Abstract
Plant growth and productivity are largely affected by environmental stresses. Therefore, plants have evolved unique adaptation mechanisms to abiotic stresses through fine-tuned adjustment of gene expression and metabolism. Recent advanced technologies, such as genome-wide transcriptome analysis, have revealed that a vast amount of non-coding RNAs (ncRNAs) apart from the well-known housekeeping ncRNAs such as rRNAs, tRNAs, small nuclear RNAs (snRNAs) and small nucleolar RNAs (snoRNAs) are expressed under abiotic stress conditions. These various types of ncRNAs are involved in chromatin regulation, modulation of RNA stability and translational repression during abiotic stress response. In this review, we summarize recent progress that has been made on ncRNA research in plant abiotic stress response.
Keywords: non-coding RNA, small RNA, antisense RNA, abiotic stress response
1. Introduction
Environmental stresses, such as drought, heat, salinity and low temperature, are major limiting factors for plant growth and productivity. Under natural conditions, plants are exposed to a variety of environmental stresses. In order to adapt and survive under the stresses, plants have evolved various molecular mechanisms for a fine-tuned control of adaptive responses [1]. Post-transcriptional regulatory mechanisms, as well as epigenetic and post-translational modifications, like ubiquitination and sumoylation, have been implicated to play an important role in the regulation of gene expression during stress conditions.
Recent genome-wide transcriptome analysis, such as tiling arrays and next generation sequencing, has revealed a large number of stress-responsive ncRNAs. Emerging evidence has revealed that ncRNAs are major products of the plant transcriptome with significant regulatory importance [2,3]. ncRNAs are transcribed from intergenic regions, antisense strands of protein-coding genes and also pseudogenes. According to their size, ncRNAs are classified as small ncRNAs (sRNAs) (<40 nt) and long ncRNAs (lncRNAs) (>200 nt). These ncRNAs are involved in the transcriptional and posttranscriptional regulation of gene expression and the modulation of RNA stability and translation under stress conditions [1,4–7].
2. Small RNAs (sRNAs)
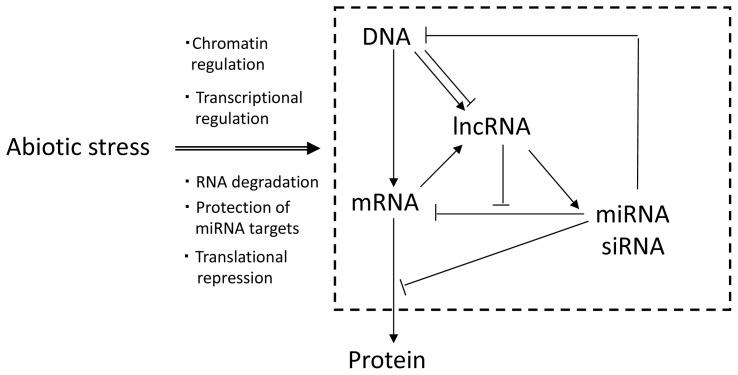
sRNAs are known to have major functional roles in eukaryotic gene regulation. In plants, knowledge regarding the biogenesis and mechanisms of action of sRNA classes including microRNAs (miRNAs), transcriptional gene silencing (TGS)-related heterochromatic small interfering RNAs (siRNAs), trans-acting siRNAs (ta-siRNAs) and natural antisense transcript siRNAs (nat-siRNA) has been primarily gained through Arabidopsis studies (Figure 1; Table 1). These sRNAs are loaded into RNA-induced silencing complexes (RISC) and negatively regulate the expression of their target genes by affecting mRNA levels, chromatin remodeling and DNA methylation.
Figure 1.
Regulation of ncRNAs in abiotic stress responses. ncRNAs (lncRNAs, miRNAs and siRNAs, etc.) are generated in response to abiotic stress, such as drought, low-temperature, heat and high-salinity. The ncRNAs are involved in various types of regulation, such as chromatin regulation, transcriptional regulation, RNA degradation, protection of miRNA targets, translational repression.
Table 1.
Classification of non-coding RNAs (ncRNAs) (1) involved in abiotic stress responses.
| Small ncRNAs (sRNAs) |
|
| Long ncRNAs (lncRNAs) |
|
ncRNAs are classified except for well-known housekeeping ncRNAs, such as rRNAs, tRNAs, snRNAs and snoRNAs.
2.1. Micro RNAs (miRNAs)
Many stress-responsive miRNAs have been identified in various plants [6,8]. miRNAs are generated from pri-miRNAs that are transcribed by RNA polymerase II and the mature miRNAs guide to cleavage target mRNAs [9]. Therefore, the expression levels of pri-miRNAs are regulated by cis-elements in a similar manner as protein-coding genes. An abundance of positive cold stress-related cis-regulatory elements, such as the Dehydration Responsive Element/Low Temperature Responsive Element (DRE/LTRE) -core (A/GCCGAC) [10], Abscisic Acid-Responsive Element (ABRE) -core (ACGTGG/TC) [11] and W-box (TTGAC) [12] are found in the promoter region of several cold-inducible MIRNA genes in Arabidopsis [13]. Several miRNAs that are upregulated in response to various abiotic stresses, including cold [14], dehydration [14,15], salinity [14] and nutrient deficiency [16] have been reported.
An Arabidopsis miRNA involved in the detoxification of reactive oxygen species (ROS) has been reported [17]. Expression of miR398 was found to be decreased by copper (Cu(II)), which is an essential nutrient in photosynthesis and response to oxidative stress and abiotic stresses [18]. miR398 was also shown to suppress superoxide dismutase (SOD) and Cu/Zinc (Zn) superoxide dismutases (CSDs) under low Cu. Interestingly, it was reported as a rare instance in plants that miR398 not only digests CSD1 and CSD2 mRNAs but also negatively regulated the translation of their protein products [19]. This miRNA-dependent translational repression is effected in part by the ARGONAUTE proteins AGO1 and AGO10 [19]. It also requires the activity of the microtubule-severing enzyme katanin and the de-capping component VARICOSE (VCS)/Ge-1, as recently suggested from animal studies. katanin1 and vcs1 mutants that did not affect CSD2 mRNA accumulation, exhibited an overaccumulation of CSD2 proteins under low Cu (II) conditions in comparison to WT plants. However, under high Cu (II) conditions, this trend was not observed [19]. This result suggested that the translational inhibition of miR398 had an important role in the regulation of CSD expression under low Cu (II) condition.
Several miRNAs function in the maintenance of phytohormone signaling during exposure to abiotic stress. An miR168-mediated feedback regulatory loop regulates AGO1 homeostasis in ABA and abiotic stress responses in Arabidopsis [20]. MIR168a-overexpressing plants and ago1 loss-of-function mutants showed ABA hypersensitivity and drought tolerance, while the mir168a mutants showed ABA hyposensitivity and drought hypersensitivity [20]. The promoter of MIR168a has four ABREs, suggesting that expression of MIR168a was directly induced by ABA. Although promoter activity of AGO1 was induced by ABA, AGO1 transcripts were negatively regulated by ABA-induced miR168a; and as a result, mRNA was maintained at a steady level during the stress response. These results suggest that a complex crosstalk exists between the global regulation of miRNA metabolism and ABA signaling functions to enable fine-tuning of abiotic stress response.
In another example of miRNA-phytohormone crosstalk in abiotic stress response, miR160 regulates the expression of Auxin Response Factors (ARF10, ARF16 and ARF17) [21]. Arabidopsis plants expressing miR160-resistant ARF10 not only showed an abnormal leaf shape but also showed hypersensitivity to ABA. Plants overexpressing miR160 showed hyposensitivity to ABA. In addition, other miRNAs targeting auxin signaling factors were also induced in response to abiotic stress [14]. Expression of miR393 was up-regulated by dehydration, salt and cold stresses and ABA [8]. A miR393 target gene, TIR1, an auxin receptor, is involved in the response to salt and oxidative stresses [22]. The promoter of miR167 contains ABREs, indicating their own regulation by ABA [14]. ARF6 and ARF8, which are targeted by miR167, are regulators of female and male reproduction [23]. TAS3-siRNA also regulates auxin signaling [24]. It is thought that miRNAs targeting auxin signaling function as mediators that connect abiotic signaling with development [7,25]. These results suggest that multistep regulation by miRNAs is required for the correct adjustment of gene expression under abiotic stress.
Nutrient deficiency under abiotic stress is known to induce or suppress various miRNAs that regulate nutrient metabolism. Previous studies have demonstrated that the expression of miR395 was increased by sulfate starvation [26]. This specific miRNA suppresses ATP sulfurylases as target mRNAs, thus resulting in catalysis of the first step of inorganic sulfate assimilation [8]. A phosphate starvation-inducible miRNA (miR399) regulates Pi homeostasis by regulating the expression of UBC24 mRNA encoding an ubiquitin-conjugating E2 enzyme [16,27]. miR399 functions as a positive regulator of Pi uptake and translocation. In addition to miR399 regulating UBC24 expression, the cleavage activity of miR399 is suppressed by a long intergenic ncRNA, Induced by Phosphate Starvation 1 (IPS1) [28]. A detailed description is provided at a later point in this chapter.
2.2. Small Interfering RNAs (siRNAs)
Recent studies have reported that small interfering RNAs (siRNAs) function in stress responses. These siRNAs are generated from long double strand RNAs through various biological processes [29].
A specific type of siRNA that is involved in RNA-directed DNA methylation suppresses the activation of retrotransposons under heat stress [30]. The transcriptional gene silencing (TGS)-related heterochromatic siRNAs are generated from RNA Polymerase IV (PolIV)-derived transcripts in repetitive DNA sequences and heterochromatin. After heat stress, a Copia-type retrotransposon in Arabidopsis, named ONSEN, becomes transcriptionally active and has been shown to result in the synthesis of extrachromosomal DNA copies in the siRNA-mediated silencing deficient mutant nrpd1 (nrpd1a), which is the largest subcomponent of PolIV. Heat-induced expression and transgenerational retrotransposition of ONSEN were suppressed by siRNA-mediated silencing. It was also reported that abiotic stresses changed the genome-wide DNA methylation status across multiple generations [31].
Trans-acting siRNAs (ta-siRNA) can be classified as a specialized case of siRNAs in plants. These siRNAs are generated from dsRNAs, which are generated from miR173, miR390 and miR828 -cleaved lncRNAs [32,33]. The long antisense non-coding RNAs (lancRNAs) of these dsRNAs were synthesized by RNA-dependent RNA polymerase 6 (RDR6), those dsRNAs were positive candidates producing siRNAs [34]. The tight feedback regulation between miR390, TAS3 ta-siRNA and ARF4, which is a target of TAS3 ta-siRNA, was required for lateral root initiation [24]. Although ta-siRNA expression has not been found to change in response to abiotic stress in Arabidopsis, the expression of a rice RDR6 homolog, which produces dsRNAs from cleaved TAS RNAs, was induced by ABA in rice [35]. These results imply that TAS3 ta-siRNA is involved in dynamic changes of root architecture during exposure to abiotic stress [36].
Borsani et al. 2005 reported that natural antisense transcript small interfering RNA (nat-siRNAs) were generated from dsRNAs produced from natural cis-antisense gene pairs of Δ1-Pyrroline-5-Carboxylate Dehydrogenase (P5CDH) and a high-salinity-stress inducible gene of unknown function (SRO5) during high-salinity stress [37]. Recent genome-wide analysis reported an accumulation of sRNAs in their overlapping region, suggesting the occurrence of an RNA interference event [38]. However, the biological process of generating nat-siRNAs is not completely understood at this time [39,40].
3. Long Non-Coding RNAs (lncRNAs)
Genome-wide tiling arrays and high-throughput sequencing have identified a vast amount of lncRNAs in plants [4,41–45]. These lncRNAs may represent alternatively spliced forms of known genes [46], products of antisense RNAs [4,38,47,48], double stranded RNAs [49], retained introns [46,50], short open reading frame [34,51,52], RNA polymerase III-derived RNAs [53] and RNA decoys mimicking miRNA targets [28]. In this manuscript, we classified the lncRNAs into long antisense non-coding RNAs (lancRNAs) and long intergenic non-coding RNAs (lincRNAs) based on its genomic locations (Figure 1; Table 1). These RNAs have various modifications that depend on each biosynthetic process.
3.1. Long Antisense Non-Coding RNAs (lancRNAs)
Over the past decade, genome-wide transcriptome analyses confirmed that approximately 30% of all annotated genes exhibited significant lancRNA expression in Arabidopsis [4,41]. These data regarding lancRNA expression are consistent with results from other organisms such as fly, human, and rice [54]. Expression profiles of lancRNAs in response to environmental stresses have been extensively characterized by an Arabidopsis tiling array analysis [4]. A certain type of lancRNAs belongs to a pair of fully overlapping sense–antisense transcripts (fSATs) in which the lancRNAs exist within the protein-coding gene regions in opposing orientations. The expression of these sense and antisense RNA transcripts are stress-responsive. On the other hand, partially overlapping sense–antisense transcripts (pSATs) do not exhibit synchronous expression patterns. These observations suggest that lancRNA were generated through multiple biosynthetic processes.
A type of lancRNA is co-expressed with sense protein-coding RNAs [4]. A large Arabidopsis tiling array analysis confirmed that more than 6000 lancRNAs were classified into the fSATs category. Interestingly, a significant linear correlation between the expression ratios (abiotic stress treated/untreated) of the sense transcripts and the ratios of the lancRNAs was observed in the fSATs. The RD29A and CYP707A1 lancRNAs that were simultaneously accumulated with sense mRNAs, were accumulated by drought- and ABA treatments. Some of the lancRNAs that were identified contained complementary sequences to those of the sense mRNAs [4], indicating that lancRNA expression is dependent upon sense mRNAs.
Co-expression of sense RNA and lancRNA of a transgene was reported as a trigger for sense post-trancriptional gene silencing (S-PTGS) [55]. In the S-PTGS process, RNA-dependent RNA polymerase 6 (RDR6), one of six Arabidopsis RDRs, generates antisense RNAs from non-canonical sense RNAs of transgenes with aberrant features, such as non-cap structure of 5′ end or poly(A) tail, to generate double stranded RNA (dsRNA) [56]. dsRNA-seq analysis showed that dsRNAs of more than 100 loci were reduced in rdr6 in relative comparison to WT [57]. RNA-seq analysis of sRNAs also revealed that mutations of ABH1 and EIN5 (XRN4), which are involved in mRNA processing and mRNA degradation, respectively, affect the level of sRNAs mapped on the antisense strand of endogenous protein-coding genes [58]. The xrn4 mutant was also screened as an enhancer of transgene silencing [59]. These results supported the observation that a certain type of lancRNA was generated from non-canonical sense RNA. Recent studies revealed that Werner Exonuclease (WEX), Silencing Defective3 (SDE3), DCL2, DCL4, Nuclear RNA Polymerase IVa (NRPD1a), RDR2 and CLASSY1 were involved in S-PTGS and downstream PTGS [60–65]. Since S-PTGS-related siRNAs are generated from dsRNAs, these results also indicate that a certain type of lancRNA is synthesized from mRNA templates via a complex amplification pathway.
It is possible that the expression of lancRNA could serve as a functional link to the chromatin regulation of epigenetic silencing [48]. Cold Induced Long Antisense Intragenic RNA (COOLAIR) in the FLC locus is a well-characterized example of this in Arabidopsis [47,66]. Exposure of plants to low temperature treatment for a 2-week period resulted in a high level of COOLAIR expression. Several weeks after the induction of COOLAIR, the transcription of FLC was significantly decreased. During this period, tri-methylated histone H3 Lys27 (H3K27me3) levels progressively increased at a region around the transcription start site [67]. The level of H3K27me3 spreading the gene body was required to maintain the repression of FLC transcription after plants were returned to warm conditions [68]. COOLAIR has been suggested to be required for a plant homeodomain-polycomb repressive complex 2 (PHD-PRC2) located at a tightly localized nucleation region within FLC. Consequently, this results in an increase in H3K27me3 levels at the FLC locus [64]. Although further analysis is necessary to elucidate the role of COOLAIR and its epigenetic silencing of FLC during the short period of vernalization [69,70], a reporter gene that was fused with COOLAIR was shown and confirmed to be capable of causing cold-induced silencing [47].
The second class of lncRNA, COLDAIR, is transcribed from a region within the first intron of FLC on the sense strand [71]. The COLDAIR transcript has been shown to interact with PRC2 and its abundance also increased during vernalization. Reduction of COLDAIR transcript levels by RNAi confirmed that it is not required for the initial repression of FLC but is necessary for subsequent maintenance of repression. These results showed that the interaction of lncRNAs and the chromatin modification complex mediates cold-inducible epigenetic regulation. Based upon bioinformatic analyses comparing lancRNAs and chromatin status, it was recently hypothesized that a type of lancRNAs from the genome was repressed by cytosine methylation and H3K36me [48]. This prediction proposed that an interaction between chromatin regulation and a certain type of lancRNAs functions in genome regulation.
A recent strand-specific RNA-seq study showed that approximately 1300 ncRNA loci exist in the antisense strand of protein-coding genes in Arabidopsis [48]. The global ratio between sense and antisense tags in exons in this study was 0.01, which was similar to a previous strand-specific RNA-seq analysis using floral tissue [43,48]. A comparison between RNA-seq and genome-wide tiling array data showed that one-half to two-thirds of the sense–antisense transcripts were only represented in one experiment [48]. Since the expression levels of lancRNAs are low compared to sense transcripts, it is possible that this difference between the two techniques may reflect technical limitations inherent to these methods. Future transcriptome analyses are required to increase our understanding of non-canonical transcripts and to clarify the types of lancRNAs that function in molecular signals, RNA decoys, guides, and scaffolds [72].
3.2. Long Intergenic Non-Coding RNAs (lincRNAs)
Several transcriptome analyses have reported that more than 1000 lincRNA loci exist between protein-coding genes in Arabidopsis [4,41–45]. A part of these lincRNAs was transcribed from the methylated DNA regions by RNA polymerase IV and are thought to be positive candidates that generate into TGS-related heterochromatic siRNAs that guide DNA methylation [64].
Another type of lincRNA is transcribed by RNA Polymerase III. In silico genome sequence analysis predicted 20 novel ncRNA candidates [53]. A specific Pol III-derived ncRNA (AtR18) responded negatively to hypoxic stress and this regulation was evidently different from that of U6 snRNA. Specifically, AtR18 was not processed into a smaller fragment and no small open reading frames (sORFs) were included. Short interspersed elements (SINEs) and 7SL (signal recognition particle) RNA for protein trafficking are known as Pol III-derived RNA, with exception of the canonical functional RNA [73,74].
Many sORFs that have not been annotated as protein-coding genes have been identified as expressed genes during developmental or environmental conditions [51,52]. A specific sORF (npc536) has a large dynamic variation of expression across a wide range of tissue and hormonal, biotic, or abiotic treatment [34]. npc536 exists in the antisense strand of a Golgi-transport complex related protein. 35S::npc536 transformants displayed heightened root growth under salt stress conditions. However, the biological function of this RNA is not still clearly understood at this time.
LincRNA is capable of regulating the cleavage activity of miRNA as a target mRNA decoy. IPS1 is a lincRNA, which was found to be induced by phosphate starvation [28,75]. Although Arabidopsis IPS1, AT4-1, AT4-2 and family members in other species share little sequence conservation with the predicted ORFs, high conservation was observed with a 22-nt sequence located in the 3′ half of the transcript [75]. This observation suggests that the IPS1 family contains the sequence that is targeted by miRNA and it does not function as a short peptide. The 22-nt sequence of IPS1 is not perfectly complementary to miR399 due to a mismatch occurring at the 10–11th position [28]. Although miR399 was capable of hybridizing to IPS1 transcript, there has been no indication of cleavage via miR399. On a whole plant level, phosphate starvation resulted in the induction of IPS1. As a result of the phosphate starvation, the miR399 target (UBC24 mRNA) accumulated. miR399 has been characterized as a phosphate starvation inducible-miRNA and a positive regulator of Pi-uptake. Interestingly, two studies implied that miR399 may function as a long-distance signal from shoots to suppress UBC24 expression in roots [76,77]. Taken together, it is reasonable to consider that the complex interactions between IPS1, miR399 and UBC24 function to maintain spatial homeostasis of Pi. Computational analyses predicted the occurrence of these possible target mimics in rice and Arabidopsis, suggesting that RNA decoys (miRNA target mimic) are conserved and functional contributors to the regulation of RNA [78].
4. Conclusions and Perspectives
Whole genome transcriptome analyses of high-density microarrays and high throughput sequencing have expanded our understanding of a novel research area pertaining to networks, which govern abiotic stress responses. Despite the extensive body of published information regarding various types of ncRNAs, their association to the adaptation and response of plants to abiotic stress is not completely understood at this time. Recent advances in whole transcriptome analyses have enabled us to gain a greater understanding regarding the mechanisms of transcriptional and posttranscriptional regulation through ncRNAs, as well as sRNAs and lancRNAs. Functional analyses of miRNAs have deciphered and unraveled complex feedback loop regulations between miRNA and target genes. In addition, the characterized interaction between miR399, IPS1 and UBC24 has been shown to be a novel type of RNA interaction system in which a lincRNA reduced a cleavage activity of phosphate starvation-inducible miRNA and regulated a target mRNA. In addition, ncRNAs are also linked to chromatin regulation. The studies regarding epigenetics in the FLC locus enabled a hypothesis to be made which suggests that an interaction of lancRNA and chromatin modification complex mediates cold-inducible epigenetic regulation. Overall, these data have revealed a complex interaction between transcriptional regulators which function to fine-tune responses to various environmental stimuli. For the future, it will be essential to investigate the biological regulations of ncRNAs in order to enable scientists to completely elucidate the entire picture of gene regulation networks in stress response.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Innovative Areas 25119724, Challenging Exploratory Research 24657041), Japan Science and Technology Agency (JST), Core Research for Evolutionary Science and Technology (CREST) and grants from the RIKEN Research Institute, Japan, to Motoaki Seki.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Hirayama T., Shinozaki K. Research on plant abiotic stress responses in the postgenome era: Past, present and future. Plant J. 2010;61:1041–1052. doi: 10.1111/j.1365-313X.2010.04124.x. [DOI] [PubMed] [Google Scholar]
- 2.Laporte P., Merchan F., Amor B.B., Wirth S., Crespi M. Riboregulators in plant development. Biochem. Soc. Trans. 2007;35:1638–1642. doi: 10.1042/BST0351638. [DOI] [PubMed] [Google Scholar]
- 3.Rymarquis L.A., Kastenmayer J.P., Huttenhofer A.G., Green P.J. Diamonds in the rough: mRNA-like non-coding RNAs. Trends Plant Sci. 2008;13:329–334. doi: 10.1016/j.tplants.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Matsui A., Ishida J., Morosawa T., Mochizuki Y., Kaminuma E., Endo T.A., Okamoto M., Nambara E., Nakajima M., Kawashima M., et al. Arabidopsis transcriptome analysis under drought, cold, high-salinity and ABA treatment conditions using a tiling array. Plant Cell Physiol. 2008;49:1135–1149. doi: 10.1093/pcp/pcn101. [DOI] [PubMed] [Google Scholar]
- 5.Mazzucotelli E., Mastrangelo A.M., Crosatti C., Guerra D., Stanca A.M., Cattivelli L. Abiotic stress response in plants: When post-transcriptional and post-translational regulations control transcription. Plant Sci. 2008;174:420–431. [Google Scholar]
- 6.Khraiwesh B., Zhu J.K., Zhu J. Role of miRNAs and siRNAs in biotic and abiotic stress responses of plants. Biochim. Biophys. Acta. 2012;1819:137–148. doi: 10.1016/j.bbagrm.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Lima J.C., Loss-Morais G., Margis R. microRNAs play critical roles during plant development and in response to abiotic stresses. Genet. Mol. Biol. 2012;35:1069–1077. doi: 10.1590/s1415-47572012000600023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sunkar R., Zhu J.K. Novel and stress-regulated MicroRNAs and other small RNA fromArabidopsis. Plant Cell. 2004;16:2001–2019. doi: 10.1105/tpc.104.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136:669–687. doi: 10.1016/j.cell.2009.01.046. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi-Shinozaki K., Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mundy J., Yamaguchi-Shinozaki K., Chua N.H. Nuclear proteins bind conserved elements in the abscisic acid-responsive promoter of a rice rab gene. Proc. Natl. Acad. Sci. USA. 1990;87:1406–1410. doi: 10.1073/pnas.87.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shang Y., Yan L., Liu Z.Q., Zheng C., Chao M., Xin Q., Wu F.Q., Wang X.F., Du S.Y., Jiang T., et al. The Mg-chelatase H subunit of Arabidopsis antagonizes a group of transcription repressors to relieve ABA-responsive genes of inhibition. Plant Cell. 2010;22:1909–1935. doi: 10.1105/tpc.110.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou X., Wang G., Sutoh K., Zhu J.K., Zhang W. Identification of cold-inducible microRNAs in plants by transcriptome analysis. Biochim. Biophys. Acta. 2008;1779:780–788. doi: 10.1016/j.bbagrm.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Liu H.H., Tian X., Li Y.U., Wu C.A., Zheng C.C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA. 2008;14:836–843. doi: 10.1261/rna.895308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou L., Liu Y., Liu Z., Kong D., Duan M., Luo L. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010;61:4157–4168. doi: 10.1093/jxb/erq237. [DOI] [PubMed] [Google Scholar]
- 16.Fuji H., Chiou T.J., Lin S.I., Aung K., Zhu J.K. A miRNA involved in phosphate starvation response in Arabidopsis. Curr. Biol. 2005;15:2038–2043. doi: 10.1016/j.cub.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Sunkar R., Kapoor A., Zhu J.K. Posttranscriptional induction of two Cu/Zn superoxide dismutase genes in Arabidopsis is mediated by downregulation of miR398 and important for oxidative stress tolerance. Plant Cell. 2006;18:2051–2065. doi: 10.1105/tpc.106.041673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamasaki H., Abdel-Ghany S.E., Cohu C.M., Kobayashi Y., Shikanai T., Pilon M. Regulation of copper homeostasis by micro-RNA in Arabidopsis. J. Biol. Chem. 2007;282:16369–16378. doi: 10.1074/jbc.M700138200. [DOI] [PubMed] [Google Scholar]
- 19.Brodersen P., Sakvarelidze-Achard L., Bruun-Rasmussen M., Dunoyer P., Yamamoto Y.Y., Sieburth L., Voinnet O. Widespread translational inhibition by plant miRNAs and siRNAs. Science. 2008;320:1185–1190. doi: 10.1126/science.1159151. [DOI] [PubMed] [Google Scholar]
- 20.Li W., Cui X., Meng Z., Huang X., Xie Q., Wu H., Jin H., Zhang D., Liang W. Transcriptional regulation of Arabidopsis miR168a and ARGONAUTE1 homeostasis in abscisic acid and abiotic stress responses. Plant Physiol. 2013;158:1279–1292. doi: 10.1104/pp.111.188789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P.P., Montgomery T.A., Fahlgren N., Kasschau K.D., Nonogaki H., Carrington J.C. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 2007;52:133–146. doi: 10.1111/j.1365-313X.2007.03218.x. [DOI] [PubMed] [Google Scholar]
- 22.Blomster T., Salojarvi J., Sipari N., Brosche M., Ahlfors R., Keinanen M., Overmyer K., Kangasjarvi J. Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response inArabidopsis. Plant Physiol. 2011;157:1866–1883. doi: 10.1104/pp.111.181883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M.F., Tian Q., Reed J.W. Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development. 2006;133:4211–4218. doi: 10.1242/dev.02602. [DOI] [PubMed] [Google Scholar]
- 24.Marin E., Jouannet V., Herz A., Lokerse A.S., Weijers D., Vaucheret H., Nussaume L., Crespi M.D., Maizel A. miR390, Arabidopsis TAS3 tasiRNAs, and their AUXIN RESPONSE FACTOR targets define an autoregulatory network quantitatively regulating lateral root growth. Plant Cell. 2010;22:1104–1117. doi: 10.1105/tpc.109.072553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglesias M.J., Terrile M.C., Bartoli C.G., D’Ippolito S., Casalongue C.A. Auxin signaling participates in the adaptative response against oxidative stress and salinity by interacting with redox metabolism inArabidopsis. Plant Mol. Biol. 2010;74:215–222. doi: 10.1007/s11103-010-9667-7. [DOI] [PubMed] [Google Scholar]
- 26.Jones-Rhoades M.W., Bartel D.P. Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell. 2004;14:787–799. doi: 10.1016/j.molcel.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 27.Aung K., Lin S.I., Wu C.C., Huang Y.T., Su C.L., Chiou T.J. pho2, a phosphate overaccumulator, is caused by a nonsense mutation in a microRNA399 target gene. Plant Physiol. 2006;141:1000–1011. doi: 10.1104/pp.106.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franco-Zorrilla J.M., Valli A., Todesco M., Mateos I., Puga M.I., Rubio-Somoza I., Leyva A., Weigel D., Garcia J.A., Paz-Ares J. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat. Genet. 2007;39:1033–1037. doi: 10.1038/ng2079. [DOI] [PubMed] [Google Scholar]
- 29.Mallory A.C., Vaucheret H. Functions of microRNAs and related small RNAs in plants. Nat. Genet. 2006;38:S31–S36. doi: 10.1038/ng1791. [DOI] [PubMed] [Google Scholar]
- 30.Ito H., Gaubert H., Bucher E., Mirouze M., Vaillant I., Paszkowski J. An siRNA pathway prevents transgenerational retrotransposition in plants subjected to stress. Nature. 2011;472:115–119. doi: 10.1038/nature09861. [DOI] [PubMed] [Google Scholar]
- 31.Boyko A., Blevins T., Yao Y., Golubov A., Bilichak A., Ilnytskyy Y., Hollunder J., Meins F., Jr., Kovalchuk I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS One. 2010;5:e9514. doi: 10.1371/journal.pone.0009514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronemus M., Vaughn M.W., Martienssen R.A. MicroRNA-targeted and small interfering RNA-mediated mRNA degradation is regulated by argonaute, dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell. 2006;18:1559–1574. doi: 10.1105/tpc.106.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allen E., Xie Z., Gustafson A.M., Carrington J.C. MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121:207–221. doi: 10.1016/j.cell.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Ben Amor B., Wirth S., Merchan F., Laporte P., d’Aubenton-Carafa Y., Hirsch J., Maizel A., Mallory A., Lucas A., Deragon J.M., et al. Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 2009;19:57–69. doi: 10.1101/gr.080275.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang J.H., Seo H.H., Han S.J., Yoon E.K., Yang M.S., Lee W.S. Phytohormone abscisic acid control RNA-dependent RNA polymerase 6 gene expression and post-transcriptional gene silencing in rice cells. Nucleic Acids Res. 2008;36:1220–1226. doi: 10.1093/nar/gkm1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartels D., Sunkar R. Drought and salt tolerance in plants. Crit. Rev. Plant. Sci. 2005;24:23–58. [Google Scholar]
- 37.Borsani O., Zhu J., Verslues P.E., Sunkar R., Zhu J.K. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in Arabidopsis. Cell. 2005;123:1279–1291. doi: 10.1016/j.cell.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X., Lii Y., Wu Z., Polishko A., Zhang H., Chinnusamy V., Lonardi S., Zhu J.K., Liu R., Jin H. Mechanisms of small RNA generation from cis-NATs in response to environmental and developmental cues. Mol. Plant. 2013;6:704–715. doi: 10.1093/mp/sst051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henz S.R., Cumbie J.S., Kasschau K.D., Lohmann J.U., Carrington J.C., Weigel D., Schmid M. Distinct expression patterns of natural antisense transcripts in Arabidopsis. Plant Physiol. 2007;144:1247–1255. doi: 10.1104/pp.107.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhan S., Lukens L. Protein-coding cis-natural antisense transcripts have high and broad expression in Arabidopsis thaliana. Plant Physiol. 2013;161:2171–2180. doi: 10.1104/pp.112.212100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamada K., Lim J., Dale J.M., Chen H., Shinn P., Palm C.J., Southwick A.M., Wu H.C., Kim C., Nguyen M., et al. Empirical analysis of transcriptional activity in the Arabidopsis genome. Science. 2003;302:842–846. doi: 10.1126/science.1088305. [DOI] [PubMed] [Google Scholar]
- 42.Li L., Wang X.F., Stolc V., Li X.Y., Zhang D.F., Su N., Tongprasit W., Li S.G., Cheng Z.K., Wang J., et al. Genome-wide transcription analyses in rice using tiling microarrays. Nat. Genet. 2006;38:124–129. doi: 10.1038/ng1704. [DOI] [PubMed] [Google Scholar]
- 43.Lister R., O’Malley R.C., Tonti-Filippini J., Gregory B.D., Berry C.C., Millar A.H., Ecker J.R. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell. 2008;133:523–536. doi: 10.1016/j.cell.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laubinger S., Zeller G., Henz S.R., Sachsenberg T., Widmer C.K., Naouar N., Vuylsteke M., Scholkopf B., Ratsch G., Weigel D. At-TAX: A whole genome tiling array resource for developmental expression analysis and transcript identification in Arabidopsis thaliana. Genome Biol. 2008;9:R112. doi: 10.1186/gb-2008-9-7-r112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazen S.P., Naef F., Quisel T., Gendron J.M., Chen H., Ecker J.R., Borevitz J.O., Kay S.A. Exploring the transcriptional landscape of plant circadian rhythms using genome tiling arrays. Genome Biol. 2009;10:R17. doi: 10.1186/gb-2009-10-2-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Filichkin S.A., Priest H.D., Givan S.A., Shen R., Bryant D.W., Fox S.E., Wong W.K., Mockler T.C. Genome-wide mapping of alternative splicing in Arabidopsis thaliana. Genome Res. 2010;20:45–58. doi: 10.1101/gr.093302.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swiezewski S., Liu F., Magusin A., Dean C. Cold-induced silencing by long antisense transcripts of an Arabidopsis Polycomb target. Nature. 2009;462:799–802. doi: 10.1038/nature08618. [DOI] [PubMed] [Google Scholar]
- 48.Luo C., Sidote D.J., Zhang Y., Kerstetter R.A., Michael T.P., Lam E. Integrative analysis of chromatin states in Arabidopsis identified potential regulatory mechanisms for natural antisense transcript production. Plant J. 2013;73:77–90. doi: 10.1111/tpj.12017. [DOI] [PubMed] [Google Scholar]
- 49.Rajeswaran R., Aregger M., Zvereva A.S., Borah B.K., Gubaeva E.G., Pooggin M.M. Sequencing of RDR6-dependent double-stranded RNAs reveals novel features of plant siRNA biogenesis. Nucleic Acids Res. 2012;40:6241–6254. doi: 10.1093/nar/gks242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ner-Gaon H., Halachmi R., Savaldi-Goldstein S., Rubin E., Ophir R., Fluhr R. Intron retention is a major phenomenon in alternative splicing in Arabidopsis. Plant J. 2004;39:877–885. doi: 10.1111/j.1365-313X.2004.02172.x. [DOI] [PubMed] [Google Scholar]
- 51.Hanada K., Higuchi-Takeuchi M., Okamoto M., Yoshizumi T., Shimizu M., Nakaminami K., Nishi R., Ohashi C., Iida K., Tanaka M., et al. Small open reading frames associated with morphogenesis are hidden in plant genomes. Proc. Natl. Acad. Sci. USA. 2013;110:2395–2400. doi: 10.1073/pnas.1213958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moghe G.D., Lehti-Shiu M.D., Seddon A.E., Yin S., Chen Y., Juntawong P., Brandizzi F., Bailey-Serres J., Shiu S.H. Characteristics and significance of intergenic polyadenylated RNA transcription in Arabidopsis. Plant Physiol. 2013;161:1210–1224. doi: 10.1104/pp.112.205245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J., Okada T., Fukushima T., Tsudzuki T., Sugiura M., Yukawa Y. A novel hypoxic stress-responsive long non-coding RNA transcribed by RNA polymerase III in Arabidopsis. RNA Biol. 2012;9:302–313. doi: 10.4161/rna.19101. [DOI] [PubMed] [Google Scholar]
- 54.Kapranov P., Willingham A.T., Gingeras T.R. Genome-wide transcription and the implications for genomic organization. Nat. Rev. Genet. 2007;8:413–423. doi: 10.1038/nrg2083. [DOI] [PubMed] [Google Scholar]
- 55.Beclin C., Boutet S., Waterhouse P., Vaucheret H. A branched pathway for transgene-induced RNA silencing in plants. Curr. Biol. 2002;12:684–688. doi: 10.1016/s0960-9822(02)00792-3. [DOI] [PubMed] [Google Scholar]
- 56.Yoshikawa M., Peragine A., Park M.Y., Poethig R.S. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 2005;19:2164–2175. doi: 10.1101/gad.1352605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng Q., Ryvkin P., Li F., Dragomir I., Valladares O., Yang J., Cao K., Wang L.S., Gregory B.D. Genome-wide double-stranded RNA sequencing reveals the functional significance of base-paired RNAs in Arabidopsis. PLoS Genet. 2010;6:e1001141. doi: 10.1371/journal.pgen.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gregory B.D., O’Malley R.C., Lister R., Urich M.A., Tonti-Filippini J., Chen H., Millar A.H., Ecker J.R. A link between RNA metabolism and silencing affecting Arabidopsis development. Dev. Cell. 2008;14:854–866. doi: 10.1016/j.devcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 59.Gazzani S., Lawrenson T., Woodward C., Headon D., Sablowski R. A link between mRNA turnover and RNA interference in Arabidopsis. Science. 2004;306:1046–1048. doi: 10.1126/science.1101092. [DOI] [PubMed] [Google Scholar]
- 60.Dunoyer P., Himber C., Voinnet O. DICER-LIKE 4 is required for RNA interference and produces the 21-nucleotide small interfering RNA component of the plant cell-to-cell silencing signal. Nat. Genet. 2005;37:1356–1360. doi: 10.1038/ng1675. [DOI] [PubMed] [Google Scholar]
- 61.Xie Z., Allen E., Fahlgren N., Calamar A., Givan S.A., Carrington J.C. Expression of Arabidopsis miRNA genes. Plant Physiol. 2005;138:2145–2154. doi: 10.1104/pp.105.062943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jauvion V., Rivard M., Bouteiller N., Elmayan T., Vaucheret H. RDR2 partially antagonizes the production of RDR6-dependent siRNA in sense transgene-mediated PTGS. PLoS One. 2012;7:e29785. doi: 10.1371/journal.pone.0029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Glazov E., Phillips K., Budziszewski G.J., Schob H., Meins F., Jr., Levin J.Z. A gene encoding an RNase D exonuclease-like protein is required for post-transcriptional silencing in Arabidopsis. Plant J. 2003;35:342–349. doi: 10.1046/j.1365-313x.2003.01810.x. [DOI] [PubMed] [Google Scholar]
- 64.Herr A.J., Jensen M.B., Dalmay T., Baulcombe D.C. RNA polymerase IV directs silencing of endogenous DNA. Science. 2005;308:118–120. doi: 10.1126/science.1106910. [DOI] [PubMed] [Google Scholar]
- 65.Dalmay T., Horsefield R., Braunstein T.H., Baulcombe D.C. SDE3 encodes an RNA helicase required for post-transcriptional gene silencing in Arabidopsis. EMBO J. 2001;20:2069–2078. doi: 10.1093/emboj/20.8.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu F., Marquardt S., Lister C., Swiezewski S., Dean C. Targeted 3′ processing of antisense transcripts triggers Arabidopsis FLC chromatin silencing. Science. 2010;327:94–97. doi: 10.1126/science.1180278. [DOI] [PubMed] [Google Scholar]
- 67.Angel A., Song J., Dean C., Howard M. A Polycomb-based switch underlying quantitative epigenetic memory. Nature. 2011;476:105–108. doi: 10.1038/nature10241. [DOI] [PubMed] [Google Scholar]
- 68.De Lucia F., Crevillen P., Jones A.M., Greb T., Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc. Natl. Acad. Sci. USA. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Helliwell C.A., Robertson M., Finnegan E.J., Buzas D.M., Dennis E.S. Vernalization-repression of Arabidopsis FLC requires promoter sequences but not antisense transcripts. PLoS One. 2011;6:e2151. doi: 10.1371/journal.pone.0021513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun Q., Csorba T., Skourti-Stathaki K., Proudfoot N.J., Dean C. R-loop stabilization represses antisense transcription at the Arabidopsis FLC locus. Science. 2013;340:619–621. doi: 10.1126/science.1234848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heo J.B., Sung S. Vernalization-mediated epigenetic silencing by a long intronic noncoding RNA. Science. 2011;331:76–79. doi: 10.1126/science.1197349. [DOI] [PubMed] [Google Scholar]
- 72.Wang K.C., Chang H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kramerov D.A., Vassetzky N.S. Short retroposons in eukaryotic genomes. Int. Rev. Cytol. 2005;247:165–221. doi: 10.1016/S0074-7696(05)47004-7. [DOI] [PubMed] [Google Scholar]
- 74.Yukawa Y., Felis M., Englert M., Stojanov M., Matousek J., Beier H., Sugiura M. Plant 7SL RNA genes belong to type 4 of RNA polymerase III- dependent genes that are composed of mixed promoters. Plant J. 2005;43:97–106. doi: 10.1111/j.1365-313X.2005.02430.x. [DOI] [PubMed] [Google Scholar]
- 75.Shin H., Shin H.S., Chen R., Harrison M.J. Loss of At4 function impacts phosphate distribution between the roots and the shoots during phosphate starvation. Plant J. 2006;45:712–726. doi: 10.1111/j.1365-313X.2005.02629.x. [DOI] [PubMed] [Google Scholar]
- 76.Lin S.I., Chiang S.F., Lin W.Y., Chen J.W., Tseng C.Y., Wu P.C., Chiou T.J. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 2008;147:732–746. doi: 10.1104/pp.108.116269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pant B.D., Buhtz A., Kehr J., Scheible W.R. MicroRNA399 is a long-distance signal for the regulation of plant phosphate homeostasis. Plant J. 2008;53:731–738. doi: 10.1111/j.1365-313X.2007.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu H.J., Wang Z.M., Wang M., Wang X.J. Wide-spread long non-coding RNAs (lncRNAs) as endogenous target mimics (eTMs) for microRNAs in plants. Plant Physiol. 2013;161:1875–1884. doi: 10.1104/pp.113.215962. [DOI] [PMC free article] [PubMed] [Google Scholar]