Pu J, Zhang B. Genetic variants in the RAB7L1 and SLC41A1 genes of the PARK16 locus in Chinese Parkinson’s disease patients. International Journal of Neuroscience. 2011;121(11):632–6. doi: 10.3109/00207454.2011.598983. [DOI] [PubMed] [Google Scholar]
- 47.Hurd TW, Otto EA, Mishima E, Gee HY, Inoue H, Inazu M, Yamada H, Halbritter J, Seki G, Konishi M, Zhou W, Yamane T, Murakami S, Caridi G, Ghiggeri G, Abe T, Hildebrandt F. Mutation of the Mg2+ Transporter SLC41A1 Results in a Nephronophthisis-Like Phenotype. Journal of the American Society of Nephrology. 2013 doi: 10.1681/ASN.2012101034. Ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]